Abstract
The successful replication of mammalian DNA viruses requires that they gain control of key cellular signalling pathways that affect broad aspects of cellular macromolecular synthesis, metabolism, growth and survival. The phosphatidylinositol 3′-kinase–Akt–mammalian target of rapamycin (PI3K–Akt–mTOR) pathway is one such pathway. Mammalian DNA viruses have evolved various mechanisms to activate this pathway to obtain the benefits of Akt activation, including the maintenance of translation through the activation of mTOR. In addition, viruses must overcome the inhibition of this pathway that results from the activation of cellular stress responses during viral infection. This Review will discuss the range of mechanisms that mammalian DNA viruses use to activate this pathway, as well as the multiple mechanisms these viruses have evolved to circumvent inhibitory stress signalling.
The successful replication of mammalian DNA viruses, such as polyomaviruses (also called papovaviruses), adenoviruses, herpesviruses and poxviruses, requires viral adaptation of the host cell to establish an environment that can accommodate the increased demands for nutrients, energy and macromolecular synthesis that accompany viral infection. All DNA viruses must gain control of key cellular signalling pathways that affect broad aspects of cellular macromolecular synthesis, metabolism, growth and survival. The phosphatidylinositol 3′-kinase–Akt–mammalian target of rapamycin (PI3K–Akt–mTOR) pathway is one such pathway. Virtually all mammalian viruses, both DNA and RNA, must regulate this pathway, either by activating or inactivating some aspect of it1. In general, mammalian DNA viruses activate this pathway at some point in their life cycle to benefit from the growth, metabolic, anti-apoptotic and translational functions that the pathway controls. However, a viral mechanism for activating this pathway is not enough; to maintain control, viruses must also overcome the many controls that are used to inhibit this pathway when cellular stress responses are activated during viral infection.
The substantial alterations in cellular physiology that are induced by a viral lytic infection — as a result of nutrient depletion, energy depletion, hypoxia and endoplasmic reticulum stress — activate cellular stress responses that alert the cell to problems with metabolic and synthetic processes. The resulting stress signalling activates mechanisms that either alleviate the problems or, if this is not possible, induce apoptosis. Stress signalling has many effects that can be either beneficial or detrimental to viral growth. One example of a detrimental effect is inhibition of translation, which is among the most common consequences of cellular stress responses2–6. Although the inhibition of this energy-intensive process permits the cell to recover from stress, it would not benefit the DNA-virus replication cycle, for which the maintenance of protein synthesis is crucial. The PI3K–Akt–mTOR pathway plays a key part in the maintenance of translation through the activation of mTOR kinase. As a result, this pathway is the target of many cellular stress responses that inactivate mTOR activity. Thus, mammalian DNA viruses must be able not only to activate the PI3K–Akt–mTOR pathway but also to counteract the inhibition of this pathway that results from the induction of stress signalling. In this Review, we discuss the various ways that mammalian DNA viruses activate and maintain control of the PI3K–Akt–mTOR signalling pathway as it relates to translation control. It should be noted, however, that the activation and maintenance of this pathway also causes many other physiological changes in the cell that can benefit a productive viral infection and contribute to viral pathogenesis.
PI3K–Akt–mTOR signalling
Akt (also called PKB) is the cellular homologue of the oncoprotein that is encoded by the AKT8 retrovirus7. Akt activation can occur in response to tropic factors, such as insulin, by several mechanisms8–10 (FIG. 1), the best understood of which involves PI3K. Akt activation depends on the phosphorylation of phosphatidylinositol (PI)-4,5-bisphosphate (PtdIns(4,5)P2), which creates PI-3,4,5-triphosphate (PtdIns(3,4,5)P3) at the plasma membrane (FIG. 1). The reversible conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 is mediated by PI3K (which converts PtdIns(4,5)P2 to PtdIns(3,4,5)P3) and phosphatase and tensin homologue (PTEN; a phosphatase that counteracts the kinase action of PI3K and converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2)11. Insulin binding to the insulin receptor leads to the phosphorylation of insulin receptor substrates (IRS) that can bind, and thus activate, PI3K. Activation of PI3K shifts the PtdIns(4,5)P2:PtdIns(3,4,5)P3 equilibrium towards PtdIns(3,4,5)P3, which binds both Akt and phosphoinositide-dependent protein kinase 1 (PDK1) and recruits them to the plasma membrane. This shift also positions PDK1 such that it can phosphorylate, and thus activate, Akt on threonine 308 (T308). Activated Akt affects multiple cellular targets that increase metabolism, growth, synthetic processes and proliferation and suppress apoptosis8,10,12–15. All of these processes are beneficial to viral lytic replication. Thus, it is not surprising that most RNA and DNA viruses have developed means to activate Akt during lytic infection1. Viruses can accomplish this by activating PI3K, repressing PTEN or both.
Figure 1. Receptor-mediated activation of phosphatidylinositol 3′-kinase (PI3K) and activation of Akt.
The example shown is insulin binding to the insulin receptor, which leads to the phosphorylation of insulin receptor substrates (IRS) that can bind, and thus activate, PI3K. In turn, activated PI3K phosphorylates phosphatidylinositol (PI)-4,5-bisphosphate (PtdIns(4,5)P2), thereby creating PI-3,4,5-triphosphate (PtdIns(3,4,5)P3) at the plasma membrane. Both Akt and phosphoinositide-dependent protein kinase 1 (PDK1) can be recruited to the membrane by binding PtdIns(3,4,5)P3. This positions PDK1 and Akt such that PDK1 can phosphorylate, and thus activate, Akt on threonine 308 (T308). PTEN, phosphatase and tensin homologue.
One of the downstream effects of activated Akt is the activation of mTOR kinase (also known as RAFT1 or FRAP) in mTOR complex 1 (mTORC1) (FIG. 2). As described below, maintaining the activity of mTORC1 is crucial for the maintenance of cap-dependent translation, in which translation initiation depends on recognition of the mRNA 5′ cap by eukaryotic initiation factor (eIF) 4F (BOX 1). Those viruses that must maintain cap-dependent translation during infection have evolved ways to either maintain mTOR kinase activity, control the activity of eIF4F or both.
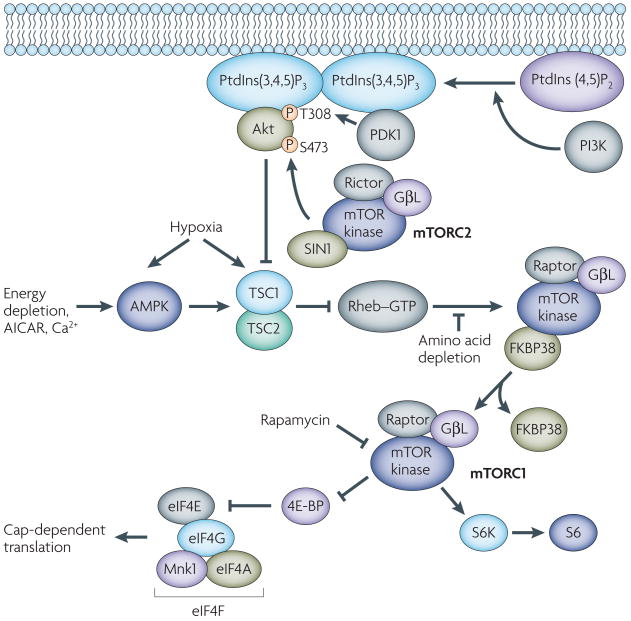
Figure 2. PI3K–Akt–mTOR signalling.
