Abstract
Regular exercise can counteract the adverse effects of aging on the musculoskeletal and cardiovascular systems. In males the normal aging process is associated with reductions in testosterone production’(Matsumoto 2002); (synder 2001) and impaired spermatogenesis, but the underlying mechanisms and their potential modification by exercise are unknown. Here we report that lifelong regular exercise (running) protects the testes against maturation as well as age related changes, and that this effect of running is associated with decreased amounts of oxidative damage to proteins, lipids and DNA in spermatogenic and Leydig cells. Six month-old male mice were divided into a sedentary group and a group which ran an average of 1.75 km/day, until the mice reached the age of 20 months. Seminiferous tubules of runners exhibited a full complement of cells at different stages of the spermatogenic process and a clear central lumen with large numbers of spermatozoa, in contrast to sedentary mice which exhibited disorganized spermatogenic cells and lacked spermatocytes in a central lumen. Levels of protein carbonyls, nitrotyrosine, lipid peroxidation products and oxidatively modified DNA were significantly greater in spermatogenic and Leydig cells of sedentary mice compared to runners. These findings suggest that lifelong regular exercise suppresses aging of the testes by a mechanism that involves reduced oxidative damage to spermatogenic and Leydig cells.
Keywords: Leydig cells, seminiferous tubules, lipid peroxidation, testosterone, spermatogenesis
Introduction
Although the aging process is complex and the underlying mechanisms poorly understood, there is considerable evidence that cumulative oxidative damage to proteins, lipids and DNA is involved (Liu, et al. 2008; Mattson and Liu 2002; Sanz, et al. 2006; Sohal and Weindruch 1996) Major reactive oxygen species (ROS) implicated in age-related tissue dysfunction and damage include superoxide anion radical, hydrogen peroxide, hydroxyl radical, and nitric oxide and peroxynitrite (Cadenas and Davies 2000; Drew and Leeuwenburgh 2002). These ROS can cause damage to membranes (lipid peroxidation) and molecular modifications of proteins including protein carbonyl formation, nitration and covalent modification by lipid aldehydes. Physical exercise is used for different purposes including the enhancement of competitive performance and disease prevention. Regular physical activity may reduce the risk of type 2 diabetes, cardiovascular diseases and some cancers (Dugan 2007). During exercise there are increased metabolic demands on the musculoskeletal and cardiovascular systems, and a concomitant increase in the production of reactive oxygen species (ROS). However, the cells respond to the oxidative stress by increasing the production of antioxidant enzymes and other defense mechanisms (Banerjee, et al. 2003; Jackson, et al. 2004). Such adaptive responses to the cellular stress associated with exercise may contribute to the beneficial effects of exercise on health.
As with other organ systems, the male reproductive organs suffer functional decline with aging characterized by reduced serum level of testosterone and reduced production of sperm ((Barnes, et al. 1998; Harman, et al. 2001; Matsumoto 2002; Vermeulen 1991)
Morphological characteristics of ageing testes include a reduced number of type-A dark spermatogonia, and increased occurrence of multinucleated spermatogonia, egalospermatocytes and giant spermatids (Schulze and Schulze 1981); (Gosden, et al. 1982); (Almahbobi, et al. 1988). Sertoli cells accumulate cytoplasmic lipid droplets and are reduced in number, as are the Leydig cells during aging (Johnson 1986). The causes of cellular dysfunction in the testes during aging are unknown, but oxidative stress is implicated (Cao, et al. 2004). Intense exercise has been reported to result in decreased production of testosterone and decreased spermatogenesis (Cumming, et al. 1989); (Hackney, et al. 2003); (Manna, et al. 2003). However, the pro-oxidative effect of acute exercise was attenuated in rats that had been exercised regularly on a treadmill for 8 weeks prior to the acute exhaustive exercise (Aksoy, et al. 2006). Although regular exercise is associated with improved health outcomes in humans, the effects of long-term regular exercise on the testes are unknown. Here we report that lifelong running protects the testes against age-related cytological alteration and oxidative stress in mice.
Material and Methods
Animals and Wheel Running
Six month-old male C57BL/6 mice were divided into a sedentary control group (n = 28) and a running group (n = 18). The running group ran an average of 1.75 km/day on a home cage running wheel (Columbus Instruments, Columbus, OH, USA) for 14 months. The wheel itself clips to the wire lid inside the cage and contains an embedded magnetic indicator. The rotation detector attaches to the lip of the plastic dome outside of the cage and the hall-effect detector counts the number of rotations of the wheel. Detectors plug into a common interface that connects to computer via RS232. Wheel rotations are converted into km automatically by software, whereas the cages of the sedentary group contained a locked running wheel. The mice were maintained for 14 months with continuous access to water and food, in a 12 h light/12 h dark cycle.
Quantification of Sertoli and Leydig cell numbers
Quantitative assessment of sertoli and leydig cell numbers was made to obtain relative differences between sedentary versus runner mice. Estimates were obtained by randomly superimposing a forbidden line unbiased counting frame (Myers, et al. 2005) on enlarged photomicrographs of stained tissues where the nuclei of Sertoli cells could be unequivocally identified by their characteristic location and appearance (eg tripartite nucleolus, (Howard CV 2005). Using this counting frame, the numbers of nuclear profiles per unit area of testis were counted. Sertoli cells were identified primarily based on nuclear morphology and based on their proximity to basement membrane. The morophological features we used to identify these cells were irregularly shaped, folded nuclear membranes and the presence of a prominent nucleolus. Cytosolic features such as lipid vacuoles and granular eosinophilic material were also considered. Leydig cells are identified based on location (interstitium) and histological features including a round vesicular nucleus with one or two eccentrically located nucleoli along with an abundant cytoplasm with lipid droplets and lipofuscin pigment.
Serum testosterone measurement
Mice were anesthetized with isoflurane and immediately dissected to access the heart. Blood was collected and spun down at 10,000 × g for 10 min at 4°C to isolate the serum. If the serum was not used immediately, it was kept at −20°C until further analysis. Serum testosterone concentrations from individual mice were measured by National Hormone & Peptide Program (NHPP) Harbor-UCLA Medical Center, Torrance, CA by radioimmunoassay after ether extraction according to published methodology (Resko, et al. 1973). The sensitivity of the assay was 7.8 pg per tube, the inter-assay variation was 8%, and the intra-assay variation was 4.3%.
