Abstract
O6-Alkylguanine-DNA alkyltransferase (alkyltransferase) provides an important source of resistance to some cancer chemotherapeutic alkylating agents. Folate ester derivatives of O6-benzyl-2′-deoxyguanosine and of O6-[4-(hydroxymethyl)benzyl]guanine were synthesized and tested for their ability to inactivate human alkyltransferase. Inactivation of alkyltransferase by the γ folate ester of O6-[4-(hydroxymethyl)benzyl]guanine was similar to that of the parent base. The γ folate esters of O6-benzyl-2′-deoxyguanosine were more potent alkyltransferase inactivators than the parent nucleoside. The 3′ ester was considerably more potent than the 5′ ester and was more than an order of magnitude more active than O6-benzylguanine, which is currently in clinical trials to enhance therapy with alkylating agents. They were also able to sensitize human tumor cells to killing by 1,3-bis(2-chloroethyl)-1-nitrosourea with O6-benzyl-3′-O-(γ-folyl)-2′-deoxyguanosine being most active. These compounds provide a new class of highly water-soluble alkyltransferase inactivators and form the basis to construct more tumor specific and potent compounds targeting this DNA repair protein.
Introduction
O6-Alkylguane-DNA alkyltransferase (alkyltransferased) is a widespread DNA repair protein that acts by transferring adducts such as methyl- or 2-chloroethyl- from the O6-position of guanine in DNA to a cysteine acceptor site 1–3. The alkyltransferase-mediated repair of DNA adducts in cells exposed to therapeutic alkylating agents such as temozolomide or 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) provides a major mechanism of resistance to these drugs. Inactivation of alkyltransferase therefore improves the response to these agents. A prototype inactivator O6-benzylguanine is undergoing clinical trials in the US 3–5 and a similar compound O6-(4-bromothenyl)guanine is being tested in the UK 6, 7. Although there have been some responses in the trials with O6-benzylguanine, it is apparent that the lack of selectivity of the drug towards the tumor alkyltransferase may be a significant factor in limiting its effectiveness. Several approaches are in progress to render alkyltransferase inactivation more tumor specific. These include attempts to make prodrugs that would be preferentially activated in tumors to generate alkyltransferase inhibitors 8 and to make compounds that would be selectively taken up by tumors. A promising candidate for the later approach is O4-benzylfolic acid (Figure 1), which is a powerful alkyltransferase inhibitor that is considerably more active than O6-benzylguanine and is likely to be taken up by the folate receptor system 9. In the present work, we describe the synthesis and the properties of a number of other folate derivatives which are also shown in Figure 1. These were γ folate esters of O6-benzyl-2′-deoxyguanosine attached through the 3′ or 5′ hydroxyl (1 and 2) and the folic acid γ ester of O6-[4-(hydroxymethyl)benzyl]guanine (3).
Fig. 1.
Structures of folate conjugates.
Previous studies with glucuronic acid derivatives of O6-benzylguanine and O6-benzyl-2′-deoxyguanosine linked via the N2- position showed that these were inactive prodrugs but were converted by β-glucuronidase to the active compounds 8. It was possible that the folate esters would act as similar prodrugs that could be accumulated via the folate transport mechanism. However, the studies described here show that all of the folate esters tested were active as alkyltransferase inactivators without need for metabolic activation and that 1 was a very potent compound. Both 1 and 2 were more active than the parent O6-benzyl-2′-deoxyguanosine in vitro. In contrast, the addition of a folic acid moiety via a γ ester to O6-[4-(hydroxymethyl)benzyl]guanine forming 3 only slightly reduced the ability to inactivate alkyltransferase. The esters were all converted to the parent compounds by esterases found in tumor cells. The ability of compounds 1–3 to sensitize cells to killing by BCNU was also investigated using tumor cells that differ in their folate receptor status.
Results and Discussion
Synthesis of folate ester derivatives
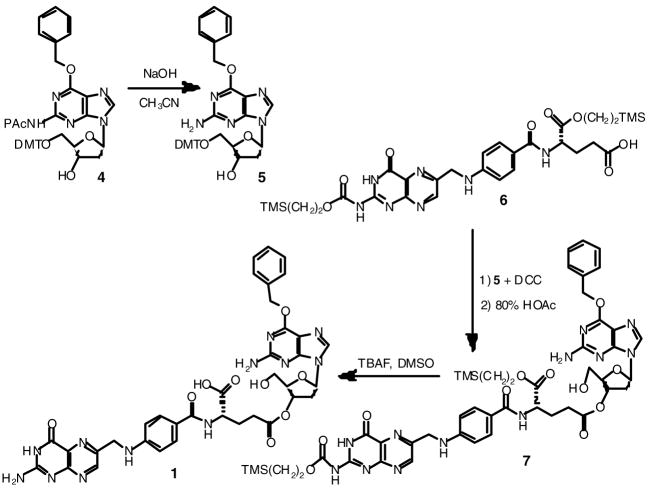
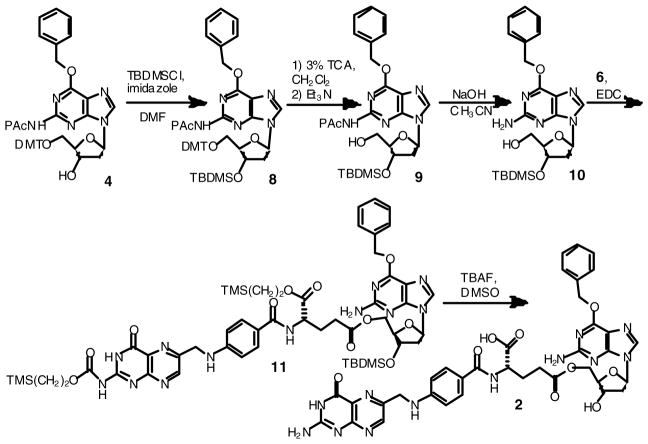
For the synthesis of the 3′ and 5′ γ folate esters of O6-benzyldeoxyguanosine (compounds 1 and 2) the starting material was O6-benzyl-5′-O-(4,4′-dimethoxytrityl)-N2-phenoxyacetyl-2′-deoxyguanosine (4). This compound had been previously prepared in this laboratory for use in the synthesis of oligonucleotides containing O6-benzyldeoxyguanosines 10. Protecting groups on the nucleoside were manipulated to give O6-benzyldeoxyguanosine having either a free 3′ hydroxyl (Scheme 1) or free 5′ hydroxyl (Scheme 2) which could be selectively coupled to the γ carboxylate of bis-silyl protected folic acid 11 using a carbodiimide.
Scheme 1.
Synthesis of 3′ γ folate ester of O6-benzyldeoxyguanosine (1). PAc indicates phenoxyacetyl
Scheme 2.
Synthesis of 5′ γ folate ester of O6-benzyldeoxyguanosine (2).