The phosphatidylinositol 3′-kinase–Akt–mammalian target of rapamycin (PI3K–Akt–mTOR) pathway is shown. The points in the pathway that transmit the inhibitory effects of cellular stress signalling (hypoxia, energy deprivation, calcium homeostasis and amino acid deprivation) and drugs (5-amino-4-imidazolecarboxamide ribose (AICAR) and rapamycin) are indicated. The extension of the pathway downstream of mTORC1 shows the effects of mTORC1 on the eukaryotic initiation factor (eIF) 4F cap-binding complex, which initiates cap-dependent translation. 4E-BP, eIF4E binding protein; AMPK, AMP-activated kinase; Mnk1, mitogen-activated protein kinase-interacting kinase 1; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; PDK1, phosphoinositide-dependent protein kinase 1; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-triphosphate; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; raptor, regulatory associated protein of TOR; rictor, rapamycin-insensitive companion of mTOR; S6, ribosomal protein S6; S6K, p70S6 kinase; S473, serine 473; T308, threonine 308; TSC, tuberous sclerosis complex.
Box 1. Viral translation strategies: caps and internal ribosome entry sites.
One of the unique aspects of viruses is their lack of constraint in using different forms of nucleic acid for their genomes. They are not limited to double-stranded DNA; they can also use single-stranded DNA (for example, parvoviruses), negative or positive single-stranded RNA (for example, orthomyxoviruses and picornaviruses, respectively) or double-stranded RNA (for example, reoviruses). Viruses that use RNA as a genome have evolved novel mechanisms for replication and transcription (for example, RNA-dependent RNA polymerases) as well as translation. In addition, many RNA viruses replicate in the cytoplasm, where they cannot use cellular RNA-processing systems that are primarily located in the nucleus. Even if these viruses entered the nucleus, RNA-dependent transcription might not be compatible with cellular mRNA processing, which is highly dependent on cellular DNA-dependent RNA polymerase type II. Thus, it is not surprising that the mRNA structures and translation strategies that are used by RNA viruses vary considerably from the general model for translation of cellular and DNA virus mRNAs, which contain 7-methylGpppG caps on the 5′ end, are spliced and contain polyA tails on the 3′ end. This mRNA structure is the basis of cap-dependent translation, in which the 5′ cap is recognized by eukaryotic initiation factor 4F (eIF4F), which identifies the mRNA for translation. Subsequently, interactions between eIF4F and polyA-binding proteins that are bound to the polyA tail further identify the mRNA and set up the structure that is needed for the 40S ribosomal subunit to attach to the 5′ end of the RNA and begin scanning for an AUG initiation codon in the appropriate context for translation initiation. Although many RNA and DNA viruses use this translation-initiation mechanism, they have also evolved means to bypass it; this is especially true for the RNA viruses. Many translatable viral mRNAs are not capped and use internal ribosome entry sites (IRESs), which are most often located in the 5′-untranslated region of viral mRNAs that are close to the AUG to be used. There are no definitive structural or sequential definitions of IRESs, although many seem to have a high degree of secondary, and possibly tertiary, RNA structure. Viral IRESs can directly bind 40S ribosomal subunits and bypass the use of many initiation factors, including eIF4F. Other viral IRESs seem to use alternative mechanisms that might not require a direct interaction with the 40S subunit, but instead require additional proteins, called IRES trans-acting factors, to attract ribosomes. Viruses that use IRESs have also evolved ways to diminish cap-dependent translation such that there will be little competition from host mRNAs for ribosomes. Often, these viral mechanisms function through the destruction of the eIF4F complex, but viruses also have other tricks. For example, if ribosomes scan a viral mRNA for an AUG, a shunting mechanism can be used in which major parts of a complex mRNA leader sequence can be bypassed and are not melted by the scanning ribosomes.
Although these specialized mechanisms were discovered and have been most extensively studied in viral systems, it must be remembered that viruses rarely create entirely unique biochemical mechanisms. Instead, they mimic or capitalize on existing cellular mechanisms. Thus, IRESs have been found in cellular genes, and there is growing evidence to suggest that their use is widespread and important, especially during times of cellular stress when cap-dependent translation might be inhibited. An additional benefit of the use of IRESs by viruses is that it might provide an additional means of avoiding the inhibitory effects of cellular stress responses. For excellent reviews on the subjects discussed above see REFS 6,118–120.
The signalling between Akt and mTORC1 involves the tuberous sclerosis complex (TSC), which comprises TSC1 and TSC2 (also known as hamartin and tuberin, respectively)16,17 and Rheb–GTP, a member of the Ras family18–21. Rheb–GTP function is mediated by the TSC, which stimulates the intrinsic GTPase activity of Rheb, thus converting it from Rheb–GTP to Rheb–GDP, which cannot activate mTOR kinase. The TSC is inactivated by Akt phosphorylation, which allows Rheb–GTP levels to remain high. Rheb–GTP displaces the mTORC1 inhibitor FKBP38, an FK506-binding-protein family member22. Hence, viruses that activate Akt will initiate mechanisms to maintain high levels of Rheb–GTP to displace FKBP38 and therefore activate mTORC1 and maintain cap-dependent translation.
mTOR kinase complexes and their activities
Two functionally distinct complexes that contain mTOR kinase are present in mammalian cells. These complexes differ in their major binding partner: raptor (regulatory-associated protein of TOR) in mTORC1 and rictor (rapamycin-insensitive companion of mTOR) in mTORC2 (REFS 23,24) (FIG. 2). In addition, the small Gβ-like protein GβL (also called mlST8) is part of each complex25 and SIN1 is found specifically in mTORC2 (REFS 26–28). Under normal conditions, the two complexes differ in their sensitivity to the drug rapamycin: mTORC1 is sensitive and mTORC2 is insensitive24.
The role of mTORC1 in the control of cap-dependent translation has been extensively studied29–31. When mTORC1 is active, it phosphorylates (activates) p70S6 kinase (S6K) and the eIF4e binding protein (4E-BP) (FIG. 2). S6K activation promotes the formation of translation initiation complexes31, which includes the phosphorylation of ribosomal protein S6. The phosphorylation of 4E-BP is a major point of control in cap-dependent translation and regulates the function of the eIF4F translation-initiation complex, which binds to the 5′ cap of an mRNA, the first step in the initiation of cap-dependent translation (FIGS 2,3). The eIF4F complex consists of eIF4E, the subunit that binds to the 5′ cap and thus brings the complex to the mRNA; eIF4G, the scaffolding protein to which the other components bind; Mnk1, an eIF4E kinase; and eIF4A, an RNA helicase. As eIF4E is the subunit that binds the 5′ cap, the functionality of the eIF4F complex relies on the association between eIF4E and eIF4G (FIG. 3). However, the binding of 4E-BP to eIF4E displaces eIF4E from eIF4G and the remainder of the complex, thus inhibiting cap-dependent translation (FIG. 3). mTORC1 regulates the binding of 4E-BP to eIF4E by controlling 4E-BP phosphorylation. Under positive growth conditions, mTORC1 is active and 4E-BP is phosphorylated and therefore unable to bind eIF4E. Consequently, eIF4E is free to bind eIF4G, thereby completing the eIF4F complex on the 5′ cap and allowing translation to proceed31. Under negative growth conditions, for example during stress or the inhibition of mTORC1 by rapamycin, mTORC1 is inactive; 4E-BP therefore becomes hypophosphorylated and binds efficiently to eIF4E, thereby removing it from the eIF4F complex and inhibiting cap-dependent translation.
Figure 3. Mechanisms by which mTORC1 activity controls cap-dependent translation.
The eukaryotic initiation factor (eIF) 4F complex consists of: eIF4E, the subunit that binds to the 5′ cap and thus brings the complex to the mRNA; eIF4G, the scaffolding protein to which the other components bind; mitogen-activated protein kinase-interacting kinase 1 (Mnk1), an eIF4E kinase; and eIF4A, an RNA helicase. The functionality of the eIF4F complex relies on the association between eIF4E and eIF4G. However, the binding of 4E-BP to eIF4E displaces eIF4E from eIF4G and the remainder of the complex, and thus inhibits cap-dependent translation. mTOR complex 1 (mTORC1) controls whether or not 4E-BP binds eIF4E by controlling 4E-BP phosphorylation. When mTORC1 is active, under positive growth conditions (a), it phosphorylates 4E-BP, which is therefore unable to bind to eIF4E. Thus, eIF4E is free to bind to eIF4G, which completes the eIF4F complex on the 5′ cap and permits translation to proceed. Under negative growth conditions, for example, during stress or the inhibition of mTORC1 by rapamycin (b), mTORC1 is inactive; 4E-BP therefore becomes hypophosphorylated and binds efficiently to eIF4E, thereby removing it from the eIF4F complex and inhibiting cap-dependent translation.
