Abstract
OBJECTIVE
To use a population-based approach to describe survival trends in patients diagnosed as having gastric or esophageal adenocarcinoma.
PATIENTS AND METHODS
A population-based complete chart review of all inpatient and outpatient records, using the resources of the Rochester Epidemiology Project, was conducted in Olmsted County, Minnesota (population 124,277), a primarily rural county with one large urban area. All residents of Olmsted County who were diagnosed as having gastric or esophageal adenocarcinoma from January 1, 1971, through December 31, 2000, were included in the study. The main outcomes were median survival and 2-year and 5-year survival rates, by decade of diagnosis.
RESULTS
From 1971 through 2000, median survival for patients with gastric adenocarcinoma (n=121) decreased from 5.5 months to 3.2 months, whereas median survival for patients with esophageal adenocarcinoma (n=65) increased from 8.5 months to 11.7 months. Decade of diagnosis was not significantly associated with patient survival for either gastric or esophageal adenocarcinoma (P>.05). There was no significant shift in stage of disease at diagnosis during the 30-year period for either gastric or esophageal adenocarcinoma (P>.05).
CONCLUSION
No significant change has occurred in the survival rates of this patient population with gastric or esophageal adenocarcinoma, which is representative of the US white population.
Since the 1970s, there have been substantial changes in the incidence of gastric and esophageal adenocarcinoma.1,2 Despite the fact that the prevalence of gastric adenocarcinoma continues to decrease, more than 21,000 persons in the United States were diagnosed as having this form of cancer in 2007, and 11,000 individuals died that year of gastric cancer.3 Numerous studies have found that the incidence of esophageal adenocarcinoma has increased steadily since the 1970s, and it now has one of the fastest growing rates among all types of cancers in the United States.4,5 The causes for these changes in cancer incidence remain much debated. Possible causes include the obesity epidemic, decreasing Helicobacter pylori prevalence, and dietary changes.4,5
In an effort to address these disturbing trends in gastric and esophageal adenocarcinoma prevalence, various surveillance and treatment protocols have been proposed or enacted. Because it is now known that esophageal adenocarcinoma arises from specialized intestinal metaplasia in Barrett esophagus (also called Barrett epithelium), routine esophagogastroduodenoscopy surveillance of high-risk patients is increasingly performed.6 Patients diagnosed as having gastric or esophageal adenocarcinoma are usually aggressively treated with surgery, radiation, and/or combination chemotherapy—and sometimes with endoscopic mucosal resection and photodynamic therapy.7 Previously, surgical interventions had been associated with significant perioperative risk, but recently this risk appears to be decreasing.8,9 The role of chemotherapy, both preoperatively and postoperatively, has been extensively studied.10–12 Despite these changes in surveillance and treatment protocols, it is unclear whether the survival of patients with gastric or esophageal adenocarcinoma has significantly improved since the 1970s.
The purpose of the current study was to use a population-based approach to describe any changes in patient survival after the diagnosis of gastric or esophageal adenocarcinoma during a 30-year period, beginning in 1971. We hypothesized that patient survival would have improved in the 1990s, compared with previous decades, as a result of advances in the quality of surgical techniques and other medical management.
PATIENTS AND METHODS
Medical care for residents of Olmsted County, situated in primarily rural southeastern Minnesota, is provided almost exclusively by 2 group practices: Mayo Clinic and Olmsted Medical Center and their affiliated hospitals and clinics. The Rochester Epidemiology Project (REP) is a medical records linkage system that allows for access to the complete medical records of these health care institutions—including inpatient, outpatient, nursing home, emergency department, pathologic, radiologic, and laboratory information dating back to the early 20th century.13 The presence of a large tertiary referral center, like Mayo Clinic, in this mostly rural area means that there has been little migration of patient populations to other health care centers. However, the REP does maintain contacts with the University of Minnesota hospitals, including the Veterans Affairs Medical Center in Minneapolis.
The unique resource of the REP has made it possible to conduct population-based research in southeastern Minnesota with unusually detailed levels of individual data and completeness of follow-up. The REP has been used extensively in the past to describe trends involving both gastric and esophageal cancers.2,5,14
In the US Census 2000, Olmsted County had a population of 124,277.15 Most people in this county reside in Rochester, the urban center of the otherwise rural county; 89% of the residents are non-Hispanic whites, a substantial portion of whom are of northern European heritage. Although 25% of county residents are employed in the health care services (vs 8% nationwide) and 30% of adults in the county have completed college (vs 21% nationwide), the residents of Olmsted County are otherwise socioeconomically similar to the US white population as a whole.13
This study was approved by the institutional review boards of both Mayo Clinic and Olmsted Medical Center. The REP database was then used to identify all patients diagnosed as having gastric or esophageal tumors from January 1, 1971, through December 31, 2000. Patients in all but 3.1% of the potential cases in the REP database had provided authorization to use their medical records for research according to Minnesota statute.
