Abstract
Optimal clonal expansion of CD4+ T cells during the primary response to Ag requires prolonged TCR recognition of peptide Ag/MHC complexes. In this study, we investigated the capacity of Cbl-b to counter-regulate late TCR signals necessary for continued cell division in vivo. During the first 24 h of a primary response to Ag, Cblb-/- 5C.C7 CD4+ T cells demonstrated no alteration in CD69, CD25, and CD71 up-regulation, or cell growth, as compared to wildtype cells. Nevertheless, beyond 24 h both the expression of CD71 and the rate of cell division were increased in the genetic absence of Cbl-b, leading to an augmented clonal expansion. This deregulation of late T cell proliferation in the absence of Cbl-b resulted in part from an inability of Cblb-/- T cells to desensitize Akt, PLCγ-1, and ERK phosphorylation events downstream of the TCR/CD3 complex, in addition to their failure to undergo a growth arrest in the absence of Ag. These observations now suggest a novel role for Cbl-b in triggering the exit from cell cycle at the end of a CD4+ T cell clonal expansion.
Keywords: T cells, cell proliferation, cell activation, rodent, E3
Introduction
Effective clonal expansion by naïve CD4+ T cells that recognize Ag for the first time in the peripheral immune system relies on a prolonged period of Ag/MHC complex presentation and TCR ligation. Such persistent Ag recognition is facilitated in part by the activation of dendritic cells via their toll-like receptors or CD40, leading to a decrease in the rate of Ag/MHC turnover (1-3). Experimental antigens designed for only transient dendritic cell expression confirm that CD4+ T cell clonal expansion and differentiation to effector or memory cell phenotypes is suboptimal when TCR ligation is terminated prematurely (4, 5). A failure of CD4+ T cells to continue to detect Ag/MHC complexes 24 h into a primary response promotes dissolution of T cell/dendritic cell tight interactions and reduces their proliferative potential (6). The use of an anti-Ag/MHC mAb to antagonize late TCR signaling has further revealed that CD4+ T cells immediately undergo a growth arrest and slow their rate of cell division once TCR ligands become unavailable (7). This counter-regulation of cell cycle progression in the absence of ongoing Ag/MHC recognition appears to underlie the clonal competition that limits clonal expansions by responder T cells already present at high frequencies (8, 9). However, there are as yet no data to support the notion that this regulation of late clonal expansion protects against the development of immunopathology.
On the other hand, Casitas B-lineage lymphoma b (Cbl-b)2 has previously been implicated in the control of T cell-mediated immunopathology. Mutation of Cblb in the Komeda rat strain leads to spontaneous diabetes development (10). Mice made genetically deficient in Cbl-b expression also demonstrate infiltrations of multiple organs with activated T and B cells and the spontaneous production of anti-dsDNA autoantibodies, in addition to heightened sensitivity to the development of experimental autoimmune encephalomyelitis (11, 12). The biochemical mechanisms by which Cbl-b molecules regulate autoimmunity development are not entirely clear; however, emerging data suggest that TCR-triggered nuclear factor kappa B (NFκB)-dependent gene expression is inhibited by Cbl-b in the absence of CD28 costimulatory signals (13). Consistent with this, Cbl-b limits the activation of protein kinase C-theta (PKC-θ) by Vav-1, thus controlling the formation of signaling rafts containing PKC-θ, card-maguk protein 1 (CARMA1), and Bcl10, that regulate the IκB kinase complex (11, 13). Cbl-b, a RING finger-containing E3 protein, has also been shown to bind to and catalyze the ubiquitination of the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K), thereby inhibiting the activation of Akt in T cells (14, 15). Interestingly, the E3 ligase activity of Cbl-b is also thought to play an important role in the blockade of TCR signaling after T cell clonal anergy induction (16-18). Nevertheless, no previous experiments have investigated the role of Cbl-b in the regulation of late TCR signals.
This study now explores the capacity of Cbl-b to influence late events in CD4+ T cell clonal expansion in vivo. The results suggest that Cbl-b has little capacity to influence the entry of naïve CD4+ T cells into cell cycle following immunization. However, the up-regulation of Cbl-b protein levels beyond 16 to 24 h of stimulation contributes to the premature growth arrest and slowing of the cell division rate that has been observed as Ag/MHC complex availability wanes. Therefore, these data support the hypothesis that Cbl-b opposes the development of immunopathology by facilitating CD4+ T cell exit from cell cycle.
Materials and Methods
Mice and Ag
Cblb+/+ as well as Cblb-/- Rag2-/- 5C.C7 mice were kindly provided by R. H. Schwartz (National Institutes of Health, Bethesda, MD) and were produced by back-crossing Cblb+/- offspring (11) to Rag2-/- 5C.C7 B10.A mice (19). Lymph node T cells from these 5C.C7 animals uniformly express Vβ3+ and CD4+, and recognize pigeon cytochrome c peptide 81-104 (PCCp)/I-Ek complexes. B10.A/Cr mice (B10.A) were purchased from Charles River Breeding Laboratories (Wilmington, MA) under a contract from the National Cancer Institute (Frederick, MD) and were used as syngeneic recipient mice for adoptive transfer studies. All mice were housed under specific pathogen-free conditions and used in accordance with guidelines put forth by the National Institutes of Health (Bethesda, MD) and the University of Minnesota Institutional Animal Care and Use Committee. Mice were sex and age matched for all experiments, ranging in age from 6-10 weeks old.
T cell activation
For studies relying on the in vitro activation of CD4+ T cells, 5C.C7 lymph node cells (>95% pure) were first labeled with 5 μM CFSE (Molecular Probes, Eugene, OR), and then were incubated in complete medium (RPMI 1640 (Mediatech, Herndon, VA) containing 10% FCS (Atlas Biologicals, Ft. Collins, CO), 2 mM L-glutamine, penicillin, streptomycin, and 5 × 10-5 M 2-ME) for 16 to 24 h at 37°C on plates, in some cases pre-coated with anti—CD3 mAb (145-2C11) (20) and in the presence or absence of soluble anti—CD28 mAb (37.51) (21), or with hamster IgG (BD Biosciences, San Diego, CA) as an irrelevant Ab control, as previously described (22). Alternatively, T cells were stimulated with ionomycin and phorbol 12-myristate 13-acetate (PMA) (Calbiochem, La Jolla, CA), or with human rIL-2 (Chiron Corporation, Emeryville, CA), as indicated. In some experiments, 10 μg/ml Brefeldin A (Calbiochem) was added to cultures to inhibit the secretion of newly synthesized IL-2, and prior to its detection by intracellular staining and flow cytometry. In other experiments, IL-2 secretion was monitored in the supernatant using an ELISA system (BD PharMingen, San Diego, CA). Finally, some cells were analyzed by western blot using Santa Cruz Biotechnology (Santa Cruz, CA) Ab to Cbl-b and actin, as well as Cell Signaling Technology (Danvers, MA) Ab to phospho-PLCγ, -Akt, -ERK, and -STAT5, as previously described (22).
Adoptive Transfer
For in vivo adoptive transfer experiments, 2 × 106 CFSE-labeled naïve 5C.C7 CD4+ T cells were injected i.v. into B10.A mice. The next day, mice were immunized with PCCp alone at the indicated dosage (or PBS as a control), to initiate a CD4+ T cell clonal expansion at relatively high responder frequency and in the absence of an adjuvant. Alternatively, in vitro pre-activated CFSE-labeled 5C.C7 CD4+ T cells were adoptively transferred into B10.A mice and then immediately challenged with PCCp.