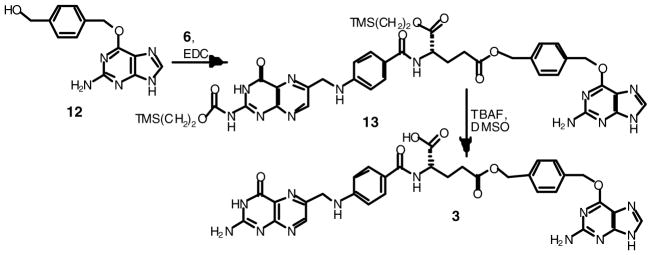
Compound 3 was prepared similarly by carbodiimide coupling of the hydroxyl group on O6-[4-(hydroxymethyl)benzyl]guanine (12) which had been prepared as described 12 to the same silyl protected folate (Scheme 3). The resulting esters were purified, deprotected and subsequently isolated by mild acid precipitation.
Scheme 3.
Synthesis of folic acid γ ester of O6-[4-(hydroxymethyl)benzyl]guanine (3).
Inactivation of purified human alkyltransferase in vitro
Previous studies have shown that O6-benzyl-2′-deoxyguanosine is about an order of magnitude less active than the free base O6-benzylguanine in the ability to inactivate purified alkyltransferase in vitro 13. When assays were conducted in the presence of DNA, this difference was considerably larger (>100-fold) since the inactivation by O6-benzylguanine is facilitated by DNA whereas it is reduced in the case of O6-benzyl-2′-deoxyguanosine 14. Both 1 and 2 were more active than O6-benzyl-2′-deoxyguanosine itself in the inactivation of alkyltransferase (Table 1). Remarkably, the 3′ ester (1) was an extremely potent alkyltransferase inactivator with an ED50 value in the absence of DNA of 16 nM, which is 125-times lower than the parent O6-benzyl-2′-deoxyguanosine and 19 times lower than O6-benzylguanine. When DNA was present, the ED50 value of 1 was increased to 0.68 μM but this is still much less than that of O6-benzyl-2′-deoxyguanosine itself (40 μM). Compound 1 was also able to inactivate the P140K mutant of alkyltransferase, which is totally resistant to O6-benzylguanine or O6-benzyl-2′-deoxyguanosine 15, 16, but quite high concentrations were required (ED50 of c. 100 μM) (results not shown).
Table 1.
Inactivation of purified human alkyltransferase in vitro
| Compound | ED50 for inactivation of alkyltransferase (μM)* | |
|---|---|---|
| − DNA | + DNA | |
| O6-benzylguanine a | 0.3 | 0.1 |
| O6-benzyl-2′-deoxyguanosine b | 2.0 | 40.0 |
| 1 | 0.016 ± 0.002 | 0.68 ± 0.02 |
| 2 | 0.64 ± 0.05 | 3.06 ± 0.18 |
| 3 | 0.70 | 0.24 |
| 12 c | 0.4 | 0.1 |
ED50 values were calculated from graphs of the percentage of remaining alkyltransferase activity against inhibitor concentration. Experiments where an S. D. is shown were repeated 3–5 times and the mean shown. Other values are the mean of two experiments.
The 5′ ester (2) was also a potent inactivator of alkyltransferase that was more effective in the absence of DNA but it was considerably less active than 1 with an ED50 value of 640 nM compared to 16 nM for 1 (Table 1). When DNA was present, the ED50 of 2 increased 5-fold to about 3 μM. These results suggest that the addition of a folate moiety increases the ability of O6-benzyl-2′-deoxyguanosine to bind to human alkyltransferase and that this binding occurs more favorably when the folate is attached to the 3′ rather than the 5′ position.
The ability of DNA to reduce the effectiveness of O6-benzyl-2′-deoxyguanosine and other 9-substituted O6-benzylguanine derivatives has been attributed to a competition between the DNA and the 9-substitutent for binding at the active site 14. Such competition would still be expected to occur with the folate esters but the fact that they are still more active than O6-benzyl-2′-deoxyguanosine itself is consistent with the concept that these folate derivatives also interact with the alkyltransferase at residues not involved in the binding of DNA. Although it was originally envisaged that 1 and 2 might act as prodrugs that would be converted to an active alkyltransferase inhibitor, it appears that this conversion to O6-benzyl-2′-deoxyguanosine is not necessary and would actually reduce their effectiveness.
Compound 3, the folic acid γ ester of 12, was slightly less effective than the free base parent compound 12 in the inactivation of alkyltransferase (Table 1). Both compounds resemble O6-benzylguanine in that inactivation is slightly greater when DNA is present. This result is in agreement with a number of studies that have shown that adducts can be added to the para position of O6-benzylguanine without greatly affecting the ability to interact with alkyltransferase 12, 19 including large fluorescent derivatives 20. This finding is consistent with models of the binding of O6-benzylguanine to alkyltransferase which shows that there is a space available around the para position of the benzyl group which is contiguous with the opening to the active site pocket 21, 22. Large adducts can therefore fit into this space without interactions with the protein. The small increase in reactivity when DNA is present, which is seen with O6-benzylguanine, 12 and 3 (Table 1) is likely to be due to an activation of the cysteine acceptor part of the protein that occurs when DNA is bound facilitating the alkyl transfer 23.
Stability and enzymatic hydrolysis of compounds 1–3
Compounds 1–3 were relatively stable when incubated in neutral solution. The rate of release of folate was approximately 2.2%/day from 1; 3.2%/day from 2; and 0.9%/day from 3. Extracts from a variety of human tumor cells and porcine liver esterase were able to release folate from 1, 2 and 3 (Fig. 2). Compound 3 was a much better substrate for porcine liver esterase than 2 or 1 with 592 pmol/h/unit converted to folate. This rate is 10–20 times that of the conjugates with O6-benzyl-2′-deoxyguanosine where 20 pmol/h/unit of 1 was converted to folate and 46 pmol/h/unit of 2 was hydrolyzed. The extent of conversion to folate by human tumor cell extracts varied slightly according to the cell type with MCF-7 cells showing the highest rate of conversion. All tumor cells tested had esterase activity towards the compounds but this activity was quite weak with more than 50% of the compound remaining after 48 h. The 5′ ester (2) was a slightly better substrate than the 3′ ester (1). There was very little cleavage (less than 2%) of either compound in culture medium containing 10% serum for 24 h.
Figure 2.

Release of folate from 1, 2 and 3 by cell extracts.
Sensitization of tumor cells to killing by BCNU
The ability of the ester inhibitors to sensitize human tumor cells to killing by BCNU was investigated using HT29, A549 and KB cells. As shown in Figure 3, both 1 and 2 were able to sensitize HT29 cells to killing by BCNU. However, their activity appears to be limited by a low uptake. Both esters were less effective than their O6-benzyl-2′-deoxyguanosine parent despite being better alkyltransferase inactivators in vitro. The observation that 1 is more active than 2 in all of the cells tested (Figure 4) is consistent with the more potent inactivation of alkyltransferase in vitro by the 3′ ester. This also supports the concept that their effects are due to the esters themselves rather than their conversion to O6-benzyl-2′-deoxyguanosine since 2 was a better esterase substrate than 1.
Figure 3.