Patients included in the study were required to be residents of Olmsted County for at least 1 year before diagnosis to avoid including referral cases. All cases were histologically identified gastric or esophageal adenocarcinomas, or disease in which no pathologic cause was identified but adenocarcinoma was the most likely diagnosis based on radiologic, endoscopic, or surgical evidence.
Patients were excluded if they were diagnosed as having gastric or esophageal adenocarcinoma before 1971 or after 2000 or if pathologic evidence was available identifying an alternative diagnosis (eg, lymphoma, squamous cell carcinoma, carcinoid tumor).
Notes from surgical procedures and results of pathologic tests were the preferred sources of information for tumor site identification. If these sources were unavailable, endoscopic or radiologic reports were evaluated. Physician documentation and descriptions of patients at the time of diagnosis were also used to determine tumor location.
The stage of disease in patients at the time of diagnosis was determined using the TNM system of the American Joint Committee on Cancer (5th ed, 1997).16 The patients’ surgical and pathologic reports were used to determine the depth of tumor invasion, the number and location of lymph nodes involved, and the presence of distant metastases. For patients in whom diagnosis was made only at autopsy, the stage of disease was determined by applying the TNM system to information obtained from the autopsy reports. Disease stages in the remaining patients who did not undergo surgery were classified by evaluating the results from imaging studies performed at the time of diagnosis.
The date of each patient’s death was recorded, if available. All patients who were believed to be still living were either verified as such through the electronic medical records or were designated as “lost to follow-up.”
Patient survival was measured from the date of diagnosis to the date of death. Patients who were lost to follow-up were censored at the last date of patient contact.
Statistical Analyses
Kaplan-Meier survival curves were used to estimate patient survival. Cox proportional hazards regression models were used to assess associations of risk factors with survival—separately for patients with gastric adenocarcinoma and patients with esophageal adenocarcinoma. The multivariate Cox models were designated a priori to include factors considered to have potential association with patient survival. These factors included age at diagnosis, sex, weighted Charlson Index of comorbidity,17,18 decade of adenocarcinoma diagnosis, and adenocarcinoma stage and site.
The association between the adenocarcinoma stage at diagnosis and the decade of diagnosis was assessed using an extension of the Fisher exact test for ordered contingency tables.
P=.05 was considered statistically significant.
RESULTS
During the 30-year study period, 186 residents of Olmsted County were diagnosed as having gastric or esophageal adenocarcinoma. Of these patients, 121 (65%) had adenocarcinomas originating in the stomach, and 65 (35%) had adenocarcinomas originating in the esophagus or esophagogastric junction.
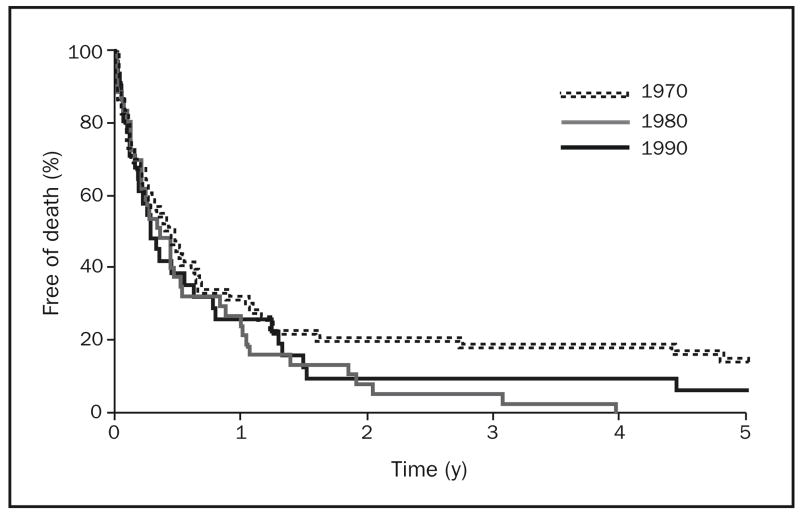
The median survival time for patients with gastric adenocarcinoma decreased during the 3 decades studied, from 5.5 months in the 1970s to 4.1 months in the 1980s to 3.2 months in the 1990s. The 2-year survival estimates for patients with gastric adenocarcinoma in the 1970s, 1980s, and 1990s were 21% (95% confidence interval [CI], 12%–35%), 8% (95% CI, 3%–24%), and 10% (95% CI, 3%–28%), respectively. Five-year survival estimates for these patients were 15% (95% CI, 8%–29%), 0% (95% CI, 0%–10%), and 7% (95% CI, 1%–25%) in the 1970s, 1980s, and 1990s, respectively. Kaplan-Meier survival curves for patients with gastric adenocarcinoma, by decade, are shown in Figure 1.
