Abstract
PURPOSE
In this study, we tested the effectiveness of a melanoma-associated antigen–derived peptide, MART-127–35, in eliciting cellular immune responses in vivo in the context of a phase I active immunization protocol. This peptide (AAGIGILTV) corresponds to residues 27–35 from the nonmutated melanoma-associated antigen MART-1/Melan A and is recognized by most melanoma-specific, HLA-A* 0201–restricted, tumor-infiltrating lymphocytes. To test the in vivo induction of cytotoxic T lymphocyte (CTL) sensitization, we compared CTL reactivity in vitro from peripheral blood mono-nuclear cell (PBMC) pools obtained before and after vaccination.
PATIENTS AND METHODS
MART-127–35 was administered to HLA-A*0201 melanoma patients subcutaneously in an emulsification with incomplete Freund’s adjuvant. A vaccination course included four inoculations of peptide at 3-week intervals. PBMC collected by leukapheresis and separated by Ficoll-Hypaque gradient before and after vaccination were analyzed in 18 patients by in vitro sensitization with MART-127–35– To induce MART-127–35–specific CTL, PBMC were incubated with 1 μM peptide (on day 0) and interleukin-2 (IL-2) (300 IU/mL, on days 1 and 4 after each stimulation). At weekly intervals, cells were harvested and an aliquot was cryopreserved for later analysis. The remaining cells were replated and restimulated using irradiated autologous PBMC pulsed with 1 μM of relevant peptide. After three restimulations, all samples from one patient were tested simultaneously for HLA-A*0201-restricted anti-MART-127–35 reactivity by microcytotoxicity and cytokine (IFN-γ) release assays.
RESULTS
Toxicities were minimal and consisted of local irritation at the site of vaccine administration. None of the patients sustained a clinical response. The first eight patients were monitored by inducing CTL reactivity from PBMC obtained preimmunization and after two and four vaccinations. Only two prevaccination cultures were reactive to MART-1, compared with five and seven cultures from PBMC obtained after two and four vaccinations, respectively. Thus, an enhancement in cytotoxic activity could be detected in postvaccination CTL cultures, and serial vaccine administrations appeared to boost the detectability of cytotoxicity in vitro. For completeness, the analysis compared prevaccination with postvaccination PBMC cultures. Specific anti–MART-127–35 cytotoxicity (≥ 10 lytic units) could be detected in two prevaccination and 12 postvaccination cultures after two in vitro stimulations. In 15 postvaccination CTL cultures, a more than threefold increase in specific release of IFN-γ was noted, compared with prevaccination.
DISCUSSION
In vivo administration of a melanoma-associated antigen peptide, emulsified in incomplete Freund’s adjuvant, could safely augment CTL reactivity against epitopes commonly expressed by melanoma cells. Although the enhancement of CTL reactivity did not achieve tumor regression, it is possible that the use of recombinant immunogens with increased immunomodulatory capabilities in future clinical trials could reach the threshold of CTL activation necessary for tumor regression.
Keywords: MART-1, melanoma, immunization
INTRODUCTION
Cytotoxic T lymphocytes (CTL) associated with in vivo tumor regression recognize nonmutated molecules expressed by most melanoma cells and normal melanocytes.1–5 These CTL recognize endogenously processed peptides (8 to 10 amino acids in length) bound to human leukocyte antigen (HLA) class I molecules on the surface of tumor cells. Identification of these peptides has led to the development of peptide vaccination protocols in several institutions with the purpose of causing tumor regression in melanoma patients by enhancing in vivo specific antitumor CTL reactivity. These clinical trials are supported also by pre-clinical data showing that vaccination with major histocompatibility complex (MHC) class I restricted epitopes can enhance cellular immunity against viruses in murine and human models6–7 and protect against tumor challenge in mice.8 Among the melanoma-associated antigens (MAA), MART-1,1 identical to Melan A,2 appears to be an immunodominant antigen in HLA-A*0201 melanoma patients, since it is recognized most frequently by lymphocytes derived from metastatic lesions9 and by peripheral blood mononuclear cells (PBMC) stimulated in vitro with matched allogeneic melanoma cells.10 We have shown that potent epitope-specific9 antimelanoma CTL activity can be generated by repetitive in vitro stimulation of PBMC with the immunodominant MART-127–35 (AAGIGILTV) peptide11 as well as epitopes derived from other MAA.12 However, evidence that the systemic administration of the same peptide can elicit antitumor CTL activity in vivo is lacking. In this study, we tested the effectiveness of MART-127–35 in eliciting cellular immune responses in vivo in the context of a phase I active immunization protocol based on the administration of MART-127–35 emulsified in incomplete Freund’s adjuvant (IFA).
