Abstract
Objectives:
A systemic inflammatory response has been reported following resuscitation from cardiac arrest. The purpose of this study was to compare the magnitude of the TNF-alpha response in two different swine models of VF arrest.
Design:
Randomized comparative trial.
Setting:
Translational research laboratory.
Subjects:
Domestic swine (n = 28, mean weight 40 kg, range 34-49 kg) of both genders.
Interventions:
Anesthetized and instrumented swine were randomized to electrically induced VF (n = 14) or spontaneous VF induced by occlusion of a coronary artery (n = 14). After 8 min of VF, countershocks were given and standard advanced cardiac life support was initiated. Resuscitated animals were observed for 3 hours and hemodynamics, base excess, and TNF-alpha concentrations were measured at intervals.
Measurements and Main Results:
TNF-alpha concentrations were significantly greater in the ischemic VF group throughout the postresuscitation period. Multivariate modeling demonstrated that the TNF-alpha level was dependent upon the method of VF induction, correlated with ischemia time (untreated VF period plus time to restoration of circulation), and the degree of postresuscitation hypoperfusion as reflected in base excess measurements.
Conclusions:
This study demonstrates that TNF-alpha concentrations increase after resuscitation from cardiac arrest and that the TNF-alpha response is more profound in animals subjected to ischemic, spontaneous VF. The observed differences may be due to a longer resuscitation time and persistent postresuscitation hypoperfusion in the ischemic VF group. These differences need to be considered in studies evaluating mechanisms of postresuscitation organ dysfunction and defining mortality markers.
Keywords: Cardiopulmonary resuscitation, cardiac arrest, ventricular fibrillation, cytokines
Introduction
There are an estimated 165,000 out-of-hospital cardiac arrests annually in the United States and approximately 77,000 to 174,000 are treated by components of organized emergency medical service systems.(1) Despite incremental efforts towards improving the “chain of survival”, the rate of survival to hospital discharge for victims of out-of-hospital cardiac arrest is approximately 6%. Approximately 75% of patients who are resuscitated, i.e., have spontaneous circulation restored, die in the hospital.(2) These deaths are usually the result of a postresuscitation syndrome characterized by progressive multi-organ failure.(3) Neurologic dysfunction and myocardial failure are prominent components of this syndrome.(4,5) Oxidant injury occurs during cardiac arrest and activation of metabolic cascades involved in reperfusion injury and an systemic inflammatory response are observed following resuscitation from cardiac arrest.(6,7) Several clinical, observational studies suggest that postresuscitation organ dysfunction may be related to a systemic inflammatory response following resuscitation.(8-11)
Most victims of out-of-hospital cardiac arrest have significant underlying coronary artery disease and more than half have an acute thrombotic occlusion of a major epicardial coronary artery.(12) To approximate this clinical scenario, several recent laboratory studies have compared short-term outcomes after resuscitation from ventricular fibrillation (VF) induced by occlusion of an epicardial coronary artery to those after VF induced by brief AC current stimulation of the myocardium, the conventional VF model in large animals.(13-15) Ischemically induced VF is more difficult to terminate, restoration of spontaneous circulation (ROSC) is less frequent, recurrent fibrillation is more common, and postresuscitation cardiac dysfunction is more profound.
Considering the differences between ischemically and electrically induced VF noted above, we hypothesized that the postresuscitation inflammatory response would be different in ischemically induced and electrically induced VF models. The purpose of this study was to compare TNF-α levels in swine subjected to ischemically induced spontaneous VF or electrically induced VF.
METHODS
This investigation was approved by the Animal Care and Utilization Review Committee of our institution and adheres to the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Yorkshire swine (n = 28, mean weight 40 ± 4 kg, range 34-49 kg) of both sexes were premedicated with ketamine (20 mg/kg) and xylazine (2 mg/kg). General anesthesia was induced with isoflurane via nose cone, and following endotracheal intubation, maintained with inhaled isoflurane (MAC 0.8-1.2%) and nitrous oxide in a 1 to 1 mixture with oxygen. End-tidal CO2 was continuously monitored using a side-stream capnometer (LifePak 12, Physio-Control Corporation, Redmond, WA) and minute ventilation was adjusted to maintain a value of 35-45 mm Hg. Standard lead II of the surface ECG was monitored continuously.