FIGURE 1.
Kaplan-Meier survival curves of patients with gastric adenocarcinoma in Olmsted County, Minnesota, by decade, 1971–2000 (n=121).
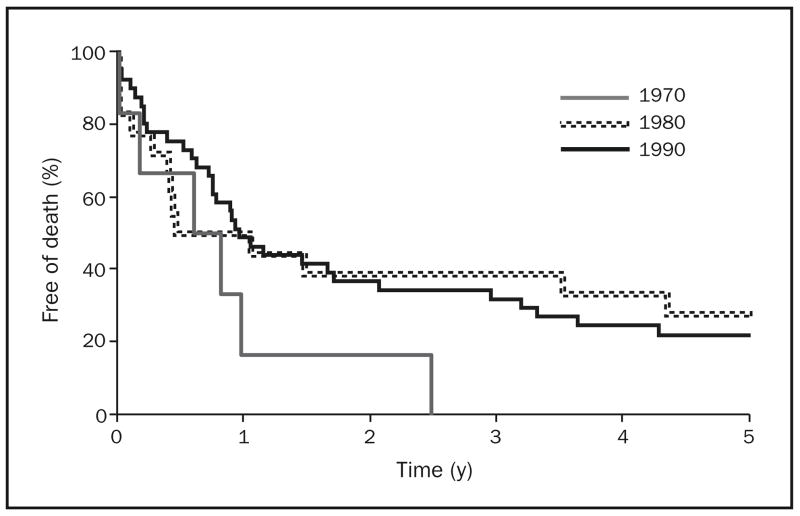
The median survival time for patients with esophageal adenocarcinoma improved during the 3 decades studied, from 8.5 months in the 1970s to 9.1 months in the 1980s to 11.7 months in the 1990s. The 2-year survival estimates for patients with esophageal adenocarcinoma in the 1970s, 1980s, and 1990s were 17% (95% CI, 3%–100%), 39% (95% CI, 22%–69%), and 37% (95% CI, 25%–55%), respectively. Five-year survival estimates for these patients were 0% (95% CI, 0%–46%), 28% (95% CI, 13%–59%), and 22% (95% CI, 12%–39%) in the 1970s, 1980s, and 1990s, respectively. Kaplan-Meier survival curves for patients with esophageal adenocarcinoma, by decade, are shown in Figure 2.
FIGURE 2.
Kaplan-Meier survival curves of patients with esophageal adenocarcinoma in Olmsted County, Minnesota, by decade, 1971–2000 (n=65).
To examine whether inclusion of the 18 cases with no available histologic results affected patient survival, we compared the overall median survival of patients across the 3 decades. When the 11 cases of presumed gastric adenocarcinoma with no histologic results were excluded from the analysis, patients’ overall median survival was 5.1 months compared with 4.1 months when these cases were included. The overall median survival of the 11 cases without histology was 3.1 months. When the 7 cases of presumed distal esophageal adenocarcinoma with no histologic results were excluded from the analysis, patients’ overall median survival was 11.5 months compared with 11.3 months when these cases were included. The overall median survival of these presumed esophageal adenocarcinoma cases without histology was 9.2 months.
In 168 cases (90%), the adenocarcinomas were histologically identified. In the remaining cases (18 [10%]), no pathologic result was obtained, but adenocarcinoma was the most likely diagnosis based on radiologic, endoscopic, or surgical evidence. These 18 cases included unspecified primary gastric tumors (n=11) and tumors described as involving only the distal third of the esophagus (n=7).
Only 1 patient with gastric adenocarcinoma and 3 patients with esophageal adenocarcinoma were lost to follow-up from the original cohort.
The distributions of patient characteristics by decade, including TNM stage at presentation, are shown in Table 1 for gastric adenocarcinoma and Table 2 for esophageal adenocarcinoma.
TABLE 1.