Viral control of mTORC1 activity and the phosphorylation of 4E-BP depends on the specific needs of the virus. Viruses that must maintain cap-dependent translation, for example the mammalian DNA viruses and many of the RNA viruses, would be expected to induce mechanisms to either keep mTORC1 active or ensure that the eIF4F complex is maintained in some other way. By contrast, viruses that use internal ribosome entry sites (IRESs), for example many of the RNA viruses, would be expected to diminish cap-dependent translation to direct ribosomes to IRESs. These viruses can inhibit mTORC1 or inactivate the eIF4F complex in some other way (BOX 1).
The functions of mTORC2 are less well characterized. The data suggest that mTORC2 has a role in the organization of the actin cytoskeleton24,32; however, its only known substrate is serine 473 (S473) of Akt9 (FIG. 2). The role of S473 phosphorylation in Akt activity is controversial, although it has been proposed to be important for the recognition and phosphorylation of Akt by PDK1 (REF. 9) (FIG. 2). As the phosphorylation of Akt on S473 seems to correlate with the full activation of Akt9, it would be expected that viruses which activate Akt must be able to activate mTORC2. The control of mTORC2 activity is not well understood. However, one study suggests that Rheb–GTP, the activator of mTORC1, does not activate mTORC2, but instead might inhibit it33. The conclusion that Rheb–GTP does not activate mTORC2 is supported by data which suggest that FKBP38 does not bind mTORC2 (REF. 22).
Inhibition by cellular stress responses
The above discussion provides a basic understanding of why a successful DNA-virus infection will involve the activation of Akt, which leads to the activation of mTORC1. However, Akt activation alone is not enough, given that the activity of mTORC1 can be inhibited by several cellular stress-induced signalling pathways that exert their effects downstream of Akt (FIG. 2). For example, hypoxia can occur during the highest rates of metabolic and synthetic activity during viral infection, which normally exceed the rates in uninfected cells. Hypoxia inhibits mTORC1 (REFS 34–36) by activating the TSC through the induction of REDD1 (REFS 34,37,38). The redd1 gene is a transcriptional target for hypoxia-inducing factor 1 (HIF1)39.
The TSC can also be activated by energy depletion through the activation of AMP-activated kinase (AMPK)40–43 (FIG. 2). ATP depletion and the consequent increases in AMP levels can occur during the highest periods of metabolic and synthetic activity during viral infection. AMPK responds both to changes in AMP concentration and to changes in the ratio of AMP to ATP. AMPK can also be activated by the AMP mimetic 5-amino-4-imidazolecarboxamide ribose (AICAR). An increased AMP to ATP ratio or the presence of AICAR allows AMPK phosphorylation and activation. This, in turn, activates the TSC, which catalyses the conversion of Rheb–GTP to Rheb–GDP and thus inhibits mTORC1 and cap-dependent translation (FIG. 2). AMPK can also be phosphorylated and activated by the calcium- and calmodulin-dependent protein kinase kinase-β (CaMKK-β) in response to alterations in intracellular calcium homeostasis44. Such changes can be triggered during viral infection, as calcium signalling and alterations in calcium homeostasis seem to be key cellular targets for many viruses45. As the TSC is involved in several stress signalling pathways, the existence of viral mechanisms that control the activation of this complex is predictable.
The crucial interactions between Rheb–GTP, FKBP38 and mTOR kinase that control the activation of mTORC1 are also a target for inhibition by stress signalling. Depletion of amino acid pools triggers a stress response that seems to inhibit the ability of Rheb–GTP to displace FKBP38 from mTORC1 (REFS 22,46). Amino acid depletion can occur during the later stages of lytic viral infections owing to the demands for amino acids that are associated with the high rates of viral protein synthesis. Another way to directly inhibit mTORC1 is to use the drug rapamycin47. However, prolonged treatment with rapamycin or its derivatives can inhibit mTORC2 (REFS 48,49).
These examples of mTORC1 inhibition by stress responses and drugs highlight the need for viruses to counteract inhibitory mechanisms at multiple points in the PI3K–Akt–mTOR pathway. In the following sections, we will examine the primary means by which mammalian DNA viruses activate this pathway and our knowledge of the molecular mechanisms that viruses use to maintain the activities that are required during times of stress. The emerging picture reveals that mammalian DNA viruses have evolved various mechanisms to activate PI3K and Akt to obtain the benefits of Akt activation, including the activation of mTORC1. In addition, mammalian DNA viruses possess multiple mechanisms to circumvent the inhibition of this pathway to relieve the effects of stress responses and maintain mTORC1, and possibly mTORC2, activity and cap-dependent translation. Some viruses have further developed ways to directly affect 4E-BP and components of the eIF4F complex, such that cap-dependent translation can be maintained even if mTORC1 activity is compromised.
PI3K–Akt–mTOR activation
BOX 2 provides a general account of the replicative strategies of DNA viruses.
Box 2. A general account of DNA-virus replicative strategies.
The mammalian DNA viruses include the parvoviruses, polyomaviruses, adenoviruses, herpesviruses and poxviruses, in ascending order of size and genetic complexity. In general, the DNA viruses share a similar replicative strategy. They must attach to and enter host cells and, with the exception of the poxviruses, deliver their genomes to the nucleus for gene expression and replication. DNA-virus gene expression is temporal. The initial viral genes that are expressed before DNA replication encode proteins that affect cellular processes to prepare the cell to execute viral DNA and protein synthesis, survive the stress of the infection and control the temporal order of subsequent viral gene expression. These are called early genes and proteins or, in the case of the herpesviruses, immediate-early genes and proteins. These proteins are often those that alter stress signalling and affect the phosphatidylinositol 3′-kinase–Akt–mammalian target of rapamycin (PI3K–Akt–mTOR) pathway and/or the integrity of the eukaryotic initiation factor 4F complex. Early or immediate-early proteins also activate the transcription of subsequent viral genes at the appropriate time; for the polyomaviruses (also known as papovaviruses) and adenoviruses these are late genes which, in general, encode virion structural proteins that encapsidate the newly synthesized viral DNA. In herpesviruses, immediate-early proteins participate in the transcriptional activation of early genes, which encode products that are primarily involved in viral DNA replication and, subsequently, late genes that encode virion structural proteins that package the viral DNA.
The unique features of the parvoviruses, the smallest DNA viruses, and the poxviruses, among the largest DNA viruses, must be noted. The parvovirus genome consists of linear single-stranded DNA. This genome structure, together with the lack of genetic complexity of the parvovirus genome, makes them the most dependent of all DNA viruses on cellular functions. For example, for parvovirus to replicate, the infected cell must either be able to enter S phase on its own or be induced into S phase by co-infection with an adenovirus or herpesvirus. The parvoviruses will not be discussed here, as the effect of parvoviruses on the PI3K–Akt–mTOR pathway has not yet been determined. In striking contrast to the parvoviruses, the poxviruses are sufficiently genetically complex that they encode all of the functions that are needed for successful replication in the cytoplasm, and little is required from the host nucleus.
Finally, most of our knowledge of DNA-virus manipulation of the PI3K–Akt–mTOR pathway comes from studies of cells in which these viruses establish lytic infections, which leads to many progeny viruses being produced and the ultimate killing of the host cell. It is important to consider, however, that in other cell types DNA viruses can use different infection strategies. In persistent viral infections, the host cells are not readily killed and progeny virus production is slow and extended over a long time period. In a latent infection, the infecting viruses become essentially dormant for extended periods of time, and produce few, if any, progeny viruses. However, upon the appropriate stimulus, the latent viruses can reactivate and produce progeny viruses. It is possible that to establish persistent and latent infections the activation and maintenance of the PI3K–Akt–mTOR pathway might be undesirable or even detrimental.