Killing of HT29 cells by BCNU plus alkyltransferase inhibitors. Results are shown for HT29 cells grown in RPMI medium and exposed to the drugs indicated for 2 h prior to addition of 40 μM BCNU for 2 h. Results are shown for treatment with O6-benzyl-2′-deoxyguanosine (BdG) and with compounds 1, 2, 3 and 12.
Figure 4.
Killing of A549, HT29 and KB cells grown in folate-free medium by BCNU plus alkyltransferase inhibitors. Cells were grown in RPMI medium minus folate for 48 h and exposed to the drugs for 8 h prior to addition of BCNU for 2 h. The amount of BCNU used was the maximum dose that gave no decrease in survival in the absence of alkyltransferase inhibition. Panel A shows results for A549 cells treated with 1 or 2 and 20 μM BCNU. Panel B shows results for HT29 cells treated with 1, 2 or 3 and 20 μM BCNU. Panel C shows results for KB cells treated with 1, 2 or 3 and 40 μM BCNU.
Compound 3 was much less effective than 1 and 2 in increasing the killing of HT29 cells by BCNU (Figure 3). In contrast, its parent 12 was very active. This result is consistent with limited uptake of 3 and poor conversion to 12 in either the cells or the culture medium.
In order to test whether compounds 1–3 entered cells via the folate receptor mechansim, studies were carried out to examine the killing by BCNU of A549, HT29 and KB cells grown for 48 h in the absence of folate prior to the addition of the inhibitors (Figure 4). The inhibitors were added for 8 h prior to treatment with BCNU to allow increased time for alkyltransferase inactivation to occur but other experiments (not shown) with 2 h or 4 h drug exposure times gave similar results. Both 1 and 2 were able to sensitize all three tumor cells to killing by BCNU and 1 was more potent than 2. Approximately the same degree of sensitization was seen with the three cell lines even though A549 cells have very low levels of folate receptors and KB cells have a very high folate receptor carrier activity. This suggests that the compounds are not taken up via a folate receptor mediated mechanism. This is supported by comparison of the effects of 8 h exposure to drugs on HT29 cells grown in the folate free medium shown in Figure 4B with the effects of 2 h exposure on HT29 cells grown in the folate containing medium in Figure 3. There was little difference in the effect under the two conditions. Experiments in which the addition of 100 μM folate 30 min prior to the inhibitor did not affect the results with 1 and 2 on KB or HT29 cells (results not shown) provide additional evidence that their effect is not dependent on a folate carrier. In contrast, previous studies with O4-benzyfolate 9 showed that growing cells in folate-free medium increased the killing effects of BCNU when O4-benzylfolate was added to KB or HT29 cells but not A549 cells and that the addition of 100 μM folate 30 min prior to the inhibitor blocked this effect.
Compound 3 was tested only in HT29 and KB cells grown in the folate free medium (Figure 4B and 4C) and this compound was very poorly active in both cells. It was slightly more effective in KB cells. This suggests that it may be taken up in a folate-carrier related manner but that such uptake is very limited. There was little enhancement of cell killing by BCNU even at 50 μM whereas 12 and O6-benzyl-2′-deoxyguanosine (Figure 2) and O6-benzylguanine itself were effective at 5 μM concentrations 15, 17, 24. This contrasts strikingly with the in vitro inactivation of alkyltransferase in which 3 is more active than O6-benzyl-2′-deoxyguanosine and only slightly less potent than O6-benzylguanine and 12. Although 3 was the best substrate for porcine liver esterase and for cellular esterases among the compounds tested, it is clear that very little of the drug added to the cell culture medium is converted to 12 by serum esterases since this metabolite was >100 x more effective than 3 itself in increasing cell killing by BCNU.
Conclusions
The in vitro studies of the inactivation of purified human alkyltransferase with compounds 1 and 2 indicate that the addition of folate adducts to the deoxyribose of O6-benzyl-2′-deoxyguanosine increases the ability to reduce alkyltransferase activity. This effect is much greater when the folate is attached to the 3′ position than when attached to the 5′ position. In fact addition of folate to the 3′ position of O6-benzyl-2′-deoxyguanosine to form 1 produces one of the most potent alkyltransferase inhibitors so far described with an ED50 of 16 nM. This value is similar to that found for O4-benzylfolate 9. At present, it is not known how these folate groups strengthen the interaction with alkyltransferase. Further study of the interaction of O4-benzylfolate and of 1 and 2 with alkyltransferase by binding studies, modelling and crystallographic investigation should provide a mechanistic explanation for this effect. This could then be tested experimentally with studies of appropriate mutants of key residues. Such an understanding would allow for the synthesis of other very potent alkyltransferase inhibitors. The addition of folate groups to O6-benzylguanine derivatives also has the important advantage of greatly increasing the water solubility of these compounds. This increased solubility is also seen with 3, the γ folate ester of 12, but addition to this position had little effect on the ability to inactivate alkyltransferase.
Surprisingly, based on the amount needed to sensitize tumor cells to killing by BCNU, the uptake of compounds 1 and 2 into tumor cells occurred to only a limited extent and was not greatly affected by the folate receptor status. Compound 3 also had much less effect on increasing sensitivity to BCNU than expected from its potency as an alkyltransferase inhibitor suggesting that uptake is poor even in the KB cells with a high folate receptor content. The results for the enhancement of cell killing by the folate esters 1–3 described here differ from those for O4-benzylfolate. This compound, which is also a highly potent alkyltransferase inhibitor, was much more potent in KB cells than in A549 cells 9, and its effects were increased by growing KB or HT29 cells in a folate free media and antagonized by the addition of folate (unpublished observations). These observations with O4-benzylfolate are consistent with its efficient uptake by a folate receptor carrier mechanism. Despite the excellent alkyltransferase inactivation activity of the folate esters described in this paper, their ability to sensitize cells to alkylating agents was limited. This is most likely to be due to a low level of uptake rather than repaid degradation since their conversion to folate by cellular esterases was quite slow (Figure 2) However, they provide a very useful lead for the design of other highly water soluble, specific and potent alkyltransferase inhibitors based on the ability to interact with additional residues in the alkyltransferase active pocket.
Experimental section
Chemistry
Chemicals were obtained from Aldrich (Milwaukee, WI) or Sigma (St. Louis, MO) and were used without further purification. UV spectra were determined on a Beckman Coulter DU 7400 spectrophotometer. 1H NMR spectra were recorded in DMSO-d6 with a Varian INOVA 400 MHz spectrometer. Chemical shifts are reported as δ values in parts per million relative to TMS as an internal standard. Splitting pattern abbreviations are as follows: s = singlet, d = doublet, dd = double doublet, ddd = a doublet of doublet of doublets, t = triplet, td = triplet of doublets, m = multiplet. Coupling constants are in hertz. Mass spectra were collected on a Thermo Finnigan TSQ Quantum spectrometer in positive ion electrospray mode scanning m/z=100 to 1500 in one second. The electrospray voltage was 3.5 kV, the transfer tube was at 350°C. Elemental analysis, performed by Atlantic Microlab, Inc. (Norcross, GA) were within 0.4% of theoretical values calculated for C, H, and N. All silica gel chromatography was carried out using Davisil, grade 633, 200–425 mesh 60 Å. Synthesis and purification of folate containing compounds was performed under reduced (yellow) light and these materials should be considered light sensitive 25.
