Abstract
Hyperpolarization of 89YCl3 and three 89Y-complexes was achieved by dynamic nuclear polarization of aqueous samples. The long T1’s of 89Y make its application as an MR imaging probe extremely promising. In addition, the wide chemical shift range for various chelates of 89Y means that agents sensitive to their biological/chemical milieu could serve as exquisite sensors of important biological events.
Hyperpolarization of nuclear spins can produce a dramatic increase in sensitivity for NMR active nuclei. Although the idea of transferring spin polarization from electrons to nuclei by dynamic nuclear polarization (DNP) to create a hyperpolarized NMR sample has been around since the mid-1950s, applications of this technology for study of liquid samples have appeared only recently. In 2003, Ardenkjaer-Larsen, et al.1 developed an automated method to polarize 13C nuclei at low temperatures in the presence of a stable trityl radical then bring the sample to room temperature very quickly to perform NMR measurements.1,2 Obviously, this method was most practical for long T1 13C nuclei such as non-protonated carbonyl or carboxyl carbons in rapidly tumbling small molecules which yield NMR signal enhancements of 10,000-fold or higher. One of the more exciting applications of this technology was reported shortly thereafter by Golman, et al.3 who demonstrated that it is practical to do real time metabolic imaging of [1-13C]pyruvate, [1-13C]lactate, and [1-13C]alanine in live animals using 13C chemical shift imaging (lactate and alanine are quickly formed from the injected hyperpolarized pyruvate through single enzyme catalyzed steps).
A commercial DNP device based on this technology now offers new opportunities for imaging nuclei that have not ordinarily been considered possible in the past. One attractive NMR nucleus for polarization is 89Y because this nucleus is difficult to detect at thermal Boltzmann polarization levels due to its small magnetic moment, low receptivity and long T1 relaxation times. 89Y does however have a favourable spin quantum number (½), sharp NMR linewidths (3–5 Hz), and a long T1 so this makes 89Y attractive as a potential in vivo imaging and spectroscopy probe. Another isotope of yttrium, 90Y (half life = 2.7 days), is an attractive radioisotope for cancer therapy because it emits high energy β electrons (average β energy = 0.93MeV) that provide relatively deep tissue penetration necessary for the treatment of larger tumors.4,5 To date, the only approved targeted 90Y radiopharmaceutical is Zevalin, used for treatment of non-Hodgkin's lymphomas.6 A second drug, 90Y-DOTA-D-Phe1-Tyr3-octreotide (90Y-DOTATOC), is in phase I/II studies in Europe for somatostatin receptor positive tumors7 and others will likely follow. Thus, a long-lived, hyperpolarized 89Y analog might be attractive for imaging the biodistribution of such drugs.
Only one 89Y NMR study was reported prior to the 70’s.8 With the advent of FT spectrometers, a few more reports appeared demonstrating that 89Y salts have unusually long T1 values and are concentration dependent.9 More interestingly, Levy, et al.,10 were first to show that complexation of 89Y3+ with crown ethers of various ring size resulted in lengthening of T1 about 4-fold over that measured for salts dissolved in DMSO. In 1990, Holz and Horrocks used 89Y3+ as a Ca2+ surrogate and reported that the chemical shift of various chelated forms of Y3+ varies widely, ranging from 36.6 ppm when bound at the EF site of parvalbumin to 129.6 ppm in Y(EDTA)−.11 This was the first demonstration that the chemical shift of 89Y3+ complexes could be used as a probe of the coordination environment of the ion. Given the potential of hyperpolarized 89Y for molecular imaging of targeted therapeutics, we recently initiated DNP studies of a few Y3+ complexes using a commercial polarizer (HyperSense, Oxford Molecular Biotools).
