Abstract
Ventricular pressure–volume relationships have become well established as the most rigorous and comprehensive ways to assess intact heart function. Thanks to advances in miniature sensor technology, this approach has been successfully translated to small rodents, allowing for detailed characterization of cardiovascular function in genetically engineered mice, testing effects of pharmacotherapies and studying disease conditions. This method is unique for providing measures of left ventricular (LV) performance that are more specific to the heart and less affected by vascular loading conditions. Here we present descriptions and movies for procedures employing this method (anesthesia, intubation and surgical techniques, calibrations). We also provide examples of hemodynamics measurements obtained from normal mice/rats, and from animals with cardiac hypertrophy/heart failure, and describe values for various useful load-dependent and load-independent indexes of LV function obtained using different types of anesthesia. The completion of the protocol takes 1–4 h (depending on the experimental design/end points).
INTRODUCTION
Around 30 years ago, Sagawa and colleagues1 embarked on a systematic and detailed analysis of canine ventricular function using pressure–volume (PV) relationships. Their work led to the appreciation that such relations provided a uniquely powerful approach to quantifying heart function, particularly in vivo1-3. Subsequent studies in large animals4 and humans5 generated PV loops in real time, both under steady-state conditions and during transient reduction of inflow to the heart. This work established the methodology as the most comprehensive yet available for assessing ventricular performance independent from loading conditions, yet simultaneously quantifying load and the interaction of heart and vasculature. The most convenient way to obtain these data was the use of an impedance (or conductance) and pressure-measuring catheter, inserted to lie along the long axis of the ventricle, to provide a real-time volume signal as well as micromanometer pressure signal. This catheter was first used in large animals and humans starting in the mid-1980s. About 15 years later, technical development in miniature sensors made it feasible to apply this approach to very small mammals (Fig. 1a)6. This method provided simultaneous measurement of both pressure and volume signals from the intact beating mouse6-16 and rat17-20 hearts. Despite its invasiveness, this sophisticated methodology has great potential for characterizing cardiac function in various genetically manipulated mouse models of cardiovascular disease, and testing the effects of various drugs under physiological and pathological conditions. Noninvasive methodologies for measuring cardiac function (echo and MRI) are limited by their application to steady-state conditions and reliance on motion parameters that can be influenced by loading conditions and thus lack specificity to the ventricle itself. Their advantage is that they can be repeated in the same animal and provide direct quantification of absolute volumes, whereas the conductance catheter signal is proportional to volume but must be appropriately calibrated to provide accurate absolute volume measurements.
Figure 1.
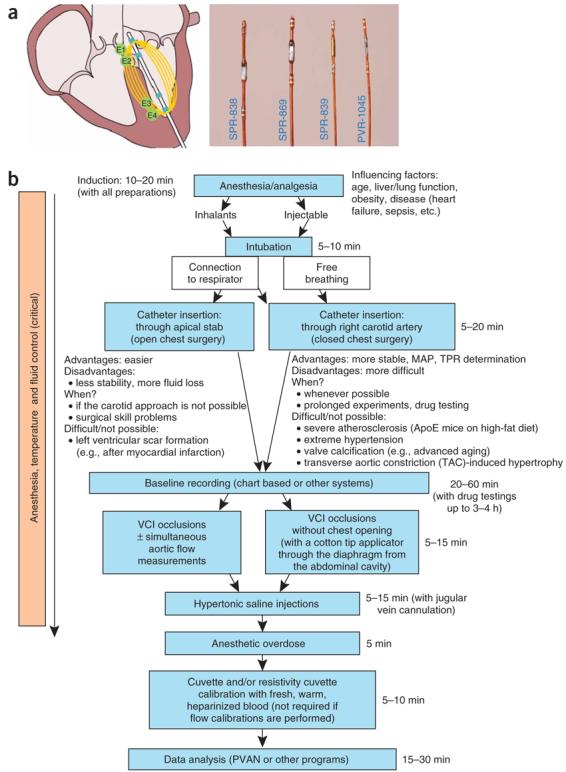
Pressure–volume (PV) catheters and main steps of the protocol. (a) Mouse and rat PV catheters (magnified image) and working principle. (b) Flow chart indicates main procedures and important considerations of the PV protocol.
Ventricular pressure measurements have been commonly used for decades, but real-time volume measurements have historically been problematic. A technique by Baan et al.21 made it possible to correlate the change in ventricular volume to a change in electrical resistance of the blood pool within the LV chamber. The conductance catheter has multiple ring electrodes placed along its length (Fig. 1a), and a high-frequency low-amplitude constant current is passed through the outer pair of electrodes to generate a local electric field between these electrodes (E4, E1). The field passes through the blood, muscle wall and surrounding structures, with field strength declining by the square of the distance from the electrodes. Electric theory indicates that if voltage potentials are measured within this field, they will be similar along planes that are perpendicular to the current field lines. The potential difference between two intervening electrodes will be inversely proportional to the amount of conductive material at that site. For the small rodent catheter, this measurement is made between two inner sensing electrodes (E2, E3), providing a time-varying signal. The resistivity of blood is about 1/3 that of the heart muscle, so the signal combines both the blood pool and chamber muscle wall. However, the latter is essentially constant12, whereas the former varies with the cardiac cycle, so the time-varying component of the conductance signal is due to blood volume changes in the cavity.
As noted earlier, this conductance signal is itself noncalibrated. There is a fairly linear relation between absolute volume and this signal, and the slope (gain) and offset are related to the geometry of the heart and surrounding structures and their conductivity. Some have used external reservoirs filled with conductive material to mimic the heart to obtain a calibration curve (cuvette calibration; see also manufacturer's instructions). However, the accuracy of this approach may be questionable under all conditions or disease models. More accurate volume calibration can be accomplished using an independent measure of cardiac output (e.g., ultrasound flow probe) from which stroke volume is derived to calculate the gain of the signal, defined as: gain = flow probe stroke volume/conductance stroke volume. The offset is due to the fact that the ventricular cavity is not a perfect insulator, and a portion of the current leaks into the muscle. This offset must be subtracted to obtain absolute volume. It is usually estimated by the hypertonic saline dilution method5,12.
The unique advantage of the PV methodology over all other available approaches to measure cardiac function is that it enables more specific measurement of the LV performance independently from loading conditions and of heart rate (for commonly used load-independent indices of systolic (Ees(Emax), dP/dtmax–end-diastolic volume; preload-recruitable stroke work (PRSW)) and diastolic function (EDPVR), see Table 1. Ees or Emax defines chamber end-systolic stiffness and can be a useful measure of contractile function, particularly to assess acute changes22,23. Chronic changes in Ees from heart disease can also reflect cardiac morphometry—that is, hypertrophy, fibrosis—and thus is not simply a reflection of ‘contractility’. The dP/dtmax–end-diastolic volume relation4 also provides a load-independent contractility index, as preload dependence of dP/dtmax is effectively reduced by using this regression. PRSW24 is a similar type of index, plotting stroke work versus end-diastolic volume for the set of load-altered loops.
TABLE 1.