O6-Benzyl-5′-O-(4,4′-dimethoxytrityl)-2′-deoxyguanosine (5)
Nucleoside 4 was synthesized according to methods reported earlier 10. NaOH (1.0 M, 50 mL) was added to a solution of nucleoside 4 (3.93 g, 4.96 mmol) in CH3CN (100 mL) and stirred at room temperature for 18 h. The reaction was neutralized with HCl (1.0 M) and extracted with CH2Cl2 (3 × 50 mL). The resulting organic layers were combined, dried with MgSO4, filtered, and the solvent was removed under reduced pressure to yield 5 as a white solid (3.18 g, 97.2 %). 1H NMR (DMSO-d6): δ 2.29 (1H, ddd, J=11.2, J=6.4, J=4.8, H-2′), 2.70 (1H, ddd, J=13.2, J=6.4, J=6.4, H-2′), 3.13 (2H, d, J=5.5, H-5′), 3.70 (3H, s, DMT-O-CH3), 3.71 (3H, s, DMT-O-CH3), 3.93 (1H, dd, J=8.8, J=4.4, H-4′), 4.39 (1H, ddd, J=9.6, J=4.4, J=4.4, H-3′), 5.32 (1H, br d, J=4.4, 3′-OH, exchanges with D2O), 5.49 (2H, s, CH2-Ph), 6.24 (1H, t, J=6.4, H-1′), 6.46 (2H, br s, N2H2, exchanges with D2O), 6.78–6.84 (4H, m, DMT Ph and DMT Ar), 7.15–7.26 (7H, m, DMT Ph and DMT Ar), 7.32–7.42 (5H, m, DMT Ar and Bn Ar), 7.51–7.94 (2H, m, Bn Ar), 7.94 (1H, s, H-8).
O6-Benzyl-3′-O-[γ-[α-[2-(trimethylsilyl)ethoxy]]-2-N-[2-(trimethylsilyl)-ethoxycarbonyl]folyl]-2′-deoxyguanosine (7)
Folate 6 was synthesized according to methods reported by Nomura et. al. 11. 4-Dimethylaminopyridine (DMAP), (0.0324 g, 0.265 mmol) and 1,3-dicyclohexylcarbodiimide (1.09 g, 5.30 mmol), were added to a solution of 6 (1.82 g, 2.65 mmol) in CH2Cl2 (75 mL) and stirred at room temperature for 1.5 h. Nucleoside 5 (1.75 g, 2.65 mmol) was then added to the solution and stirred for an additional 18 h. The solvent was removed under reduced pressure to yield a yellow foam. The solid foam was dissolved in EtOAc and filtered to remove insoluble dicyclohexylurea and dried under reduced pressure to afford a yellow solid. This solid was dissolved in 80% acetic acid (25 mL) and stirred for 30 min. Ethanol (250 mL) was added and the solvent was removed under reduced pressure to afford an orange foam which was purified by column chromatography (silica gel, 7:3:0.25 CH2Cl2:EtOAc:MeOH) to give 7 (1.1 g, 40.4%). 1H NMR (DMSO-d6): δ 0.003 (9H, s, Si(CH3)3), 0.058 (9H, s, Si(CH3)3), 0.92–0.96 (2H, m, TMS-CH2CH2), 1.03–1.07 (2H, m, TMS-CH2CH2), 1.94–2.03 (1H, m, gluβ-CH2b), 2.11 (1H, ddd, J=20.8, J=8.0, J=6.0, gluβ-CH2a), 2.40 (1H, br dd, J=13.2, J=5.6, H-2′), 2.48 (2H, br t, J=7.6, gluγ-CH2), 2.84 (1H, ddd, J=14.8, J=9.2, J=6.0, H-2′), 3.52–3.62 (2H, m, H-5′), 4.00 (1H, td, J=4.4, J=1.6, H-4′), 4.11–4.15 (2H, m, TMS-CH2CH2), 4.27–4.32 (2H, m, TMS-CH2CH2), 4.41 (1H, ddd, J=12.8, J=7.6, J=5.2, gluα-CH), 4.59 (2H, br d, J=6.0, folate 6-CH2NH, s in D2O), 5.16 (1H, br t, J=5.6, 5′-OH, exchanges with D2O), 5.32 (1H, br d, J=6.0, H-3′), 5.50 (2H, s, CH2-Ph), 6.19 (1H, dd, J=9.2, J=5.6, H-1′), 6.50 (2H, br s, guanine N2H2, exchanges with D2O), 6.66 (2H, d, J=8.8, pAB Ar), 7.03 (1H, t, J=6.0, folate 6-CH2NH, exchanges with D2O), 7.32–7.42 (3H, m, Bn Ar), 7.50 (2H, br dd, J=8.4, J=1.6, Bn Ar), 7.66 (2H, br d, J=8.8, pAB Ar), 8.10 (1H, s, guanine H-8), 8.26 (1H, br d, J=7.6, glu NH, exchanges with D2O), 8.84 (1H, s, folate H-7), 11.71 (2H, br s, folate N3H and folate N2H, exchanges with D2O). MS m/z 1025.6 [M+H]+; Anal. (C47H60N12O11Si2) C, H, N.
O6-Benzyl-3′-O-(γ-folyl)-2′-deoxyguanosine (1)
Tetrabutylammonium fluoride (TBAF), (1.0 M in THF, 2.15 mL) was added to a solution of 7 (0.220 g, 0.215 mmol) dissolved in DMSO (2.15 mL) and stirred at room temperature for 2 h. Water (25 mL) was added to the reaction and the pH of the solution was adjusted to 3 with HCl. The yellow precipitate was filtered off and suspended in 2:1 H2O/MeOH (50 mL). NaHCO3 (0.036 g, 0.430 mmol) was added to the suspension and stirred until the solid was completely dissolved (~2 h). The solution was acidified to pH 3 with HCl and the resulting solid was filtered and dried under vacuum (0.161 g, 95.8 %). UV (0.05 M phosphate, pH 7.4) λmax= 253 nm (ε=1.23 × 104), λmax= 284 nm (ε=2.00 × 104), λmax= 362 nm (ε =5.20 × 103). 1H NMR (DMSO-d6): δ 1.91–2.02 (1H, m, gluβ-CH2b), 2.09–2.18 (1H, m, gluβ-CH2a), 2.41 (1H, br dd, J=12.8, J=5.6, H-2′), 2.47 (2H, br t, J=7.2, gluγ-CH2), 2.84 (1H, ddd, J=14.8, J=9.2, J=6.0, H-2′), 3.57 (2H, br s, H-5′), 4.01 (1H, td, J=4.0, J=1.2, H-4′), 4.35–4.40 (1H, m, gluα-CH), 4.48 (2H, br d, J=5.2, folate 6-CH2NH, s in D2O), 5.16 (1H, br t, J=5.6, 5′-OH, exchanges with D2O), 5.32 (1H, br d, J=6.0, H-3′), 5.50 (2H, s, CH2-Ph), 6.19 (1H, dd, J=9.2, J=5.6, H-1′), 6.50 (2H, br s, guanine N2H2, exchanges with D2O), 6.66 (2H, d, J=8.8, pAB Ar), 6.92 (3H, t, J=6.0, folate 6-CH2NH and folate N2H2, exchanges with D2O ), 7.33–7.42 (3H, m, Bn Ar), 7.50 (2H, br dd, J=8.4, J=1.6, Bn Ar), 7.65 (2H, br d, J=8.8, pAB Ar), 8.10 (1H, s, guanine H-8), 8.13–8.14 (1H, m, glu-NH, exchanges with D2O), 8.65 (1H, s, folate H-7), 11.48 (1H, br s, folate N3H exchanges in D2O), 12.54 (1H, br s, CO2H, exchanges in D2O). MS m/z 781.3 [M+H]+; Anal. (C36H36N12O9·1.6 H2O) C, H, N.