Baseline Characteristics of Patients With Gastric Adenocarcinoma, by Decade of Diagnosisa
| Decade
|
||||
|---|---|---|---|---|
| Characteristic | 1970–1979 (n=53) | 1980–1989 (n=37) | 1990–1999 (n=31) | Overall (N=121) |
| Male | 28 (53) | 19 (51) | 17 (55) | 64 (53) |
| TNM stage | ||||
| I | 6 (11) | 2 (5) | 4 (13) | 12 (10) |
| II | 9 (17) | 5 (14) | 2 (7) | 16 (13) |
| III | 8 (15) | 10 (27) | 4 (13) | 22 (18) |
| IV | 27 (51) | 15 (41) | 13 (42) | 55 (45) |
| Unknown | 3 (6) | 5 (14) | 8 (26) | 16 (13) |
| Surgical intervention | ||||
| None | 26 (49) | 20 (54) | 16 (52) | 62 (51) |
| Palliative | 5 (9) | 3 (8) | 5 (16) | 13 (11) |
| Curative | 22 (42) | 14 (38) | 10 (32) | 46 (38) |
| Radiation therapy | 5 (9) | 4 (11) | 0 (0) | 9 (7) |
| Chemotherapy | 17 (32) | 7 (19) | 7 (23) | 31 (26) |
| Metastasis | ||||
| Localized | 7 (13) | 1 (3) | 3 (10) | 11 (9) |
| Lymph nodes | 35 (66) | 22 (59) | 16 (52) | 73 (60) |
| Distant | 27 (51) | 14 (38) | 13 (42) | 54 (45) |
| Charlson Index, weighted | ||||
| Median (IQR) | 8 (0–15) | 9 (2–13) | 8 (0–14) | 8 (0–15) |
| Mean ± SD | 6.2±3.7 | 8.1±2.8 | 7.5±3.2 | 7.1±3.4 |
Values are number (percentage) unless indicated otherwise. IQR = interquartile range.
TABLE 2.
Baseline Characteristics of Patients With Esophageal Adenocarcinoma, by Decade of Diagnosisa
| Decade
|
||||
|---|---|---|---|---|
| Characteristic | 1970–1979 (n=6) | 1980–1989 (n=18) | 1990–1999 (n=41) | Overall (N=65) |
| Male | 6 (100) | 13 (72) | 35 (85) | 54 (83) |
| TNM stage | ||||
| I | 0 (0) | 3 (17) | 7 (17) | 10 (15) |
| II | 0 (0) | 2 (11) | 3 (7) | 5 (8) |
| III | 1 (17) | 6 (33) | 12 (29) | 19 (29) |
| IV | 3 (50) | 4 (22) | 11 (27) | 18 (28) |
| Unknown | 2 (33) | 3 (17) | 8 (20) | 13 (20) |
| Surgical intervention | ||||
| None | 2 (33) | 6 (33) | 18 (44) | 26 (40) |
| Palliative | 3 (50) | 4 (22) | 14 (34) | 21 (32) |
| Curative | 1 (17) | 8 (44) | 9 (22) | 18 (28) |
| Radiation therapy | 4 (67) | 3 (17) | 13 (32) | 20 (31) |
| Chemotherapy | 3 (50) | 3 (17) | 14 (34) | 20 (31) |
| Metastasis | ||||
| Localized | 0 (0) | 3 (17) | 7 (17) | 10 (15) |
| Lymph nodes | 4 (67) | 9 (50) | 12 (29) | 25 (38) |
| Distant | 3 (50) | 2 (11) | 5 (12) | 10 (15) |
| Charlson Index, weighted | ||||
| Median (IQR) | 8 (4–10) | 9 (2–13) | 8 (0–12) | 8 (0–13) |
| Mean ± SD | 7.0±2.5 | 7.9±3.4 | 6.4±3.8 | 6.8±3.6 |
Values are number (percentage) unless indicated otherwise. IQR = interquartile range.
The multivariate Cox proportional hazards models for gastric and esophageal adenocarcinoma are shown in Table 3. In the Cox model for gastric adenocarcinoma, adjusting for age, sex, Charlson Index, and TNM stage at diagnosis, there was no significant association between decade of diagnosis and patient survival (P=.23). For the decade of the 1980s relative to the 1970s, the hazard ratio for gastric adenocarcinoma cases was 1.5 (95% CI, 0.9–2.6), and for the decade of the 1990s relative to the 1970s, the hazard ratio was 1.4 (95% CI, 0.8–2.5). In the Cox model for esophageal adenocarcinoma, adjusting for the same factors, there was again no significant association between decade of diagnosis and patient survival (P=.75). For the decade of the 1980s relative to the 1970s, the hazard ratio for esophageal adenocarcinoma cases was 1.3 (95% CI, 0.4–5.0), and for the decade of the 1990s relative to the 1970s, the hazard ratio was 1.0 (95% CI, 0.3–3.2).
TABLE 3.