Hemodynamic parameters and indices of systolic and diastolic function derived from PV relations in mice and rats.
| Mouse1 | Mouse2 |
Rat2 | Rat2 | |||
|---|---|---|---|---|---|---|
| Anesthesia used | Urethane + etomidate + morphine |
Isoflurane | Ketamine + xylazine |
Pentobarbital sodium |
Isoflurane | PB |
| Parameter | ||||||
| BW (g) | 20–30 | 20–34 | 20–34 | 20–34 | 250–500 | 300–450 |
| HR (min−1) | 490–655 | 470–620 | 340–510 | 365–550 | 370–420 | 350–440 |
| MAP (mm Hg) | 81–105 | 93–109 | 72–90 | 100–124 | 93–120 | |
| ESP (mm Hg) | 80–120 | 92–118 | 104–125 | 90–110 | 119–146 | 113–142 |
| EDP (mm Hg) | 2–8 | 1–6 | 1–9 | 2–8 | 0.5–8 | 1–7 |
| ESV (μl) | 2–12 | 7–21 | 9–20 | 10–29 | 21–141 | 36–160 |
| EDV (μl) | 20–33 | 25–53 | 25–39 | 28–54 | 161–303 | 170–266 |
| SV (μl) | 14–26 | 17–30 | 14–21 | 17–25 | 103–191 | 101–154 |
| CO (ml min−1) | 7–16 | 8–16 | 6–10 | 6–13 | 49–70 | 42–62 |
| CI (ml min−1 × kg) | 280–557 | 350–580 | 225–400 | 226–480 | 114–227 | 110–172 |
| Ea (mm Hg μl−1) | 4–6 | 3–7 | 5–9 | 4–6 | 0.5–1.1 | 0.5–1.1 |
| TPR (mm Hg μl−1 × min) | — | 6–12 | 10–19 | 7–14 | 1.5–2.5 | 1.9–2.5 |
| Systolic indices | ||||||
| EF (%) | 50–88 | 55–72 | 49–63 | 44–62 | 49–89 | 42–87 |
| dP/dtmax (mm Hg s−1) | 9,500–16,000 | 8,200–14,200 | 7,700–14,480 | 6,900–11,000 | 8,900–13,100 | 7,600–11,500 |
| SW (mm Hg × ml) | 1,200–2,700 | 1,500–2,600 | 1,600–2,200 | 1,100–2,100 | 13,500–22,900 | 12,800–21,000 |
| Ees(Emax)(mm Hg μl−1) | 6–14 | 7–14 | − | 6–9 | 2–7 | 1–4 |
| dP/dtmax−EDV (mm Hg s−1 ml−1) | 360–600 | 180–470 | — | 160–390 | 40–120 | 28–90 |
| PRSW (mm Hg) | 70–130 | 58–93 | — | 51–86 | 50–170 | 50–140 |
| Efficiency (%) | 70–85 | 60–75 | — | 55–68 | 60–90 | 50–80 |
| Diastolic indices | ||||||
| −dP/dtmin (mm Hg s−1) | 6,000–12,000 | 6,700–10,500 | 6,900–10,400 | 5,400–9,500 | 5,900–11,900 | 5,970–9,970 |
| Tau (W) (ms) | — | 4.4–7.6 | 4.8–8.5 | 4.9–10 | 7–9.6 | 7–9.9 |
| Tau (G) (ms) | 7–9 | 7–12 | 8–13 | 7–15 | 10–13 | 10–13 |
| EDPVR | — | 0.04–0.12 | — | 0.06–0.2 | 0.01–0.04 | 0.01–0.04 |
BW, body weight; CI, cardiac index, CO/BW; CO, cardiac output; dP/dt−EDV, dP/dtmax−end diastolic volume relation; dP/dtmax, peak rate of pressure rise; −dP/dtmin, peak rate of pressure decline; Ea, arterial elastance (measure of ventricular afterload); EDP, end-diastolic pressure; EDPVR slope, end-diastolic PV relation slope; EDV, end-diastolic volume; Ees/max, end-systolic elastance (slope of the end-systolic relationship); EF, ejection fraction; Efficiency (SW/PV area); ESP, end-systolic pressure; ESV, end-systolic volume; HR, heart rate; MAP, mean arterial pressure; PRSW, preload recruited stroke work (slope of stroke work−EDV relationship); SV, stroke volume; SW, stroke work; Tau (G), relaxation time constant calculated by Glantz method (regression of dP/dt versus pressure); Tau (W), relaxation time constant calculated by Weiss method (regression of log(pressure)); TPR, total peripheral resistance, MAP/CO. The given wide ranges of variables were derived from various control mouse and rat strains and represent large number of experiments over the past 5 years.
The following protocol describes the procedures (summarized in Fig. 1b) for this method (anesthesia/analgesia, intubation techniques (Fig. 2), surgical techniques for LV catheterization (open and closed chest approaches; Fig. 3), vena cava inferior occlusion methods (Fig. 4a) and calibrations (Figs. 4b,c and 5) to convert the raw conductance signals to true volumes), provides movies of the key processes/steps performed in our laboratories (Supplementary Movies 1-5 online), presents representative examples of PV loops and various calculated useful hemodynamics indices (Table 1 and Figs. 5-7) and gives troubleshooting advice (see TROUBLESHOOTING).
Figure 2.

Intubation techniques. (a) Shows a less invasive technique without tracheal incision which requires more experience; (b) shows a more invasive, but simpler technique. The technique shown in (b) is recommended, because the tracheal tube is more secured allowing less chance for accidental sliding out from the trachea during the surgery/measurements. Sequentially numbered panels indicate stages of the procedure for intubation (see also Supplementary Movie 1 online).
Figure 3.
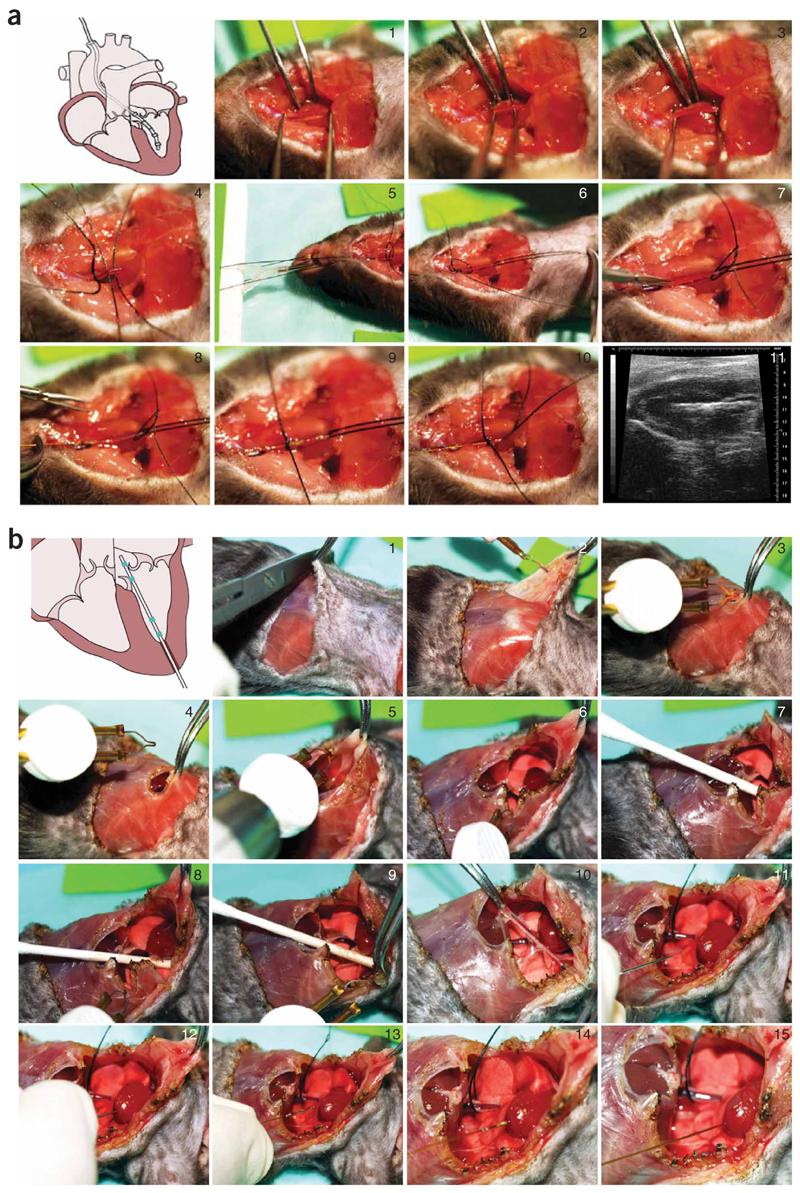
Surgical procedures for LV catheterization. (a) Closed-chest approach: insertion of the catheter into the LV through right carotid artery (see also Supplementary Movie 2 online). Sequentially numbered panels indicate stages of procedure. Image 11 shows mouse PV catheter in the LV on an echo image. (b) Open-chest approach: insertion of the catheter into the LV following stabbing of the apex with a 25–30 gauge needle through the stab wound (see also Supplementary Movie 3 online). Sequentially numbered panels indicate stages of procedure.