Flow Cytometry
Lymphocytes were harvested from either tissue culture plates or from the spleen and lymph nodes of adoptive transfer recipient animals, and then immediately fixed with 2% formaldehyde (Sigma-Aldrich). Cells were then mixed with 2 × 105 counting beads (Caltag Laboratories, Inc., Burlingame, CA) and stained with an antibody cocktail containing CD71- or CD69-PE, CD25-APC, and CD4-PerCP-Cy5.5 (BD PharMingen). Alternatively, cells were permeabilized with 0.5% saponin (Sigma-Aldrich) and stained with IL-2-PE and CD4-APC (BD PharMingen) as previously described (23). The majority of 5C.C7 CD4+ T cells were then distinguished from the endogenous polyclonal CD4+ T cell population based on a persistence of CFSE fluorescence in the FL1 channel and CD4 staining, using either a BD FACSCalibur or BD LSR II flow cytometer (BD Biosciences, San Diego, CA) and FlowJo analysis software (Tree Star, San Carlos, CA).
Measurement of cell cycle progression in vivo
5C.C7 CD4+ T cell G0—>G1 cell cycle entry was monitored by flow cytometry using the forward scatter (FSC) determination. The average cell division rate for the CFSE-labeled 5C.C7 CD4+ T cell population at various times after stimulation was calculated as previously described (7, 24). Briefly, based on the peaks of CFSE fluorescence intensity within the population, each T cell was assigned to a particular cell division group d (with d = 0 to n cell divisions), and the number of T cell events (E) observed within each cell division group (Ed) was determined. Average cell division (D) was then calculated using the following equation:
Note that this calculation of the average number of cell divisions is corrected for the 2-fold increase in cell number that is associated with each division. To allow for a comparison of D values between groups within an experiment, the standard error of the mean (SEM) was determined for each sample using the following formula:
Results
Cbl-b counter-regulates the primary CD4+ T cell response to Ag stimulation in vivo
Mice made genetically deficient for Cbl-b have been shown to spontaneously produce autoantibodies and develop an immunopathology that is associated with multi-organ T and B cell infiltration (11, 12). Whether this immunopathology stems from a reduced threshold for the activation of the naïve autoreactive lymphocytes in the absence of Cblb, or from an inability of the clonal anergy tolerance mechanism to control the clonal expansion and effector function of Cblb-/- self Ag-specific lymphocytes is uncertain. Furthermore, it remains unclear what effects a selection of autoreactive T cells and resulting immunopathology might have on the phenotype and function of CD4+ T cells taken from these mice.
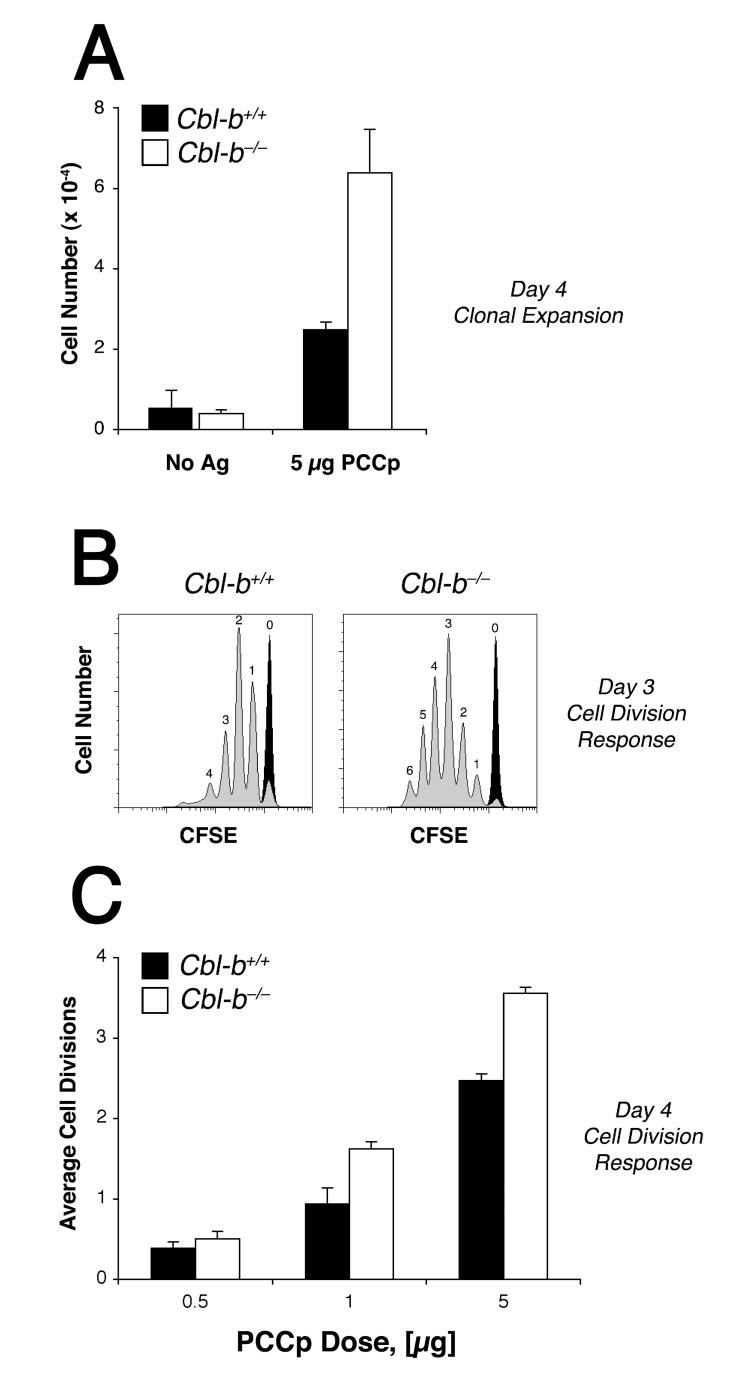
To investigate the role of Cbl-b in the control of the CD4+ T cell primary response to Ag in the absence of confounding T cell autoreactivity or autoimmunity, we examined the proliferation of Rag2-/- TCR-transgenic (TCR-Tg) 5C.C7 CD4+ T cells following their adoptive transfer into wildtype B10.A mice and stimulation with PCCp. Donor 5C.C7 mice lack the capacity to rearrange endogenous TCR gene loci and, therefore, avoid any self reactivity. For these experiments, we also took advantage of an immunization regimen capable of only sub-optimal CD4+ T cell priming and known to induce a partial peripheral tolerance to Ag (23): PCCp was administered in the absence of adjuvant as a single i.v. bolus to wildtype recipient mice that had been adoptively transferred with 5C.C7 CD4+ T cells at a relatively high precursor frequency (2 × 106). Exposure of the naïve 5C.C7 CD4+ T cell population to 5 μg PCCp under these conditions elicited only a weak (∼4-fold) clonal expansion by 96 h that on average was associated with about two rounds of cell division (Fig. 1). Interestingly, Cbl-b acted in this system to moderate the response, as naïve Rag2-/- 5C.C7 CD4+ T cells made genetically deficient for Cblb demonstrated on average one additional round of cell division and accumulated to higher numbers following the immunization. Therefore, these data provided support for the hypothesis that Cbl-b acts within CD4+ T cells to counter-regulate Ag-induced proliferation in vivo even in the absence of systemic autoimmunity or lymphoproliferative disease.