Oxidative stress markers
Testes were homogenized in T-PER Reagent according to manufacturer’s instructions. (Pierce, Rockford, IL. Cat # 78510) Samples were centrifuged to pellet tissue debris. For each assay, 40 ug of protein was used. An Amplex Red Hydrogen peroxide/Peroxidase Assay Kit (Molecular Probes, Eugene, Oregon) was used to measure H2O2 levels using the protocol provided with the kit. Briefly, 5 μM Amplex Red and 15 μg/ml horseradish peroxidase (3.75 Units) were included in the incubations. H2O2 was detected by the formation of the fluorescent Amplex Red oxidation product resorufin using excitation and emission wavelengths of 563 and 587 nm, respectively. Lipid peroxidation levels were assessed using an 8-Isoprostane Assay Kit (OxisResearch, Portland, OR). Briefly, protein fractions (100 μl) were added to a 96-well plate and incubated with 100 μl of horseradish peroxidase-conjugated antibody at room temperature for 1 h, 200 μl of substrate was added to each well, and after a 30 minute incubation the reaction was stopped by adding 50 μl of 3 M sulfuric acid and the absorbance was read at 450 nm. Protein carbonyl content was determined as described previously (Lyras, et al. 1996), except that the final protein pellets were dissolved in 1 ml of 6 M guanidinium hydrochloride. Carbonyl content was calculated as nmol/mg of protein. Measurement of protein-bound nitrotyrosine content was performed using a Nitrotyrosine Assay Kit (OxisResearch) using the protocol provided with the kit.
Relative quantification of gene specific mRNA by real-time PCR
Total RNA was isolated from the mice testes in sedentary and runners group with Tri kit (Sigma). First-strand cDNA was generated with random hexamers as primers using AMV and M-MLV reverse transcriptases H plus (ABgene), according to the manufacturers recommendations. To compare specific messenger expression in different samples, the relative abundance of gene specific mRNA was normalized against the Glyceraldehyde 3 phosphate. Raw data were processed by means of the comparative Ct method by formula 2−ΔΔct. The relative gene specific mRNA levels recorded in testes. For RT-PCR, gene specific forward and reverse primers (Table 1) were designed based on the know sequence obtained from gene bank. The PCR mixture consisted of 0.5 ng of a cDNA template for gene specific cDNA amplification or for Gly-3-P amplification, respectively; 625 nM of each primer; and 10 μl of 2X SYBR green Master mix (Applied Biosystems) in a final volume of 20 μl. Amplification was carried out under the following conditions: initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C, and annealing followed by extension at 60 °C. Amplification of gene specific cDNA and Gly-3-P cDNAs of the same sample was performed simultaneously in separate tubes in duplicate. Each plate contained repeated reference samples, and the pooled results for all plates were analyzed with the ABI Prism 7000 Sequence Detection System software version 1.1 (PE Applied Biosystems). Raw data for each plate were entered into a Microsoft Excel spreadsheet and the relative expression values of for all gene specific samples were calculated manually to confirm the results.
Table 1.
| Primer name | Primer sequence from 5′→ 3′ |
|---|---|
| SOD1 F | GATGAAGAGAGGCATGTTGGAGA |
| SOD1 R | ATGGTTTGAGGGTAGCAGATGAG |
| SOD2 F | GGCCTACGTGAACAATCTCAAC |
| SOD2 R | GACCTTGCTCCTTATTGAAGCC |
| GPX1 F | CTACACCGAGATGAACGATCTG |
| GPX1 R | CTCAAAGTTCCAGGCAATGTCG |
| GPX4 F | TTTCGTGTGCATCGTCACCA |
| GPX4 R | CACGCAGCCGTTCTTATCAATG |
| GAPDH F | TGGAGTCTACTGGTGTCTTCAC |
| GAPDH R | GTGGATGCAGGGATGATGTTCT |
Histology
Testes collected at the end of experiment and were Bouin fixed and paraffin embedded. Sections (5 microns) were stained with hematoxylin and eosin and viewed by transmitted light microscopy. Histopathological damage was assessed by examination of 100 seminiferous tubule cross sections per testis.
Immunoblot analysis
After tissue homogenization tissue lysates were obtained by resuspending tissue in RIPA buffer (50 mM Tris-HCl, 0.5% Triton X-100, 0.25% Nadeoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, and 5 mM MgCl2) containing a 1:1000 dilution of protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO). A clear supernatant was obtained by centrifugation of lysates at 17,000 × g for 10 min. Protein content was determined using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Proteins (50 μg) were separated by SDS-PAGE (8–12%) and transferred to a nitrocellulose membrane. Primary antibodies included: rabbit polyclonal against SOD1, rabbit polyclonal against HO-1 and goat polyclonal against GPX1 (Santa Cruz Biotechnology Inc CA); anti-phospho-histone H2AX (Ser139), clone JBW 301 (Upstate, Temecula, CA); rabbit polyclonal against HNE-Michael Adducts, reduced rabbit pAb (Calbiochem, EMD Chemicals Inc. San Diego, CA); and a mouse monoclonal against β-actin (Sigma-Aldrich, St. Louis, MO).
Immunohistochemistry
Briefly, sections (5-μm thick) of paraffin-embedded mice testes were deparaffinized before being exposed to citrate buffer (0.01 m, pH 6.0) heated in a microwave for 3 min. The sections were treated with 0.3% hydrogen peroxide in methyl alcohol for 20 min to block endogenous peroxidase activity. After three washes in phosphate-buffered saline (PBS), the sections were incubated with appropriate blocking serum before being incubated over night at 4 degrees C with one of the following primary antisera: mouse anti-nitrotyrosine antibody (Zymed Laboratories, South San Francisco CA 94080), monoclonal antibody against 8-hydroxy-2′-deoxyguanosine (Genox Corporation, Baltimore MD) and anti-HNE- Michael Adducts, reduced rabbit pAb (Calbiochem, EMD Chemicals Inc. San Diego, CA 92121). After three washes in PBS, the appropriate biotinylated secondary antibody and avidin–biotin peroxidase complex (Vector Elite Kit; Vector, Burlingame, CA, USA) were added sequentially. The peroxidase reaction was developed using a peroxidase substrate kit (DAB, SK-4100; Vector). Before being mounted, the sections were counterstained with haematoxylin. For controls, each of the primary antisera was omitted from some sections.