Figure 4.
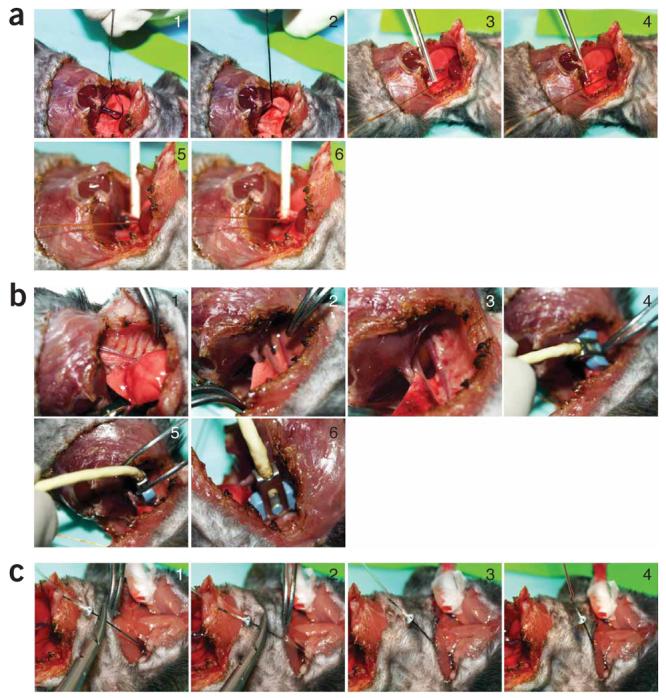
Occlusion techniques, aortic flow measurements and jugular vein injection. (a) Vena cava inferior occlusion techniques. (b) Aortic flow measurements. (c) Jugular vein injection (see also Supplementary Movies 4 and 5 online). Sequentially numbered panels indicate stages of procedure.
Figure 5.
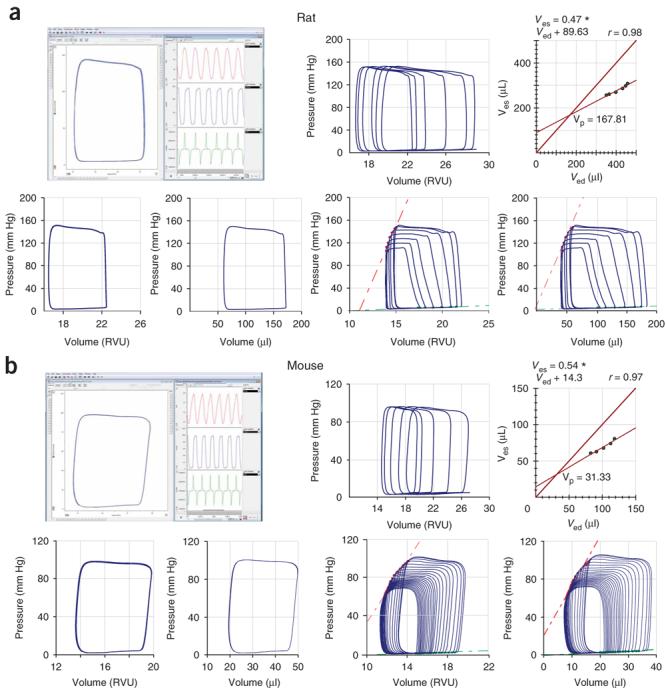
Representative examples of rat and mouse baseline PV loops and occlusions. Examples show representative (a) rat and (b) mouse PV loops before the calibration (in RVUs) and following cuvette and saline calibrations (in microliters) obtained by closed-chest approach with Millar PV system and analyzed by PVAN3.5. Upper rows (a and b), left-hand panels show examples of baseline uncalibrated PV loops (rectangular shape, left side of the panel), volume signal (red trace), pressure signal below volume (blue trace) and +dP/dt and −dP/dt derived from the pressure signal (green trace). Upper rows (a and b) in middle and right show examples of hypertonic saline injections (rightward ship of PV loops without decrease in the amplitude) and derived Vp values. Lower rows (a and b) show uncalibrated (in RVUs) and calibrated (in microliters) normal rat and mouse baseline PV loops (left two panels) and loops following inferior vena cava occlusions (right two panels) See also Supplementary Movies 2 and 4 online, and Figure 6a.
Figure 7.
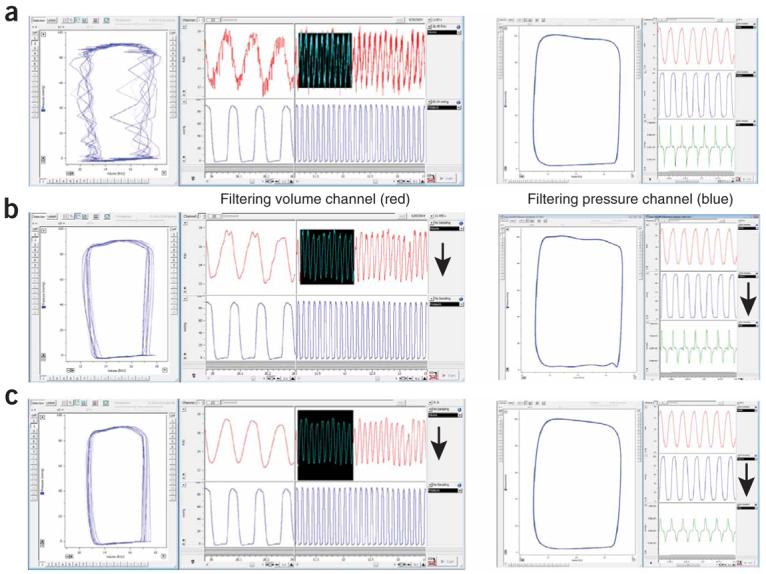
Representative example of noise and effects of filtration of volume/pressure signal on PV relations. Left column: (a) baseline PV loops with noise showing regular pattern at volume channel, (b) the same following the use of a low-pass 60-Hz digital filter (auto adjust function) or (c) smoothing filter (Triangular (Barlett) window with 25 points) applied to the volume channel (left: PV loops (blue trace), right up and down volume (red trace) and pressure (blue trace) signals, respectively. Figure shows that filtering volume channel may significantly improve the signal without major changes in derived parameters. In this particular case, the noise was originated from electrical network and replugging the system into a stabilized circuit/outlet completely solved the problem (the nature of the noise was also indicated by the noise pattern and the effect of the 60-Hz filter). Right column: (a) baseline normal PV loops, (b) the same following the use of a low-pass 60-Hz digital filter (auto adjust function) or (c) smoothing filter (Triangular (Barlett) window with 25 points) applied to the pressure channel (left: PV loops (blue trace), right up volume (red trace), right middle pressure (blue trace) signals and right down (green trace) +dP/dt and −dP/dt, respectively. The figure shows that filtering of pressure signal may profoundly affect important derived parameters (e.g., +dP/dt and −dP/dt, green traces), which is not desirable. Arrows indicate channels at which filters were applied.
Experimental design
Anesthesia, body temperature control and intubation
The following injectable or gas anesthetics/analgesics can be used (see also ref. 25):
Injectable
Ketamine (50 mg kg−1)
Ketamine (50 mg kg−1 + fentanyl 250 μg kg−1)
Ketamine/diazepam (40–80/5–10 mg kg−1)
Ketamine/xylazine (80–100/10 mg kg−1)
Chloral hydrate (300–400 mg kg−1)
Alpha-chloralose (55 mg kg−1)
Pentobarbital sodium (40–80 mg kg−1)
Urethane (800–1,200 mg kg−1)
Etomidate (5–10 mg kg−1)
Inhalants (induction 3–4%, maintenance 1.5% mixed with 100% oxygen)
Halothane
Isoflurane
Methoxyflurane
Analgesia: morphine (1 mg kg−1) or fentanyl (50–250 μg kg−1)
Muscle relaxant: pancuronium (2 mg kg−1)
Euthanasia: pentobarbital
In obese animals, considerable differences may occur in the distribution of the injectable anesthetics, similar to that in aging animals or in animals with impaired liver function. Animals with various models of heart failure and shock may be more sensitive to the cardiodepressive effects of these agents. Even a slight overdose, especially with ketamine/xylazine or pentobarbital sodium, may profoundly decrease the heart rate and cardiac function. For most injectable anesthetics, the intubation of the animals will significantly improve the hemodynamic variables (this is especially critical with pentobarbital, which markedly increases mucus secretion in the respiratory tract). With the proper use and careful optimization, it is possible to achieve reasonable results with almost all anesthetic agents, and we will show examples with ketamine/xylazine, pentobarbital sodium, urethane+etomidate+morphine and isoflurane in our protocol (see Table 1). However, because of the ease of overdosing and decreasing heart rate with some of the above-mentioned agents, we recommend using urethane + etomidate + morphine/fentanyl or isoflurane for anesthesia. There are reports describing extreme cardiodepressive effects of various anesthetics (e.g., ketamine/xylazine or pentobarbital), but most likely a significant part of these effects may actually be attributed to the lack of proper temperature control, intubation and overdosing.