Figure 1. Cbl-b limits CD4+ T cell clonal expansion in response to primary Ag stimulation.
A, 2 × 106 CFSE-labeled Rag2-/- 5C.C7 CD4+ T cells, either Cblb+/+ (filled bars) or Cblb-/- (open bars), were adoptively transferred into normal B10.A recipient mice and treated either with PBS alone or 5 μg PCCp 81-104. Four days later animals were sacrificed and the LN cells were mixed with counting beads, and then analyzed by flow cytometry. Numbers of CFSE+ CD4+ T cells recovered from the LN are shown. B, Adoptive transfer recipient animals were immunized either with PBS alone (black tracing) or with 1μg PCCp (gray tracing), and 3 d later CFSE dye dilution by wildtype (left panel) or Cblb-/- (right panel) spleen 5C.C7 CD4+ T cells was measured. C, Average cell division response for either wildtype (filled bars) or Cblb-/- (open bars) LN 5C.C7 CD4+ T cells determined 4 d after immunization with PCCp as indicated. Error bars indicate the standard error of the mean for at least 6 animals per group. LN and spleen T cells gave similar results in these experiments.
Normal basal Cbl-b levels do not influence the recognition of rare peptide Ag/MHC complexes by naïve CD4+ T cells
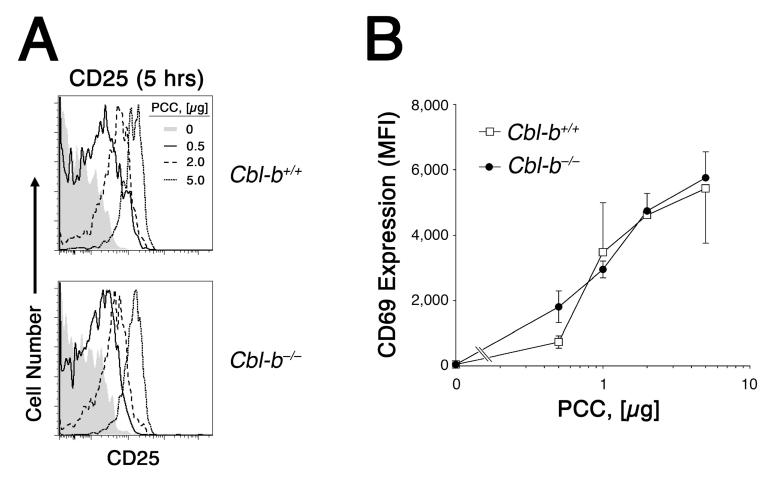
To better understand the mechanism by which Cbl-b antagonizes Ag-dependent cell cycle progression in vivo, we examined the dose response curve for Ag recognition by wildtype and Cblb-/- 5C.C7 CD4+ T cells. A titration of Ag in the immunization regimen revealed that at higher doses of PCCp cell divisions were inhibited in wildtype cells as compared to the Cbl-b-deficient CD4+ T cells (Fig. 1C). However, at limiting Ag concentration Cblb played virtually no role in the regulation of the cell division rate. Furthermore, Cblb-/- CD4+ T cells demonstrated no increased sensitivity to Ag, using CD69 and CD25 as markers of productive TCR engagement (Fig. 2). Consequently, our data offered no support for a model in which basal Cbl-b expression in naïve CD4+ T cells raises the threshold for initial Ag recognition. Rather, the data were more compatible with the hypothesis that Cbl-b expression or activity is increased only in response to intensified or prolonged TCR stimulation.
Figure 2.
Cbl-b does not raise the threshold for initial Ag recognition. 2 × 106 CFSE-labeled Rag2-/- 5C.C7 CD4+ T cells were adoptively transferred into B10.A recipients and exposed to varying doses of PCCp. 5 h later, animals were sacrificed and CFSE-labelled lymph node CD4+ T cells were examined for expression of activation markers. A, CD25 expression by T cells from wildtype (top) and Cbl-b-deficient (bottom) donor mice exposed to either 0 μg (shaded histogram), 0.5 μg (solid tracing), 2.0 μg (dashed tracing), or 5 μg (dotted tracing) PCCp infusion. B, CD69 expression by wildtype (open square) and Cbl-b-deficient (closed circle) CD4+ T cells following immunization with the indicated dose of PCCp. Two to three animals were examined at each dose of Ag, and data are expressed as the mean fluorescence intensity ± SEM.
Cbl-b counter-regulates late cell division by inhibiting TCR-dependent CD71 expression and blastogenesis
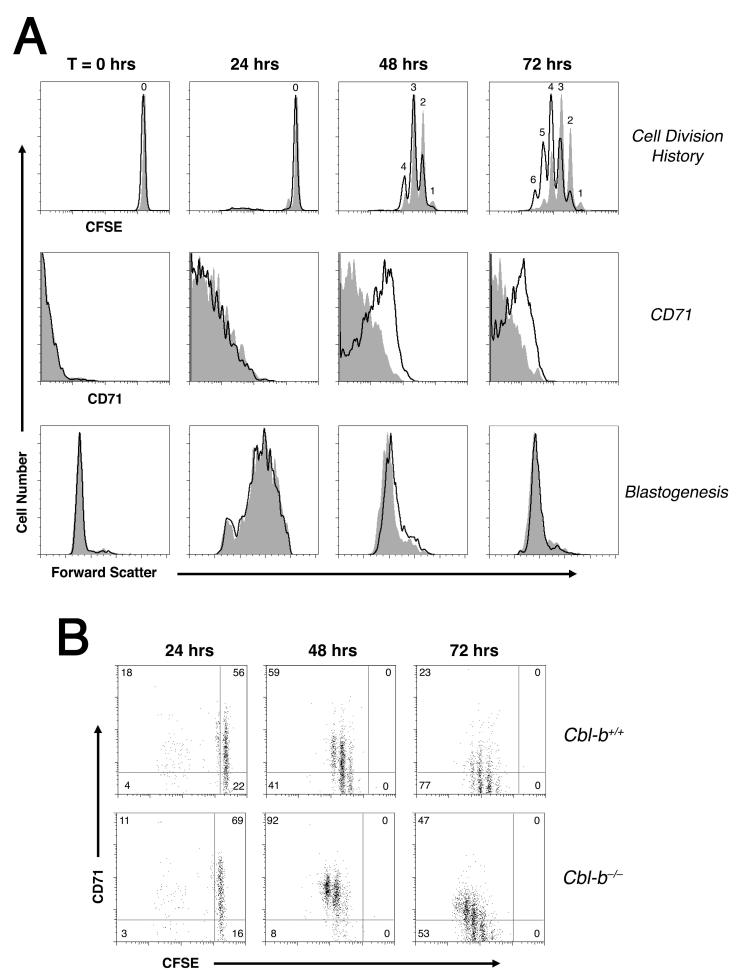
Previously, we observed that toward the end of a primary CD4+ T cell response to Ag stimulation in vivo, the rate of cell division and mean cell size were highly correlated (7). Based on this, we have postulated that a T cell’s ability to achieve a blastogenesis after each successive round of cell division determines the subsequent cell division time. Given that in our experiments Cbl-b appeared to play no role in the regulation of the threshold for initial Ag recognition, we hypothesized that Cbl-b does not increase the time to first cell division, but rather slows subsequent cell divisions by inhibiting the rate of G1—>S phase transition. A careful time course of in vivo CD4+ T cell activation demonstrated that wildtype 5C.C7 CD4+ T cells initiated and completed the majority of their cell divisions between 24 and 48 hours (Fig. 3). Cbl-b-deficient T cells also initiated their proliferative response between 24 and 48 hours, and demonstrated only a modestly increased cell division rate during this period as compared to the wildtype controls. Nevertheless, between 48 and 72 h of stimulation the Cbl-b knockout T cells divided at least once more, suggesting that Cbl-b activity was most important to suppress cell divisions at the end of a response.