Multivariate Cox Proportional Hazards Models for Survival of 186 Patients With Gastric and Esophageal Cancera
| Gastric adenocarcinoma (n=121)
|
Esophageal adenocarcinoma (n=65)
|
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 1.2 (1.1–1.5) | .01 | 1.2 (0.9–1.6) | .27 |
| Maleb | 1.3 (0.9–2.0) | .18 | 3.7 (1.2–12.0) | .03 |
| Charlson Index, weighted | 1.0 (0.9–1.1) | .52 | 1.1 (0.98–1.3) | .10 |
| Diagnosis decadec | ||||
| 1980s | 1.5 (0.9–2.6) | 1.3 (0.4–5.0) | ||
| 1990s | 1.4 (0.8–2.5) | .23 | 1.0 (0.3–3.2) | .75 |
| TNM stage at diagnosisd | ||||
| II | 1.7 (0.7–3.9) | <.001 | 4.9 (0.98–25.0) | <.001 |
| III | 3.0 (1.2–7.1) | 4.6 (1.1–19.0) | ||
| IV | 7.8 (3.2–19.0) | 28.0 (6.1–129.0) | ||
| Gastric sitee | ||||
| Diffuse | 0.7 (0.3–1.4) | .21 | NA | NA |
| Distal | 0.6 (0.4–1.05) | NA | NA | |
| Esophageal sitef | ||||
| EGJ | NA | NA | 1.3 (0.6–2.8) | .58 |
P value is for the overall association between the variable and patient survival. CI = confidence interval; EGJ = esophagogastric junction; HR = hazard ratio; NA = not applicable.
HR is based on female reference value of 1.0.
HR is based on 1970s reference value of 1.0.
HR is based on stage I reference value of 1.0.
HR is based on cardia/fundus reference value of 1.0.
HR is based on esophagus reference value of 1.0.
When we included only the 168 patients with known histologic results in the analysis, the associations in the multivariate Cox proportional hazards models remained essentially unchanged.
No significant shift was detected in the distribution of TNM stage of disease at diagnosis during the 3 decades studied—either among cases of gastric adenocarcinoma (P=.10) or cases of esophageal adenocarcinoma (P=.96). Most cases were diagnosed at advanced stages throughout the 3-decade period.
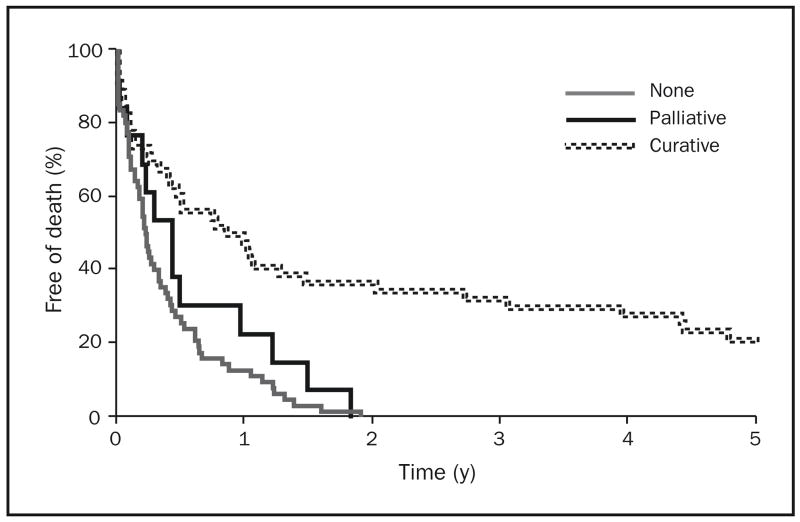
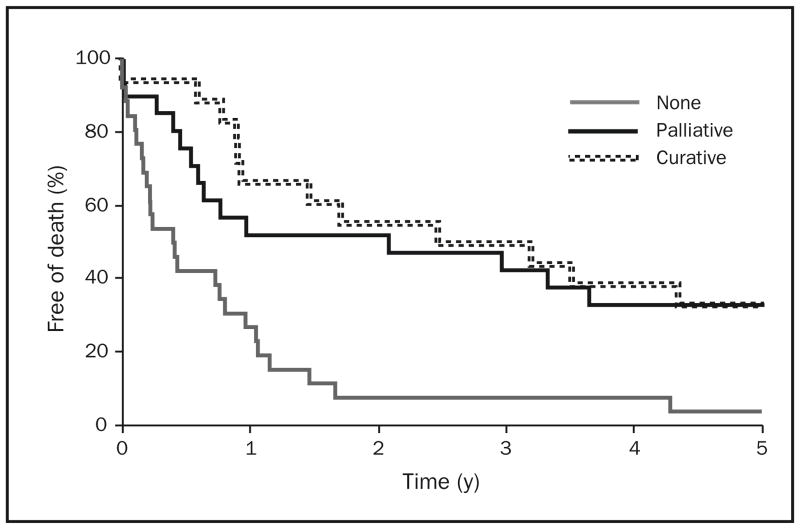
Figure 3 illustrates the Kaplan-Meier survival curves of patients with gastric adenocarcinoma, by surgical intervention (none, palliative, curative). Figure 4 shows the Kaplan-Meier survival curves of patients with esophageal adenocarcinoma, by surgical intervention. Patient survival was significantly associated with surgical extent for both cancer sites (P<.001). Cases involving curative surgery were associated with prolonged survival for both types of cancer. Palliative surgery for cases of esophageal adenocarcinoma—but not cases of gastric adenocarcinoma—appeared to be associated with longer survival. Prognosis remained poor for patients with gastric adenocarcinoma throughout the 3 decades studied.