Most of the above-mentioned agents require special handling because of the drug regulation laws and possible toxic effects. Urethane is carcinogenic (avoid contact with the skin) and prolonged use in animals may lead to hemolysis, making urine red. Similarly, avoid contact with and inhalation of gas anesthetics (especially methoxyflurane). Use downdraft table to avoid exposure to waste gases.
Surgical procedures for LV catheterization
For drug testing and more prolonged experiments, the closed-chest approach (see Step 8A, Fig. 3a and Supplementary Movie 2 online) is more suitable because it is less invasive and animals are more stable for a longer period. An additional advantage of this approach in various animal models (e.g., in heart failure) is that arterial pressure records can easily be obtained from the carotid artery at the start or at the end of the experiment, allowing the calculation of total peripheral resistance (TPR) later (TPR = (mean arterial pressure–mean venous pressure)/cardiac output). The carotid approach should also be used in a chronic heart failure model induced by ligation of the left anterior coronary artery because of the scar formation in the apex area. Retrograde insertion via the LV apex does have some methodological advantages even for drug testing or other protocols as proper placement of the catheter in the LV is easier to confirm, and the procedure is done very quickly. In addition, if the carotid artery is severely atherosclerotic (e.g., in ApoE mice fed with a high-fat diet), or when the aortic valve is calcified (e.g., in advanced aging models) or in transverse aortic constriction (TAC)-induced hypertrophy and heart failure models, the open chest approach (Step 8B, Fig. 3b and Supplementary Movie 3 online) is appropriate.
Conductance catheter calibration
The conductance signal is itself noncalibrated and must be carefully converted to absolute volumes if such information is required. The primary equation relating conductance to volume is V = 1/α (ρL2)(G–Gp), where ρ is the blood resistivity, L is the distance between sensing electrodes, G is the conductance (measured as a voltage and utilizing a constant current circuit, this is directly proportional to G), Gp is the parallel conductance due to conductivity of the muscle wall and surrounding tissues and α is a gain coefficient (volume correction/calibration factor). The simplistic model of this approach is that the electric field is as if applied from parallel plates, so the current lines are straight and parallel to the catheter shaft. The fact that we use point source electrodes means that the field lines are curved, and this introduces a value of α that is not unity, and some nonlinearity to the volume signal calibration. This nonlinear behavior is more problematic the larger the heart, and actually, the mouse heart may be the best designed for this technology, given the small distances from the catheter that are involved. In this mammal, the relationship between catheter and Doppler-measured stroke volume, for example, is highly linear over a broad loading range12. The small heart and local electric field distribution also have implications for the parallel conductance, which can be quite large in larger mammals, as right ventricular (RV) volumes are clearly registered in the signal. In the mouse, Gp appears mostly due to the muscle wall, and there is little far field (i.e., RV or other chamber volume) contribution. This was tested and previously demonstrated6,12.
With regards to the actual calibration procedure, there are two approaches generally taken. One is to use mock-up cylinders with known volumes, and the catheter is placed in each using fluid matching the conductivity of blood (or blood), and a calibration curve generated. In our experience, this can yield inaccurate values as the cylinders do not exactly replicate the field distribution in the mouse heart, and calibrations can vary between different types of hearts (e.g., heart failure models, hypertrophy and infarction). Rather, we prefer the direct approach where both the gain and offset of the calibration are determined. The gain is assessed by measuring the cardiac output by Doppler flow probe. This can be done with a flow-velocity probe that can be placed on the ascending (or descending) aorta (Craig Hartley, Houston company) multiplied by a measure of cross-sectional diameter or using a volume flow probe (Transonics) that provides flow without requiring area assessment. For practical reasons, we prefer the latter and use the descending thoracic aorta as the placement position. Although this clearly excludes some relevant blood flow, the anatomy of the mouse (small upper body) implies that fairly little flow is missed, and this proportion is fairly constant among animals6,12.
Data acquisition, analysis, noise filtering
In our experience, sampling data at 1–2 kHz is optimal; higher rates simply increase the file size. If concomitant electrical analysis is being performed, higher digitization rates would be useful. The default setting of the PVAN in PowerLab (PV analysis program) uses 1 kHz. The data analysis using PVAN module integrated into Chart is straightforward and quick (see the manufacturer's instructions). More experienced users may also create algorithms in chart to calculate various selected parameters, but as PVAN is included with the Millar PV system, this is not required. Some users have developed their own custom software for PV analysis7,9.
Noise filtering may be a critical issue under some circumstances. In general, we do not recommend using noise filters. In 99% of cases, noise (mostly appearing at volume channel) can be prevented by very simple things (see TROUBLESHOOTING for details). In most of these cases, noise at volume channel actually originates from electric network and replugging the system into stabilized circuit/outlet may solve the problem. If the noise problem cannot be solved and the noise pattern is regular, most likely one of the filters built into PowerLab/Chart may improve the signal. In these cases, filtering of volume signal may be acceptable (this will only minimally affect derived parameters). However, do not try to filter the pressure signal because it may profoundly affect derived parameters (e.g., +dP\dt) (see Fig. 7 for representative examples). However, if any filter is applied, it should be used for all conditions.
MATERIALS
REAGENTS
Anesthesia/analgesia
Urethane (Sigma-Aldrich, cat. no. U2500) ! CAUTION Use gloves during handling.
Etomidate injection (Bedford Laboratories, cat. no. Div-ETMP02)
Morphine sulfate injection (Baxter Healthcare Corp.)
Nembutal 50 mg ml−1 pentobarbital sodium, USP (Ovation Pharmaceuticals, cat. no. NDC no. 67386-501-55)
Ketaved 100 mg ml−1 ketamine, USP (Vedco, cat. no. NDC no. 50989-248-06)
AnaSed 20 mg kg−1 xylazine solution (Lloyd Laboratories, cat. no. NADA no. 139-236)
Forane isoflurane, USP (Baxter, cat. no. NDC no. 10019-360-40) ! CAUTION Do not inhale; use downdraft table during handling.
Other
0.9% (wt/vol) sodium chloride injection, USP (Hospira, cat. no. NDC no. 0409-4888-50)
Surgical tape
Alconox (Alconox Inc.) for catheter cleaning
EQUIPMENT
Tabletop anesthesia machine, single (Harvard Apparatus, cat. no. 72-3012)
MiniVent ventilator for mice (Harvard Apparatus, cat. no. 73-0043) Harvard ventilator model 683 for rats (Harvard Apparatus, cat. no. 55-0000)
Tracheotomy cannula, 1.3 mm outer diameter (o.d.) for mice (Harvard Apparatus, cat. no. 73-2730)
Tracheotomy cannula, 3.0 mm o.d. for rats (Harvard Apparatus, cat. no. 73-2733)
Tubing kit (Harvard Apparatus, cat. no. 72-1049)
Gaymar T/Pump circulating water blanket (Gaymar Industries, cat. no. TP-400) or Homeothermic Blanket (Harvard Apparatus, cat. no. 507214-16)
Stereo microscope (Carl Zeiss Optical Inc., cat. no. Stemi 2000)
Boom stand for microscope (Diagnostic Instruments Inc., cat. no. SMS6B)
Cole–Parmer Illuminator 50 W (Cole–Parmer, cat. no. 41720)
Battery-operated Thermal Cautery Unit (Fine Science Tools Inc., cat. no. 18015-00)
Dumont no. 55 Dumostar Forceps (Fine Science Tools Inc., cat. no. 11295-51)
Graefe forceps, curved (Fine Science Tools Inc., cat. no. 11052-10)
Moria MC31 forceps (Fine Science Tools Inc., cat. no. 11370-31)
Mayo scissors (Fine Science Tools Inc., cat. no. 14512-15)
Iris scissors (Fine Science Tools Inc., cat. no. 14041-10)
Halsey needle holder (Fine Science Tools Inc., cat. no. 12501-13)
Olsen–Hegar needle holder (Fine Science Tools Inc., cat. no. 12002-12)
Vannas–Tubingen spring scissors, titanium (Fine Science Tools Inc., cat. no. 15610-08)
Durasorb disposable underpads (Kendall/Tyco Healthcare, cat. no. 1038)
Gauze sponges, sterile (Dukal, cat. no. 62208)
Cotton-tipped applicators, sterile (Solon, cat. no. 368)
Surgical suture, black braided silk, 4.0 (Surgical Specialties Corp., cat. no.