Quantification of immunohistochemistry by the positive pixel count algorithm
The immune complex was visualized with DAB and counterstained with hematoxylene and positive stained cells were detected in presence and absence of distinct staining by using positive pixel count algorithm which quantifies the amount of a specific stain in a scanner slide image using tissue microarray software from Aperio Technologies Inc., Vista CA, 92081 (Liu et al. 2008). Briefly, brown color of DAB is specified (range of hues and saturation) and three intensity ranges (weak, positive, and strong), for pixels which satisfy the color specification, the algorithm counts the number and intensity-sum in each intensity range, along with three additional quantities: average intensity, ratio of strong/total number, and average intensity of weak positive pixels. The algorithm has been set of default input parameters when first selected—these inputs have been pre-configured for brown color quantification in the three intensity ranges (220-175, 175-100, and 100-0). Pixels which are stained, but do not fall into the positive-color specification, are considered negative stained pixels—these pixels are counted as well, so that the fraction of positive to total stained pixels is determined. The algorithm is applied to an image by using ImageScope. This program allows to select an image region of analysis specify the input parameters, run the algorithm, and view/save the algorithm results. When using the ImageScope program, a pseudo-color markup image is also shown as an algorithm result. The markup image allows the user to confirm that specified inputs are measuring the desired color and intensity ranges. Once a set of algorithm inputs has been confirmed, the settings can be saved in a macro file for subsequent repeated use.
Results
Lifelong Running Protects the Testes against Age-Related Histological Changes
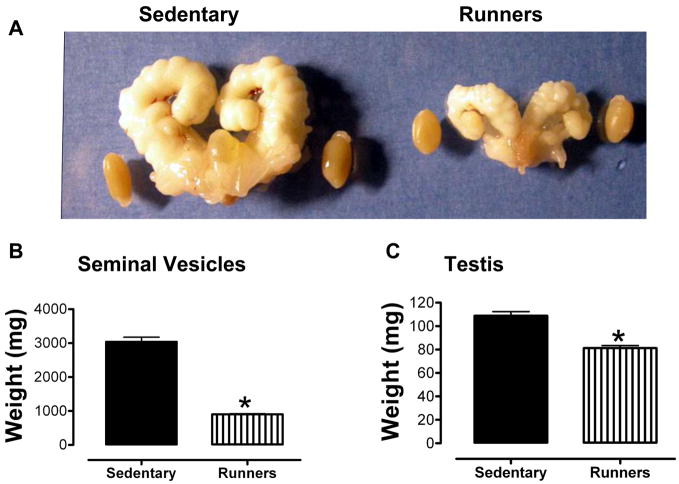
All mice were housed in identical cages in the same room within the animal facility, and were provided food and water ad libitum. When the mice reached 20 months of age we collected their reproductive organs (excluding the epididymis). Gross examination revealed enlarged seminal vesicles and testes in the sedentary animals compared to the runners (Fig. 1A). Weights of seminal vesicles (Fig. 1B) and testes (Fig. 1C) of sedentary mice were significantly greater than those of runners. The serum testosterone level of runners was significantly greater than that of sedentary animals (Fig. 1D).
Figure 1.
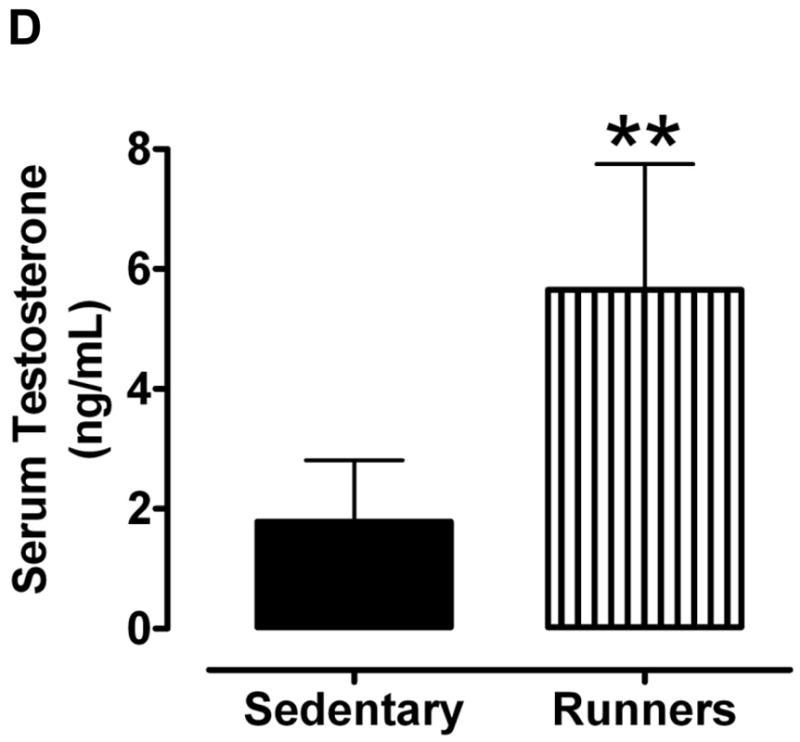
Aged sedentary mice exhibit enlarged seminal vesicles and testes compared to lifelong runners. A. Photographs of the seminal vesicles and testes of a representative sedentary mouse (left) and lifelong runner mouse (right). B and C. Weights of seminal vesicles (B) and testes (C) of sedentary mice and runners. Values are the mean and SEM (n = 6 mice per group). *p<0.001. D. Serum testosterone levels. Values are the mean and SEM (n = 6 mice per group). *p<0.001.