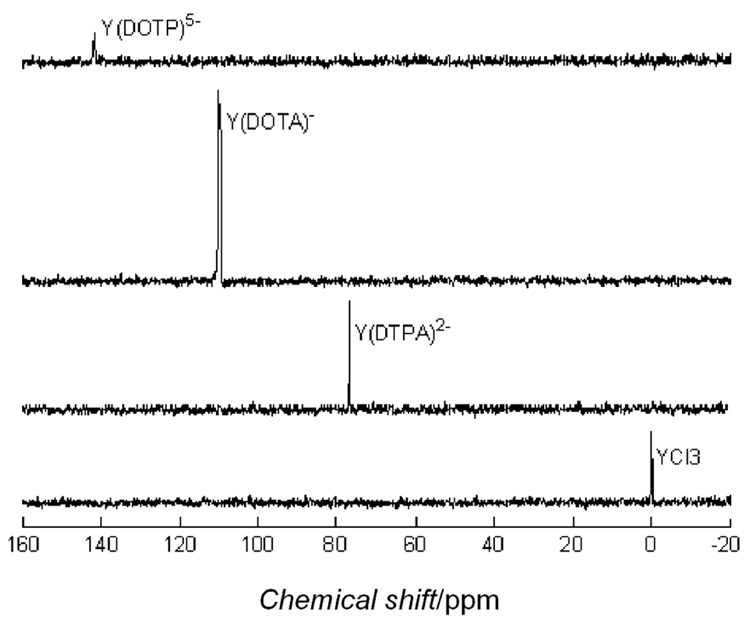
Dynamic nuclear polarization of frozen solutions consisting of YCl3 and three different chelated forms of Y3+ in the presence of a common stable trityl radical resulted in polarization enhancements that varied from 246 to 1527-fold above thermal equilibrium at 310K. Although these polarizations are small compared to recently reported 13C samples where polarizations of 5,000 – 10,000-fold have been achieved, they were sufficient to allow easy detection of the 89Y NMR signal using a single 10° pulse (Figure 1). From an experimental standpoint, the necessity of reducing the sample volume by taking only a portion of the effluent from the dissolution process undoubtedly resulted in a loss of some magnetization due to T1 processes before the NMR data collection ensued (see Supporting Information). Here, our goal was to measure T1 values on each sample by following the decay process so the sample volume was limited to 1.5 mL to ensure that the entire volume was sampled with each 10° pulse. If the volume of the sample is not restricted to the sample coil, diffusion will cause erroneous estimates of the T1 due to the cos(n-1) term in equation 1 (Supporting information), making the T1 appear artificially long. Also, the linewidth of the trityl radical used here is much too wide to match the Larmor frequency of 89Y at the polarizing field (6.89 MHz) so other more favourable radicals should produce substantially higher polarizations. The polarization time used here (2.5 hours) was arbitrary and chosen simply for experimental convenience. In general, the DNP effect is mediated by the electron-nuclear dipolar interactions and the low gyromagnetic ratio of 89Y means that the polarization time constant is likely very long. Thus, it is likely that higher polarization levels could have been achieved by polarizing the samples for a longer period.
Figure 1.
The polarization enhancements reported in Table 1 were determined by comparison of the intensity of the polarized 89Y signal after the first 10° pulse to a 3M YCl3 standard (single 90° pulse). Comparison of the signal from hyperpolarized samples after full relaxation (thermal polarization) was impossible at these concentrations (mM). The polarization predicted for a sample cooled to 1.4K is 221 times greater than the Boltzmann thermal value at 310K. The measured enhancements of YCl3 and Y(DOTP)5− were only slightly above this value so the effect due to DNP is only marginal in those cases. The remaining samples had polarizations well above that predicted for a cooled sample. It is interesting to note that the more highly charged species (Y3+ and Y(DOTP)5−) show the lowest polarization and, as the charge is reduced (Y(DTPA)2− > Y(DOTA)−), polarization increases. This may reflect less than optimal glass formation at 1.4K with the more highly charged species.
Table 1.
Hyperpolarized 89Y data for YCl3 and YIII complexes.
| Compound | Conc. in NMR tube (mM) | Measured enhancement | Measured T1 (s) | Chemical shift (ppm) |
|---|---|---|---|---|
| YCl3 | 15 | 246 | 620 | 0 (ref) |
| Y(DTPA)2− | 7.6 | 566 | 451 | 76 |
| Y(DOTA)− | 7.9 | 1527 | 499 | 109 |
| Y(DOTP)5− | 10 | 298 | 264 | 141 |
| Y(DOTP)5− | 5 | 1042 | 277 | 141 |
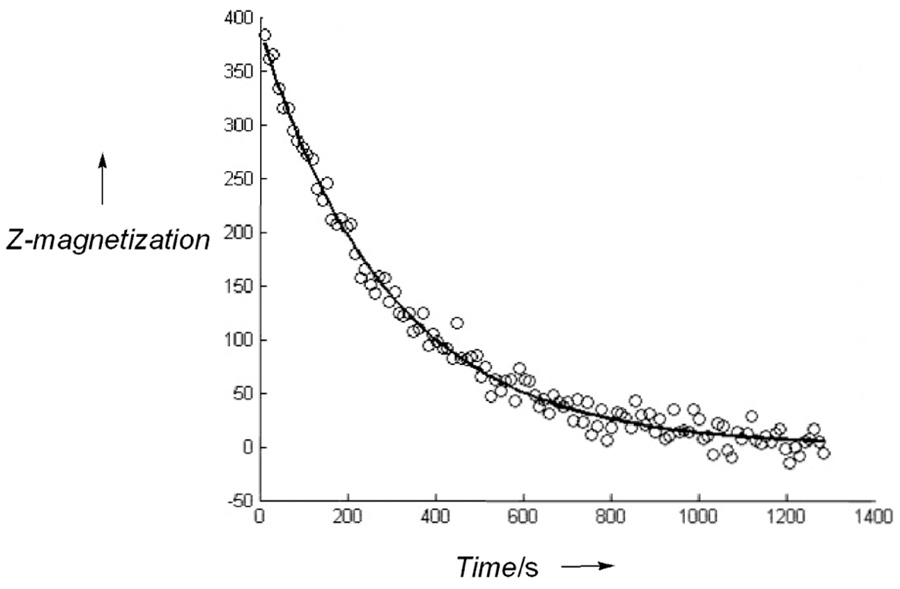