MATERIALS AND METHODS
Clinical protocol and patient population
In a phase I dose-escalating protocol, 23 HLA-A*0201 patients with metastatic melanoma received the MART-127–35 peptide subcutaneously at doses ranging from 0.1 to 10 mg. The nine amino acid peptide was emulsified in incomplete Freund’s adjuvant. A vaccination course included four inoculations of peptide at 3-week intervals. Peripheral blood monocytes were collected by leukapheresis before vaccination (condition A) and 3 weeks after the second vaccination (condition B) and fourth vaccination (condition C). PBMC from 18 of the 23 patients were analyzed. Two of the patients excluded from the analysis had rapid progression of disease, which did not allow for a complete collection of PBMC at the end of at least two immunizations. Another patient had an insufficient number of PBMC to study, and a fourth patient developed multiple infections because of his cancer. Finally, one patient was added to this protocol at a later date. All exclusions from the analysis were done a priori. A partial response was defined as a reduction of tumor burden ≥ 50% of the sum of the perpendicular diameters of all lesions without any tumor sites progressing in size. The protocol was approved by the Clinical Research Committee of the National Cancer Institute. Patients signed a written informed consent before enrollment in the protocol. For the analysis of T-cell sensitization by the vaccine, each patient’s preimmunization PBMC count was used as a control in a paired analysis. The patient population consisted of 6 women and 12 men, who ranged in age from 27 to 68 years; 10 patients had received previous therapy with immunologically active substances, including seven patients who had previously received high-dose interleukin-2 (IL-2) therapy. All patients had a performance status of ECOG (Eastern Cooperative Oncology Group) 0 or 1. No correlation was noted between any of these variables and toxicity, response, or T-cell activation (data not shown but available upon request).
HLA typing and subtyping
HLA class I and class II type was established on PBMC as previously described.13 Because minimal cross-reactivity of MART-127–35 with other natural HLA-A2 allelic variants has been shown,14 all patients were HLA-A2 subtyped to ensure the appropriateness of their enrollment, using a high-resolution nested sequence polymerase chain reaction set to resolve the HLA-A*0201 through the HLA-A*0217alleles.14
Peptides
MART-127–35 (AAGIGILTV) used for the in vitro analysis was synthesized by Peptide Technologies Inc. (Gaithersburg, Maryland) by a solid-phase method and purified by high-pressure liquid chromatography (> 95% pure).9 The binding affinity of MART-127–35 to HLA-A*0201 has been previously reported.11 The control Flu M158–66 peptide (GILGFVFTL) from the influenza matrix protein was synthesized by Multiple Peptide Systems (San Diego). All peptides were used at a final concentration of 1 μM from aliquots dissolved in 100% dimethyl sulfoxide and stored at −70°C.
Generation of lymphocytes
We obtained 2 to 5 × 109 PBMC from melanoma patients by leukapheresis and separated them in Ficoll-Hypaque gradients (LSMR, Organon Teknika, Durham, North Carolina). All PBMC preparations were frozen in human AB serum with 10% dimethyl sulfoxide (Sigma Chemical Co, St. Louis) and stored in liquid nitrogen. To induce MART-127–35–specific or Flu M158–66–specific CTL,11 5 × 106 PBMC/well were plated in 24-well plates (Costar, Cambridge, Massachusetts) in 2 mL of complete medium (CM) consisting of Iscove’s (Biofluids, Rockville, Maryland) plus 0.03% L-glutamine, 100 U/mL penicillin (both from National Institutes of Health [NIH] media unit), 10% heat-inactivated human AB serum (Biofluids), and 25 mM Hepes (Biofluids). On the same day, 1 μM of peptide was added, and IL-2, 300 IU/mL (Chiron Co, Emeryville, California), was added 24 hours (day 1) and 96 hours (day 4) after each stimulation. At weekly intervals, cells were harvested and an aliquot was cryopreserved for later analysis. The remaining cells were replated at 1.0 × 106 cells/well and stimulated using 2.5 to 8 × 106 irradiated (3000 rads) autologous PBMC/well previously incubated for 1 to 2 hours at 37°C in CM plus 1 μM of relevant peptide. Cultures were continued for 28 days, collecting and cryopreserving samples weekly for analysis.