Monoject 1 cc tuberculin syringes (Sherwood Medical, cat. no. 501400)
Lo-dose insulin syringe 1/2 cc (Becton Dickinson, cat. no. 329461)
Millar PV system MPVS-300/400 or MPVS Ultra (Millar Instruments Inc.). The system includes calibration cuvette for mice and rats; MPVS Ultra includes resistivity calibration cuvette.
For mice: PVR-1045, sensor tip PV catheter, 1F; or SPR-839, 1.4F (Millar Instruments Inc.); FT111B, 1.2F (SciSense)
For rats: SPR-869, Sensor Tip PV Catheter, 2F small rat; or SPR-838, 2F normal/large rat (Millar Instruments Inc.); FT211B, 1.6 F (SciSense)
PowerLab 4/30 with Chart Pro (AD Instruments Inc., cat. no. ML866/P) (not required for MPVS-400 system)
MiniARCO trimmer (animal clipper; Wahl, cat. no. 8787-450A)
Intradermic tubing PE-10 (Becton Dickinson, cat. no. 427401)
Intradermic tubing PE-50 (Becton Dickinson, cat. no. 427411)
Needle assortment (18, 25 and 30 gauge; Thomas Scientific)
Various-sized syringes
Perivascular flow module TS420 (Transonic Systems Inc.)
1-mm perivascular probes (Transonic Systems Inc., cat. no. 1PRB)
Visual Sonic Echo system (optional; Vevo 770 High-Resolution In Vivo Imaging System; RMV 707B High Frame Rate Scanhead; RMV 716 Scanhead, Vevo Integrated Rail System III, Imaging Kit, Aquasonic ultrasound gel)
PROCEDURE
▲ CRITICAL All animal procedures described or shown in videos were approved by the Institutional Animal Care and Use Committees of NIAAA or Johns Hopkins Medical Institutions and were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Anesthesia, body temperature control and intubation ● TIMING 10–30 min
1| Deliver intraperitoneal injection of chosen anesthetics in 0.5 ml of physiological saline solution (we recommend urethane (800 mg kg−1) + etomidate (5–10 mg kg−1) + morphine (2 mg kg−1)) or put animal into the anesthesia chamber containing gas anesthetic (we recommend isoflurane 3–4% for induction). See Experimental design for further information on anaesthetics/analgesics.
▲ CRITICAL STEP It is critical to optimize the dose of any anesthetic used for the given mouse/rat strain and the pathological model evaluated (see Experimental design for further details).
2| When the animal is anesthetized (not responding to tail or ear pinch), shave the neck and chest area with a clipper and carefully move the animal to the heating pad with the water temperature set to 40 °C; in the case of a servo-controlled homeothermic blanket, insert the temperature feedback probe into the rectum and set the desired body temperature to 37–37.5 °C.
▲ CRITICAL STEP The temperature control in anesthetized mice and rats is critical (especially in mice). Without proper heating, the heart rate in anesthetized mice can reduce almost to half of the normal rate.
3| Using surgical tape, tape down the front paws and one distal paw of the mouse to the heating blanket (use the remaining distal paw to monitor the depth of anesthesia: movement of the paw can indicate decrease of the depth of the anesthesia). Also, place a thin piece of tape across the tip of the snout, and tape this down to pull the head back slightly to create traction on the trachea. Instead of the tape for the latter procedure, a needle holder can also be used.
▲ CRITICAL STEP Do not obstruct the nostrils because mice are obligate nose breathers.
4| Following midline neck incision, pull the skin away from the underlying muscles and cut it off. Pull the pretracheal muscles apart gently with forceps and dissect the underneath of the trachea to free it from surrounding tissues.
▲ CRITICAL STEP Be careful not to damage carotid arteries and vagus nerves, which run alongside the trachea.
5| Pass a #4 surgical silk suture underneath the trachea, make a small cut onto the surface, insert the tracheotomy cannula (1.3 mm o.d. for mice and 3.0 mm for rats) and tie down with the suture (Fig. 2b; Supplementary Movie 1 online). Alternatively, intubation can also be performed without a tracheal incision as shown in Fig. 2a; Supplementary Movie 1 online, but this procedure requires more experience.
6| Connect the tracheotomy cannula to the respirator in case of gas anesthesia providing mixture of 100% oxygen and 2% isoflurane (later, isoflurane can be decreased to 1–1.5%).
7| Calculate and set the recommended ventilation settings for mice or rats, depending on the animal weight, according the following formulas: tidal volume (Vt, ml) = 6.2 M1.01 (M = animal mass, kg); respiration rate (RR, min−1) = 53.5 × M−0.26. For example, in a 30-g mouse, Vt = 179.6 μl and RR is 133 min−1. In a 400-g rat, Vt = 2,457 μl and RR is 68. Regularly monitor the depth of the anesthesia by checking the response to a tail pinch and make necessary adjustments if required. In the case of an injected anesthetic, after 20–40 min if required, inject approximately 15–20% of the initial amount i.p. or i.v. to maintain the level of anesthesia.
Surgical procedures for LV caxstheterization ● TIMING 10–30 min
8| The PV catheter can be inserted to the LV through right carotid artery without opening the chest cavity (closed-chest approach, option A; see Supplementary Movie 2 online, and Fig. 3a) or after the chest opening and stabbing the apex of the left ventricle with a 25–30 gauge needle through the stab wound (open-chest approach, option B; see Supplementary Movie 3 online, and Fig. 3b). Perform these procedures (see outlined below) under a stereomicroscope (×10 magnification).
(A) Closed-chest approach (right carotid artery catheter insertion)
(i) In an immobilized anesthetized animal, make an inverted T-shaped middle-neck incision from mandible to the sternum. Move aside parotid glands and with forceps, bluntly dissect the thin muscle layer around the throat to expose and isolate the right carotid artery (Fig. 3a, panels 1–3; Supplementary Movie 2 online). ▲ CRITICAL STEP It is very important to separate carotid artery from vagus before ligation.
(ii) Secure suture around the proximal end of the artery, gently pull it and tape it to the table (or use a needle holder instead of the tape) and insert two additional sutures beneath carotid artery. Place a very loose knot to the middle suture and gently pull the distal suture (without securing it) with a needle holder and clamp it to the skin of the animal to fix it in the desired position (Fig. 3a, panels 4–6).
(iii) Put a couple of drops of physiological saline in the vessel area, make a tiny incision near the proximal end of the artery with a microincision scissors and extend the incision longitudinally for a short distance (Fig. 3a, panel 7).
(iv) Pull back slightly on the arterial flap while inserting the catheter tip into the vessel followed by gently securing the middle suture (Fig. 3a, panels 7–9).