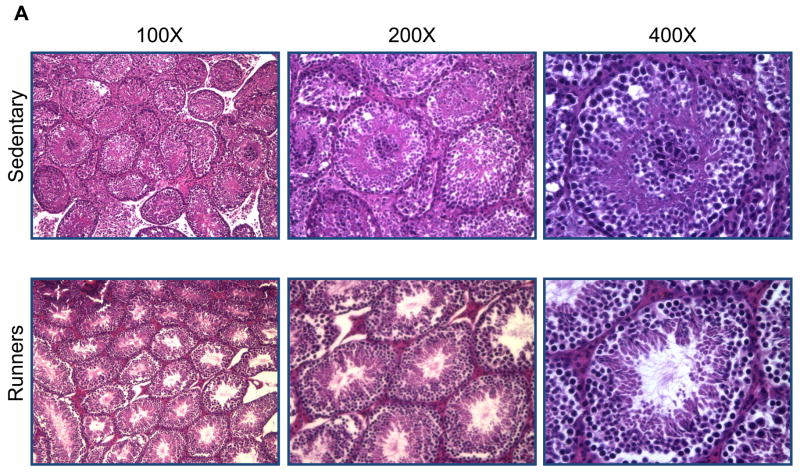
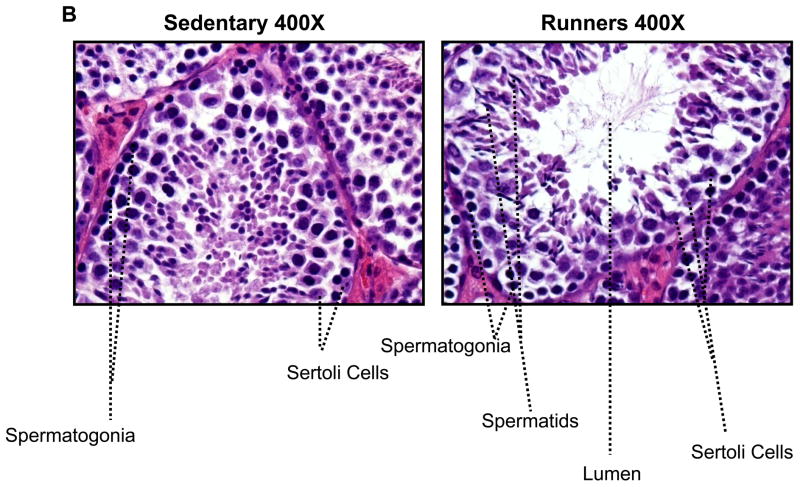
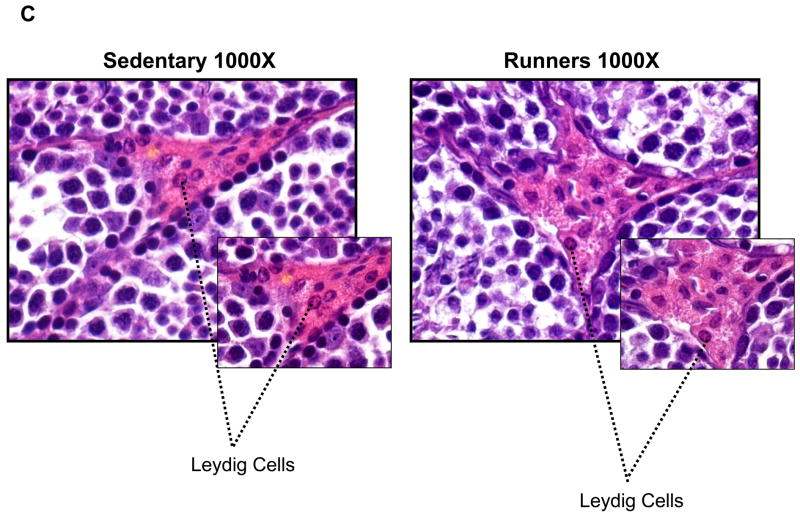
To investigate possible differences in the cellular organization of the testes, they were Bouin fixed and paraffin embedded, and then sections were stained with hematoxylin and eosin. There were markedly fewer spermatogonia in the seminiferous tubules of sedentary mice compared to runners (Fig. 2A). High magnification micrographs revealed cells at different stages of the spermatogenic process which were clearly visible in single sections from runners, compared to sedentary mice (Fig. 2B); different stages of spermatogonia, spermatids were abundant in seminiferous tubules from runners. Testes from sedentary mice exhibited focal and diffuse areas of sclerosis which were not evident in runners (Fig. 2). Non spermatogenic Sertoli cells, which support and nourish spermatozoa, were significantly more in the seminiferous tubules of runners, but were sparse in sedentary mice (Fig. 2B). These cells divided the seminiferous epithelium into basal and adluminal compartments in runners, a compartmentalization that was lacking in testes from sedentary mice. At higher magnification the interstitial supporting tissue between the seminiferous tubules showed a significant reduction in Leydig cell number and size in sedentary mice compared to runners (Fig. 2C and 2D). In runners Leydig cells were present as single cells and cell clusters.
Figure 2.
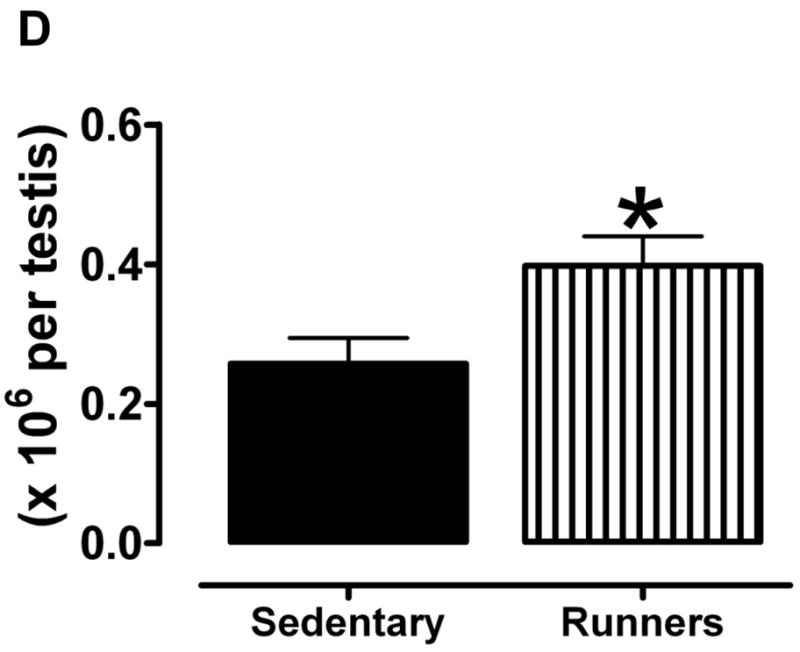
Testes of old lifelong runners exhibit preserved cellular composition and integrity compared to sedentary mice. A. Micrographs of H & E stained sections of testes from sedentary mice and runners shown at three different magnifications. The micrographs illustrate transverse sections through seminiferous tubules. Loss of spermatogonia was prominent in sedentary mice, whereas cells at all stages of the spermatogenic process are clearly visible in runners. B. High magnification micrographs reveal focal and diffuse areas of sclerosis, and a sparcity of Sertoli cells in seminiferous tubules of sedentary mice. In contrast, cells at different stages of spermatogenesis, as well as Sertoli cells, are evident and organized in the normal outside-in progression within the seminiferous tubules of runners. Clear lumen, numerous spermatids and more number of sertoli cells are also hallmark in runners. C. At higher magnification interstitial supporting tissue between the seminiferous tubules reveals a reduction in Leydig cell number and size in sedentary mice compared to runners. D. Leydig cell number in sedentary mice compared to runners. Values are the mean and SEM (n = 6 mice per group). *p<0.001.