Assessment ofAg recognition by CTL
At the end of the 4 weeks, all samples were simultaneously thawed and incubated for 24 to 48 hours in CM with 300 IU/ mL IL-2. HLA-A*0201-restricted anti-MART-127–35 CTL specificity was tested by the standard 4-hour 51Cr release microcytotoxicity assay and 24-hour cytokine (IFN-γ) release assay. Cytotoxicity was tested against T2 cells15 pulsed with 1 μM of relevant (MART-127–35) or irrelevant (Flu M158–66) peptide.11,16 The T2 cell line was selected as a target because it expresses only the HLA-A*0201 allele, which was the restriction element for this vaccination. To assess interassay variability, anti–MART-127–35 CTL effectors of known potency were used as positive controls. T2 cells were prepared by overnight incubation in 0.1 mCu of 51Cr/mL and plated at a concentration of 2 × 103 cells/well (U bottom 96-well plate, Costar). Effector: target (E:T) ratios of 25, 6.25, and 1.5:1 were used. Specific secretion of IFN-γ was analyzed after three in vitro stimulations; 5 × 105 effector cells/mL were cocultured for 24 hours in 200 μL of CM at 37°C with the same number of T2 cells pulsed with relevant or irrelevant peptide. Depending on CTL availability, the most relevant cultures were tested for release of IFN-γ in response to naturally processed MART-127–35 epitope by coculturing effectors with the melanoma clones 624.38 and 624.28 derived from the 624. MEL bulk cell line. Both clones express comparable amounts of MART-1, but 624.38 expresses HLA-A2,3 alleles and 624.28 expresses HLA-A-,3.17 In standard culture conditions, these clones do not express any other HLA class I or class II alleles13; therefore, they differ only in HLA-A2 expression. Supernatants from these cocultures were tested by enzyme-linked immunosorbent assay (R+D ELISA kit, Minneapolis).11
CD4/CD8 phenotyping
CD8+ or CD4+ pheno-type expression was tested by direct immunofluorescence on a FACScan (Becton Dickinson, San Jose, California) using 1:10 diluted phycoerythrin conjugated anti-CD4 and fluorescein isothiocyanate conjugated anti-CD8 murine mAb (Becton Dickinson).
Statistical analysis
Lytic data are presented as lytic units (LU)/106 effector cells (1 LU = number of effectors required to induce 30% lysis of 2 × 103 targets). Specific CTL activity was defined by subtracting the LU of the irrelevant target from the LU of the relevant target (e.g., anti-MART-127–35-specific activity = LU(T2 + MART) – LU(T2 + FLU)) as described by Vitiello et al7 Evidence of in vitro CTL induction was arbitrarily defined as ≥ 10 specific LU after two or three stimulations. Non-parametric Fishers exact test was used to compare the frequency of in vitro CTL induction between prevaccination and postvaccination cultures (two-tailed P values are shown). Specific release of IFN-γ by a PBMC culture was arbitrarily defined as (1) threefold or higher difference in IFN-γ production in response to relevant (T2 + MART-127–35) vs irrelevant (T2 + Flu M158–66) stimulation, and (2) at least 100 pg/5 × 105 cells/24 hours production of IFN-γ A threefold increase in specific release between prevaccination and postvaccination cultures was arbitrarily chosen as evidence of differences in CTL reactivity and statistically compared by Fishers exact test. Production of IFN-γ was also compared parametrically between prevaccination and post-vaccination cultures using a two-tailed paired sample Student’s test.
RESULTS
Clinical effects of the administration of MART-127–35 peptide in IFA in a phase I clinical trial
The administration of the MART-127–35 immunodominant peptide to melanoma patients permitted the first immunologic assessment of this new modality of cancer treatment. Toxicities were minimal and consisted of local irritation at the site of vaccine administration. None of the 23 patients treated had a clinical response, although three patients demonstrated a decrease in the size of some lesions.