(v) Simultaneously, release the distal suture and quickly advance the presoaked catheter (for 30 min into physiological saline solution) into the left ventricle until the PV signal is displayed in the monitor (Fig. 3a, panel 10, Figs 5a,b; also see Supplementary Movie 2 online). ▲ CRITICAL STEP Never force the catheter and never grab the sensors by forceps (this can damage it and perforate vessel or ventricle). If some resistance is encountered while advancing the catheter tip, gently pull back and try advancing again until the catheter is inside the ventricle; be patient. If required, gently rotate the catheter shaft to achieve optimal placement of the tip along the axis of the left ventricle (Fig. 3a, panel 10). After stabilization of the signal for 10–15 min, record the baseline PV loops at a steady state or at varying preloads during the vena cava inferior occlusions (see below and also Figs. 4a and 5).
(vi) It is advisable to cannulate left jugular vein at this point by inserting a plastic tube (P10 in mice) or cannulation needle connected to tubing for later possible fluid/drug or saline injections/infusions as shown in Figure 4c and Supplementary Movie 5 online.
(vii) To test the effect of a new drug that may also affect peripheral resistance, it is advisable to cannulate one of the femoral arteries with a plastic tube and connect it to a pressure transducer recording on separate channel at PowerLab. This way, you can also calculate the time course of the TPR changes during the experiment.
(B) Open-chest approach (catheter insertion after the chest opening and stabbing the apex of the left ventricle with a 25–30 gauge needle through the stab wound)
(i) In anesthetized, fixed and intubated/respirated animals, make an incision over the xyphoid process and separate the skin from the chest wall by blunt lateral dissections with scissors and/or heat cauterization. Starting around the xyphoid process, cut through the chest wall moving laterally on both sides with the cauter until the diaphragm is clearly visible from beneath. Cut through the diaphragm to expose the apex of the heart. Alternatively, begin the chest incision (anterior thoracotomy) above the xyphoid process, cut across the sternum to fully expose the chest cavity and retract the chest walls either by pulling sutures through the rib cage or by using a chest retractor (Fig. 3b, panels 1–9 and Supplementary Movie 3 online).
(ii) Gently remove the pericardium from the heart with forceps (Fig. 3b, panel 10).
(iii) Using a 25–30 gauge needle, make a stab wound near the apex of the heart into the left ventricle and remove the needle. Do not push the needle in more than 2–4 mm (Fig. 3b, panels 11 and 12).
(iv) Insert the catheter tip retrograde into the left ventricle until the proximal electrode on the catheter (E4) is just inside the ventricular wall. Adjust the position of the catheter to obtain rectangular-shaped pressure volume (in diseased animals, the shape may not be rectangular) loops (Figs. 5 and 6).
(v) After stabilization of the signal for 10–15 min, record baseline PV loops at steady state or at varying preloads during the inferior vena cava occlusions. This latter procedure is used to derive various load-independent indices of systolic function.
(vi) Vena cava inferior occlusions can be performed in open-chest respirated animals by pulling of a suture placed around the vessel, by lifting the vessel with a small stick or compressing it with a forceps (Fig. 4a, and Supplementary Movie 4 online). In case of the closed-chest approach, it is possible to obtain reasonable vena cava occlusions by compression of the inferior vena cava through diaphragm with a cotton tip applicator from the opened abdominal cavity (without chest opening). However, this requires considerable experience and may not be as reproducible as the other above-mentioned methods. During the data collection, shut off the small animal respirator for a few seconds to acquire data without lung motion artifact.
(vii) At the conclusion of the experiment, remove the catheter by gently pulling it back through the stab wound and euthanize the animal by an overdose of pentobarbital. Upon removal, immediately place the tip of the catheter into a syringe filled with saline to prevent clotting.
(viii) Clean the catheter with the detergent provided (Alconox) according to Millar's instructions (see also Millar training CD). Proper care of the catheter will considerably prolong its useful life.
Figure 6.
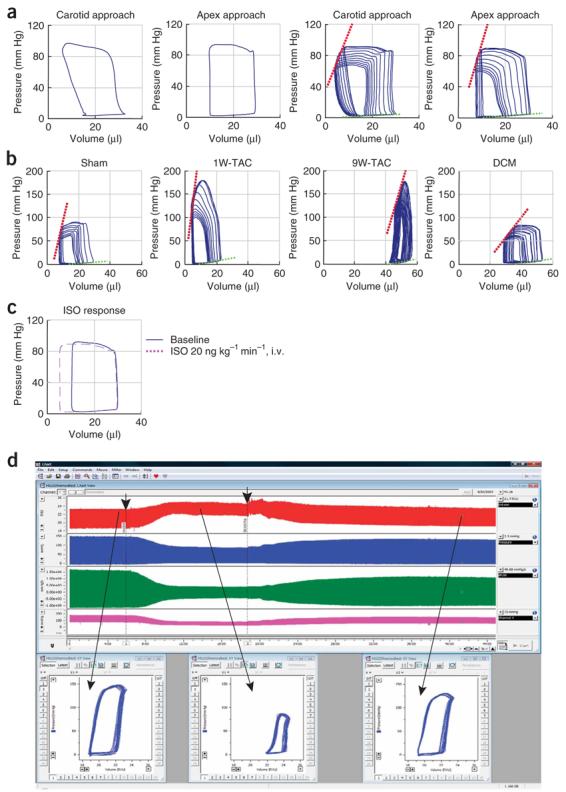
Representative normal and pathological mouse baseline PV loops and occlusions, and example of hemodynamic effects of drug in mice and rats. (a) Normal calibrated baseline PV loops and occlusions (calibration was performed on the basis of flow measurements and hypertonic saline injections). Normal baseline PV loops from mice (left two panels) and loops during vena cava inferior occlusions (right two panels) obtained from LV catheterization through right carotid artery (carotid approach) or through ventricular apex (see also Fig. 5 and Supplementary Movies 2-3 online). (b) Characteristic changes in PV relationships obtained by vena cava inferior occlusions in control (sham)mice, 1 and 9 weeks following TAC, and in dilated cardiomyopathy (DCM). (c) Characteristic changes in baseline (blue continuous trace) PV relationships during isoproterenol (ISO, red) infusion. (d) Characteristic changes in rat LV volume (red trace) and pressure (blue trace), +dP/dt and −dP/dt derived from LV pressure signal (green trace), and arterial pressure (purple trace) before (at baseline) or after an administration of a drug with known cardiodepressive properties (a cannabinoid type 1 (CB1) receptor agonist Hu210), followed by the recovery after the administration of the CB1 antagonist SR141716 (drug administrations are indicated by arrows). Lower panels show characteristic PV loops at baseline and following the drug administrations. Note that even without any calibrations, the volume traces and PV relationships are very informative.
Catheter calibrations, data analysis ● TIMING 15–60 min
9| Catheter calibrations and data analysis can be carried out by relative volume unit (RVU) calibration (option A), cuvette calibration with fresh heparinized, warm blood (option B), saline calibration (option C) or aortic flow calibration (option D) (see Supplementary Movie 5 online, and Figs. 4b and 5a,b).
(A) RVU calibration
(i) This is the default built-in calibration in the Millar PV systems (converts conductance to RVUs). Perform the calibration according to the manufacturer's instructions and always record raw data in raw RVUs (do not attempt to create charts with predefined formulas that immediately convert the RVUs to true volume signal based on cuvette and saline calibrations during the online streaming because if there is a mistake in calibrations, original data may not be retrievable). Analysis of RVU signals even without conversion to absolute volumes can be very informative in most pathological models.
(B) Cuvette calibration with fresh heparinized, warm blood
(i) With the calibration cuvette, an actual blood sample from the animal is used to make a more accurate assessment of LV blood volume and convert the volume data from RVUs to units of true volume (μl): use a mouse or rat insulator-type calibration cuvette with known diameter provided by manufacturer, place it on a heating pad or into a 37 °C prewarmed water bath and fill quickly the first 4–5 holes with freshly taken warm blood from heparinized animals (Supplementary Movie 5 online).
(ii) Hold the catheter tip centered above the well of the cuvette (guides provided by manufacturer may be used), quickly submerge it in the blood (all 4 electrodes should be submerged) and hold it as steady as possible for 10–20 s in each well (normally using the first 1–5 wells is sufficient).