Figure 3. Reduced CD71 expression in Cblb-expressing CD4+ T cells predicts a premature cessation of cell cycle progression.
A, Wildtype (gray tracing) and Cblb-/- (open tracing) LN 5C.C7 CD4+ T cells were examined for CFSE dye dilution, CD71 expression, and cell size at various times after immunization with 5 μg PCCp. B, Spleen 5C.C7 CD4+ T cell CD71 levels expressed as a function of CFSE content. Plots are representative of 4 individual animals. The LN and spleen results were found to be similar.
Consistent with these results, Cblb-/- T cells were on average bigger than wildtype at the 48 h time point, indicating an inhibition by Cbl-b of late G1—>S phase transitions (Fig. 3A). Cbl-b-deficient T cells also demonstrated a stronger and more durable expression of the transferrin receptor CD71. CD71 expression during cell cycle progression has previously been shown to be a useful marker of PI3K- and mammalian target of rapamycin (mTOR)-dependent G1—>S phase cell cycle progression (25). In our experiments, CD71 expression proved to be a better predictor of the rate of cell division than blastogenesis itself. The highest CD71 expression was always observed in T cells demonstrating the fastest cell division rate during the immunization (Fig. 3B). Interestingly, CD71 expression fell to near background level in wildtype CD4+ T cells after 72 h of stimulation, whereas Cblb-/- T cells exhibiting the fastest rate of cell division continued to express CD71 even at that late time point. Thus, these data suggested a new model where Cbl-b antagonizes the activity of PI3K and limits mTOR-dependent blastogenesis late during a primary response to Ag in the absence of infection or adjuvant.
The induction of Cbl-b both inhibits programming for autonomous cell division in the absence of ongoing TCR stimulation, and desensitizes CD4+ T cells to low numbers of Ag/MHC complexes
Several reports have now demonstrated that CD4+ T cells remain fully aware of the continued presence of peptide Ag/MHC complexes throughout their primary clonal expansion. Intense competition for limiting Ag/MHC complexes at late times after initial Ag encounter leads to dissolution of CD4+ T cell/dendritic cell conjugates, and eventually exit of T cells from the cell cycle (4-7). Given that Cbl-b does not appear to raise the threshold for initial Ag/MHC recognition in vivo, but instead promotes the cessation of cell division, we hypothesized that Cbl-b molecules are induced during the course of a primary Ag encounter to play a counter-regulatory role.
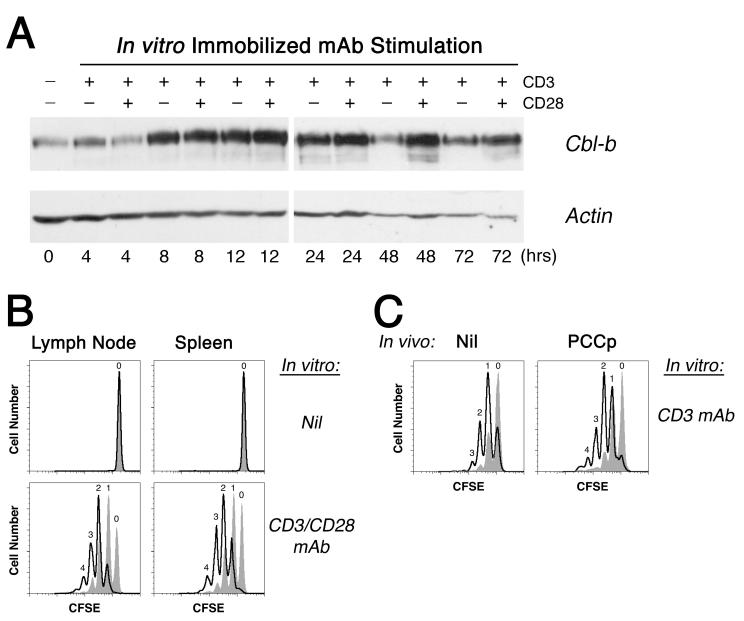
Consistent with this model, the activation of wildtype 5C.C7 CD4+ T cells in vitro led to a large increase in Cbl-b protein expression by 8 h of incubation that was sustained beyond 72 h (Fig. 4A). Ligation of the TCR/CD3 complex with CD3 mAb alone proved sufficient to induce this initial rise in Cbl-b protein levels. It was previously suggested that CD28 signaling increases the rate of Cbl-b turnover in T cells and reduces protein expression (26). We, too, found some evidence for this CD28—Cbl-b antagonism at the 4 h time point; nevertheless, T cells stimulated with a combination of CD3 and CD28 mAbs ultimately demonstrated an increased Cbl-b protein level at the latest time points, perhaps as a consequence of sustained blastogenesis and improved in vitro T cell viability in the presence of CD28 costimulatory signaling (Fig. 4A and data not shown). Based on these data, it appeared that Cbl-b would have the greatest opportunity to exert its counter-regulatory effects at late times during T cell activation.
Figure 4. Cbl-b up-regulation is associated with decreased proliferative potential.
A, Cbl-b and actin protein levels were compared in normal 5C.C7 CD4+ T cells over time following stimulation in vitro with 1 μg/ml immobilized CD3 and/or 1 μg/ml soluble CD28 mAb as indicated. B, CFSE-labeled wildtype (gray tracing) and Cblb-/- (open tracing) 5C.C7 CD4+ T cells were stimulated in vitro for 24 h with a combination of 1 μg/ml immobilized CD3 mAb and 5 μg/ml soluble CD28 mAb (or with no mAb as a control), and then were adoptively transferred into normal B10.A mice without immunization. CFSE dye dilution 3 d later is shown for LN and spleen 5C.C7 CD4+ T cells. C, As in (B), the T cells were stimulated with 1 μg/ml immobilized CD3 mAb alone and subsequently adoptively transferred either in the absence (left panel) or presence (right panel) of 1 μg PCCp, and then LN 5C.C7 CD4+ T cells were analyzed for CFSE dye dilution 4 d later. Results were reproduced in 3 independent experiments. Spleen T cells gave similar findings.