Under fluoroscopic guidance, high fidelity, micro-manometer tipped catheters (Millar Instruments, Houston, TX) were positioned in the ascending aorta via a femoral artery and in the right atrium (RA) via a jugular vein. A standard 7F bipolar pacing lead was positioned in the right ventricular apex via a jugular vein. Electrocardiographic and hemodynamic data were recorded and stored on a lap-top computer using PowerLab Chart v. 5.2 (ADInstruments, Castle Hill, Australia). Standard adhesive defibrillation electrode patches (Quick-Combo, Physio-Control Corporation, Redmond, WA) were applied to the left and right lateral aspects of the thorax.
Following instrumentation, animals were randomized by permuted block design to undergo electrically induced VF or ischemically induced VF. Electrical VF was induced by passing 60 Hz AC current for approximately 0.5 sec through the electrodes of the right ventricular bipolar lead. Ischemic VF was induced by occlusion of the left anterior descending coronary artery distal to the first septal perforator using a standard PTCA catheter and balloon introduced into a guiding catheter inserted via a carotid artery. The site of coronary occlusion and the occurrence of complete cessation of coronary flow were confirmed angiographically.
After 7 min of untreated VF, manual closed-chest compressions were begun with the animal in the supine position and were administered at a rate of approximately 100/min with force sufficient to depress the sternum 1.5 to 2.0 inches. In the ischemic group, the occluding balloon remained inflated throughout resuscitative efforts. One minute after starting chest compressions, a transthoracic countershock at 200 J was given using an impedance compensating, truncated exponential biphasic defibrillation waveform defibrillator (LifePak 12, Physio-Control Corporation, Redmond, WA). Successful defibrillation was defined as absence of VF at 5 sec after shock delivery.(17) If VF persisted, additional shocks in an escalating energy sequence (300, 360J) were administered. Chest compressions were performed between shocks and positive pressure ventilations (FiO2=1.00) were performed at a rate of 8 to 12 ventilations/min. If VF persisted or recurred after three shocks, epinephrine at a dose of 0.0.1 mg/kg mg was administered and shocks repeated. If necessary, epinephrine was repeated at 4-5 min intervals and amiodarone, 150 mg, was given, CPR continued, and shocks repeated until VF was terminated or for 15 minutes. If asystole or pulseless electrical activity (PEA) followed shocks, CPR was continued and additional epinephrine (0.01 mg/kg every 4-5 min) was administered until spontaneous arterial pressures of 60 mm Hg appeared or for 15 min. At the end of 15 min of CPR, animals remaining in VF, PEA, or asystole were considered resuscitation failures and resuscitative efforts terminated.
In those animals achieving return of spontaneous circulation (ROSC), defined as an arterial systolic pressure >60 mm Hg for 10 min(18), hemodynamic and blood gas measurements were made at intervals for 3 hours. In the ischemic group the LAD balloon was deflated 60 min after ROSC. Dopamine, 5-15 μg/kg/min, was infused, if needed, to maintain a systolic arterial pressure >90 mmHg following resuscitation.
Five minutes prior to electrical VF induction or LAD balloon inflation and at 15, 30, 60 min following restoration of circulation and at hourly intervals for 2 hours thereafter, arterial blood was sampled, placed in sterile, chilled (0° C), heparinized tubes, and centrifuged at 5000 rpm for 10 min. Plasma was immediately separated and stored at −80° C until analysis. TNF-α, was determined by a quantitative sandwich ELISA using a commercially available kit specific for this porcine cytokine (R&D Systems, Inc., Minneapolis, MN).
Data were entered into an Excel spreadsheet (Excel 2003, Microsoft Corporation, Redmond, WA) and translated into native SAS format using DBMS/Copy (version 8, DataFlux Corporation, Cary, NC). All statistical analyses were conducted using SAS version 9.1.3 (SAS Institute, Cary, NC). Summaries of numerical variables are presented as medians with interquartile ranges (IQRs). Numerical variables were compared using the Wilcoxon rank sum test, both when comparing the two groups of animals as a whole or when comparing hemodynamic or laboratory measurements between the two groups at specific time points (i.e., without accounting for the repeated measures design of the study).