(iii) Record the conductance changes in the volume channel in RVUs. Calculate the volume of the wells (or check in the manufacturer's instructions) using the equation for the volume of a cylinder, where the radius is that of the cuvette well and the length is based on the length between the inner two sensing electrodes (E2 and E3) on the catheter tip. The conductance output from dipping the catheter tip in the wells can be correlated with the known volumes to develop a calibration equation that converts the data from RVUs into units of true volume (μl). According to our experience, the calculated slope of the equation in mice based on 2–5 point cuvette calibrations varies from 4 to 7 and the intercept varies from −10 to −25; in rats, these values are from 10 to 20 and from −14 to −80, respectively.
(iv) Enter the well volume and corresponding blood conductance values into PVAN (or the slope and intercept values of the equation) and make conversion of RVUs to more realistic first estimate of true volumes (μl). However, these estimated volume signals are still larger than expected because of the parallel conductance, which refers to the conductivity of the heart muscle that surrounds the LV blood pool. To account and correct for this change, perform intravenous hypertonic saline calibration as described below.
(C) Saline calibration
(i) Ideally, the current applied to the excitation electrodes on the catheter should go through the blood only, but in reality, some of the applied current flows into the surrounding muscle, which is also a conductor, often causing an overestimation of the blood volume within the ventricle. As the heart muscle acts as a shunt to the applied current, this effect is referred to as parallel conductance or parallel resistance, or in volume calculations as parallel volume (Vp). To obtain a value for Vp, perform a saline bolus calibration with hypertonic saline (30%) bolus injection into the animal at the conclusion of the experiment as follows: firstly, cannulate jugular vein as shown in Supplementary Movie 5 online, and Figure 4c.
(ii) After turning of the respirator for a few seconds (and during the injection), inject 5–10 μl hypertonic (30% saline solution) i.v. into mice and 20–40 μl into rats to obtain visible shift to the right in PV loops (change in conductance) without significant decrease in the pressure signal amplitude (e.g., see Supplementary Movie 5 online, and Fig. 5a,b). The parallel volume is calculated by solving a system of linear equations to locate the intersection of two lines, one represented by the saline calibration data (plotting Ved versus Ves for each beat during the phase where the volume signal appears to rise following the hypertonic saline bolus) and the other by Ved = Ves. The Ved = Ves line represents equal end-systolic and end-diastolic volumes or the equivalent of a heart chamber devoid of blood. The value at the intersection of the Ved = Ves line and the saline calibration line represents the parallel volume of muscle tissue only (Vp) and is calculated by PVAN program. According to our experience, Vp values in mice (depending on size and strain) vary from 17 to 42 μl and in rats from 130 to 280 μl. Details of the method and its theory have been reported previously6,21.
(iii) Because of the possible variability, perform at least 2–3 saline calibrations in each animal. Every time, wait a few minutes for the volume signal to recover. Use only the data during the rising phase of this intervention.
(iv) Enter the calculated Vp value from saline injection to PVAN together with the parameters derived from cuvette calibrations and convert volume to true volumes in microliters (see Fig. 5 and Table 1 for examples).
(D) Aortic flow calibration
(i) Following the collection of the data, the conductance signal can also be converted to true volumes by performing the saline calibration as described above in conjunction with aortic flow determinations8. Perform open chest surgery as described previously (Step 8B).
(ii) Slowly turn the animal onto its left side, paying attention to only minimally disturb the volume signal. Make a lateral thoracotomy between T3 and T5 to create a small window and gently dissect a small part of the thoracic aorta running parallel to the spinal column with forceps (see Supplementary Movie 5 online, Fig. 3b, panels 6–9, and Fig. 4b).
(iii) Use either a perivascular flow probe (Transonics) placed around the aorta to assess cardiac output or a Doppler flow to provide flow velocity. The latter is then multiplied by the aortic cross-sectional area (echo image) to determine volume flow. The phasic flow data are then integrated to determine stroke volume, and this is assessed while simultaneous measurements of the PV loops are made. The latter provides stroke volume (SV) from the catheter, determined by the mean width of the PV loop. The ratio provides the calibration gain. ? TROUBLESHOOTING
● TIMING
The total amount of time necessary for anesthesia, surgery and calibrations for one animal may largely depend on the experience and surgical skills of the investigator and experimental protocol used. This time can vary from 1 to several hours. Plan around 1 week for a study with several groups with 8–14 animals each. It is advisable to start with a few control animals on the day of the experiment to make sure that everything is optimized and working well, followed by measurements in pathological states or groups. This will also minimize the potential errors in case the catheter needs to be replaced in the middle of the study by another one with slightly different properties.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table.
| Potential problem(s) | Possible source(s) and solution(s) |
|---|---|
| From the beginning of the experiment, the uncalibrated volume signal is in the normal range (in mice 4–7; in rats 5–10 RVUs), pressure signal and dP/dt is in the lower range, the heart rate is very low (see Table 1 for normal values) |
The temperature of the animal may have dropped or the anesthetic is overdosed. Check the temperature of the animal and the temperature control unit; also check the depth of the anesthesia and make sure that the anesthetic was not overdosed. If so, in case of inhalation anesthesia, decrease the concentration by 1–1.5%, or for a short period, use just 100% oxygen. Make adjustments and allow 10–15 min for stabilization. In case of injectable anesthetic overdose, i.v. fluid injection and 100% oxygen can help recovery. Make sure that animals are always intubated if injectable anesthesia is performed and trachea tubes are not blocked by mucus. If so, insert a small plastic tube (P10 in mice) to the tube connected to a 10–20 ml syringe and suck out obstructing mucus |
| From the beginning of the experiment, pressure signal and dP/dt is low |
Animal may have lost too much blood during the surgery and/or fluid via evaporation through the surgical surface. Anesthesia/analgesia may also be overdosed or not sufficient. Use battery-operated electrocautery during the surgery to minimize blood loss and always moisturize the surgical surface with physiological saline to decrease drying out and evaporation. If the blood/fluid loss is significant, slowly inject/infuse physiological saline solution into the jugular or femoral veins; in more severe cases, inject albumin- containing solution. If the abdominal cavity is intact, saline solution can also be injected i.p. Check the anesthesia/analgesia depth. Paradoxically, if animals feel pain and distress, it can also lead to hemodynamic instability; if so, adjust the drug concentrations |
| From the beginning of the experiment, pressure signal and dP/dt is normal, but volume signal is very low |
Most likely, the catheter is not in the right position. Try to improve the volume signal by gently readjusting the position of the catheter |
| The pressure and/or volume signal is noisy, regular 50 Hz (Europe) to 60 Hz (USA) noise pattern |
The most likely cause is electric interference, which can originate from pumps, electrocautery devices, ungrounded operating tables, ventilators/respirators, fluorescent lamps, transformers, fans and electric warming blankets. Try to isolate any sources of electrical interference by moving them away from the catheter and by sequentially unplugging the possible interfering appliances. If the noise is coming from the power supply, sometimes just unplugging the MPVS system and plugging into different outlet, preferably with stabilized power supply for the laboratory equipment, can eliminate the problem. If the noise problem from the electrical network cannot be resolved for any reasons, the built-in 50- and 60-Hz filter in PowerLab can sometimes improve results |
| The pressure and/or volume signal is noisy and shows irregular pattern |
If all of the above fail and the noise is irregular, check if it disappears with another catheter. If so, the first catheter is most likely damaged. Check catheter for damage under the microscope, and if the coating is disrupted, clean and send it immediately to the manufacturer for further evaluation and repair. Always have at least one or two backup catheters before starting a study |
| The pressure sensor shows an excessive amount of drift, and/or it is not possible to ‘zero balance’ the pressure transducer output |
Fluid may have gotten inside the catheter, or the pressure sensor diaphragm may be cracked or broken. It is possible to check the functionality of the pressure sensor by connecting an ohmmeter (resistance gauge) across the pins of the pressure sensor connector. The bridge on the Millar sensor should have symmetrical input and output impedances of approximately 1,000 ohms. If either reading is dramatically different from 1,000 ohms, there are chances that the pressure sensor is broken because of the above-mentioned reasons. Please contact Millar Instruments' customer support |