To further test the role of Cbl-b in the control of late cell cycle progression, we performed an overnight stimulation of the 5C.C7 CD4+ T cells in vitro with a combination of CD3 and CD28 mAbs, and then examined their capacity to undergo cell divisions in the absence of Ag following an adoptive transfer into normal B10.A hosts. As previously reported (7), wildtype CD4+ T cells failed to demonstrate a durable proliferative response after adoptive transfer into animals that lacked their Ag, with the T cells only infrequently dividing more than once, and often not dividing at all (Fig. 4B). 5C.C7 CD4+ T cells made genetically deficient for Cbl-b, however, always divided at least once after adoptive transfer and frequently divided 2, 3, or 4 times. Thus, a 24 h period of CD3/CD28 stimulation was sufficient to up-regulate Cbl-b molecules that appeared to act as a brake on subsequent in vivo cell cycle progression in the absence of Ag. The expression of Cbl-b in the CD4+ T cells in effect prevented the development of a more durable proliferative “program”.
In addition to its effects on the programming of T cells for Ag-independent cell cycle progression, Cbl-b also reduced the sensitivity of primed CD4+ T cells to low doses of Ag at late times during their activation response. Following an overnight period of in vitro activation with CD3 mAb alone, the Cblb-/- 5C.C7 CD4+ T cells typically divided only once after adoptive transfer into unimmunized hosts (Fig. 4B). Transfer into a recipient animal that was also immunized with a low dose (1 μg) of PCCp, however, led to another round of cell division. In contrast, wildtype 5C.C7 CD4+ T cells demonstrated essentially no increased proliferation after transfer into PCCp immunized mice. Taken together, the results indicated that Cbl-b molecules induced during the first 24 h of TCR/CD3 ligation both put a brake on subsequent cell division in the absence of Ag as well as desensitize the CD4+ T cells to further TCR signaling.
Cbl-b dampens IL-2 production and CD25 expression during the first 24 h of stimulation
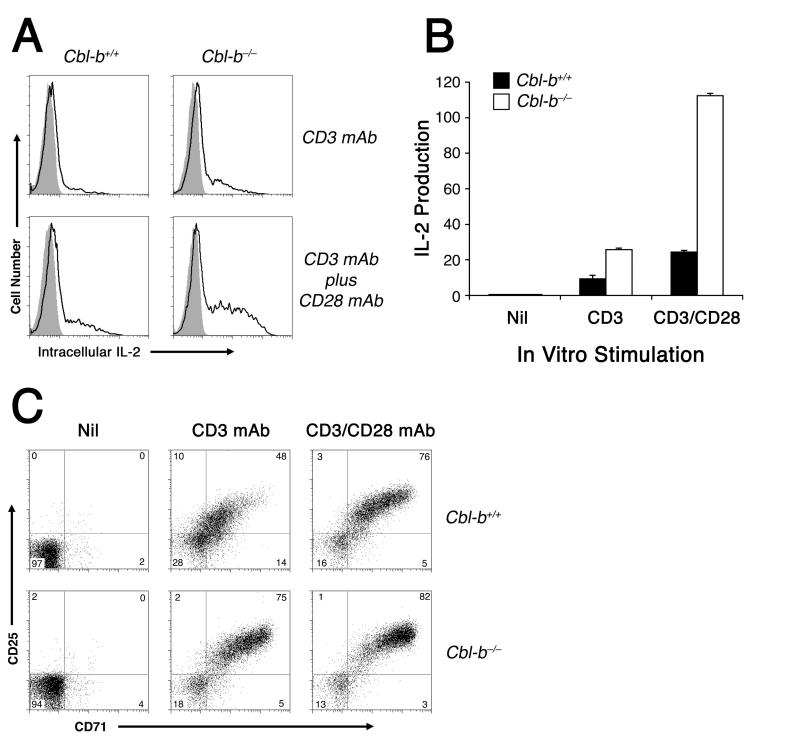
To investigate further this capacity of Cbl-b to regulate programming for proliferation in the absence ongoing Ag stimulation, we examined autocrine IL-2 production and response in vitro. Consistent with previous reports (11, 12), Cbl-b-deficient Rag2-/- 5C.C7 CD4+ T cells demonstrated heightened IL-2 synthesis upon stimulation with either CD3 mAb alone, or with the combination of CD3 and CD28 mAbs (Fig. 5A,B).
Figure 5. Cbl-b limits the induction of IL-2, CD25, and CD71 expression during the first 22 h of T cell activation.
A, Cblb+/+ or Cblb-/- 5C.C7 CD4+ T cells were stimulated 22 h in vitro with either 2 μg/ml immobilized CD3 mAb alone or a combination of immobilized CD3 mAb plus 2 μg/ml soluble CD28 mAb, as indicated. Brefeldin A (10 μg/ml) was added 9 h into the stimulation period. At the end of the experiment, 5C.C7 CD4+ T cells were examined by flow cytometry for intracellular IL-2 accumulation using IL-2-PE (open tracing) or isotype control PE-coupled mAb (gray tracing). B, Cumulative IL-2 production data for the experiment shown in (A), expressed as the product %IL-2+ x MFI. Error bars indicate the SEM for 4 replicate samples per group. C, Stimulation of 5C.C7 CD4+ T cells (either Cblb+/+, top, or Cblb-/-, bottom) for 22 h with either medium alone (nil), or with 0.5 μg/ml immobilized CD3 mAb alone, or with immobilized CD3 mAb plus 0.5 μg/ml soluble CD28 mAb. At the end of the in vitro stimulation, viable CD4+ T cells were examined for CD25 and CD71 expression. All results shown were reproduced in at least one additional experiment.
Furthermore, the pattern of in vitro CD25 and CD71 expression was altered by the Cblb gene deletion. Therefore, it appeared that newly synthesized Cbl-b molecules could antagonize the production of IL-2 and functional IL-2 receptors in vitro, as well as inhibit the expression of CD71 during the first 24 h of Ag stimulation. Accordingly, a reduction in IL-2 receptor signaling and limiting iron stores following Cbl-b induction may lead to eventual growth arrest late during a clonal expansion.
Cbl-b inhibits late TCR/CD3-dependent proximal signal transduction, but cannot regulate growth factor receptor-dependent STAT5 phosphorylation
The finding that Cbl-b is expressed and can inhibit TCR-triggered activation events within the first 24 h of stimulation complicated our initial interpretation of blocked late cell cycle progression. TCR antagonism by Cbl-b molecules during the first 24 h of activation may have simply blunted the blastogenesis response and ensured a premature exit from cell cycle by rapidly dividing T cells. Alternatively, up-regulated Cbl-b molecules (or their downstream target proteins) effectively antagonize the TCR throughout clonal expansion.