FIGURE 3.
Kaplan-Meier survival curves of patients with gastric adenocarcinoma in Olmsted County, Minnesota, by surgical intervention (none, palliative, curative), 1971–2000 (n=121).
FIGURE 4.
Kaplan-Meier survival curves of patients with esophageal adenocarcinoma in Olmsted County, Minnesota, by surgical intervention (none, palliative, curative), 1971–2000 (n=65).
In this collection of cases, a total of only 10 (8%) of the 121 patients with gastric adenocarcinoma and only 9 (14%) of the 65 patients with esophageal adenocarcinoma had undergone endoscopy within the 5 years before diagnosis.
DISCUSSION
In this population-based study of all cases of gastric and esophageal adenocarcinoma diagnosed in Olmsted County, we found no significant change in patient survival during 3 decades. There was no significant improvement in survival despite advances in screening, diagnosis, and treatment during this same period.
Median survival of patients with gastric adenocarcinoma actually appeared to decrease between 1971 and 2000. Patient survival for both diseases has remained dismal. Median survival of patients with esophageal adenocarcinoma appeared to have slightly improved between 1971 and 2000, but this change was not deemed to be significant in the current study. Although curative surgical attempts were associated with prolonged survival, this result may be a reflection of patients’ functional status, which determined at time of diagnosis whether patients were surgical candidates.
The discouraging results of the current study are in contrast to several other studies that have reported improved survival in patients with gastric or esophageal cancer. Studies published in Sweden and the United Kingdom have noted improved survival of patients with gastric cancer during portions of the same period covered by the current study.19,20 Hansson et al19 described increasing 5-year survival rates in their population-based study of the Swedish population from 1960 to 1989. Newnham et al20 noted that 5-year survival rates of a patient population in England and Wales increased through the 1990s.
The less encouraging results of the current study may be due to different populations with different risk factors or to different data collection practices. For example, large database studies could conceivably miss cases in which patients died quickly after diagnosis or in which presumptive diagnosis was made on the basis of imaging but no tissue sample was obtained because of a patient’s poor performance status. Such factors would lead to significant ascertainment bias.
Screening as a method for reducing mortality from both gastric and esophageal adenocarcinoma is performed routinely in some countries.21 In Japan, where the incidence of gastric cancer is much higher than in the United States, survival of patients with gastric adenocarcinoma does seem to be improving. This improvement appears to be the result, at least in part, of the frequent diagnosis of early-stage gastric cancer in a mass population screening program in Japan.21 Of note, in the collection of cases in the current study in which diagnosis was made in a community with ready access to specialized medical care, only 10 of the 121 patients with gastric adenocarcinoma and only 9 of the 65 patients with esophageal adenocarcinoma had undergone endoscopy within the 5 years before diagnosis. Given the much lower rate of gastric cancer in the United States than in Japan, the cost of a mass screening program for this cancer would likely be prohibitive.
Several other studies based on large databases or on populations have found conflicting results in survival rates for patients with gastric or esophageal adenocarcinoma. Studies in the United States conducted with the Surveillance Epidemiology and End Results (SEER) databases of the National Cancer Institute have suggested a significant improvement in survival of patients with esophageal adenocarcinoma from 1973 to 1998.22 The EUROCARE study conducted in the European Union noted a slight improvement in patient survival from 1978 to 1989 with significant variation among European countries, but that study did not select for adenocarcinomas and included all upper gastrointestinal tract cancers except lymphomas.23 A population-based study of more than 2000 patients in France between 1976 and 2002 noted no significant improvement in survival of patients with esophageal adenocarcinoma.24
Despite differences in findings regarding whether survival rates are changing significantly, these and other studies agree that survival rates remain poor—particularly among patients who are not candidates for resection.25 This conclusion is in complete agreement with our findings.