The T1’s were measured by fitting the polarization decay curves (Figure 2) to equation 1 (Supporting Information). The T1 values reported previously for Y(NO3)3 on thermally polarized samples varied from 63 – 270 s depending upon concentration (1M samples gave longer T1 values than 3M samples).10 Our estimates of T1 obtained by following the decay of hyperpolarized 89Y are even longer than those reported by Levy, et al. by another factor of ~2.3 but the concentration of the hyperpolarized sample was also lower by a factor of ~67 so the trend reported by Levy, et al. appears to hold over a very wide concentration range. It is unclear however whether this is simply an effect due to concentration or whether it partially reflects the experimental difficulty in measuring such long T1 values for such an insensitive nucleus. In general, the T1’s of the 89Y-chelates were found to be lower than that of the YCl3 sample, also in contradiction to earlier results.10 One possible explanation for this observation is the higher B0 field used here (14.1 Tesla) may have an additional chemical shift anisotropy contribution to the T1 relaxation mechanism in the asymmetric environment of the chelates. It is also possible that other ligand spins in these samples (14N, 31P) contribute to the relaxation of 89Y.
Figure 2.
NMR signal of 89Y(DOTA)− collected as a function of time after the sample was ejected from the polarizer, then inserted into the magnet. Total time following dissolution until the first acquisition was 30 seconds. The pH was 7 and final concentration of Y(DOTA)− in the NMR tube was 7.9 mM. The fitted line gave a T1 of 499 seconds.
In conclusion, hyperpolarization of 89Y salts and 89Y complexes is feasible with currently available commercial hardware. The long T1’s of 89Y make its application as an MR imaging probe quite promising. In addition, the wide chemical shift range for 89Y means that contrast agents sensitive to a variety of biological/chemical milieu could serve as exquisite sensors of important biological events.
Supplementary Material
Experimental procedures as well as the equation used to fit the NMR data to obtain T1. This material is available free of charge inline at http://pubs.acs.org.
Acknowledgements
This research was supported in part by grants from the National Institutes of Health (CA-115531, DK-058398 and RR-02584) and the Robert A. Welch Foundation (AT-584).
REFERENCES
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc. Natl. Acad. Sci., USA. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Proc. Natl. Acad. Sci., USA. 2003;100:10435–10439. doi: 10.1073/pnas.1733836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golman K, Zandt R, Thaning M. Proc. Natl. Acad. Sci., USA. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Golman K, Petersson JS. Acad. Radiol. 2006;13:932–942. doi: 10.1016/j.acra.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Neves M, Kling A, Oliveira A. J. Radioanal. Nucl. Chem. 2005;266:377–384. [Google Scholar]
- 5.Zweit J. Phys. Med. Biol. 1996;41:1905–1914. doi: 10.1088/0031-9155/41/10/004. [DOI] [PubMed] [Google Scholar]
- 6.Cheson BD. Curr. Drug Targets. 2006;7:1293–1300. doi: 10.2174/138945006778559157. [DOI] [PubMed] [Google Scholar]
- 7.de Jong M, Valkema R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WA, Bakker WH, Smith C, Pauwels S, Krenning EP. Semin. Nucl. Med. 2002;32:133–140. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 8.Brun E, Oeser J, Staub HH, Telschow CG. Phys. Rev. 1949;76:1528. [Google Scholar]
- 9.Hassler C, Kronenbitter J, Schwenk A. Z. Phys. 1977;280:117. [Google Scholar]; Adam RM, Fazakerley GV, Reid DG. J. Magn. Reson. 1979;33:655. [Google Scholar]
- 10.Levy GC, Rinaldi PL, Bailey JT. J. Magn Reson. 1980;40:167–173. [Google Scholar]
- 11.Holz RC, DeW. Horrocks W. J. Magn Reson. 1990;89:627–631. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures as well as the equation used to fit the NMR data to obtain T1. This material is available free of charge inline at http://pubs.acs.org.