A mixed linear modeling approach was used to analyze repeated TNF-α measurements, while accounting both for the correlations between repeated measurements within each animal and the multiple comparisons of post-resuscitation measurements to time zero. Prior to the modeling analysis, each TNF-α value was transformed by taking the logarithm of one plus the measured cytokine level. This transformation was used to improve the normality of the distribution of cytokine values. The repeated measures analysis was conducted using SAS Proc MIXED, using only fixed effects and assuming a covariance matrix with compound symmetry. For all models, the time of measurement was treated as an unordered categorical variable, and each time point was compared to the control value at time zero using a Dunnet-Hsu adjustment for multiple comparisons. Confidence intervals for effect magnitude were not corrected for multiple comparisons, however, as the purpose of such intervals is estimation rather than hypothesis testing. In addition to the time after resuscitation, additional fixed predictor variables included treatment group (electrical versus ischemic VF), total ischemia time, and base deficit. Treatment group and total ischemia time was constant for each animal, while base deficit was incorporated as a time-dependent predictor. Treatment group assignment and total ischemia time demonstrated substantial colinearity, in that animals with ischemically-induced VF had uniformly longer total ischemia times than those with electrically-induced VF. Thus, models could not be fit that included both treatment group assignment and total ischemia time simultaneously as independent predictor variables. Base excess and treatment group assignment also demonstrated colinearity. Other than the Dunnett-Hsu adjustment mentioned above, no adjustments were made for multiple comparisons and a maximum p value of 0.05 was considered statistically significant.
RESULTS
Prearrest hemodynamic and arterial blood gas values were not significantly different between groups (table 1). For the ischemic group, VF occurred spontaneously within 1076 ± 559 sec of balloon occlusion of the LAD in all animals. Resuscitation variables are shown in table 2. The number of countershocks required to initially terminate VF was not significantly different between groups. However, the ischemic VF group received a greater number of shocks and epinephrine during resuscitative efforts due to recurrent VF, and required a longer period of time to restore spontaneous circulation (ROSC). No animal in the electrical VF group received amiodarone compared to 12/14 in the ischemic VF group. All animals in the electrical induction group achieved ROSC compared to 43% in the ischemic group. All animals in the ischemic VF group required vasopressor support during the postresuscitation observation period. No animal required pressor support in the electrical VF group. Left ventricular stroke work for the groups is shown in table 3.
Table 1.
Control, prearrest measurements
| Electrical VF (n=14) | Ischemic VF (n=14) | |
|---|---|---|
| Weight (kg) | 40 ± 4 | 39 ± 4 |
| Male/Female | 10/4 | 9/5 |
| Heart rate (beats/min) | 107 ± 21 | 114 ± 14 |
| Mean aortic pressure (mmHg) |
88 ± 9 | 94 ± 11 |
| Mean right atrial pressure (mmHg) |
5 ± 4 | 5 ± 2 |
| Arterial pO2 (mmHg) | 222 ± 50 | 213 ± 55 |
| Arterial CO2 (mmHg) | 40 ± 4 | 40 ± 3 |
| Arterial pH | 7.52 ± 0.0.6 | 7.51 ± 0.06 |
| Base Excess (mmol/L) | 8 ± 4 | 9 ± 2 |
Values are mean ± SD.
Table 2.
Resuscitation Variables for Groups
| CS to Defib |
Refibs | Total CS |
Total Joules |
Epi Dose (mg) |
Total Ischemia Time (sec) |
% ROSC | |
|---|---|---|---|---|---|---|---|
| EVF | 1.5 ± 0.7 |
0.7 ± 1.0 |
2.2 ± 1.5 |
564 ± 493 |
0.6 ± 0.4 |
607 ± 90 | 14/14 |
| IVF | 2.7 ± 2.3 |
6 ± 4 * | 11.0 ± 5.0* |
3342 ± 1639* |
1.9 ± 0.5* |
1070 ± 151* |
6/14* |
p < 0.001; values are mean ± SD.
CS = countershocks; Defib = defibrillation; Epi Dose = total epinephrine dose; refibs = refibrillations; ROSC = Restoration of spontaneous circulation; Total Ischemia time = Untreated VF duration (7 min) plus time to ROSC.
Table 3.