Culture expansion and T-cellphenotype
The first eight patients tested (#1–#8) were monitored by expanding PBMC obtained preimmunization (condition A), after two vaccinations (condition B), and after four vaccinations (condition C). PBMC from the remaining patients were analyzed only prevaccination and postvaccination. Table 1 shows the expansion and phenotype of the PBMC cultures after three restimulations. Neither of these variables appeared to be consistently affected by vaccination with the MART-127–35 peptide. T-cell phenotype was also not significantly predictive of the specificity of the cultures.
Table 1.
Expansion Rate and Phenotype of PBMC Cultures Sensitized in Vitro with MART-127–35
| Fold Expansiona |
CD4+ (%)
|
CD8+ (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Patient # | Vaccine Dose (mg) | A | B | C | A | C | A | C |
| 1 | 0.1 | 4 | 29 | 66 | nd | nd | nd | nd |
| 2 | 0.3 | 47 | 42 | 5 | 47 | 64 | 9 | 9 |
| 3 | 0.3 | 22 | 15 | 38 | nd | nd | nd | nd |
| 4 | 1.0 | 122 | 37 | 150 | 7 | 35 | 55 | 38 |
| 5 | 1.0 | 207 | 357 | 228 | 36 | 36 | 39 | 36 |
| 6 | 3.0 | 50 | 43 | 20 | 51 | 22 | 24 | 60 |
| 7 | 3.0 | 6 | 7 | 8 | 59 | 38 | 13 | 24 |
| 8 | 10.0 | 2 | 4 | 5 | nd | nd | nd | nd |
| 9 | 1.0 | 2 | 7 | 66 | 44 | 14 | 15 | |
| 10 | 0.1 | 130 | 115 | 66 | 42 | 18 | 41 | |
| 11 | 0.1 | 11 | 8 | 24 | 17 | 35 | 36 | |
| 12 | 0.1 | 22 | 151 | 45 | 35 | 28 | 35 | |
| 13 | 3.0 | 35 | 49 | 35 | 49 | 44 | 31 | |
| 14 | 3.0 | 11 | 9 | 56 | 40 | 21 | 29 | |
| 15 | 10.0 | 7 | 3 | nd | nd | nd | nd | |
| 16 | 10.0 | 32 | 34 | 65 | 35 | 28 | 52 | |
| 17 | 10.0 | 323 | 2170 | 30 | 39 | 45 | 52 | |
| 18 | 10.0 | 3 | 5 | 32 | 4 | 45 | 80 | |
Cumulative expansion at 3 weeks.
Abbreviations: nd = not done; A = preimmunization CTL culture; B = CTL culture from PBMC obtained after two immunizations; C = postimmunization PBMC.
Enhancement of CTL reactivity by active immunization with MART-127–35 peptide emulsified in IFA
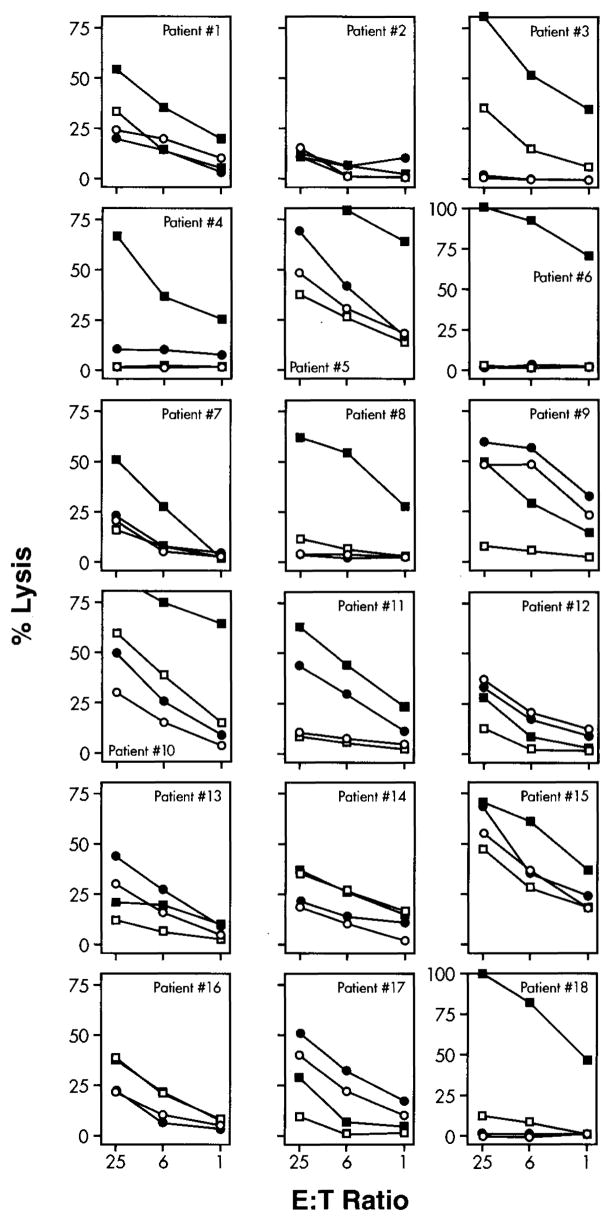
Lytic activity of each CTL culture is shown in detail in Figure 1. The same data are summarized as specific lytic units in Table 2. Among the initial eight patients, specific lytic activity was noted after two stimulations in five postimmunization (condition C) PBMC cultures. After the same number of in vitro stimulations, five cultures of PBMC obtained after two in vivo vaccinations (condition B) and none of the preimmunization cultures (condition A) had detectable lytic activity. After a third round of in vitro stimulation, two additional postvaccination cultures became specific, compared