The use of the REP in this and other population-based studies results in several unique advantages over larger database studies. Unlike other hospital or database studies of cancer outcomes, which may be seriously affected by referral and selection biases, the current study was able to accurately evaluate survival for the entire inception cohort of patients who were diagnosed as having cancer in the target population. Dates of diagnosis were remarkably accurate on the basis of procedure documentation. Follow-up was also complete, with few patients lost to follow-up, allowing for unusually complete survival information.
The primary limitation of our study was the relatively small size of the background population and thus the number of included cases. This factor may have limited the power of the study, but the overall finding that there was no clinically meaningful change in survival during 3 decades remains unchanged.
Another potential limitation is that the population of Olmsted County remained primarily white during the study period, despite recent demographic changes. This factor may raise concerns regarding the generalizability of this study’s results to the US population. However, because cancers involving the upper gastrointestinal tract, particularly esophageal adenocarcinomas, have a much higher prevalence in the US white population than in populations of other racial or ethnic groups,26 our results are likely to be representative for at least the US white population.
Patients with gastric or esophageal adenocarcinomas continue to have a very poor prognosis, even if they appear to be eligible for surgical cure. Moreover, the number of patients achieving cure has not improved appreciably in the past 3 decades despite general advances in surgical techniques and other medical care. Nevertheless, surgical cure remains the only intervention that may significantly improve a patient’s chance of survival, as described by our data. Without surgical intervention, 2-year survival for patients with either gastric or esophageal adenocarcinoma remained essentially zero in the current study. However, it is possible that much of the gain seen with surgery was due to lead-time bias resulting from cases that were diagnosed at earlier TNM stages and therefore deemed to be operable. Our study did not examine that possibility.
Our general approach to stage III and stage IV esophageal adenocarcinoma began to change after 2000, with the publication of several studies that suggested a survival benefit for patients treated with neoadjuvant combination chemoradiation therapy.27–31 We look forward to reporting the population-based impact of this therapy after 2010.
Likewise, the National Cancer Research Institute’s Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial, in the United Kingdom, has impacted clinical practice in the management of patients with gastric adenocarcinoma.32 A 10-year population-based assessment of the impact of the MAGIC trial on patient survival will be possible in 2017.
CONCLUSION
In this population-based study, we have shown that, despite advances in diagnostic tools and refinements in surgical techniques, survival of patients with adenocarcinoma of the stomach or esophagus has not improved during the past 3 decades. Current efforts at cancer prevention and early screening of high-risk populations for premalignant lesions, such as Barrett esophagus, have not resulted in a significant change in the stage of presentation of disease in the studied community, possibly because of the low number of screened patients. Increased efforts at refining prevention and early diagnosis of gastric and esophageal cancer are essential because resection offers the best hope for cure.
Acknowledgments
This study was supported by a clinical research grant from the American College of Gastroenterology. The Rochester Epidemiology Project is supported by National Institutes of Health grant RO 1AR30582. Dr Romero’s participation was supported in part by National Institutes of Health grant NIDDK 02956.
- CI
confidence interval
- REP
Rochester Epidemiology Project
Footnotes
Individual reprints of this article are not available.
References
- 1.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11(2):235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Locke GR, III, Talley NJ, Carpenter HA, Harmsen WS, Zinsmeister AR, Melton LJ., III Changes in the site- and histology-specific incidence of gastric cancer during a 50-year period. Gastroenterology. 1995;109(6):1750–1756. doi: 10.1016/0016-5085(95)90740-8. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. SEER Cancer Statistics Review 1975–2004. Bethesda, MD: National Cancer Institute; [Accessed August 20, 2008]. Estimated new cancer cases and deaths for 2007. http://seer.cancer.gov/csr/1975_2004/results_single/sect_01_table.01.pdf. [Google Scholar]
- 4.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–1289. [PubMed] [Google Scholar]
- 5.Crane SJ, Locke GR, III, Harmsen WS, et al. The changing incidence of oesophageal and gastric adenocarcinoma by anatomic sub-site. Aliment Pharmacol Ther. 2007;25(4):447–453. doi: 10.1111/j.1365-2036.2006.03229.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SJ, Dickey W, Hughes D, O’Connor FA. Surveillance for Barrett’s oesophagus: results from a programme in Northern Ireland. Eur J Gastroenterol Hepatol. 2005;17(10):1029–1035. doi: 10.1097/00042737-200510000-00005. [DOI] [PubMed] [Google Scholar]
- 7.McKian KP, Miller RC, Cassivi SD, Jatoi A. Curing patients with locally advanced esophageal cancer: an update on multimodality therapy. Dis Esophagus. 2006;19(6):448–453. doi: 10.1111/j.1442-2050.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalrymple-Hay MJ, Evans KB, Lea RE. Oesophagectomy for carcinoma of the oesophagus and oesophagogastric junction. Eur J Cardiothorac Surg. 1999;15(5):626–630. doi: 10.1016/s1010-7940(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 9.Jensen LS, Pilegaard HK, Puho E, Pahle E, Melsen NC. Outcome after transthoracic resection of carcinoma of the oesophagus and oesophagogastric junction. Scand J Surg. 2005;94(3):191–196. doi: 10.1177/145749690509400303. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Allum WH, Stenning SP, et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 11.Bosset JF, Lorchel F, Mantion G, et al. Radiation and chemoradiation therapy for esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):239–245. doi: 10.1002/jso.20365. [DOI] [PubMed] [Google Scholar]
- 12.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185(6):538–543. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104(2):510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- 15.US Census Bureau. Fact sheet: Olmsted County, Minnesota. US Census Bureau. [Accessed August 20, 2008];2006 Web site. http://factfinder.census.gov/servlet/ACSSAFFFacts?_event=Search&geo_id=&_geoContext=&_street=&_county=olmsted&_cityTown=olmsted&_state=04000US27&_zip=&_lang=en&_sse=on&pctxt=fph&pgsl=010.