Post Resuscitation Left Ventricular Stroke Work (gm-m)
| Time (min) | Electrical VF | Ischemic VF | P |
|---|---|---|---|
| 0 | 45 ± 8 | 50 ± 11 | 0.178 |
| 15 | 27 ± 15 | 13 ± 22 | 0.095 |
| 30 | 10 ± 7 | 6 ± 5 | 0.342 |
| 60 | 16 ± 7 | 7 ± 6 | 0.071 |
| 90 | 22 ± 7 | 8 ± 6 | 0.005 |
| 120 | 27 ± 7 | 13 ± 11 | 0.010 |
| 180 | 33 ± 9 | 18 ± 1 | 0.033 |
Values = mean ± SD
Time 0 = control, other times represent time after restoration of spontaneous circulation.
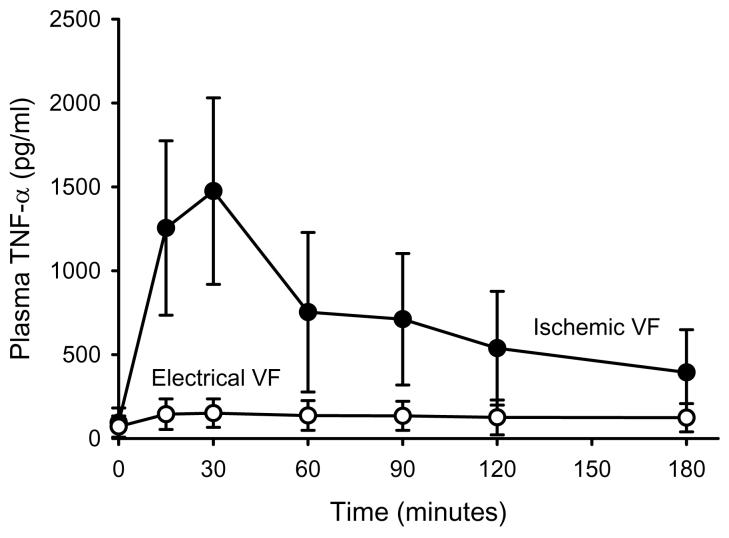
TNF-α concentrations measured at intervals are shown in figure 1. At all time points, TNF-α concentrations were significantly greater in those animals subjected to ischemic VF. The multivariable model for TNF-α demonstrated that plasma concentration was highly dependent upon the time it was measured after resuscitation and the method of VF induction (table 4).
Figure 1.
Plasma TNF versus Time
Plasma levels of TNF-α prearrest (time = 0) and at timed intervals following resuscitation are shown are shown for the two VF groups. Statistical analysis of these data is presented in table 4.
Table 4.
Multivariable modeling results for TNF
| Multivariable Model of TNF† | |||
|---|---|---|---|
| Predictor | Estimate (95% CI) | P Value | |
| Time (min) | 0 | Reference | |
| 15 | 4.9 (3.2, 7.4) | < 0.0001‡ | |
| 30 | 4.6 (2.9, 7.3) | < 0.0001‡ | |
| 60 | 3.6 (2.2, 5.9) | < 0.0001‡ | |
| 90 | 3.1 (1.9, 5.1) | < 0.0001‡ | |
| 120 | 3.0 (1.8, 4.9) | < 0.0001‡ | |
| 180 | 2.5 (1.5, 4.1) | 0.0018‡ | |
| Gender | Male | Reference | |
| Female | 0.8 (0.6, 1.1) | 0.21 | |
| VF Type | Electrical | Reference | |
| Ischemic | 3.5 (2.6, 4.6) | < 0.0001 | |
Estimates are multiplicative factors, relative to a reference measurement of TNF in a male animal, undergoing electrical VF induction, at time zero. Estimates are based on models of log transformed values of TNF measurement plus one (see text for details).
P Values corrected for multiple comparisons to baseline at time zero, using the Dunnett-Hsu correction.