| The pressure sensor appears to be functional, but the readings are off by approximately 25 or 100 mm Hg |
The pressure calibration in the data acquisition hardware is incorrect. Recalibrate the pressure signal in the data acquisition software according to the manufacturer's instructions. Briefly, the pressure control side of the MPVS-300/400 has three calibration settings. The settings are 0, 25 and 100 mm Hg. The standardized pressure output from the MPVS-300/400 is 1 V per 100 mm Hg. Therefore, the 0 mm Hg calibration setting corresponds to 0 V output, 25 mm Hg corresponds to 250 mV (0.25 V) output, and 100 mm Hg corresponds to 1 V output. In the case where the pressure readings are off by exactly 25 or 100 mm Hg, the wrong units label has been assigned to the voltage output coming out of the MPVS-300/400 for a particular calibration setting (e.g., the 25 mm Hg units label was assigned to 0 V out rather than 0 mm Hg units label) |
| The zero balanced pressure sensor in saline or distilled water drifts a few mm Hg after insertion into the biological environment |
The most likely reason is that the catheter was not properly prepared before inserting it to the animal. Presoak the catheter tip in saline at body temperature for at least 30 min before use (e.g., by inserting the tip (but not the connectors) through the tip of a 1-ml syringe containing physiological saline solution) |
| The volume/conductance signal output from the MPVS-300/400 appears to drift |
Electronic hardware has some drift present in the signal as a result of the electronic components heating up. To minimize conductance signal drift from the MPVS-300/400, turn it on and let it warm up at least 30 min before calibrating the output and using the catheter to collect PV data |
| The calibrated volume signal is larger than expected | Following the cuvette calibration, the volume signal (converted from RVUs to uLs) is larger than expected. The reason for this is that the parallel volume contribution of the myocardium (parallel conductance, which refers to the conductivity of the heart muscle that surrounds the left ventricular blood pool) has not been taken into account. Perform hypertonic saline calibration to correct for Vp |
| The conductance/volume signal is out of range | In larger animals with dilated cardiomyopathy, the conductance signal may go out of the range. Measure the ventricular length of the animal in question and make sure that the catheter being used has electrode spacing that matches this length (e.g., a 6-mm signal electrode spacing is appropriate for small-sized rats and a 9-mm signal electrode spacing is appropriate for larger rats). In some cases, custom catheter design may be required; contact the manufacturer with such request |
| The volume/conductance outputs from the cuvette calibration are not consistent |
Make sure that the cuvette with fresh heparinized blood (to avoid clotting) is at body temperature. Always properly clean the cuvette between uses. Center the catheter tip within the cuvette and submerge it (all four electrodes should be submerged) in the blood, and hold it as steady as possible for 10–20 s in each well (normally using the first 1–5 wells is sufficient) |
ANTICIPATED RESULTS
Using miniature PV catheters advanced in the left ventricle via right carotid artery or ventricular apex following stabbing with a needle, this sophisticated methodology allows simultaneous measurements of both pressure and volume signals from the mouse with intact heart beat and rat hearts both at steady state and during manipulation or decreasing of the preload through VCI occlusions. The estimation of the absolute volume from the raw conductance measurements is possible by various calibrations (RVU (built into the Millar PV systems), external cuvette with blood and hypertonic saline calibrations in live animals) or by combination of the use of independent measure of cardiac output and hypertonic saline calibration as described in this protocol (for representative examples of baseline PV loops and occlusions before and after saline calibrations, see Figs. 5 and 6; Supplementary Movies 2, 4 and 5 online). With both surgical techniques, very similar results can be obtained, given the careful optimization of the temperature regulation, fluid balance and anesthesia/analgesia during the surgery and ultimately the surgical procedures, which are the major keys to the success of this whole approach (see Supplementary Movies 1-5 online, and Figs. 2-4).
A large number of hemodynamic parameters and indices of systolic and diastolic function can be derived from PV relations both from steady state data and from data measured at decreasing preloads (for normal ranges of selected, commonly used both load-dependent and load-independent hemodynamics variables obtained in our laboratories with various anesthesia protocols and approaches, see Table 1). For representative mouse PV loops and occlusions at baseline and during the development of myocardial hypertrophy and failure 1 and 9 weeks following TAC, in dilated cardiomyopathy and following drug additions, see Figure 6.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of NIH/NIAAA (to P.P.). NIH-NHLBI HL-077180; HL-59408. We are indebted to Huntly Millar and Tim Daugherty for reading the protocol and valuable suggestions and acknowledge Millar Instruments for the permission to use animations in the movies and for providing background information on the use of Millar PV systems. P.P. dedicates this protocol to his beloved mother Iren Bolfert and grandmother Ilona Kerenyi.
Footnotes
Supplementary information is available via the HTML version of this article.
References
- 1.Sagawa K, Maughan WL, Suga H, Sunagawa K. Cardiac Contraction and the Pressure–Volume Relationship. Oxford University Press; New York: 1988. [Google Scholar]
- 2.Kass DA, Yamazaki T, Burkhoff D, Maughan WL, Sagawa K. Determination of left ventricular end-systolic pressure–volume relationships by the conductance (volume) catheter technique. Circulation. 1986;73:586–595. doi: 10.1161/01.cir.73.3.586. [DOI] [PubMed] [Google Scholar]
- 3.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure–volume analysis: a guide for clinical, translational, and basic researchers. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 4.Little WC, Cheng CP. Effect of exercise on left ventricular-arterial coupling assessed in the pressure–volume plane. Am. J. Physiol. 1993;264:H1629–H1633. doi: 10.1152/ajpheart.1993.264.5.H1629. [DOI] [PubMed] [Google Scholar]
- 5.Kass DA, Midei M, Graves W, Brinker JA, Maughan WL. Use of a conductance (volume) catheter and transient inferior vena caval occlusion for rapid determination of pressure–volume relationships in man. Cathet. Cardiovasc. Diagn. 1988;15:192–202. doi: 10.1002/ccd.1810150314. [DOI] [PubMed] [Google Scholar]
- 6.Georgakopoulos D, et al. In vivo murine left ventricular pressure–volume relations by miniaturized conductance micromanometry. Am. J. Physiol. 1998;274:H1416–H1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- 7.Georgakopoulos D, et al. The pathogenesis of familial hypertrophic cardiomyopathy: early and evolving effects from an alpha-cardiac myosin heavy chain missense mutation. Nat. Med. 1999;5:327–330. doi: 10.1038/6549. [DOI] [PubMed] [Google Scholar]
- 8.Nagayama T, et al. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation. 2007;116:2399–2408. doi: 10.1161/CIRCULATIONAHA.107.706523. [DOI] [PubMed] [Google Scholar]
- 9.Takimoto E, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 10.Takimoto E, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacher P, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 12.Georgakopoulos D, Kass DA. Estimation of parallel conductance by dual-frequency conductance catheter in mice. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H443–H450. doi: 10.1152/ajpheart.2000.279.1.H443. [DOI] [PubMed] [Google Scholar]
- 13.Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacher P, et al. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int. J. Mol. Med. 2006;17:369–375. [PMC free article] [PubMed] [Google Scholar]
- 15.Batkai S, et al. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H909–H918. doi: 10.1152/ajpheart.00373.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay P, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacher P, et al. Left ventricular pressure–volume relationship in a rat model of advanced aging-associated heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2132–H2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacher P, et al. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J. Pharmacol. Exp. Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batkai S, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batkai S, et al. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1689–H1695. doi: 10.1152/ajpheart.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baan J, et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70:812–823. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]
- 22.Kass DA, Maughan WL. From ‘Emax’ to pressure–volume relations: a broader view. Circulation. 1988;77:1203–1212. doi: 10.1161/01.cir.77.6.1203. [DOI] [PubMed] [Google Scholar]
- 23.Kass DA, et al. Influence of contractile state on curvilinearity of in situ end-systolic pressure–volume relations. Circulation. 1989;79:167–178. doi: 10.1161/01.cir.79.1.167. [DOI] [PubMed] [Google Scholar]
- 24.Glower DD, et al. Linearity of the Frank–Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 25.Hanusch C, Hoeger S, Beck GC. Anaesthesia of small rodents during magnetic resonance imaging. Methods. 2007;43:68–78. doi: 10.1016/j.ymeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