- 16.Yamada T, Alpers DH. Textbook of Gastroenterology. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Hansson LE, Sparen P, Nyren O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230(2):162–169. doi: 10.1097/00000658-199908000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newnham A, Quinn MJ, Babb P, Kang JY, Majeed A. Trends in oesophageal and gastric cancer incidence, mortality and survival in England and Wales 1971–1998/1999. Aliment Pharmacol Ther. 2003;17(5):655–664. doi: 10.1046/j.1365-2036.2003.01520.x. [DOI] [PubMed] [Google Scholar]
- 21.Arisue T, Tamura K, Tebayashi A. End results of gastric cancer detected by mass survey: analysis using the relative survival rate curve [in Japanese] Gan To Kagaku Ryoho. 1998;15(4 pt 21):929–936. [PubMed] [Google Scholar]
- 22.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37(12):1359–1365. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 23.Faivre J, Forman D, Esteve J, Gatta G EUROCARE Working Group. Survival of patients with oesophageal and gastric cancers in Europe. Eur J Cancer. 1998;34(14):2167–2175. doi: 10.1016/s0959-8049(98)00329-3. [DOI] [PubMed] [Google Scholar]
- 24.Bouvier AM, Binquet C, Gagnaire A, Jouve JL, Faivre J, Bedenne L. Management and prognosis of esophageal cancers: has progress been made? Eur J Cancer. 2006 Jan;42(2):228–233. doi: 10.1016/j.ejca.2005.08.038. Epub 2005 Dec 7. [DOI] [PubMed] [Google Scholar]
- 25.Sihvo EI, Luostarinen ME, Salo JA. Fate of patients with adenocarcinoma of the esophagus and the esophagogastric junction: a population-based analysis. Am J Gastroenterol. 2004;99(3):419–424. doi: 10.1111/j.1572-0241.2004.04094.x. [DOI] [PubMed] [Google Scholar]
- 26.Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99(4):582–588. doi: 10.1111/j.1572-0241.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 27.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–467. doi: 10.1056/NEJM199608153350702. published correction appears in N Engl J Med. 1999;341(5):384. [DOI] [PubMed] [Google Scholar]
- 28.Shah MA, Kelsen DP. Combined modality therapy of esophageal cancer: changes in the standard of care [editorial]? Ann Surg Oncol. 2004 Jul;11(7):641–643. doi: 10.1245/ASO.2004.04.907. Epub 2004 Jun 14. [DOI] [PubMed] [Google Scholar]
- 29.Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53(7):925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crosby TD, Brewster AE, Borley A, et al. Definitive chemoradiation in patients with inoperable oesophageal carcinoma. Br J Cancer. 2004;90(1):70–75. doi: 10.1038/sj.bjc.6601461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005 Jul 1;23(19):4330–4337. doi: 10.1200/JCO.2005.05.017. Epub 2005 Mar 21. [DOI] [PubMed] [Google Scholar]
- 32.Chua YJ, Cunningham D. The UK NCRI MAGIC trial of perioperative chemotherapy in resectable gastric cancer: implications for clinical practice [editorial] Ann Surg Oncol. 2007 Oct;14(10):2687–2690. doi: 10.1245/s10434-007-9423-7. Epub 2007 Jul 27. [DOI] [PubMed] [Google Scholar]