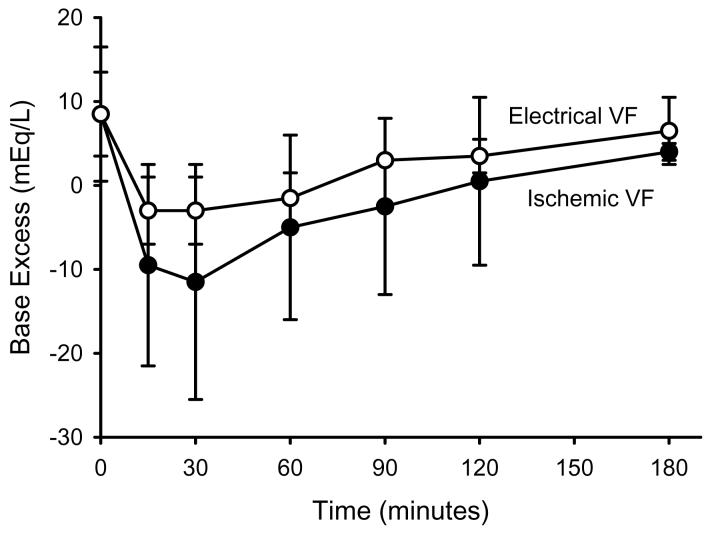
Base excess for the two VF groups during the postresuscitation period is shown in figure 2. Base excess was significantly lower than prearrest values in both groups during the 3 hours following ROSC, but was significantly lower in the ischemic VF group compared with the electrical VF group at all time points. Multivariable modeling demonstrated base excess was dependent upon time of measurement, method of VF induction, and gender (table 5).
Figure 2.
Base Excess versus Time
BE measure prearrest (time = 0) and at timed intervals following resuscitation are shown for ischemically and electrically induced VF groups. Statistical analysis of these data is shown in table 5.
Table 5.
Multivariable modeling results for base excess
| Multivariable Model of Base Excess† | |||
|---|---|---|---|
| Predictor | Estimate (95% CI) | P Value | |
| Time (min) | 0 | Reference | |
| 15 | −14.3 (−16.3, −12.3) | < 0.0001‡ | |
| 30 | −14.4 (−16.6, −12.2) | < 0.0001‡ | |
| 60 | −12.4 (−14.7, −10.1) | < 0.0001‡ | |
| 90 | −9.4 (−11.6, −7.1) | < 0.0001‡ | |
| 120 | −7.7 (−10.0, −5.5) | < 0.0001‡ | |
| 180 | −4.6 (−7.0, −2.3) | 0.0008‡ | |
| Gender | Male | Reference | |
| Female | −2.2 (−3.6, −0.8) | 0.0016 | |
| VF Type | Electrical | Reference | |
| Ischemic | −4.8 (−6.2, −3.4) | < 0.0001 | |
Estimates in mEq/L of base excess, relative to a reference measurement of base excess in a male animal, undergoing electrical VF induction, at time zero.
P Values corrected for multiple comparisons to baseline at time zero, using the Dunnett-Hsu correction.
DISCUSSION
Oxidant injury occurs during cardiac arrest and metabolic cascades, well described in other ischemia models, are likely to be involved in the reperfusion injury that is observed following successful resuscitation.(6,7) In the present study comparing ischemically induced and electrically induced VF cardiac arrest in a swine model, the reperfusion TNF-α response was significantly greater following resuscitation from ischemic VF. This difference was evident within 15 minutes of return of circulation and persisted during the first hours following resuscitation. In separate analyses, the TNF-α response was dependent on the method of VF induction, the duration of ischemia (time to restoration of spontaneous circulation), and associated with the degree of postresuscitation hypoperfusion as reflected in measured base excess over time. These differences are likely to be important in investigations of the postresuscitation syndrome, particularly the study of the inflammatory cytokines as potential mediators of postresuscitation organ dysfunction and markers of outcome.
Limited clinical investigations suggest that there is activation of a systemic inflammatory response syndrome, similar to that of sepsis, in patients resuscitated from out-of-hospital cardiac arrest. TNF-α and IL-8 levels have been shown to peak at 6 and 12 hours, respectively, after arrest and a correlation has been demonstrated between TNF-α and IL-8 levels and outcome.(8) The levels of these cytokines were also correlated with the dose of epinephrine administered during and after resuscitation suggesting that higher levels were observed in patients undergoing prolonged resuscitation efforts or with persistent hypotension following resuscitation. A marked increase in cytokines has been demonstrated within 2-5 hours of resuscitation from cardiac arrest with greater elevations observed in patients who eventually died in the hospital.(9) These differences were most striking in nonsurviving patients who required catecholamine pressor support during the postresuscitation period, suggesting a relationship between the severity of postresuscitation hypoperfusion, i.e., shock, and sustained cytokine production.