Running decreases levels of lipid peroxidation and oxidative stress in the testes
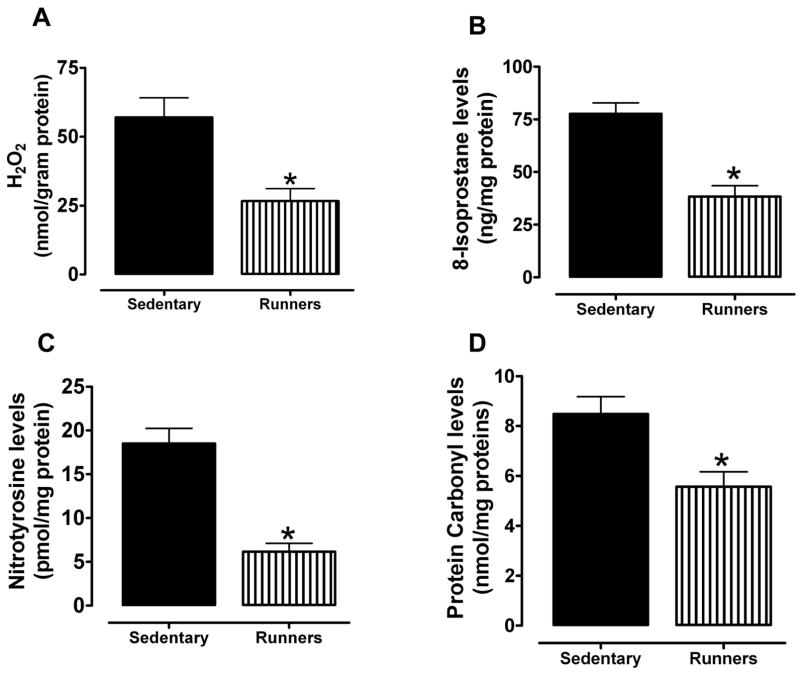
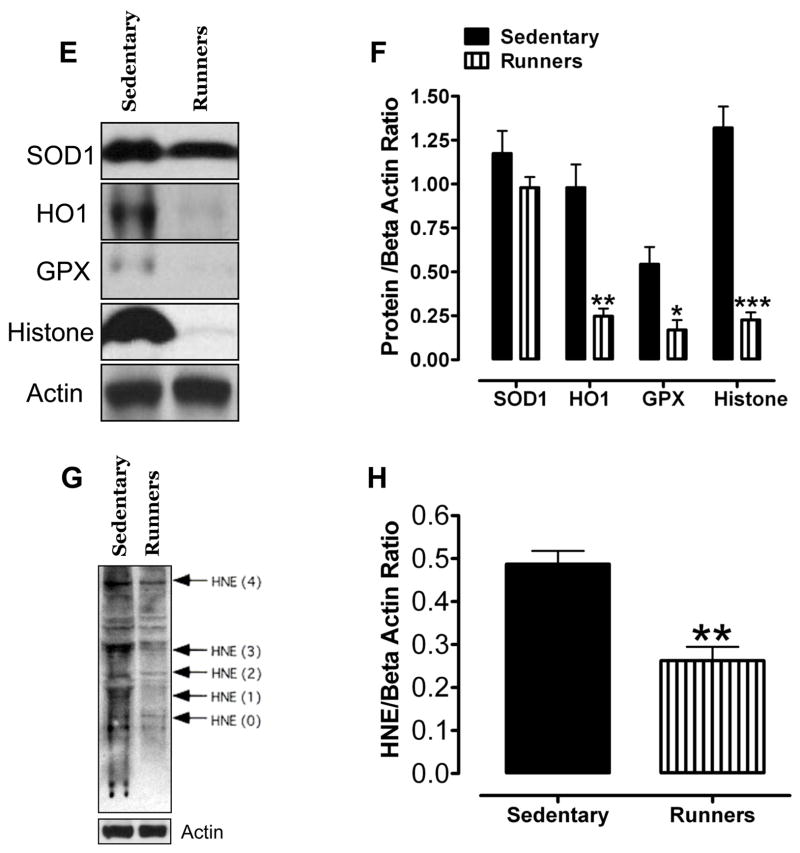
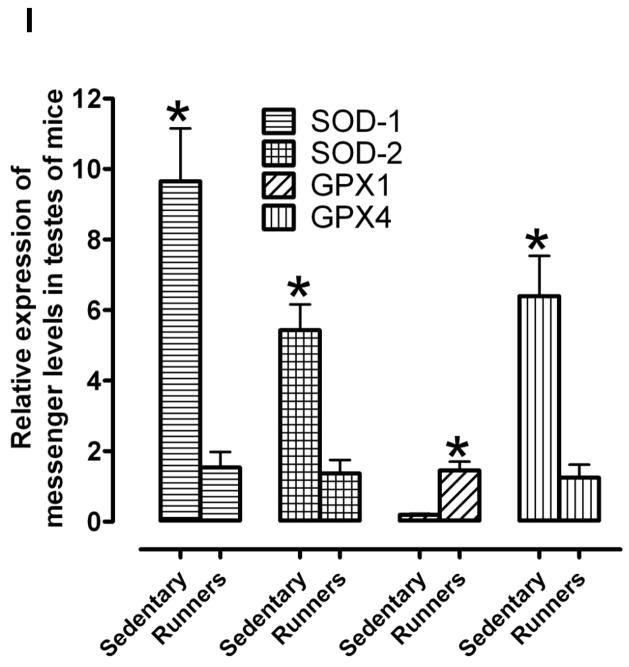
Hydrogen peroxide is one of the reactive oxygen species (ROS) secreted by macrophages that are seen closely aligned with Leydig cells in the testicular interstitium (Gautam, et al. 2006). We first measured levels of H2O2 in the testes and found that the amount of H2O2 was significantly less in runners compared to sedentary mice (Fig. 3A). The levels of 8-isoprostane, a marker of lipid peroxidation (Morrow 2006) (Johnson, et al. 2007), were also significantly less in the testes of runners compared to sedentary mice (Fig 3B). Levels of nitrotyrosine (a marker of reactive nitrogen species) and protein carbonyl levels (a biomarker of protein oxidation) (Beal 2002) were significantly lower in testes of runners compared to sedentary mice (Fig. 3C, D). To assess the effect of lifelong running on oxidative stress-responsive enzymes in the testes, we measured the levels of superoxide dismutase (SOD1), hemeoxygenase-1 (HO1), glutathione peroxidase (GPX), and phosphorylated histone γ-H2AX in the testes from both groups of mice. Immunoblot analysis showed that HO1, GPX and γ-H2AX were significantly reduced in testes from runners compared to sedentary mice (Fig. 3E and F). We also measured the level of the lipid peroxidation product 4-hydroxy-2-nonenal (HNE), a toxic aldehyde implicated in various age-related diseases (Keller and Mattson 1998), in the testes from both groups. HNE levels were significantly lower in the testes from runners compared to sedentary mice (Fig. 3G and H). In order to see the effect of running on mRNA activity levels of all major antioxidant enzymes such as SOD1, SOD2, GPX-1 and GPX-4, we have performed real time PCR. Our data showed that SOD1, SOD2 and GPX-4 levels were significantly high in runners compared to sedentary animals (Fig. 3 and Table 1). However, GPX-1 level was significantly lower in runners compared to sedentary animals.
Figure 3.