with only one prevaccination culture. Patient #2 failed to develop anti–MART-127–35 reactivity in prevaccination and postvaccination cultures in three separate attempts, suggesting a specific unresponsiveness to the MART-127–35 epitope. The same PBMC preparations could easily be sensitized against Flu M158–66 in paired culture conditions, excluding the possibility that the in- ability to elicit anti-MART-1 reactivity was because of the quality of the PBMC preparation. The data obtained in these eight patients suggested that an enhancement in cytotoxic activity could be detected in postvaccination CTL cultures and that serial vaccine administrations could boost the detectability of cytotoxicity in vitro. Since the peak effect of the vaccination protocol occurred after the full four-vaccination treatment course, we tested the remaining patients at one time point (condition C).
Figure 1.
Lytic activity of CTL cultures induced in vitro by three weekly stimulations with MART-127–35 peptide and tested after 21 days of culture. Anti-MART-1 CTL were tested in a standard 4-hour 51Cr release assay. For each patient tested, data are presented for prevaccination CTL cultures tested against relevant target (● = T2 + MART-127–35) and irrelevant target (○ = T2 + Flu M158–66) and postvaccination cultures (■ = T2 + MART-127–35 and □ = T2 + Flu M158–66).
Table 2.
HLA-A*0201–Restricted, Specific Lytic Activity of PBMC Cultures Sensitized in Vitro with MART-127–35
| Patient # | LUa at Week 2
|
LUb at Week 3
|
||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| 1 | 0 | 4 | 34 | 0 | 0 | 33 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 5 | 26 | 47 | 12 | 55 | >100 |
| 4 | 0 | 17 | 0 | 0 | 20 | 50 |
| 5 | 0 | 22 | 28 | 30 | >100 | >100 |
| 6 | 0 | 14 | 15 | 0 | >100 | >100 |
| 7 | 0 | 0 | 2 | 0 | 7 | 15 |
| 8 | 0 | 13 | 90 | 0 | 15 | >100 |
| 9 | 0 | 20 | 5 | 14 | ||
| 10 | 9 | 60 | 13 | >100 | ||
| 11 | 5 | 7 | 14 | 67 | ||
| 12 | 0 | 0 | 0 | 0 | ||
| 13 | 8 | 0 | 12 | 0 | ||
| 14 | 53 | 17 | 0 | 1 | ||
| 15 | 0 | >100 | 0 | >100 | ||
| 16 | 11 | 20 | 0 | 0 | ||
| 17 | 7 | 13 | 20 | 0 | ||
| 18 | 0 | 55 | 0 | >100 | ||
LU = Specific lytic units/106 effector cells calculated from the data presented in Figure 1, as previously reported.7
Patients 1–8 were evaluated after both two and four immunizations; the following patients (9–18) were evaluated after four inoculations only (P2 value A vs C < 0.01 for both week 2 and week 3).
Abbreviations: A = preimmunization CTL culture; B = CTL culture from PBMC obtained after two immunizations; C = postimmunization PBMC.