O6-Benzyl-3′-O-(t-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)-N2-phenoxyacetyl-2′-deoxyguanosine (8)
Imidazole (0.607 g, 8.92 mmol) was added to a solution of 4 (1.77 g, 2.23 mmol) in DMF (6 mL) and stirred until completely dissolved. tert-Butyldimethylsilyl chloride (1.01 g, 6.70 mmol) was added and the reaction was stirred at room temperature for 18 h. The solvent was removed under reduced pressure, water (20 mL) was added to the residue and extracted with CH2Cl2 (3×30 mL). The organic extracts were combined, dried over MgSO4, filtered and the solvent was removed under reduced pressure. The resulting oil was purified by column chromatography (silica, 70:30 EtOAc:Hex) to yield 8 as a white solid (1.84 g, 90.9%). 1H NMR (DMSO-d6): δ −0.031 (3H, s, Si-CH3), 0.035 (3H, s, Si-CH3), 0.816 (9H, s, Si-C(CH3)3), 2.38 (1H, ddd, J=12.8, J=6.8, J=5.2, H-2′), 2.96 (1H, ddd, J=13.2, J=6.4, J=6.4, H-2′), 3.20–3.28 (2H, m, H-5′), 3.73 (6H, s, DMT-(O-CH3)2), 3.88 (1H, br dd, J=9.6, J=5.2, H-4′), 4.69 (1H, br dd, J=10.8, J=5.2, H-3′), 5.04 (1H, d, J=18.0, diastereotopic CH2-Ph), 5.05 (1H, d, J=18.0, diastereotopic CH2-Ph), 5.66 (2H, s, Pac-CH2), 6.40 (1H, br dd, J=6.0, J=6.8, H-1′), 6.77–6.83 (4H, m DMT Ar), 6.94–7.10 (3H, m, Pac Ar), 7.17–7.25 (7H, m, DMT Ph and DMT Ar), 7.29–7.35 (4H, m, Bn Ar and DMT Ar), 7.38–7.46 (3H, m, Bn Ar), 7.57–7.60 (2H, m, Pac Ar), 8.44 (1H, s, H-8), 10.69 (1H, br s, N2H, exchanges with D2O). Anal. (C52H57N5O8Si) C, H, N.
O6-Benzyl-3′-O-(t-butyldimethylsilyl)-N2-phenoxyacetyl-2′-deoxyguanosine (9)
A solution of 3% TCA (67.8 mL, 19.9 mmol) in CH2Cl2 was added to a solution of 8 (4.51 g, 4.97 mmol) dissolved in CH2Cl2 (100 mL) and stirred at room temperature for 4 minutes. Et3N (2.77 mL, 19.9 mmol) was added to the solution and the solvent was removed under reduced pressure. The resulting solid was purified by column chromatography (silica, 90:10 CH2Cl2: EtOAc) to afford 9 as an off-white foam (2.56, 85.0%). 1H NMR (DMSO-d6): δ 0.088 (3H, s, Si-CH3), 0.094 (3H, s, Si-CH3), 0.875 (9H, s, Si-C(CH3)3), 2.28 (1H, ddd, J=13.2, J=6.0, J=3.2, H-2′), 2.82 (1H, ddd, J=13.2, J=6.0, J=2.0, H-2′), 3.51 (1H, J=11.2, J=5.2, J=4.8, H-5′), 3.57 (1H, ddd, J=11.6, J=5.6, J=5.6, H-5′), 3.84 (1H, ddd, J=4.8, J=4.8, J=2.8, H-4′), 4.60 (1H, ddd, J=5.6, J=2.8, J=2.8, H-3′), 4.94 (1H, t, J=5.4, 5′-OH, exchanges with D2O), 5.04 (2H, s, Pac-CH2), 5.63 (2H, s, CH2-Ph), 6.33 (1H, t, J=6.8, H-1′), 6.92–6.97 (3H, m, PAc Ar), 7.26–7.31 (2H, m, Bn Ar), 7.34–7.42 (3H, m, Bn Ar), 7.54 (2H, dd, J=8.4, J=1.6, PAc Ar), 8.47 (1H, s, H-8), 10.69 (1H, s, N2H, exchanges with D2O). Anal. (C31H39N5O6Si) C, H, N.
O6-Benzyl-3′-O-(t-butyldimethylsilyl)-2′-deoxyguanosine (10)
NaOH (2 M, 21 mL, 42.3 mmol) was added to a solution of 9 (2.56 g, 4.23 mmol) dissolved in CH3CN (13 mL) and stirred at room temperature for 21 h. Water (20 mL) was added to the reaction and the pH was adjusted to 7 with HCl. The solution was extracted with CH2Cl2 (2 × 30 mL) and the organic extracts were combined, dried over MgSO4, and filtered. The solvent was removed under reduced pressure to yield 10 as a white solid (1.86 g, 93.4 %). 1H NMR (DMSO-d6): δ 0.11 (6H, s, Si-(CH3)2), 0.90 (9H, s, Si-C(CH3)3), 2.21 (1H, ddd, J=12.8, J=5.6, J=2.4, H-2′), 2.70 (1H, ddd, J=13.2, J=8.0, J=5.6, H-2′), 3.49 (1H, ddd, J=11.6, J=5.2, J=4.8, H-5′), 3.55 (1H, ddd, J=11.6, J=5.2, J=5.2, H-5′), 3.82 (1H, ddd, J=4.4, J=4.4, J=2.4, H-4′), 4.53 (1H, ddd, J=5.2, J=2.4, J=2.4, H-3′), 5.04 (1H, t, J=5.6, 5′-OH, exchanges with D2O), 5.50 (2H, s, CH2-Ph), 6.21 (1H, dd, J=8.0, J=6.0, H-1′), 6.50 (2H, br s, N2H2, exchanges with D2O), 7.33–7.42 (3H, m, Bn Ar), 7.48–7.51 (2H, m, Bn Ar), 8.11 (1H, s, H-8). Anal. (C23H33N5O4Si) C, H, N.