For complete details of the infection strategies and replicative cycles of the DNA viruses, and all things virological, see REFS 121,122.
The Polyomaviridae (Papovaviridae)
The polyoma (papova) viruses are small viruses that are most commonly represented by the mouse polyoma (Py) virus, simian virus 40 (SV40) and the papillomaviruses. They contain covalently closed, circular, double-stranded (ds) DNA genomes of 5–8,000 base pairs that encode 5–8 temporally expressed early and late proteins.
Differential splicing of the Py virus early-gene transcript produces mRNAs for three early proteins: large tumour antigen (PyLT), middle tumour antigen (PyMT) and small tumour antigen (PyST)50. The plasma-membrane-bound PyMT is a potent oncogene that alters the activities of key cellular signalling proteins50,51, including PI3K, which is important for PyMT-mediated transformation52. PI3K activation leads to the phosphorylation of several cellular targets, including Akt50–56. Presumably, the activation of Akt leads to the activation of mTORC1, although this has not been examined directly. Importantly, PyMT associates with the plasma membrane to activate the pathway. However, little is known about the mechanisms that the Py virus uses to counteract the stress responses that inhibit mTORC1.
Recent evidence suggests that, depending on the presence of growth factors in the medium, PyST can switch Akt between anti- and pro-apoptotic states by interacting with protein phosphatase 2A (PP2A). This is dependent on PyST–PP2A differentially controlling the phosphorylation status of Akt T308 and S473, which, in turn, modulates the phosphorylation pattern of Akt substrates57.
The early region of SV40 encodes two oncoproteins: the large tumour (SVLT) and small tumour (SVST) antigens. SVLT transforms mouse and human cells primarily by binding and inactivating p53 and the retinoblastoma (Rb) family proteins58. Additionally, it has been reported that SVLT transformation requires insulin receptor substrate 1 (IRS1)59. SVST binds to PP2A, a major phosphatase that controls many cellular functions. This interaction is required for SV40-mediated transformation of human cells60. For most PP2A substrates, the SVST–PP2A interaction inhibits dephosphorylation; however, in some cases, the interaction with SVST can direct PP2A-mediated dephosphorylation to specific substrates61–63.
Akt and mTOR are activated early during SV40 infection. Both SVLT63,64 and SVST65 have been shown to activate Akt, although the mechanisms that are involved remain unclear. Activation by SVLT can be inhibited by PI3K inhibitors, which indicates that SVLT activates PI3K. Our unpublished data suggest that SVLT requires IRS1 to increase Akt phosphorylation, IRS1 functions as an adaptor for the insulin receptor (FIG.1) and, on activation by the receptor, IRS1 can bind and activate PI3K66. However, the link between SVLT, which is predominately a nuclear protein, and IRS1 and PI3K at the plasma membrane is not clear. Despite early reports of a cell-surface form of SVLT67,68, there is no definitive evidence for an SV40 protein that inserts into the plasma membrane to activate PI3K, as is the case for PyMT.
The interaction between SVST and PP2A is required for the SVST-mediated activation of Akt65. This association might inhibit the function of PP2A in dephosphorylating and inactivating Akt. Paradoxically, although Akt and mTORC1 are activated early in an SV40 infection, the mTORC1 substrates 4E-BP and S6K become hypophosphorylated late in infection63. Hypophosphorylation is mediated by SVST and requires the PP2A binding region. It is therefore possible that SVST targets the phosphatase activity to 4E-BP and S6K. In any case, hypophosphorylation inhibits cap-dependent translation late in the infection63. This is counter-intuitive, as late in infection there is extensive translation of SV40 late mRNAs, which encode three virion structural proteins. Interestingly, the late mRNAs are polycistronic, and recent evidence has indicated that IRESs are used for their translation69. This finding suggests that late in SV40 lytic infection the hypophosphorylated 4E-BP reduces cap-dependent translation such that ribosomes can be diverted to IRES utilization. This would favour translation from the IRESs of the SV40 late mRNAs and the synthesis of virion structural proteins69.
The papillomaviruses are the aetiological agents of papillomas (warts), and several members of this virus family cause cancer, including the high-risk human papillomaviruses (HPVs), which cause cervical cancer70,71. HPVs express several early proteins, two of which, E6 and E7, are required for the maintenance of HPV-related oncogenesis through interactions with p53 and Rb, respectively. It has also been shown that HPV E7, similar to SVST, can interact with PP2A, thus interfering with the ability of PP2A to dephosphorylate and inhibit Akt72. This might be a mechanism by which HPV can maintain Akt phosphorylation on both T308 and S473 in the absence of active PI3K72. The observed phosphorylation of Akt S473 suggests that HPV activates mTORC2, but this has not been formally demonstrated. It has also been suggested that the ability of E7 to upregulate Akt activity is linked to its ability to bind to and inactivate the Rb protein family73, although the mechanism that is involved has not been defined.
The ability of HPV to maintain active Akt, and thus activate mTORC1, might be crucial for the successful initiation of HPV replication, which occurs exclusively in differentiated cells and requires HPV E7. In cells that have been induced to differentiate, E7 protein levels are increased owing to enhanced translation of E7 mRNA, and this correlates with increased levels of phosphorylated 4E-BP74. One interpretation of these data is that as E7 accumulates in differentiated cells it begins to activate Akt and mTORC1, thereby increasing phosphorylation of 4E-BP, which, in turn, promotes E7 translation and creates a positive-feedback mechanism. It has also been reported that the HPV16 E5 protein might be required for the activation of PI3K and Akt75, although the specific nature of the contribution of E5 is not fully understood.
As a papillomavirus infection progresses, the need to activate Akt specifically to maintain mTOR activity can be circumvented by the E6 protein, which binds to tuberin in the TSC and thereby directs tuberin to proteasome-mediated degradation76. As a result, the levels of Rheb–GTP remain high, which activates mTORC1 and maintains the phosphorylation of 4E-BP and S6K. This ability to inactivate the TSC would also circumvent the inhibitory effects of any cellular stress responses that exert their effects through the inactivation of Akt or activation of the TSC (FIG. 2).
The Adenoviridae
Adenoviruses possess linear dsDNA genomes of 30–38 kilobases (kb) that encode 30–40 proteins. Similar to the polyomaviruses, adenovirus gene expression during replication occurs in two phases: early and late. The early proteins are responsible for preparing the cell to execute viral replication by overriding cellular checkpoints, driving quiescent cells into DNA replication, activating other viral genes and blocking apoptosis77. Adenovirus early proteins target several cellular pathways that are crucial for cell growth and survival. Two early adenovirus proteins, E1A and E1B-55K, target Rb and p53, respectively78–80. In addition to manipulating the cell cycle, adenoviruses have also been shown to activate host protein synthesis through the activation of mTORC1 (REF. 81). Early reports suggested that the E1A protein mediates the increased phosphorylation of 4E-BP and S6K82,83. More recent studies in infected airway epithelial cells indicate that the early adenoviral proteins E4-ORF1 and E4-ORF4 are involved in the activation of PI3K, which results in the activation of Akt and mTORC1 (REF. 81).