Specific anti-MART-127–35 cytotoxicity (≥ 10 LU) could be generated after two in vitro stimulations in two prevaccination and 12 postvaccination cultures (P2 < 0.01). After three restimulations, six and 12 prevaccination and postvaccination cultures were specific (P2 < 0.01). Because of the limited number of CTL available, HLA-A*0201-expressing melanoma targets were not used to test for recognition of naturally processed MART-127–35 epitope, since in previous experiments, we noted that CTL generated in vitro with this MART-127–35 peptide can invariably recognize melanoma cells provided that they express sufficient amounts of HLA-A*0201 and/or MART-1.1,9,11,16 Furthermore, in a previous study, no significant differences were noted between melanoma and T2 targets in cytotoxicity assays in which anti-MART-127–35 CTL from 12 different melanoma patients were used.16
Cytokine release by anti-MART-127–35 PBMC cultures
T-cell reactivity was tested for IFN-γ release in an HLA-A*0201-restricted assay by pulsing MART-127–35 on T2 cells expressing HLA-A*0201 molecules (Table 3). This assay excludes non-HLA-A*0201 -restricted secretion of IFN-γ, since the T2 line does not express any other HLA class I or class II alleles. Therefore, this assay is aimed at analyzing specifically HLA-A*0201-restricted secretion of IFN-γ. Specific cytokine release (see Materials and Methods, above) was noted in six of 18 prevaccination and 16 of 18 postvaccination cultures. In a two-tailed paired analysis, the postvaccination cultures secreted significantly more IFN-γ than the prevaccination cultures (paired sample Student’s t test: P2 < 0.001), and 15 of the 16 postvaccination cultures considered to be specific demonstrated at least a threefold increase in cytokine release compared with prevaccination PBMC. Cytokine release, therefore, was more sensitive than the cytotoxicity assay in detecting CTL activation by the vaccine. Among the seven post-vaccination cultures considered nonspecific by cytotoxicity assay, four were specific according to the IFN-γ release assay (patients 9, 12, 13, and 17). It is important to note that these four relatively young PBMC cultures were cytotoxic, but specificity could have been masked by the presence of LAK-like, non-MHC restricted killing. Depending on availability of effector cells, the specific cultures were tested for release of cytokine in response to naturally processed MART-127–35 (Table 4). This was done by coculturing the PBMC with either 624.38 or 624.28 clones derived by limiting dilution from the 624-MEL bulk melanoma cell line. These clones have previously been extensively characterized.17 Both express comparable amounts of MART-1 mRNA and protein; however, 624.28 has lost expression of functional HLA-A2 while retaining the remaining parental HLA-phenotype. Eleven of 11 specific postvaccination cultures secreted IFN-γ in response to 624.38 but not 624.28, while only one prevaccination culture showed significant, though modest, secretion of IFN-γ (paired sample Student’s t test: P2 < 0.001).
Table 3.
HLA-A*0201–Restricted Cytokine Release by PBMC Cultures Sensitized in Vitro with MART-127–35
| IFN-γ release (pg/5 × 105 effectors/24 hours)
|
||||
|---|---|---|---|---|
| A
|
C
|
|||
| Patient # | T2+MART-1 | T2+Flu M1 | T2+MART-1 | T2+Flu M1 |
| 1 | 476 | 229 | 7839 | 273 |
| 2 | 10 | 0 | 360 | 0 |
| 3 | 498 | 460 | 6785 | 322 |
| 4 | 1614 | 88 | 8580 | 0 |
| 5 | 5976 | 310 | 27,080 | 0 |
| 6 | 262 | 0 | 25,620 | 0 |
| 7 | 131 | 83 | 5952 | 0 |
| 8 | 0 | 0 | 7499 | 0 |
| 9 | 12 | 18 | 960 | 436 |
| 10 | 696 | 68 | 23,760 | 80 |
| 11 | 1146 | 893 | 3240 | 0 |
| 12 | 82 | 0 | 1570 | 0 |
| 13 | 1664 | 44 | 2668 | 0 |
| 14 | 46 | 0 | 192 | 0 |
| 15 | 540 | 0 | 4336 | 0 |
| 16 | 0 | 0 | 64 | 0 |
| 17 | 1864 | 956 | 3264 | 56 |
| 18 | 60 | 0 | 8848 | 0 |
Release of cytokine is expressed as the amount of IFN-γ (pg/mL) secreted by 5 × 105 effector cells/mL CTL cocultured for 24 hours in the presence of 5 × 105 relevant (T2 + MART-127–35) or irrelevant (T2 + Flu M158–66) stimulators per milliliter. The assay was performed after three in vitro stimulations.