O6-Benzyl-3′-O-(t-butyldimethylsilyl)-5′-O-[γ-[ α-[2-(trimethylsilyl)ethoxy]]-2-N-[2-(trimethylsilyl)-ethoxycarbonyl]folyl]-2′-deoxyguanosine (11)
DMAP (0.764 g, 6.25 mmol), 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (EDC) (1.20 g, 6.25 mmol), and 10 (2.67 g, 5.68 mmol) were added to a solution of protected folate 6 (4.29 g, 6.25 mmol) in CH2Cl2 (180 mL) and stirred at room temperature for 3 h. Water (100 mL) was added to the solution, the organic layer was extracted off and subsequently dried over MgSO4 and filtered. The solution was dried under reduced pressure and the resulting yellow foam was purified by column chromatography (silica gel, EtOAc) to afford 11 (2.62 g, 40.5%). 1H NMR (DMSO-d6): δ 0.012 (9H, s, Si-(CH3)3), 0.070 (9H, s, Si-(CH3)3), 0.080 (6H, s, Si-(CH3)2), 0.86 (9H, s, Si-C(CH3)3), 0.90–0.94 (2H, m, TMS-CH2CH2), 1.04–1.08 (2H, m, TMS-CH2CH2), 1.91–1.99 (1H, m, gluβ-CH2b), 2.03–2.12 (1H, m, gluβ-CH2a), 2.25 (1H, ddd, J=13.6, J=6.0, J=3.6, H-2′), 2.44 (2H, br t, J=7.4, gluγ-CH2), 2.83 (1H, ddd, J=7.6, J=3.6, J=2.8, H-2′), 3.96 (1H, ddd, J=6.0, J=6.0, J=6.0, H-4′), 4.10–4.16 (3H, m, TMS-CH2CH2 and H-5′), 4.23 (1H, dd, J=11.6, J=6.0, H-5′), 4.29–4.33 (2H, m, TMS-CH2CH2), 4.37 (1H, ddd, J=12.8, J=5.2, J=2.0, gluα-CH), 4.53 (1H, ddd, J=6.0, J=2.8, J=2.8, H-3′), 4.59 (2H, d, J=6.0, folate 6-CH2-NH, s in D2O), 5.50 (2H, s, CH2-Ph), 6.22 (1H, br t, J=7.0, H-1′), 6.52 (2H, br s, guanine N2H2, exchanges with D2O), 6.66 (2H, d, J=8.8, pAB Ar), 7.04 (1H, t, J=6.2, folate 6-CH2NH, exchanges with D2O), 7.33–7.43 (3H, m, Bn Ar), 7.50 (2H, br dd, J=8.4, J=1.6, Bn Ar), 7.66 (2H, d, J=8.8, pAB Ar), 8.09 (1H, s, guanine H-8), 8.26 (1H, d, J=7.6, glu-NH, exchanges with D2O), 8.84 (1H, s, folate H-7), 11.72 (2H, br s, folate N3H and N2H, exchanges with D2O). Anal. (C53H74N12O11Si3) C, H, N.
O6-Benzyl-5′-O-(γ-folyl)-2′-deoxyguanosine (2)
TBAF (1.0 M in THF, 6.71 mL) was added to 11 (0.510 g, 0.448 mmol) dissolved in DMSO (5.0 mL). The reaction was stirred at room temperature for 3 h. Water (140 mL) was added to the reaction and the pH of the solution was adjusted to 3 with HCl. The yellow precipitate was filtered off and suspended in H2O (150 mL). NaHCO3 (1M, 0.896 ml) was added to the suspension and stirred until the solid was completely dissolved (~2 h). The solution was acidified to pH 3 with HCl and the resulting solid was filtered (0.297 g, 85.0 %). The crude product, dissolved in 0.1 M NaHCO3 at 10 mg/mL, was purified on a Sephadex LH-20 column eluted with 0.1 M NaCl at a flow rate of 1 mL/min. UV absorption was continuously monitored at 280 nm and 10 mL fractions were collected. The combined fractions (110–140) were reduced to approximately 50 ml and the pH of the solution was adjusted to 3 with HCl. The resulting yellow precipitate was filtered and dried to afford 2 as a yellow solid (0.110 g, 31.5 % overall yield). UV (0.05 M phosphate, pH 7.4) λmax= 253 nm (ε=1.23 × 104), λmax= 284 nm (ε =2.00 × 104), λmax= 362 nm (ε =5.20 × 103). 1H NMR (DMSO-d6): δ 1.90–1.98 (1H, m, gluβ-CH2b), 2.05–2.11 (1H, m, gluβ-CH2a), 2.25 (1H, ddd, J=13.2, J=6.0, J=3.6, H-2′), 2.41 (2H, br t, J=7.6, gluγ-CH2), 2.70 (1H, ddd, J=7.6, J=6.4, J=6.4, H-2′), 3.93–3.97 (1H, m, H-4′), 4.13 (1H, dd, J=11.6, J=6.4, H-5′), 4.24 (1H, dd, J=11.6, J=4.8, H-5′), 4.29–4.35 (1H, m, gluα-CH), 4.37–4.40 (1H, m, H-3′), 4.48 (2H, d, J=5.6, folate 6-CH2-NH, s in D2O), 5.44 (1H, br s, 3′-OH, exchanges with D2O), 5.49 (2H, s, CH2-Ph), 6.21 (1H, t, J=6.8, H-1′), 6.51 (2H, br s, guanine N2H2, exchanges with D2O), 6.64 (2H, d, J=8.8, pAB Ar), 6.92 (3H, br t, J=6.0, folate N2H2 and folate 6-CH2NH, exchanges with D2O), 7.32–7.41 (3H, m, Bn Ar), 7.49 (2H, dd, J=8.4, J=1.6, Bn Ar), 7.64 (2H, d, J=8.8, pAB Ar), 8.04 (1H, s, guanine H-8), 8.12 (1H, br d, J=6.4, glu-NH, exchanges with D2O), 8.64 (1H, s, folate H-7), 11.49 (1H, br s, folate N3H, exchanges with D2O), 12.51 (1H, br s, CO2H, exchanges with D2O). MS m/z 781.3 [M+H]+; Anal. (C36H36N12O9·1.5 H2O) C, H, N.