Lifelong running results in reduced levels of lipid peroxidation, and protein carbonylation and nitration in the testes. Levels of H2O2 (A), isoprostanes (B), nitrotyrosine (C) and protein carbonyls (D) were measured in testicular samples sedentary mice and runners. Values are the mean and SEM (n = 6 mice per group) *p< 0.01. Proteins isolated from testis of sedentary mice and runners were used to measure levels of antioxidant enzymes (SOD, Cu/Zn-superoxide dismutase; HO-1, heme oxygenase; GPX, glutathione peroxidase) and γ-H2AX (E and F) and proteins modified by the lipid peroxidation product 4-hydroxynonenal (HNE) (G and H). Values are the mean and SEM (n = 6 mice per group) *p<0.05. ***p<0.001, **p<0.01. I. mRNA levels of SOD1, SOD2, GPX-1 and GPX-4 in sedentary mice compared to runners. Values are the mean and SEM (n = 6 mice per group). *p<0.001.
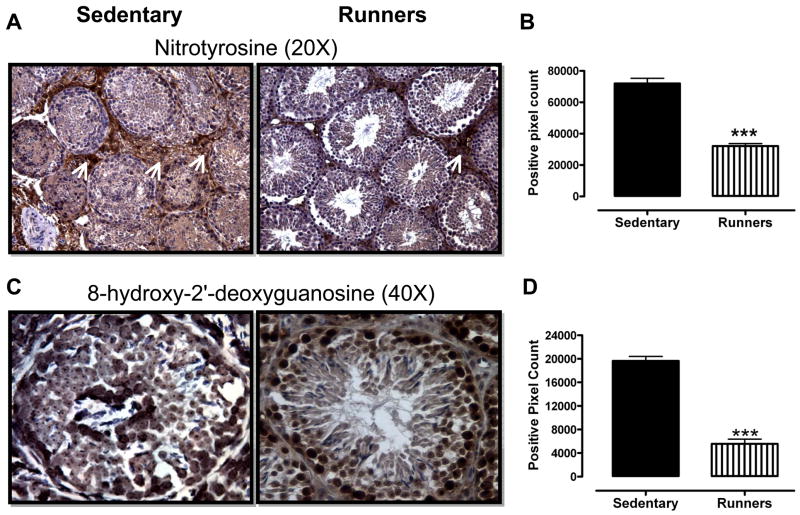
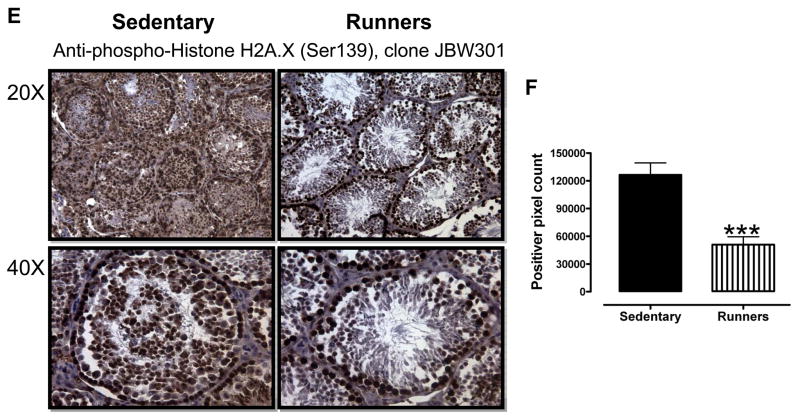
In order to gain insight into which cell types within the testes suffer oxidative stress as a result of a sedentary lifestyle, we performed immunohistochemical staining of tissue sections of testes from runners and sedentary mice using antibodies against nitrotyrosine, γ-H2AX and 8-hydroxy-2′-deoxyguanosine (a marker of oxidative DNA damage) (Yarborough, et al. 1996). As expected, overall levels of nitrotyrosine immunoreactivity were significantly greater in sections of testes from sedentary mice, compared to runners (Fig. 4A and 4B). Ledyig cells and spermatogonia exhibited the highest levels of nitrotryosine immunoreactivity (Fig. 4A), suggesting that these cell types were subjected to particularly high levels of nitrosative stress in sedentary mice. The levels of 8-hydroxy-2′-deoxyguanosine immunoreactivity were significantly greater in sections of testes from sedentary mice, compared to runners (Fig. 4C and D), with all cell types within the seminiferous tubules, as well as leydig cells, exhibiting robust immunoreactivity (Fig. 4C). The levels of γ-H2AX immunoreactivity were significantly greater in sections of testes from sedentary mice, compared to runners (Fig. 4E and F). Leydig cells and all cell types within the seminiferous tubules exhibited robust γ-H2AX immunoreactivity in the testes of sedentary mice; only spermatogonia exhibited strong γ-H2AX immunoreactivity in the testes of runners (Fig. 4E).