E4-ORF1 and E4-ORF4 are derived from the E4 region of human adenoviruses. The primary transcript of this region is subject to a complex pattern of differential splicing that produces as many as 24 distinct mRNAs, all of which share common terminal sequences84. Thus, a range of related proteins that have potentially overlapping functions are produced. The E4-ORF1 protein activates growth factor and PI3K signalling, possibly through a direct interaction with PI3K, which has been shown to correlate with increased Rheb–GTP and mTOR kinase activation81,85. However, even in the absence of E4-ORF1, mTORC1 substrates can be maintained in their phosphorylated states owing to the activity of E4-ORF4. This effect occurs in the absence of increased Rheb–GTP, which suggests that E4-ORF4 has a more direct effect on mTORC1 or its substrates. It has been suggested that this might be mediated, in part, by the inhibitory interaction between E4-ORF4 and PP2A, which would inhibit the dephosphorylation of mTORC1 substrates81. Thus, two adenovirus proteins can affect the phosphorylation of mTORC1 substrates indirectly, using E4-ORF1 and PI3K, and more directly, using E4-ORF4. The more direct effects of E4-ORF4 would be predicted to maintain the phosphorylation of mTORC1 substrates even during cell stress responses that inhibit Akt or activate the TSC.
The Herpesviridae
The human herpesviruses (HHV) include herpes simplex virus (HSV) 1 and 2, varicella zoster virus (VZV), human cytomegalovirus (HCMV), Epstein–Barr virus (EBV), HHV-6, HHV-7 and Kaposi's sarcoma herpesvirus (KSHV; also known as HHV-8). These large DNA viruses have linear dsDNA genomes that can be up to 230 kb in size and are capable of encoding up to 200 proteins, as is the case for HCMV, the largest HHV. The host ranges and rates of the replicative cycles of the various HHVs differ. For example, HSV-1, which can infect many species, replicates rapidly and might be able to complete the bulk of its replicative cycle before the induction of stress signalling becomes pronounced or inhibitory. Conversely, HCMV, which has a strict tropism for human cells, has a slow replicative cycle and must deal with the inhibitory effects of stress signalling. Data suggest that HCMV does this effectively by affecting many cellular signalling pathways in a way that inhibits stress signals or circumvents their inhibitory effects86–93. HCMV infection can be sustained under stress conditions that would kill uninfected cells90,94, which suggests that infection can be cytoprotective for the host. Regarding the effects of HHVs on the PI3K–Akt–mTOR pathway, most is known about HCMV and HSV.
HCMV infection activates Akt by stimulating T308 phosphorylation, which occurs through the activation of PI3K64,95, and S473 phosphorylation, which occurs through the activation of mTORC2 (REF. 88) (FIG. 2). Transient activation of PI3K–Akt results from receptor-mediated signalling owing to HCMV attachment to viral receptors95. Longer-term activation requires the expression of HCMV-encoded proteins64,88. It has been suggested that either of the two major immediate-early proteins of HCMV (the 72 kDa IE1 and 86 kDa IE2 proteins) can stimulate the phosphorylation of Akt at both T308 and S473 (REF. 64); however, the mechanism that is used is unknown.
The effects of HCMV on the PI3K–Akt–mTOR pathway extend well beyond Akt activation. AMPK can be phosphorylated and activated in HCMV-infected cells. However, its ability to inhibit mTORC1 is blocked87. Data suggest that HCMV targets AMPK and the TSC, thereby limiting the levels of phosphorylated (activated) AMPK87 and inactivating the TSC through binding of the HCMV immediate-early protein pUL38 (N. J. moorman et. al., personal communication). It is important to note that inhibition of the TSC by pUL38 would not only block the effects of activated AMPK but also the effects of other stress responses that signal by activating the TSC, for example, hypoxia and calcium homeostasis.
Viral-mediated inhibition of the TSC is probably sufficient to protect mTORC1 activity and cap-dependent translation from the inhibitory effects of most stress responses. However, HCMV infection also targets the mTOR complexes and functionally alters them to maintain 4E-BP phosphorylation and cap-dependent translation. This is indicated by the virus-mediated resistance of mTOR kinase activity to rapamycin in HCMV-infected human fibroblasts, which correlates with the rapamycin-insensitive phosphorylation of 4E-BP86. However, this resistance is specific for 4E-BP, as the phosphorylation of S6K by mTOR kinase remains sensitive to rapamycin86. Thus, the viral infection induces a substrate-specific alteration in mTORC1 activity. Similarly, HCMV infection alters the substrate specificity of mTORC2, which gains the ability to phosphorylate 4E-BP and S6K86. These data suggest that HCMV targets and alters both mTOR complexes to facilitate 4E-BP and S6K phosphorylation and maintain translation.
HCMV also acts downstream of mTOR by inducing Mnk1-dependent phosphorylation of eIF4E (FIG. 2) and increasing the levels of eIF4E93. An increase in eIF4E levels might enable hypophosphorylated 4E-BP to be titrated out, but still allow the maintenance of sufficient free eIF4E to bind eIF4G and preserve the integrity of the eIF4F complex. Thus, HCMV maintains redundant mechanisms to control the signalling pathway at this point — first, by hyperphosphorylating 4E-BP to lower its affinity for eIF4E and, second, by increasing the levels of eIF4E to titrate out hypophosphorylated 4E-BP. These two mechanisms might be required to maintain cap-dependent translation in different cell types and under different growth conditions.
The studies discussed above of how HCMV affects the PI3K–Akt–mTOR pathway were performed in cells in which the virus can produce a robust, lytic infection. It should be noted that in other cell types, in which the virus can produce a chronic, persistent or latent infection, the need to activate this pathway might not be as crucial and could even be detrimental for the virus (BOX 2); in such cells, it has been reported that HCMV infection can activate PTEN, thereby inhibiting Akt activation. This might be necessary to set up conditions to establish persistent or latent infections96.
HSVs also exert multiple effects on the PI3K–Akt–mTOR pathway. The HSV-2 UL39 gene encodes the large subunit of HSV ribonucleotide reductase, which is also known as ICP10. It has been proposed that HSV-2 ICP10 has a serine–threonine protein kinase (PK) activity located within the first 411 amino acids (ICP10PK). ICP10PK is a constitutively activated growth-factor receptor that signals through both the Ras–MEK (mitogen-activated protein kinase kinase)–ERK (extracellular signal-regulated kinase) and PI3K–Akt–mTOR pathways97. This is a controversial model, however, as other data suggest that the kinase activity of ICP10PK is not intrinsic98,99. If this mechanism of Akt activation is functional, it would be specific for HSV-2, as the HSV-1 ribonucleotide reductase subunit does not contain the putative kinase activity. Thus, other HSV mechanisms for activating Akt early in infection remain to be elucidated. However, conditions change as the HSV infection progresses and the HSV kinase US3 accumulates in effective amounts. Under these conditions, Akt becomes dephosphorylated and inactivated. One interpretation of these data is that activated Akt is needed for its anti-apoptotic function early in infection and, presumably, activates mTOR before accumulation of the US3 kinase. However, late in infection, Akt might not be needed or might be deleterious to HSV infection100.
Inactivation of Akt activity could result in loss of mTORC1 activity. However, similar to HCMV, HSV-1 exerts effects downstream in the pathway that would circumvent the loss of Akt and the inhibitory effects of stress signalling. Specifically, HSV-1 directly regulates formation of the eIF4F translation-initiation complex. The HSV-1 protein ICP0 stimulates the phosphorylation of eIF4E and 4E-BP1, which leads to the degradation of 4E-BP1 and facilitates formation of the eIF4F complex101. In addition, eIF4F-complex formation is stimulated by the chaperone-like activity of the HSV protein ICP6, which interacts directly with eIF4G102. Such a direct effect on translation might allow HSV to circumvent most of the inhibitory stress signalling that is induced during its rapid life cycle.
Other members of the herpesvirus family that are known to activate the pathway include: VZV, using pORF47 and pORF66 (REF. 103); EBV, using latent membrane protein 2A104–106; and KSHV, using the viral G-protein-coupled receptor107. For EBV and KSHV, viral activation of the pathway is thought to contribute to oncogenesis.