O6-[4-[γ-[[α-[2-(trimethylsilyl)ethoxy]]-2-N-[2-(trimethylsilyl)-ethoxycarbonyl]folyl]-oxymethyl]benzyl]guanine (13)
O6-[4-(Hydroxymethyl)benzyl]guanine (12) was synthesized as described 12. Bis-silyl protected folic acid 6, (600 mg, 0.88 mmol), 12 (270 mg, 1.0 mmol) and DMAP (109 mg, 0.90 mmol) were combined in 15 ml of DMF. EDC (173 mg, 0.9 mmol) was added and the reaction was stirred at room temperature for two hours. The solvent was evaporated under reduced pressure. The residue was dissolved in 100 ml of CH2Cl2 and extracted with an equal volume of 0.05 M HCl in water followed by pure water. These extractions gave emulsions that required centrifugation to separate. The CH2Cl2 was then evaporated under reduced pressure. The resulting material was purified by column chromatography (silica gel, 19:1 chloroform:methanol). Upon evaporation of the solvent under reduced pressure, the desired bis-silyl protected product 13 was isolated as a yellow solid (300 mg, 36%). 1H NMR (DMSO-d6) with no TMS standard: δ 0.01 (9H, s, TMS-CH3), 0.05 (9H, s, TMS-CH3), 0.92 (2H, ddd, J=8.4, J=6.8, J=4.4, TMS-CH 2CH2), 1.04 (2H, ddd, J=8.8, J=6.8, J=4.0, TMS-CH2CH2), 1.94–2.02 (1H, m, gluβ-CH2b), 2.04–2.13 (1H, m, gluβ-CH2a ), 2.47 (2H, t, J=7.6, gluγ-CH2), 4.09–4.13 (2H, m, TMS-CH2CH2), 4.27–4.31 (2H, m, TMS-CH2CH2), 4.36 (1H, ddd, J=12.4, J=7.6, J=5.2, gluα-CH), 4.58 (2H, d, J=5.6, folate 6-CH2-NH, s in D2O), 5.08 (2H, s, benzyl-CH2-folate), 5.47 (2H, s, benzyl-CH2-guanine), 6.28 (2H, s, guanine N2H2, exchanges with D2O), 6.65 (2H, d, J=8.4, pAB Ar), 7.03 (1H, t, J=6.0, folate 6-CH2-NH, exchanges with D2O), 7.35 (2H, d, J=8.0, Bn Ar), 7.48 (2H, d, J=8.0, Bn Ar), 7.64 (2H, d, J=8.4, pAB Ar), 7.81 (1H, s, guanine H-8), 8.24 (1H, d, J=7.6, glu-NH, exchanges with D2O), 8.83 (1H, s, folate H-7), 11.7 (2H, br s, folate N3H and folate N2H, exchanges with D2O), 12.4 (1H, br s, guanine N9H, exchanges with D2O). MS m/z 939.4 [M+H]+.
O6-[4-[(γ-folyl)-oxymethyl]benzyl]guanine (3)
The above bis-protected O6-[4-(hydroxymethyl)benzyl]guanine γ-folate ester 13, (290 mg, 0.31 mmol) was dissolved in 45 ml of dimethyl sulfoxide. TBAF (1 M in THF, 5 ml) was added and the reaction was stirred for two hours at room temperature. The reaction was terminated with the addition of 450 ml of water and the suspension was acidified to pH 3 with HCl. Centrifugation of the resulting gelatinous mixture pelleted the product. The pellet was dissolved in 100 ml of 1 mM NaHCO3 and precipitated by acidifying to pH 3 with HCl. The material was then repeatedly washed by suspending in water and pelleting by centrifugation prior to final drying under high vacuum to give 3 as a yellow powder (210 mg, 97%). UV in 0.1 M HCl, λmax = 290 nm (ε=2.95 × 104) and 364 nm (ε =2.90 × 103), at pH 7 in 0.05 M phosphate buffer, λmax = 283 nm (ε =3.23 × 104) and 347 nm (ε =6.60 × 103), in 0.1 M NaOH, λmax= 256 nm (ε =2.79 × 104), 285 nm (ε =3.16 × 104) and 366 nm (ε =8.30 × 103), with decomposition in acid and base. 1H NMR (DMSO-d6): δ 1.91–2.01 (1H, m, gluβ-CH2b). 2.06–2.15 (1H, m, gluβ-CH2a), 2.47 (2H, t, J=8.0, gluγ-CH2), 4.35 (1H, ddd, J=12.8, J=8.0, J=4.8, gluα-CH), 4.48 (2H, d, J=6.0, folate 6-CH2-NH, s in D2O), 5.08 (2H, s, benzyl-CH2-folate), 5.48 (2H, s, benzyl-CH2-guanine), 6.30 (2H, s, guanine N2H2, exchanges with D2O), 6.64 (2H, d, J=8.8, pAB Ar), 6.90 (2H, br s, folate N2H2, exchanges with D2O), 6.94 (1H, t, J=6.0, folate 6-CH2-NH, exchanges with D2O ), 7.36 (2H, d, J=8.0, Bn Ar), 7.48 (2H, d, J=8.0, Bn Ar), 7.65 (2H, d, J=8.8, pAB Ar), 7.82 (1H, s, guanine H-8), 8.13 (1H, d, J=7.6, glu-NH, exchanges with D2O), 8.65 (1H, s, folate H-7), 11.5 (1H, br s, folate N3H, exchanges with D2O), 12.4 (2H, br s, guanine N9H and folic acid CO2H, exchanges with D2O). MS m/z 695.2 [M+H]+; Anal. (C32H30N12O7·1H2O) C, H, N.
Inactivation of purified recombinant human alkyltransferase
ED50 values for the inactivation of purified human alkyltransferase in vitro were obtained as previously described 9, 14. Purified recombinant human alkyltransferase was incubated with different concentrations of prodrugs in 0.5 mL of reaction buffer (50 mM Tris-HCl, pH 7.6, 0.1 mM EDTA, 5.0 mM dithiothreitol) containing 50 μg of hemocyanin or 10 μg calf thymus DNA for 30 min at 37°C.
Sensitization of cells to killing by BCNU
KB, HT29 and A549 cells were grown in RPMI 1640 medium or in RPMI medium lacking folate in the presence of 10% fetal bovine serum. The effect of alkyltransferase inactivators on the sensitivity of cells to BCNU was determined using a colony-forming assay 8, 9, 12, 17. Cells were plated at a density of 106 in 25 cm2 flasks and 24 h later were incubated with different concentrations of potential inhibitors for 2–8 h as indicated before exposure to 20 or 40 μM BCNU for 2 h as previously described 8, 9. After 2 h, the medium was replaced with fresh medium containing the drug but no BCNU and the cells were left to grow for an additional 16–18 h. The cells were then replated at densities of 250–1000 cells per 25 cm2 flask and grown for 8 days until discrete colonies had formed. The colonies were washed with 0.9% saline solution, stained with 0.5% crystal violet in ethanol, and counted.
Conversion of esters to folate
Cells were trypsinized, washed with Hank’s balanced salt solution, counted and pelleted. On ice, the cell pellets were resuspended in 50 mM sodium phosphate, 5mM DTT, pH 7.4 at a concentration of 108 cells/ml and disrupted by sonication. The sonicated cells were centrifuged at 12,000 x g for 10 min and the supernatant was removed. Complete mini protease inhibitor (Roche, Mannheim Germany) was added as directed by the supplier. Lysate total protein concentration was determined and the lysates were frozen until used at −20°C. Reactions (600 μL), containing one of the ester substrates (200 μM) and 2 mg of lysate protein in 50 mM phosphate buffer (pH 7.4) were incubated at 37°C. At various times 50 μL were removed for analysis by HPLC. The amount of folic acid liberated was used to determine the extent of ester hydrolysis. The susceptibility of the compounds to hydrolysis by porcine liver esterase (Sigma, St. Louis, MO) was also measured in the same way.