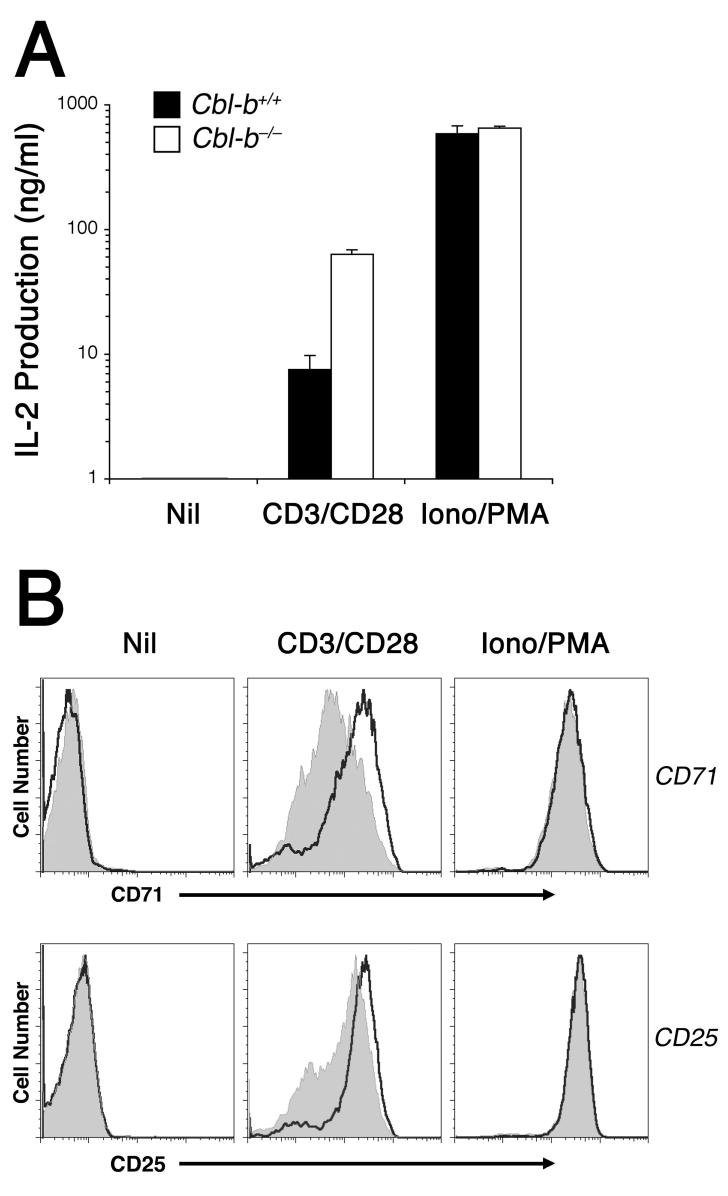
To differentiate these two possibilities, we took advantage of the calcium ionophore ionomycin and the PKC-activating phorbol ester PMA to initiate a strong G0—>G1 phase progression that was independent of regulation by Cbl-b. As shown in figure 6, the combination of ionomycin and PMA strongly induced IL-2 production by CD4+ T cells, independent of the status of the Cblb gene. Similarly, 16 h of ionomycin plus PMA stimulation was sufficient to up-regulate an equivalently high level of CD71 and CD25 in both the wildtype and Cbl-b-deficient CD4+ T cells. Cbl-b protein expression was also strongly induced by 16 h of ionomycin and PMA stimulation (data not shown); therefore, the downstream signaling pathways triggered by these two agents and necessary for IL-2, CD71, and CD25 protein induction appeared to be insensitive to counter regulation by Cbl-b. This failure of Cbl-b to act as an inhibitor in the absence of TCR/CD3 signals was revealing, and further supported the model that Cbl-b targets TCR proximal signaling pathways for counter-regulation.
Figure 6. Cbl-b counter-regulation of TCR/CD3 signaling is bypassed by ionomycin and PMA.
Wildtype and Cbl-b-deficient 5C.C7 CD4+ T cells were stimulated 16 h in vitro either with medium alone (nil), 1μg/ml immobilized CD3 mAb plus 2 μg/ml soluble CD28 mAb (CD3/CD28), or with the combination of 0.5 μM ionomycin and 10 ng/ml PMA (Iono/PMA). A, IL-2 secretion into the supernatant, with error bars indicating the SEM of triplicate samples. B, CD71 and CD25 expression of wildtype (gray tracing) and Cbl-b-deficient (open tracing) CD4+ T cells, determined in parallel by flow cytometry. The experiments were repeated twice more with similar results.
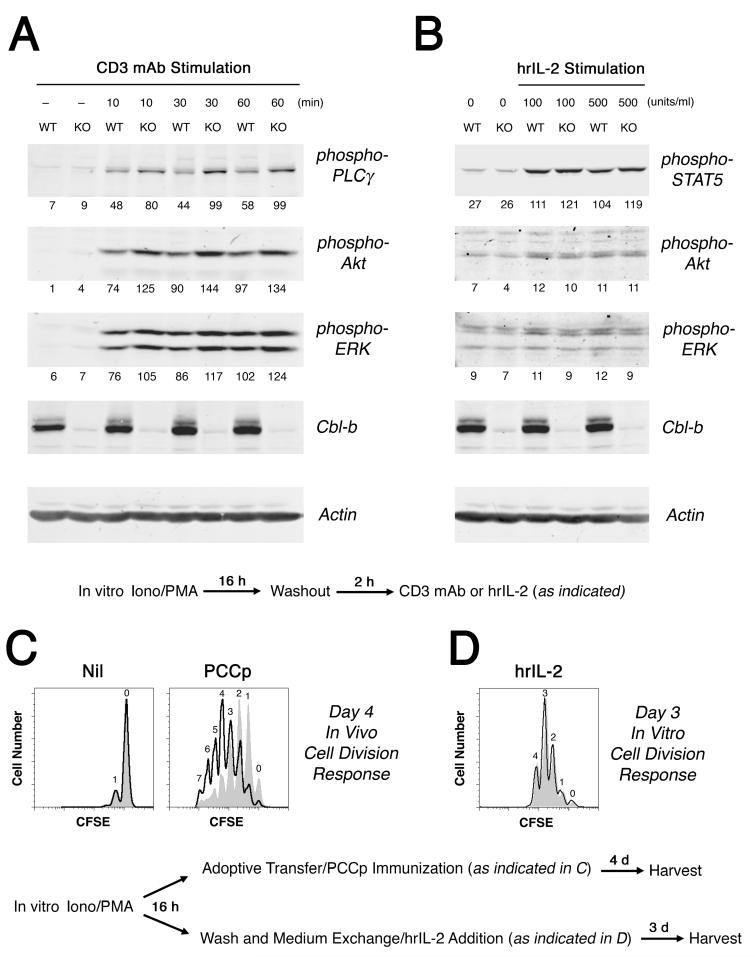
Using this ionomycin and PMA pharmacological system to activate equally the Cbl-b-sufficient and Cbl-b-deficient T cells, we then examined their late signaling characteristics and proliferative responsiveness. After a 16 h period of pre-activation in the presence of ionomycin and PMA, and following a 2 h washout period, Cblb knockout 5C.C7 CD4+ T cells exhibited an enhanced level of anti-CD3 mAb-induced PLCγ-1, Akt, and ERK phosphorylation, as compared to wildtype T cells (Fig. 7A). Similarly, the adoptive transfer of these ionomycin plus PMA pre-treated T cells into normal hosts revealed that the Cblb-/- CD4+ T cells could undergo more rounds of cell division following challenge with Ag (Fig. 7C). In contrast, these ionomycin plus PMA pre-treated T cells demonstrated no capacity of Cbl-b to counter-regulate IL-2 receptor-triggered STAT5 phosphorylation and in vitro proliferation (Fig. 7B and D). Therefore, the up-regulation of Cbl-b during CD4+ T cell activation leads to a selective antagonism of TCR-dependent proximal signaling events at late time points during the primary response to Ag, but leaves T cells free to respond to cytokine stimulation.
Figure 7. Cbl-b inhibits TCR/CD3 downstream signaling at late times after initial CD4+ T cell activation.