The Poxviridae
The poxviruses are large DNA viruses that contain linear dsDNA genomes of 130–230 kb. They are unique among mammalian DNA viruses in that they replicate exclusively within the host cell cytoplasm108. Discrete cytoplasmic regions are organized into replication compartments, termed factories, during the productive growth cycle109. Despite this independence from most nuclear functions, poxviruses remain dependent on the cellular translational machinery. Poxvirus mRNAs are capped on their 5′ end through the action of a viral methyl-transferase com-plex110 and therefore use eIF4F to initiate cap-dependent translation. However, studies that used the model poxvirus vaccinia virus (VV) indicated that poxviruses might be able to circumvent mTORC1, and the stress signalling that might inhibit this complex, through their ability to trigger the destruction of 4E-BP and thus remove the main inhibitor of eIF4F-complex formation. In addition, cellular signalling pathways were activated that stimulate the Mnk1 kinase to phosphorylate and activate eIF4E. Both events seem to be crucial for successful VV replication. Further, eIF4E and eIF4G are relocated into the cytosolic viral-replication compartments111.
This apparent circumvention of mTORC1 for translation does not mean that the PI3K–Akt–mTOR pathway is not activated by poxviruses. Myxoma virus (MV) is a member of the poxvirus family that causes a fatal disease known as myxomatosis in European rabbits112, although it is non-pathogenic in other vertebrates, including humans113–115. Interestingly, MV can productively infect many human tumour-cell lines116. This ability is associated with the MV host-range gene product M-T5 (REF. 116), an ankyrin-repeat protein that complexes with Akt and upregulates Akt kinase activity117. The ability of MV to grow in human tumour cells depends on the cells either having a high activation state of Akt at the time of infection or the ability of M-T5 to induce high levels of activated Akt117.
Summary and conclusions
FIGURE 4 illustrates the ways in which mammalian DNA viruses affect the PI3K–Akt–mTOR pathway, the mTORC1 substrates and the components of the eIF4F complex. The evidence shows that all mammalian DNA-virus families have inherent capacities to modulate PI3K activity to affect the PtdIns(4,5)P2 to PtdIns(3,4,5)P3 ratio. How some viruses accomplish this activation remains unclear. Given that PyMT and, possibly, adenovirus E4-ORF1 directly interact with PI3K at the plasma membrane, it could be predicted that other DNA viruses either encode proteins or induce cellular proteins that have similar functions. Repression of PTEN activity would be an alternative way for a virus to modulate the PtdIns(4,5)P2 to PtdIns(3,4,5)P3 ratio; however, this has not been well studied in the DNA viruses.
Figure 4. Summary of the effects of mammalian DNA viruses on the components of the PI3K–Akt–mTOR pathway, the substrates of mTORC1 and the eIF4F complex.
The many activating and inhibitory mechanisms that are used by DNA viruses to maintain mTOR complex 1 (mTORC1) activity and cap-dependent translation are summarized. However, it should be noted that these effects might also provide many other physiological changes in the cell that can benefit a productive viral infection and contribute to viral pathogenesis. 4E-BP, eIF4E binding protein; AD, adenovirus; eIF, eukaryotic elongation factor; HCMV, human cytomegalovirus; HPV, human papillomavirus; HSV, herpes simplex virus; Mnk1, mitogen-activated protein kinase-interacting kinase 1; mTOR, mammalian target of rapamycin; MV, myxoma virus; PDK1, phosphoinositide-dependent protein kinase 1; PI3K, phosphatidylinositol 3′ kinase; PP2A, protein phosphatase 2A; S6, ribosomal protein S6; S6K, p70S6 kinase; ST, small tumour antigen; SV, simian virus 40; TSC, tuberous sclerosis complex; VV, vaccinia virus.
Abrogation of PP2A function by viral proteins affects the PI3K–Akt–mTOR pathway at several levels. For example, PyST, SVST and HPV E7 control of Akt; SVST-mediated hypophosphorylation of 4E-BP and S6K; and adenovirus E4-ORF4 and PP2A might be involved in Rheb–GTP-independent activation of mTOR. PP2A is a key regulator of many cellular processes and therefore it would not be surprising to find that most DNA viruses gain control of PP2A as a means to control Akt and mTOR activity.
The general need for viruses to control Akt activity suggests that direct interactions between viral proteins and Akt would be a good control strategy. However, this has been clearly demonstrated for only one virus protein, M-T5, which raises the question of why a direct interaction has not been shown for the nuclear-replicating viruses. Are they more restricted than poxviruses in their ability to directly manipulate Akt or is the mechanism more subtle and has therefore not yet been detected?
The TSC is a crucial point in the pathway where stress signalling can initiate inhibitory effects on mTORC1 by converting Rheb–GTP to Rheb–GDP. Thus, it is not surprising that this complex is targeted for inactivation by HPV E6 and HCMV UL38. Given its crucial regulatory position, it is likely that the TSC will be found to be a target for other DNA viruses. It is also likely that some viruses induce other means to maintain Rheb–GTP or antagonize FKBP38, the mTORC1 inhibitory protein.
The mTOR complexes are targets for several viruses. The evidence from studies of HCMV shows that both mTORC1 and mTORC2 are activated and can be structurally altered during infection, which results in increased phosphorylation of 4E-BP and rapamycin resistance. That most DNA-virus infections activate mTORC2 is suggested from the experiments that document Akt activation. Specifically, most analyses that detected Akt activation involved western analysis of the phosphorylation of S473, the mTORC2 phosphorylation site. The control of mTORC2 activity is not well understood, but one study suggested that Rheb–GTP, the activator of mTORC1, inhibits mTORC2 (REF. 34). If this is the case, then how are both mTORC1 and mTORC2 activated during infection with viruses such as HCMV? It is possible that virus-mediated alterations of the mTOR complexes alter the response to Rheb–GTP or FKBP38. Alternatively, Rheb–GTP or FKBP38 might be a direct target for some DNA viruses to control the activity of the mTOR complexes.
Several DNA viruses function downstream of mTOR and target 4E-BP and components of the eIF4F complex. These viral-mediated effects would seem to be the ultimate way to counteract the inhibitory effects of stress responses, all of which inhibit the pathway upstream of mTOR (FIG. 2). However, the viruses retain multiple mechanisms to maintain the activity of the pathway from PI3K to the mTOR complexes. This Review has mainly used the maintenance of cap-dependent translation as the focus for why mammalian DNA viruses target the PI3K–Akt–mTOR pathway. This is, of course, a narrow view. The many interactions that the DNA viruses have with this pathway suggest additional effects on cellular physiology. These might not only benefit viral infection, but also translate into viral pathogenesis. For example, nearly all of the targets in FIG. 4 can be an oncoprotein if mutated or inappropriately activated or expressed. Some of the mammalian DNA viruses discussed above are true transforming viruses (for example, SV40, high-risk HPVs, some adenoviruses, EBV and KSHV), but others are not. even if a virus has not been shown to be a frank transforming agent, it might still serve as a co-factor with other agents or mutations to promote transformation. For example, the effects of any DNA virus on oncoproteins such as PI3K, Akt, TSC, mTOR and mTOR effectors could increase the oncogenic potential of the cell, thereby functioning as one factor among several that ultimately result in transformation.
Viruses have long served as a means to study and decipher complex cellular phenomena. Understanding how viruses manipulate key signalling pathways and circumvent stress signalling will provide valuable insight into not only how viruses manipulate these pathways, and thereby cause pathogenesis, but also the normal cellular controls of these pathways that may arise under special growth and metabolic conditions.
Acknowledgments
The authors thank S. Adams, A. Diehl, C. Simon, B. Keith, C. Thompson and T. Shenk for many discussions and help in the preparation of this manuscript. J.C.A. is funded by Public Health Services grants R01 CA28379-27 and R01 GM45773-16 from the National Institutes of Health and the Abramson Family Cancer Research Institute.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
UL39
Entrez Genome: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genome
EBV | HHV-7 | HSV1 | HSV2 | KSHV | MV | Py virus | SV40 | VV
Entrez Protein: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=protein
ICP0 | ICP10 | M-T5 | PyLT | PyMT | PyST | SVLT
FURTHER INFORMATION
James C. Alwine's homepage: http://www.med.upenn.edu/camb/faculty/mv/alwine.html
All Links are Active in the Online PDF
References
- 1.Cooray S. The pivotal role of phosphatidylinositol-3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]; An overview of the importance of the control of Akt to most viruses.