Supplementary Material
Elemental analyses for compounds 1–3. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Work in AEP’s laboratory was supported by grants CA-018137 and CA-071976 from the National Cancer Institute, National Institutes of Health, USA.
Footnotes
Abbreviations: alkyltransferase, O6-alkylguane-DNA alkyltransferase; PAc, phenoxyacetyl; DMAP, 4-dimethylaminopyridine, TBAF, tetrabutylammonium fluoride; EDC, 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride.
References
- 1.Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutation Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 2.Margison G, Povey AC, Kaina B, Santibáñez-Koref MF. Variability and regulation of O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 3.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 4.Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE, 2nd, Bigner DD, Friedman HS. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23:7178–7187. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 5.Warren KE, Aikin AA, Libucha M, Widemann BC, Fox E, Packer RJ, Balis FM. Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J Clin Oncol. 2005;23:7646–7653. doi: 10.1200/JCO.2005.02.0024. [DOI] [PubMed] [Google Scholar]
- 6.Middleton MR, Margison GP. Improvement of chemotherapy efficacy by inactivation of a DNA-repair pathway. Lancet Oncol. 2003;4:37–44. doi: 10.1016/s1470-2045(03)00959-8. [DOI] [PubMed] [Google Scholar]
- 7.Ranson M, Middleton MR, Bridgewater J, Lee SM, Dawson M, Jowle D, Halbert G, Waller S, McGrath H, Gumbrell L, McElhinney RS, Donnelly D, McMurry TB, Margison GP. Lomeguatrib, a potent inhibitor of O6-alkylguanine-DNA-alkyltransferase: phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2006;12:1577–1584. doi: 10.1158/1078-0432.CCR-05-2198. [DOI] [PubMed] [Google Scholar]
- 8.Wei G, Loktionova NA, Pegg AE, Moschel RC. Beta-glucuronidase-cleavable prodrugs of O6-benzylguanine and O6-benzyl-2′-deoxyguanosine. J Med Chem. 2005;48:256–261. doi: 10.1021/jm0493865. [DOI] [PubMed] [Google Scholar]
- 9.Nelson ME, Loktionova NA, Pegg AE, Moschel RC. 2-Amino-O4-benzylpteridine derivatives: potent inactivators of O6-alkylguanine-DNA alkyltransferase. J Med Chem. 2004;47:3887–3891. doi: 10.1021/jm049758+. [DOI] [PubMed] [Google Scholar]
- 10.Luu KX, Kanugula S, Pegg AE, Pauly GT, Moschel RC. Repair of oligodeoxyribonucleotides by O6-alkylguanine-DNA alkyltransferase. Biochemistry. 2002;41:8689–8697. doi: 10.1021/bi025857i. [DOI] [PubMed] [Google Scholar]
- 11.Nomura M, Shuto S, Matsuda A. Development of an efficient intermediate, alpha-[2-(trimethylsilyl)ethoxy]-2-N-[2-(trimethylsilyl)ethoxycarbonyl]folic acid, for the synthesis of folate (gamma)-conjugates, and its application to the synthesis of folate-nucleoside conjugates. J Org Chem. 2000;11:5016–5021. doi: 10.1021/jo000132a. [DOI] [PubMed] [Google Scholar]
- 12.Chae MY, McDougall MG, Dolan ME, Swenn K, Pegg AE, Moschel RC. Substituted O6-benzylguanine derivatives and their inactivation of human O6-alkylguanine-DNA alkyltransferase. J Med Chem. 1994;37:342–347. doi: 10.1021/jm00029a005. [DOI] [PubMed] [Google Scholar]
- 13.Moschel RC, McDougall MG, Dolan ME, Stine L, Pegg AE. Structural features of substituted purine derivatives compatible with depletion of human O6-alkylguanine-DNA alkyltransferase. J Med Chem. 1992;35:4486–4491. doi: 10.1021/jm00101a028. [DOI] [PubMed] [Google Scholar]
- 14.Pegg AE, Chung L, Moschel RC. Effect of DNA on the inactivation of O6-alkylguanine-DNA alkyltransferase by 9-substituted O6-benzylguanine derivatives. Biochem Pharmacol. 1997;53:1559–1564. doi: 10.1016/s0006-2952(97)00060-9. [DOI] [PubMed] [Google Scholar]
- 15.Loktionova NA, Xu-Welliver M, Crone T, Kanugula S, Pegg AE. Mutant forms of O6-alkylguanine-DNA alkyltransferase protect CHO cells from killing by BCNU plus O6-benzylguanine or O6-8-oxo-benzylguanine. Biochem Pharmacol. 1999;58:237–244. doi: 10.1016/s0006-2952(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 16.Xu-Welliver M, Kanugula S, Pegg AE. Isolation of human O6-alkylguanine-DNA alkyltransferase mutants highly resistant to inactivation by O6-benzylguanine. Cancer Res. 1998;58:1936–1945. [PubMed] [Google Scholar]
- 17.Dolan ME, Moschel RC, Pegg AE. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990;87:5368–5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciocco GM, Pegg AE, Moschel RC, Chae MY, McLaughlin PJ, Zagon IS, Pegg AE. Specific labeling of O6-alkylguanine-DNA alkyltransferase by reaction with O6-(p-hydroxy[3H]methylbenzyl)guanine. Cancer Res. 1995;55:4085–4091. [PubMed] [Google Scholar]
- 19.Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 20.Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc Natl Acad Sci US A. 2004;101:9955–9959. doi: 10.1073/pnas.0401923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels DS, Mol CD, Arvai AS, Kanugula S, Pegg AE, Tainer JA. Active and alkylated human AGT structures: a novel zinc site, inhibitor and extrahelical binding. DNA damage reversal revealed by mutants and structures of active and alkylated human AGT. EMBO J. 2000;19:1719–1730. doi: 10.1093/emboj/19.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wibley JEA, Pegg AE, Moody PCE. Crystal structure of the human O6-alkylguanine-DNA alkyltransferase. Nucleic Acid Res. 2000;28:393–401. doi: 10.1093/nar/28.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegg AE, Boosalis M, Samson L, Moschel RC, Byers TL, Swenn K, Dolan ME. Mechanism of inactivation of human O6-alkylguanine-DNA alkyltransferase by O6-benzylguanine. Biochemistry. 1993;32:11998–12006. doi: 10.1021/bi00096a009. [DOI] [PubMed] [Google Scholar]
- 24.Pegg AE, Swenn K, Dolan ME, Moschel RC. Increased killing of prostate, breast, colon and lung tumor cells by the combination of inactivators of O6-alkylguanine-DNA alkyltransferase and N, N-bis(2-chloroethyl)-N-nitrosourea. Biochem Pharmacol. 1995;50:1141–1148. doi: 10.1016/0006-2952(95)00249-y. [DOI] [PubMed] [Google Scholar]
- 25.Thomas AH, Suárez G, Cabrerizo FM, Martino R, Capparelli AL. Study of the photolysis of folic acid and 6-formylpterin in acid aqueous solutions. J Photochem Photobiol A: Chem. 2000;135:147–154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Elemental analyses for compounds 1–3. This material is available free of charge via the Internet at http://pubs.acs.org.