Figure 4.
Lifelong running results in reduced levels of nitrotyrosine (A and B), 8-OHdG (C and D) and γ-H2AX (E and F) immunoreactivities in testicular cells. ***p<0.001.
Discussion
Degenerative and sclerotic changes were prominent in the testes of old sedentary mice, but were absent from testes of old lifelong runners. Associated with reduced levels of testosterone (Matsumoto 2002; Vermeulen 1991); (Harman et al. 2001) and sperm production in aging males are reductions in the number of type A dark spermatogonia, Sertoli and Leydig cells, and increased numbers of multinucleated spermatogonia, megalospermatocytes, giant spermatids and multilayered spermatogonia (Horn, et al. 1996); (Sampson, et al. 2007). Consistent with the latter findings, we observed very low numbers of type A spermatogonia and spermatocytes in the semiferous tubules of aged sedentary mice. In contrast, the seminiferous tubules of age-matched lifelong runners exhibited cytological features similar to those of young males including a well-organized stratification of the spermatogenic cells and luminal sperm. Sertoli cells provide nutritive and trophic support for spermatogenic cells (Griswold 1998), and a decrease in Sertoli cell number with aging is correlated with a decreased sperm count (Neaves, et al. 1984; Paniagua, et al. 1987). Our study found that Sertoli cells are abundant in seminiferous tubules of runners, whereas they are diminished in sedentary aged animals. Similarly, Leydig cell number was lower in the testes of mice in the sedentary group compared to the runners.
Our findings are consistent with a major role for accumulated free radical damage in the disruption of spermatogenic process during aging. Levels of H2O2, protein carbonyls, nitrotyrosine, lipid peroxidation products, and oxidatively modified DNA were all present in relatively high amounts in the testes of sedentary mice compared to runners. Immunostaining of testes tissue sections with antibodies against nitrotyrosine, 8-hydroxy-2′-deoxyguanosine and γ-H2AX indicated high levels of oxidative damage to proteins and DNA in spermatogonia and Leydig cells in sedentary mice compared to runners. These findings suggest that lifelong regular exercise can counteract age related oxidative damage to cells critical for sperm and testosterone production. The testicular cells of old sedentary mice may respond to the oxidative stress by up-regulating their production of antioxidant enzymes as indicated by higher levels of SOD1, HO1 and GPX in testes of sedentary mice compared to runners.
The specific mechanism(s) by which running protects the testes against aging remains to be established. However, previous studies of the effects of exercise on other tissues suggest several possibilities. Exercise may increase the resistance of cells to stress by activating adaptive stress response pathways including those involving transcription factors such as NF-κB and mitogen-activated protein kinases, resulting in increased expression of genes that encode cytoprotective proteins such as heat-shock proteins, phase 2 enzymes and antioxidant enzymes (Ji 2007); (Marini, et al. 2007); (Quindry, et al. 2007). Indeed, exercise training increases superoxide dismutase and HSP-72 content in the myocardium as well as in other organs (Yamashita, et al. 1999); (Starnes and Taylor 2007). In proliferative tissues prone to cancer, exercise can upregulate the expression of proteins that induce apoptosis of damaged (potentially cancerous) cells (Campbell, et al. 2007). Regular exercise is believed to reduce the risk of several types of cancer including those of the colon (Slattery 2004), prostate gland (Giovannucci, et al. 2005) and breast cancer (Monninkhof, et al. 2007). Results of epidemiological studies have also suggested a protective effect of exercise against testicular cancer. The beneficial effects of exercise in reducing oxidative damage and preserving the cellular normalcy in the testes during aging might be expected to also preserve the function and prevent pathology of the testes during aging in humans. Although not examined in the present study, it is reasonable to consider that, in addition to counteracting the aging process, regular exercise might protect the male reproductive system against diseases that involve oxidative stress. It was shown that 4- HNE–modified proteins that oxidative stress is generated in testes with varicocele (Shiraishi and Naito 2005). We found that along with above mentioned oxidative stress makers, runners testes had very little amount of 4-HNE levels compared to sedentary animals, which may indicate that running protects against aging related oxidative stress and oxidative stress induced lipid peroxidation in testes. This was also true for protein oxidation as we noticed that protein carbonyl levels, a biomarker of protein oxidation were also significantly less in runners. As oxidative damage is implicated in many age-related diseases, by reducing oxidative stress, exercise may protect the testes against dysfunction and disease during aging.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging. Tissues and serum samples were kindly provided by Alexis. M. Stranahan.
Footnotes
Author contributions: S.C., T.G.S., D.H.H., J.D.L., M.M. and T.V.A. designed and performed the research; S.C., T.V.A., T.G.S. and D.H.H. analyzed data; and S.C. and M.P.M. wrote the manuscript.
The authors declare no conflict of interest.