In our laboratory study, the TNF-α response following reperfusion in ischemic VF animals was similar to the cytokine pattern observed in the clinical population. TNF-α concentration was increased in all ischemic VF animals within 15 min of resuscitation when compared to prearrest levels. This difference from control was sustained for 60-90 min. At all postarrest time points, TNF-α was greater in the ischemic VF group when compared to electrically induced VF animals. This difference appears associated with the more profound tissue hypoperfusion in the ischemic VF group, as reflected in the base excess differences between groups and the need for pressor support in resuscitated ischemic VF animals. Additionally, the time to restoration of spontaneous circulation was longer in the ischemic VF group, thereby extending total ischemia time.
The role that the proinflammatory cytokines play in the production and progression of the postresuscitation syndrome following cardiac arrest is not understood. Observational clinical reports indicate that one or more of the cytokines may predict survival or neurologic outcome following resuscitation.(8-11) A laboratory study has demonstrated a relationship between TNF-α concentrations and cardiac function in the early postresuscitation period.(19) Other laboratory models of clinical syndromes characterized by systemic hypoperfusion and reperfusion, e.g., sepsis and hemorrhagic shock, support the role of cytokines in organ dysfunction during ischemia and after resuscitation and reperfusion.(20,21) It is likely that the proinflammatory cytokines play a similar role in the postresuscitation syndrome. The choice of arrest model will be important in defining mediators of reperfusion injury following resuscitation as well as methods or interventions to minimize or suppress those mediators that may impact eventual outcome.
This study has several limitations. Firstly, we evaluated only one untreated VF duration in this study. We did not attempt to determine if we could duplicate the TNF-α response observed in ischemic VF animals by prolonging the untreated VF time following electrical VF induction and our findings may not be generalizable to all VF durations. However, it is unlikely that prolonging the duration of electrically induced VF would replicate the metabolic, pathologic, or hemodynamic effects of ischemically induced VF followed by resuscitation. “Ischemia” in the coronary occlusion model includes three phases: 1) regional LV ischemia followed by, 2) multiorgan or “whole body” ischemia during VF, then 3) persistent whole body hypoperfusion following resuscitation due to myocardial infarction and LV dysfunction typically requiring vasopressor and inotropic support. In laboratory studies of electrically induced VF durations of 10-15 min, few animals are resuscitated or survive long enough for extended monitoring.(22,23) In the single study of 10 min untreated electrically induced VF duration in which extensive invasive hemodynamic data are available, hemodynamic indicators of systemic perfusion at 2 hours were not significantly different from prearrest values.(22) Secondly, we measured only TNF-α following resuscitation and reperfusion as a biomarker of the systemic inflammatory response. Due to the severity of the ischemic insult in the ischemic VF model and published data in heterogeneous clinical populations, it is likely that other cytokines will also be increased. Additionally, there are likely to be differences between tissue levels and blood levels of TNF-α which were not assessed in this study. Thirdly, epinephrine was administered during resuscitation and animals in the ischemic VF group received a greater dose of epinephrine. It has been suggested in other models that epinephrine decreases TNF-α production.(24) Despite possible inhibition of TNF-α, animals in the ischemic VF group demonstrated significantly greater plasma TNF-α levels. Fourthly, we used base excess as a marker of metabolic acidosis and “metabolic” measure of systemic perfusion after resuscitation. Base excess is an accepted surrogate for lactic acidosis, is superior to pH determination alone, and, in the selected study model, other metabolic derangements that might effect the measurement were unlikely to be present, e.g., hyperchloremic acidosis, renal failure, ketoacidosis.(25)
This study demonstrates that TNF-α concentrations increase after resuscitation from cardiac arrest and that the TNF-α response is more profound in animals subjected to ischemic, spontaneous VF. The observed differences may be due to a longer resuscitation time and persistent postresuscitation hypoperfusion in the ischemic VF group. These differences need to be considered in studies evaluating mechanisms of postresuscitation organ dysfunction and defining mortality markers in conventional laboratory cardiac arrest models.