Wildtype (WT) and Cblb gene knockout (KO) 5C.C7 CD4+ T cells were activated in vitro for 16 h in the presence of 0.4 μM ionomycin plus 10 ng/ml PMA. One aliquot of pre-activated T cells was washed free of pharmacological agents and rested in culture for an additional 2 h, after which the T cells were then exposed to 1 μg/ml immobilized CD3 mAb for the times indicated (A) or incubated with human recombinant IL-2 for 30 m at the concentrations shown (B). Cbl-b and actin, as well as phospho-PLCγ, -Akt, -ERK, and -STAT5 levels were determined by western blotting. Note that the phospho-Akt and -ERK bands shown in panel B have been linearly amplified for clarity. Average band densities for individual samples (without amplification) are shown beneath each band. In a second aliquot of pre-activated Cblb+/+ (gray tracing) and Cblb-/- (open tracing) 5C.C7 CD4+ T cells, CFSE dye dilution was measured either 4 days after adoptive transfer into mice immunized with either PBS alone (left panel) or 5 μg PCCp (right panel) (C), or 3 days after washing and in vitro cultivation in the presence of hrIL-2 (D).
Discussion
This work has explored the role of one counter-regulatory protein, Cbl-b, in the control of late Ag-driven cell cycle progression. During their primary response to Ag, CD4+ T cells rely on continuous TCR signaling to achieve the highest rate of cell division, maximal clonal expansion, and optimal differentiation to a helper phenotype (4, 5, 7). Prolonged clustering with Ag-bearing dendritic cells facilitates this durable TCR engagement, but also forces CD4+ T cells with identical Ag specificities to compete for peptide Ag/MHC ligands if they are to remain in cell cycle (27). Despite this new appreciation of the necessity for continuous TCR signaling to prolong CD4+ T cell clonal expansion, the biochemical pathways involved in mediating this signal in vivo remain unknown.
Cbl-b has previously been implicated in the inhibition of p85 PI3K downstream signaling to NFκB (11, 13-15). We, therefore, chose to investigate this molecule in these studies based on our own in vitro data indicating a requirement for TCR- and CD28-dependent PI3K signaling to maintain an optimal rate of cell division at late time points after the initial Ag encounter (22). This study now establishes that during a primary response to Ag stimulation in the absence of adjuvant or infection, the rate of cell division by CD4+ T cells that have already committed to a proliferative response becomes limited by their induction of Cbl-b protein, and its subsequent inhibition of TCR-dependent proximal signaling events. Cbl-b molecules (and/or their target proteins) not only desensitize CD4+ T cell blasts to low numbers of Ag/MHC complexes at late times after the initiation of priming, but also promote early exit from the cell cycle in the absence of ongoing TCR signaling.
These experiments were designed to address the concern that changes in T cell signaling observed in the absence of Cblb are a consequence, rather than cause, of the spontaneous autoimmune disease that has sometimes been observed in these mice (11, 12). Use of the 5C.C7 TCR-Tg on the Rag2-/- background precluded the development of an autoreactive CD4+ T cell repertoire in the Cblb-/- animals, and prevented disease in our experiments. Adoptive transfer of these CD4+ T cells into normal hosts also eliminated the effect of a loss of Cbl-b expression in other immune cells or tissues. Therefore, one can be confident that these results have not been confounded by some alteration in the specificity or differentiation state of the responder CD4+ T cell population, or by a secondary effect of systemic inflammation or non-T cell Cbl-b deficiency.
It is most important to note that this investigation did not reveal in vivo evidence for control of naïve CD4+ T cell initial recognition of Ag by Cbl-b. This result is remarkable given previous studies that have reported a selective capacity of Cbl-b molecules in naïve T cells to interrupt signaling between p85 PI3K and NFκB, even within the first 30 minutes of in vitro activation (11-15). We, too, have observed a capacity of Cbl-b to selectively antagonize NFκB translocation to the nucleus 4 h into an in vitro activation, particularly when CD28 costimulatory signals are lacking (R.Z., unpublished observation). Nevertheless, such alterations in PI3K and NFκB activation may be relatively unimportant to establishing the threshold for T cell activation in vivo, at least as measured by the early induction of CD69 and CD25 expression. Alternatively, early B7/CD28 interactions (even in the absence of infection) may be sufficient to overcome any inhibitory effect of a few Cbl-b molecules, and allow for adequate early PI3K and NFκB signaling in vivo. Consistent with this latter theory, previously published in vitro activation studies have suggested that the costimulation of T cell cultures with anti-CD28 mAb alleviates much of the counter-regulatory effect of Cbl-b (26). The induction of CD69 and CD25 is thought to depend at least in part on the activation of NFκB (28, 29). Therefore, it is more likely that early during Ag priming of CD4+ T cells in vivo there is insufficient Cbl-b activity to overcome the effects of CD28 costimulatory signaling and inhibit TCR signaling to PI3K, Akt, and NFκB.
In contrast to its inability to effectively antagonize initial Ag/MHC recognition in vivo, or prevent an early increase in cell size and CD71 surface expression by the Ag-specific CD4+ T cells, Cbl-b did prove important in the exit of these T cells from cell cycle during the period between 48 and 72 hours. Although the mechanism of this counter-regulation remains uncertain, Cbl-b expression was associated with a premature reduction in cell size and CD71 by 48 hours. Our previous studies have implicated a CD28/PI3K/Akt/mTOR signaling pathway as necessary to maintain CD4+ T cells in cell cycle following Ag stimulation in vitro (22, 30). Others have shown that the expression CD71 relies on signals mediated by PI3K and mTOR (25), and our own unpublished experiments confirm this (R.Z.). Therefore, we now speculate that during the in vivo primary response to Ag, PI3K, mTOR, and NFκB activities wane as Cbl-b protein levels rise and desensitize the CD4+ T cells to further Ag stimulation, thus quenching their remaining proliferative program. It also follows that during a primary response to Ag in the presence of an adjuvant or infection, or in the clinical setting of systemic inflammation, increased PI3K activity associated with enhanced CD28 and OX40 costimulatory receptor signaling overcomes the negative effects of Cbl-b and facilitates a more durable clonal expansion response via the induction of mTOR regulators such as survivin and aurora B (31, 32).
Results obtained by others using P14 TCR-transgenic CD8+ T cells primed during lymphocytic choriomeningitis virus infection support the conclusions that Cbl-b cannot interrupt early Ag recognition or entry into cell cycle by naïve T cells, but instead puts a brake on late TCR signaling (33). Because CD8+ T cells can be programmed for a durable clonal expansion, without need for persistent Ag recognition to maintain a high rate of cell division, the up-regulation of Cbl-b and subsequent TCR down-modulation are not accompanied by a slowing of the rate of proliferation (33, 34). On the other hand, Cblb-/- P14 CD8+ T cells do demonstrate augmented IFN-γ production on Ag rechallenge, consistent with a need for continuous TCR signaling to ensure optimal effector T cell generation and a capacity for Cbl-b to interfere with this TCR-dependent differentiation event (33, 35).