References
- Aksoy Y, Yapanoglu T, Aksoy H, Demircan B, Oztasan N, Canakci E, Malkoc I. Effects of endurance training on antioxidant defense mechanisms and lipid peroxidation in testis of rats. Arch Androl. 2006;52:319–323. doi: 10.1080/01485010500503587. [DOI] [PubMed] [Google Scholar]
- Almahbobi G, Papadopoulos V, Carreau S, Silberzahn P. Age-related morphological and functional changes in the Leydig cells of the horse. Biol Reprod. 1988;38:653–665. doi: 10.1095/biolreprod38.3.653. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- Barnes CJ, Covington BWt, Cameron IL, Lee M. Effect of aging on spontaneous and induced mouse testicular germ cell apoptosis. Aging (Milano) 1998;10:497–501. doi: 10.1007/BF03340164. [DOI] [PubMed] [Google Scholar]
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Campbell KL, McTiernan A, Li SS, Sorensen BE, Yasui Y, Lampe JW, King IB, Ulrich CM, Rudolph RE, Irwin ML, et al. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16:1767–1774. doi: 10.1158/1055-9965.EPI-07-0291. [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Cumming DC, Wheeler GD, McColl EM. The effects of exercise on reproductive function in men. Sports Med. 1989;7:1–17. doi: 10.2165/00007256-198907010-00001. [DOI] [PubMed] [Google Scholar]
- Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann N Y Acad Sci. 2002;959:66–81. doi: 10.1111/j.1749-6632.2002.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Dugan SA. Exercise for health and wellness at midlife and beyond: balancing benefits and risks. Phys Med Rehabil Clin N Am. 2007;18:555–575. xi. doi: 10.1016/j.pmr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gautam DK, Misro MM, Chaki SP, Sehgal N. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis. Apoptosis. 2006;11:39–46. doi: 10.1007/s10495-005-3087-1. [DOI] [PubMed] [Google Scholar]
- Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Richardson DW, Brown N, Davidson DW. Structure and gametogenic potential of seminiferous tubules in ageing mice. J Reprod Fertil. 1982;64:127–133. doi: 10.1530/jrf.0.0640127. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Hackney AC, Szczepanowska E, Viru AM. Basal testicular testosterone production in endurance-trained men is suppressed. Eur J Appl Physiol. 2003;89:198–201. doi: 10.1007/s00421-003-0794-6. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Horn R, Pastor LM, Moreno E, Calvo A, Canteras M, Pallares J. Morphological and morphometric study of early changes in the ageing golden hamster testis. J Anat. 1996;188(Pt 1):109–117. [PMC free article] [PubMed] [Google Scholar]
- Howard CVRM, editor. Three-Dimensional Measurement in Microscopy. Abingdon: Garland Science/BIOS; 2005. [Google Scholar]
- Jackson MJ, Khassaf M, Vasilaki A, McArdle F, McArdle A. Vitamin E and the oxidative stress of exercise. Ann N Y Acad Sci. 2004;1031:158–168. doi: 10.1196/annals.1331.015. [DOI] [PubMed] [Google Scholar]
- Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. Spermatogenesis and aging in the human. J Androl. 1986;7:331–354. doi: 10.1002/j.1939-4640.1986.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mattson MP. Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction, and death in the nervous system. Rev Neurosci. 1998;9:105–116. doi: 10.1515/revneuro.1998.9.2.105. [DOI] [PubMed] [Google Scholar]
- Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute Kidney Injury Leads to Inflammation and Functional Changes in the Brain. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyras L, Evans PJ, Shaw PJ, Ince PG, Halliwell B. Oxidative damage and motor neurone disease difficulties in the measurement of protein carbonyls in human brain tissue. Free Radic Res. 1996;24:397–406. doi: 10.3109/10715769609088038. [DOI] [PubMed] [Google Scholar]
- Manna I, Jana K, Samanta PK. Effect of intensive exercise-induced testicular gametogenic and steroidogenic disorders in mature male Wistar strain rats: a correlative approach to oxidative stress. Acta Physiol Scand. 2003;178:33–40. doi: 10.1046/j.1365-201X.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- Marini M, Lapalombella R, Margonato V, Ronchi R, Samaja M, Scapin C, Gorza L, Maraldi T, Carinci P, Ventura C, et al. Mild exercise training, cardioprotection and stress genes profile. Eur J Appl Physiol. 2007;99:503–510. doi: 10.1007/s00421-006-0369-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- Morrow JD. The isoprostanes - unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr Pharm Des. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- Myers M, Ebling FJ, Nwagwu M, Boulton R, Wadhwa K, Stewart J, Kerr JB. Atypical development of Sertoli cells and impairment of spermatogenesis in the hypogonadal (hpg) mouse. J Anat. 2005;207:797–811. doi: 10.1111/j.1469-7580.2005.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaves WB, Johnson L, Porter JC, Parker CR, Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–763. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- Paniagua R, Martin A, Nistal M, Amat P. Testicular involution in elderly men: comparison of histologic quantitative studies with hormone patterns. Fertil Steril. 1987;47:671–679. doi: 10.1016/s0015-0282(16)59120-1. [DOI] [PubMed] [Google Scholar]
- Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol. 2007;103:1056–1062. doi: 10.1152/japplphysiol.00263.2007. [DOI] [PubMed] [Google Scholar]
- Resko JA, Malley A, Begley D, Hess DL. Radioimmunoassay of testosterone during fetal development of the rhesus monkey. Endocrinology. 1973;93:156–161. doi: 10.1210/endo-93-1-156. [DOI] [PubMed] [Google Scholar]
- Sampson N, Untergasser G, Plas E, Berger P. The ageing male reproductive tract. J Pathol. 2007;211:206–218. doi: 10.1002/path.2077. [DOI] [PubMed] [Google Scholar]
- Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze C. Multinucleate Sertoli cells in aged human testis. Cell Tissue Res. 1981;217:259–266. doi: 10.1007/BF00233579. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Naito K. Increased expression of Leydig cell haem oxygenase-1 preserves spermatogenesis in varicocele. Hum Reprod. 2005;20:2608–2613. doi: 10.1093/humrep/dei063. [DOI] [PubMed] [Google Scholar]
- Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–252. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- Snyder PJ. Effects of age on testicular function and consequences of testosterone treatment. J Clin Endocrinol Metab. 2001;86:2369–2372. doi: 10.1210/jcem.86.6.7602. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes JW, Taylor RP. Exercise-induced cardioprotection: endogenous mechanisms. Med Sci Sports Exerc. 2007;39:1537–1543. doi: 10.1249/mss.0b013e3180d099d4. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Clinical review 24: Androgens in the aging male. J Clin Endocrinol Metab. 1991;73:221–224. doi: 10.1210/jcem-73-2-221. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough A, Zhang YJ, Hsu TM, Santella RM. Immunoperoxidase detection of 8-hydroxydeoxyguanosine in aflatoxin B1-treated rat liver and human oral mucosal cells. Cancer Res. 1996;56:683–688. [PubMed] [Google Scholar]