Acknowledgments
Supported, in part, by a grant from the National Institutes of Health, R01 HL076671.
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics— 2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Steen P, Herlitz J, et al. International resuscitation network registry: design, rationale and preliminary results. Resuscitation. 2005;65:265–277. doi: 10.1016/j.resuscitation.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Negovsky VA. Postresuscitation disease. Crit Care Med. 1988;16:942–946. doi: 10.1097/00003246-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Edgren E, Hedstrand U, Kelsey S, Sutton-Tyrrell K, Safar P, the BRCT I Study Group Assessment of neurologic prognosis in comatose survivors of cardiac arrest. Lancet. 1994;343:1055–1059. doi: 10.1016/s0140-6736(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 5.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Nozari A, Liu XL, Rubertsson S, Wiklund L. Development of a novel biomarker of free radical damage in reperfusion injury after cardiac arrest. FEBS Let. 2000;470:1–6. doi: 10.1016/s0014-5793(00)01279-5. [DOI] [PubMed] [Google Scholar]
- 7.Idris AH, Roberts J, II, Caruso L, et al. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med. 2005;33:2043–2048. doi: 10.1097/01.ccm.0000174104.50799.bd. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Saitoh D, Fukuzuka K, et al. Significance of elevated serum interleukin-8 in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2001;51:47–53. doi: 10.1016/s0300-9572(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 9.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 10.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou J, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 11.Mussack T, Biberthaler P, Kanz KG, et al. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med. 2002;30:2669–2674. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Niemann JT, Rosborough JP, Walker RG. A model of ischemically induced ventricular fibrillation for comparison of fixed-dose and escalating-dose defibrillation strategies. Acad Emerg Med. 2004;11:619–624. [PubMed] [Google Scholar]
- 14.Niemann JT, Rosborough JP, Youngquist S, Thomas J, Lewis RJ. Is all ventricular fibrillation the same? A comparison of ischemically induced with electrically induced ventricular fibrillation in a porcine cardiac arrest and resuscitation model. Crit Care Med. 2007;35:1356–1361. doi: 10.1097/01.CCM.0000261882.47616.7D. [DOI] [PubMed] [Google Scholar]
- 15.Wang, Weil MH, Tang W, Chang YT, Lei Huang. A comparison of electrically induced cardiac arrest with cardiac arrest produced by coronary occlusion. Resuscitation. 2007;72:477–483. doi: 10.1016/j.resuscitation.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Ristagno G, Tang W, Xu TY, Sun S, Weil MH. Outcomes of CPR in the presence of partial occlusion of left anterior descending coronary artery. Resuscitation. 2007 doi: 10.1016/j.resuscitation.2007.04.005. in press. [DOI] [PubMed] [Google Scholar]
- 17.Gliner BE, White RD. Electrocardiographic evaluation of defibrillation shocks delivered to out-of-hospital sudden cardiac arrest patients. Resuscitation. 1999;41:133–144. doi: 10.1016/s0300-9572(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 18.Idris AH, Becker LB, Ornato JP, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. Circulation. 1996;94:2324–2336. doi: 10.1161/01.cir.94.9.2324. [DOI] [PubMed] [Google Scholar]
- 19.Niemann JT, Garner D, Lewis RJ. Tumor necrosis factor-α is associated with early postresuscitation myocardial dysfunction. Crit Care Med. 2004;32:1753–1758. doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29(Suppl):S99–S106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 21.Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006;21:160–172. doi: 10.1177/0885066605284330. [DOI] [PubMed] [Google Scholar]
- 22.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 23.Menegazzi JJ, Wang HE, Lightfoot CB, et al. Immediate defibrillation versus interventions first in a swine model of prolonged ventricular fibrillation. Resuscitation. 2003;59:261–270. doi: 10.1016/s0300-9572(03)00212-0. [DOI] [PubMed] [Google Scholar]
- 24.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann NY Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 25.Englehart MS, Schreiber MA. Measurement of acid-base resuscitation endpoints: lactate, base deficit, or what? Curr Opin Crit Care. 2006;12:569–574. doi: 10.1097/MCC.0b013e328010ba4f. [DOI] [PubMed] [Google Scholar]