- 2.Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RJ, et al. The unfolded protein response in nutrient sensing and differentiation. Nature Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 4.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 5.Wouters BG, et al. Control of the hypoxic response through regulation of mRNA translation. Sem Cell Dev Biol. 2005;16:487–501. doi: 10.1016/j.semcdb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nature Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]; Reviews the various ways that stress can affect translation.
- 7.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine–threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 8.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]; An older review that outlines the many functions of Akt.
- 9.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 11.Baker SJ. PTEN enters the nuclear age. Cell. 2007;128:25–28. doi: 10.1016/j.cell.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Cass LA, et al. Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol. 1999;19:5882–5891. doi: 10.1128/mcb.19.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill MM, et al. A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1999;19:7771–7781. doi: 10.1128/mcb.19.11.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueki K, et al. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 16.Jozwiak J. Hamartin and tuberin: working together for tumour suppression. Int J Cancer. 2006;118:1–5. doi: 10.1002/ijc.21542. [DOI] [PubMed] [Google Scholar]
- 17.Krymskaya VP. Tumour suppressors hamartin and tuberin: intracellular signalling. Cell Signal. 2003;15:729–739. doi: 10.1016/s0898-6568(03)00040-8. [DOI] [PubMed] [Google Scholar]
- 18.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 20.Astrinidis A, Henske EP. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene. 2005;24:7475–7481. doi: 10.1038/sj.onc.1209090. [DOI] [PubMed] [Google Scholar]
- 21.Avruch J, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 22.Bai X, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, et al. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 26.Jacinto E, et al. SIN1/MIP1 maintains rictor–mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Inoki K, Ikenoue T, Guan KL, Iaccheri L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polak P, Hall MN. mTORC2 caught in a SINful. Akt Dev Cell. 2006;11:433–434. doi: 10.1016/j.devcel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]; An overview of the effects of stress on mTOR signalling and translation.
- 31.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci USA. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arsham AM, Plas DR, Thompson CB, Simon MC. PI3-K/Akt signaling is neither required for hypoxic stabilization of HIF-1 nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162–15170. doi: 10.1074/jbc.M111162200. [DOI] [PubMed] [Google Scholar]
- 36.Cai SL, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Beucken T, Koritzinsky M, Wouters BG. Translational control of gene expression during hypoxia. Cancer Biol Ther. 2006;5:749–755. doi: 10.4161/cbt.5.7.2972. [DOI] [PubMed] [Google Scholar]
- 38.Ellisen LW. Growth control under stress: mTOR regulation through the REDD1–TSC pathway. Cell Cycle. 2005;4:1500–1502. doi: 10.4161/cc.4.11.2139. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzer R, et al. REDD1 integrates hypoxia-mediated survival signaling downstream of phosphatidylinositol 3-kinase. Oncogene. 2005;24:1138–1149. doi: 10.1038/sj.onc.1208236. [DOI] [PubMed] [Google Scholar]
- 40.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz–Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw RJ, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Kimble SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 43.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 45.Chami M, Oules B, Paterlini-Brechot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta. 2006;1763:1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Avruch J, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 47.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 48.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Zeng Z, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb KA, Villarreal LP. Natural biology of polyomavirus middle T antigen. Microbiol Mol Biol Rev. 2001;65:288–318. doi: 10.1128/MMBR.65.2.288-318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichaso N, Dilworth SM. Cell transformation by the middle T-antigen of polyoma virus. Oncogene. 2001;20:7908–7916. doi: 10.1038/sj.onc.1204859. [DOI] [PubMed] [Google Scholar]
- 52.Utermark T, Schaffhausen BS, Roberts TM, Zhao JJ. The p110α isoform of phosphatidylinositol 3-kinase is essential for polyomavirus middle T antigen-mediated transformation. J Virol. 2007;81:7069–7076. doi: 10.1128/JVI.00115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Summers SA, Lipfert L, Birnbaum MJ. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI3-kinase-dependent manner. Biochem Biophys Res Commun. 1998;246:76–81. doi: 10.1006/bbrc.1998.8575. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan DR, et al. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987;50:1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan DR, et al. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci USA. 1986;83:3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 57.Andrabi S, Gjoerup OV, Kean JA, Roberts TM, Schaffhausen B. Protein phosphatase 2A regulates life and death decisions via Akt in a context-dependent manner. Proc Natl Acad Sci USA. 2007;104:19011–19016. doi: 10.1073/pnas.0706696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmons DT. SV40 large T antigen functions in DNA replication and transformation. Adv Virus Res. 2000;55:75–134. doi: 10.1016/s0065-3527(00)55002-7. [DOI] [PubMed] [Google Scholar]; A comprehensive review of SV40 large-T-antigen functions.
- 59.DeAngelis T, Chen J, Wu A, Prisco M, Baserga R. Transformation by the simian virus 40 T antigen is regulated by IGF-I receptor and IRS-1 signaling. Oncogene. 2006;25:32–42. doi: 10.1038/sj.onc.1209013. [DOI] [PubMed] [Google Scholar]
- 60.Rundell K, Parakati R. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin Cancer Biol. 2001;11:5–13. doi: 10.1006/scbi.2000.0341. [DOI] [PubMed] [Google Scholar]; A review of the function of the SV40 small-t antigen.
- 61.Yang SI, et al. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang CS, et al. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol Cell Biol. 2005;25:1298–1308. doi: 10.1128/MCB.25.4.1298-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Y, Kudchodkar SB, Alwine JC. Effects of simian virus 40 large and small tumor antigens on mammalian target of rapamycin (mTOR) signaling: small tumor antigen mediates hypophosphorylation of eIF4E-binding protein 1 late in infection. J Virol. 2005;79:6882–6889. doi: 10.1128/JVI.79.11.6882-6889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu Y, Alwine JC. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and cellular kinase Akt. J Virol. 2002;76:3731–3738. doi: 10.1128/JVI.76.8.3731-3738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan H, Veldman T, Rundell K, Schlegel R. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J Virol. 2002;76:10685–10691. doi: 10.1128/JVI.76.21.10685-10691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Backer JM, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lange-Mutschler J, Deppert W, Hanke K, Hennin R. Detection of simian virus 40 T-antigen-related antigens by a 125I-protein A-binding assay and by immunofluorescence microscopy on the surface of SV40 transformed monolayer cells. J Gen Virol. 1981;52:301–312. doi: 10.1099/0022-1317-52-2-301. [DOI] [PubMed] [Google Scholar]
- 68.Lange-Mutschler J, Henning R. A subclass of simian virus 40 T antigen with a high cell surface binding affinity. Virology. 1983;127:333–344. doi: 10.1016/0042-6822(83)90148-4. [DOI] [PubMed] [Google Scholar]
- 69.Yu Y, Alwine JC. 19S late mRNAs of simian virus 40 have an internal ribosome entry site upstream of the virion structural protein 3 coding sequence. J Virol. 2006;80:6553–6558. doi: 10.1128/JVI.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirzamani N, Salehian P, Farhadi M, Tehran EA. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp Mol Pathol. 2006;81:231–234. doi: 10.1016/j.yexmp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 72.Pim D, Massimi P, Dilworth SM, Banks L. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene. 2005;24:7830–7838. doi: 10.1038/sj.onc.1208935. [DOI] [PubMed] [Google Scholar]
- 73.Menges CW, Baglia LA, Lapoint R, McCance DJ. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006;66:5555–5559. doi: 10.1158/0008-5472.CAN-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh KJ, Kalinina A, Park NH, Bagchi S. Deregulation of eIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein. J Virol. 2006;80:7079–7088. doi: 10.1128/JVI.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim SH, et al. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ ERK1,2 and PI3K/Akt. Cell Mol Life Sci. 2006;63:930–938. doi: 10.1007/s00018-005-5561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu Z, et al. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279:35664–35670. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 77.O'Shea CC, Choi S, McCormick F, Stokoe D. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle. 2005;4:883–888. doi: 10.4161/cc.4.7.1791. [DOI] [PubMed] [Google Scholar]; A thorough study of adenovirus effects on several cell-signalling pathways.