It is interesting that Cbl-b counter-regulates TCR/CD3-dependent activation signals, but does not inhibit IL-2 receptor signaling in blasting CD4+ T cells. Like the TCR, IL-2 receptors are thought to activate PI3K, with PI3K activity necessary for expression of cyclin D3 and degradation of p27kip1 prior to entry into cell cycle (36). Therefore, it might have been reasonable to expect that Cbl-b would also antagonize late IL-2-dependent PI3K signaling and cell cycle progression by 5C.C7 CD4+ T cell blasts. However, experiments shown here utilizing ionomycin plus PMA-generated CD4+ T cell blasts (or T cells pre-treated with CD3 and CD28 mAb; data not shown) did not demonstrate a significant increase in Akt phosphorylation during the first 30 m of exposure to the cytokine. In contrast, IL-2 was sufficient to stimulate equally the phosphorylation of STAT5 in both Cbl-b-expressing and Cbl-b-deficient CD4+ T cell blasts. IL-2 treatment was also observed to protect both Cblb-/- and wildtype CD4+ T cell blasts from growth factor withdrawal-induced cell death (R.Z., unpublished observation). Perhaps consistent with a dominant role for phospho-STAT5-dependent and Cbl-b/PI3K-independent signaling pathways in IL-2-triggered cell growth and survival in vitro, the PI3K inhibitor LY294002 blocks neither the expression of cyclin D3 and cyclin E, nor the down-regulation of p27kip1 at late time points after treatment with IL-2 (37). Taken together, the data suggest there is little opportunity for Cbl-b to antagonize IL-2-dependent late cell signaling events. This capacity of IL-2 receptors to bypass the counter-regulatory effects of Cbl-b may help explain how IL-2 can promote a reversal of clonal anergy in CD4+ T cells despite their high expression of Cbl-b (16-18, 38). Thus, the ability of Cbl-b to counter-regulate late cell cycle progression in our adoptive transfer system is most consistent with the model that IL-2 is neither necessary nor sufficient to mediate CD4+ T cell proliferation in vivo (39).
In summary, this work details a new role for Cbl-b in the late regulation of CD4+ T cell clonal expansion following a primary recognition of Ag. The accumulation of Cblb protein hours to days into an immune response antagonizes TCR-dependent proximal signal transduction and imposes a requirement for continued strong TCR signaling to maintain a high rate of cell division. When Ag/MHC complexes can no longer be detected during the course of a clonal expansion as a consequence of this Cbl-b-dependent desensitization, CD4+ T cells then fail to maintain their expression of transferrin receptors (CD71) and undergo a premature growth arrest that prevents additional G1—>S phase transitions. Such counter-regulation of TCR-dependent late cell growth by Cbl-b may underlie its ability to act as a barrier to the development of autoimmune disease following self Ag recognition.
Acknowledgements
We wish to thank M. Jenkins and S. Jameson for their reading of the manuscript and thoughtful comments.
Footnotes
This study was supported by grants from the North Central Chapter Arthritis Foundation and NIH grants P01 AI35296 and R01 GM54706.
- Cbl-b
- Casitas B-lineage lymphoma b
- mTOR
- mammalian target of rapamycin
- NFκB
- nuclear factor kappa B
- PCCp
- pigeon cytochrome c peptide 81-104
- PI3K
- phosphatidylinositol 3-kinase
- PKC-θ
- protein kinase C-theta
- PMA
- phorbol 12-myristate 13-acetate
- TCR-Tg
- TCR-transgenic
Disclosures
The authors have no financial conflict of interests to disclose.
References
- 1.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 3.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 4.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obst R, van Santen HM, Melamed R, Kamphorst AO, Benoist C, Mathis D. Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation. Proc. Natl. Acad. Sci. USA. 2007;104:15460–15465. doi: 10.1073/pnas.0707331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Yarke CA, Dalheimer SL, Zhang N, Catron DM, Jenkins MK, Mueller DL. Proliferating CD4+ T Cells Undergo Immediate Growth Arrest upon Cessation of TCR Signaling In Vivo. J. Immunol. 2008;180:156–162. doi: 10.4049/jimmunol.180.1.156. [DOI] [PubMed] [Google Scholar]
- 8.Smith AL, Wikstrom ME, de St Groth B. Fazekas. Visualizing T cell competition for peptide/MHC complexes: a specific mechanism to minimize the effect of precursor frequency. Immunity. 2000;13:783–794. doi: 10.1016/s1074-7613(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 9.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 10.Yokoi N, Komeda K, Wang HY, Yano H, Kitada K, Saitoh Y, Seino Y, Yasuda K, Serikawa T, Seino S. Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nat. Genet. 2002;31:391–394. doi: 10.1038/ng927. [DOI] [PubMed] [Google Scholar]
- 11.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 12.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveirados-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 13.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol. Cell. Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J. Biol. Chem. 2001;276:4872–4878. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- 16.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 17.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 19.Kaye J, Hedrick SM. Analysis of specificity for antigen, Mls, and allogenic MHC by transfer of T-cell receptor alpha- and beta-chain genes. Nature. 1988;336:580–583. doi: 10.1038/336580a0. [DOI] [PubMed] [Google Scholar]
- 20.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. USA. 1987;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 22.Bonnevier JL, Yarke CA, Mueller DL. Sustained B7/CD28 interactions and resultant phosphatidylinositol 3-kinase activity maintain G(1)-->S phase transitions at an optimal rate. Eur. J. Immunol. 2006;36:1583–1597. doi: 10.1002/eji.200535626. [DOI] [PubMed] [Google Scholar]
- 23.Vanasek TL, Khoruts A, Zell T, Mueller DL. Antagonistic roles for CTLA-4 and the mammalian target of rapamycin in the regulation of clonal anergy: enhanced cell cycle progression promotes recall antigen responsiveness. J. Immunol. 2001;167:5636–5644. doi: 10.4049/jimmunol.167.10.5636. [DOI] [PubMed] [Google Scholar]
- 24.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A Role for Mammalian Target of Rapamycin in Regulating T Cell Activation versus Anergy. J. Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J. Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 27.Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc. Natl. Acad. Sci. USA. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruben S, Poteat H, Tan TH, Kawakami K, Roeder R, Haseltine W, Rosen CA. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988;241:89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Cabrera M, Munoz E, Blazquez MV, Ursa MA, Santis AG, Sanchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J. Biol. Chem. 1995;270:21545–21551. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- 30.Bonnevier JL, Mueller DL. Cutting edge: B7/CD28 interactions regulate cell cycle progression independent of the strength of TCR signaling. J. Immunol. 2002;169:6659–6663. doi: 10.4049/jimmunol.169.12.6659. [DOI] [PubMed] [Google Scholar]
- 31.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat. Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 32.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Shamim M, Nanjappa SG, Singh A, Plisch EH, LeBlanc SE, Walent J, Svaren J, Seroogy C, Suresh M. Cbl-b regulates antigen-induced TCR down-regulation and IFN-gamma production by effector CD8 T cells without affecting functional avidity. J. Immunol. 2007;179:7233–7243. doi: 10.4049/jimmunol.179.11.7233. [DOI] [PubMed] [Google Scholar]
- 34.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J. Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 35.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 36.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 37.Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J. Immunol. 2006;176:2730–2738. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- 38.Colombetti S, Benigni F, Basso V, Mondino A. Clonal anergy is maintained independently of T cell proliferation. J. Immunol. 2002;169:6178–6186. doi: 10.4049/jimmunol.169.11.6178. [DOI] [PubMed] [Google Scholar]
- 39.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J. Exp. Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]