- 78.Helt AM, Galloway DA. Mechanims by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 79.Querido E, et al. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 2001;15:3104–3117. doi: 10.1101/gad.926401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Querido E, et al. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Shea C, et al. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J. 2005;24:1211–1221. doi: 10.1038/sj.emboj.7600597. [DOI] [PMC free article] [PubMed] [Google Scholar]; Studies of the effects of adenoviruses on mTOR.
- 82.Gingras AC, Sonenburg N. Adenovirus infection inactivates the translational inhibitors 4E-BP1 and 4E-PB2. Virology. 1997;237:182–186. doi: 10.1006/viro.1997.8757. [DOI] [PubMed] [Google Scholar]
- 83.de Groot RP, et al. Induction of the mitogen-activated p70 S6 kinase by adenovirus E1A. Oncogene. 1995;10:543–548. [PubMed] [Google Scholar]
- 84.Boyer J, Ketner G. Manipulation of early region 4. Methods Mol Med. 2007;130:1–17. doi: 10.1385/1-59745-166-5:1. [DOI] [PubMed] [Google Scholar]
- 85.Frese KK, et al. Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene. 2003;22:710–721. doi: 10.1038/sj.onc.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kudchodkar S, Yu Y, Maguire T, Alwine JC. Human cytomegalovirus infection induces rapamycin insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol. 2004;78:11030–11039. doi: 10.1128/JVI.78.20.11030-11039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol. 2007;81:3649–3651. doi: 10.1128/JVI.02079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc Natl Acad Sci USA. 2006;103:14182–14187. doi: 10.1073/pnas.0605825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isler JA, Maguire TG, Alwine JC. Production of infectious HCMV virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. J Virol. 2005;79:15338–15397. doi: 10.1128/JVI.79.24.15388-15397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alwine JC. In: Current Topics in Microbiology and Immunology. Shenk TE, Stinski MF, editors. Vol. 325. Springer; New York: 2008. pp. 263–279. [DOI] [PubMed] [Google Scholar]; A complete overview of the effects of lytic HCMV infection on the PI3K–Akt–mTOR pathway.
- 92.Hakki M, Geballe AP. Double-stranded RNA binding by human cytomegalovirus pTRS1. J Virol. 2005;79:7311–7318. doi: 10.1128/JVI.79.12.7311-7318.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walsh D, Perez C, Notary J, Mohr I. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J Virol. 2005;79:8057–8064. doi: 10.1128/JVI.79.13.8057-8064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buchkovich NJ, et al. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J Virol. 2008;82:31–39. doi: 10.1128/JVI.01881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen YH, et al. Human cytomegalovirus inhibits Akt-mediated eNOS activation through upregulating PTEN (phosphatase and tensin homolog deleted on chromosome 10) Cardiovasc Res. 2006;69:502–511. doi: 10.1016/j.cardiores.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Smith CC. The herpes simplex virus type 2 protein ICP10PK: a master of versatility. Front Biosci. 2005;10:2820–2831. doi: 10.2741/1738. [DOI] [PubMed] [Google Scholar]
- 98.Langelier Y, et al. The R1 subunit of herpes simplex virus ribonucleotide reductase is a good substrate for host cell protein kinases but is not itself a protein kinase. J Biol Chem. 1998;273:1435–1443. doi: 10.1074/jbc.273.3.1435. [DOI] [PubMed] [Google Scholar]
- 99.Conner J. The unique N terminus of herpes simplex virus type 1 ribonucleotide reductase large subunit is phosphorylated by casein kinase 2, which may have a homologue in Escherichia coli. J Gen Virol. 1999;80:1471–1476. doi: 10.1099/0022-1317-80-6-1471. [DOI] [PubMed] [Google Scholar]
- 100.Benetti L, Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of ΔUS3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol. 2006;80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh D, Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18:660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walsh D, Mohr I. Assembly of an active translation initiation factor complex by a viral protein. Genes Dev. 2006;20:461–472. doi: 10.1101/gad.1375006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Reference 101, examined the ways that herpesviruses can activate cap-dependent translation at points downstream of mTOR.
- 103.Rahaus M, Desloges N, Wolff MH. Varicella-zoster virus requires a functional PI3K/Akt/GSK-3 signaling cascade for efficient replication. Cell Signal. 2007;19:312–318. doi: 10.1016/j.cellsig.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Moody CA, et al. Modulation of the cell growth regulator mTOR by Epstein–Barr virus-encoded LMP2A. J Virol. 2005;79:5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scholle F, Bendt KM, Raab-Traub N. Epstein–Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J Virol. 2000;74:10838–10845. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sodhi A, et al. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10:133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 108.Moss B. In: Field's Virology. Knipe DM, et al., editors. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2849–2883. [Google Scholar]
- 109.Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- 110.Venkatesan S, Gershowitz A, Moss B. Modification of the 5′ end of mRNA: association of RNA triphosphatase with the RNA guanylyl-transferase-RNA (guanine-7) methyltransferase complex from vaccinia virus. J Biol Chem. 1980;255:903–908. [PubMed] [Google Scholar]
- 111.Walsh D, et al. eIF4F architectural alterations accompany cellular translation initiation factor redistribution in poxvirus-infected cells. Mol Cell Biol. 2008 Feb 4; doi: 10.1128/MCB.01631-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kerr P, McFadden G. Immune responses to myxoma virus. Viral Immunol. 2002;15:229–246. doi: 10.1089/08828240260066198. [DOI] [PubMed] [Google Scholar]
- 113.Fenner F. Adventures with poxviruses of vertebrates. FEMS Microbiol Rev. 2000;24:123–133. doi: 10.1016/S0168-6445(00)00027-9. [DOI] [PubMed] [Google Scholar]
- 114.Jackson EW, Dorn CR, Saito JK, McKercher DG. Absence of serological evidence of myxoma virus infection in humans exposed during an outbreak of myxomatosis. Nature. 1966;211:313–314. doi: 10.1038/211313a0. [DOI] [PubMed] [Google Scholar]
- 115.McFadden G. Poxvirus tropism. Nature Rev Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sypula J, Wang F, Ma Y, Bell JC, McFadden G. Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol. 2004;8:108–114. [Google Scholar]
- 117.Wang G, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Reference 116, investigated the growth of MV in human tumour cells and the requirement for the activation of Akt.
- 118.Schneider RJ, Mohr I. Translation initiation and viral tricks. Trends Biochem Sci. 2003;28:130–136. doi: 10.1016/S0968-0004(03)00029-X. [DOI] [PubMed] [Google Scholar]
- 119.Ryabova LA, Pooggin MM, Hohn T. Viral strategies of translation initiation: ribosomal shunt and reinitiation. Prog Nucleic Acid Res Mol Biol. 2002;72:1–39. doi: 10.1016/S0079-6603(02)72066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Reference 118, discusses the intriguing translational mechanisms that are exploited by viruses
- 120.Hellen CUT, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 2001;15:1593–1612. doi: 10.1101/gad.891101. [DOI] [PubMed] [Google Scholar]
- 121.Flint SJ, Enquist LW, Krug RM, Racaniello VR, Skalka AM, editors. Principles of Virology. American Society for Microbiology; Washington D. C.: 2004. [Google Scholar]
- 122.Knipe DM, et al., editors. Field's Virology. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]; Together with Reference 121, provides a wealth of information on viral replication and all things virological.