Specific release of IFN-γ by a PBMC culture was defined as (1) threefold or higher difference in IFN-γ production in the presence of relevant (T2 + MART-127–35) vs irrelevant (T2 + Flu M158–66) stimulation and (2) ≥ 100 pg/106 cells/24 hours production of IFN-γ. Difference in CTL reactivity was considered a threefold increase in specific release between prevaccination and postvaccination cultures.
Abbreviations: A = preimmunization CTL culture; C = postimmunization PBMC.
Table 4.
HLA-A*0201–Restricted Recognition of Naturally Processed MART-1 Antigen by PBMC Cultures Sensitized in Vitro with MART-127–35
| IFN-γ release (pg/5 × 105 effectors/24 hours)
|
||||
|---|---|---|---|---|
| A
|
C
|
|||
| Patient # | 624.38 (HLA-A2+) | 624.28 (HLA-A2−) | 624.38 (HLA-A2+) | 624.28 (HLA-A2−) |
| 1 | 0 | 0 | 6708 | 0 |
| 3 | 131 | 38 | 6215 | 0 |
| 6 | 0 | 0 | 16,936 | 480 |
| 7 | 32 | 0 | 4998 | 16 |
| 8 | 0 | 0 | 4098 | 0 |
| 10 | 2180 | 1372 | 24,356 | 240 |
| 11 | 0 | 0 | 5806 | 27 |
| 12 | 0 | 0 | 2384 | 0 |
| 15 | 0 | 0 | 6761 | 0 |
| 17 | 0 | 0 | 5009 | 202 |
| 18 | 0 | 0 | 7454 | 0 |
IFN-γ release by PBMC cultures in the presence of the melanoma cell clones 624.38 (MART-1 + and HLA-A*0201+) and 624.28 (MART-1 + and HLA-A*0201−).17
Specific release of IFN-γ by a PBMC culture was defined as (1) threefold or higher difference in IFN-γ production in the presence of relevant (T2 + MART-127–35) vs irrelevant (T2 + Flu M158–66) stimulation and (2) ≥ 100 pg/106 cells/24 hours production of IFN-γ. Difference in CTL reactivity was considered a threefold increase in specific release between prevaccination and postvaccination cultures.
Abbreviations: A = preimmunization CTL culture; C = postimmunization PBMC.
DISCUSSION
The MART-127–35 peptide derived from the nonmutated melanoma-associated antigen MART-1/Melan A1,2 was used for vaccination of patients with advanced melanoma in a phase I protocol. No clinically significant toxicities were observed, and none of the patients vaccinated demonstrated an objective clinical response to the treatment.
Because of the lack of clinical effectiveness, it became important to analyze the direct effect of the vaccine on its immunologic target. We thus attempted to develop a method able to quantitate epitope-specific sensitization against MAA. In viral systems, Vitiello et al were able to monitor T-cell sensitization to the in vivo administration of hepatitis B vaccine (HBVc18-27) lipopeptide by comparing cytotoxic activity in prevaccination and postvaccination PBMC cultures induced in vitro by stimulation with HBVc18–27 (10 (μg/mL).7 T-cell sensitization in vivo against MAA, however, differs significantly from sensitization against viral antigens. Although the purpose of vaccination against a virus is to induce a primary in vivo activation of naive T-cells, most MAA (including MART-1) are nonmutated molecules constitutively expressed by normal cells.1,4 As a consequence, normal, non-tumor-bearing individuals and melanoma patients can develop T-cells reactive against MAA,11,16 and the monitoring method requires a resolution sufficient to detect enhancement of a constitutively expressed immune reactivity. The standard immunologic approach for addressing this problem is a paired analysis of the frequency of CTL precursors.18 However, extensive work in our laboratory failed to reliably detect anti-MART-127–35 CTL precursors in standard limiting dilution assays, although it could quantitate anti-Flu M158–66 precursor frequencies (unpublished observations). To monitor the effects of the MART-127–35 vaccine on CTL activity, we compared epitope-specific reactivity between bulk PBMC cultures. With this assay, we were able to identify a significant proportion of patients in whom specific anti–MART-127–35 reactivity was enhanced by the vaccination. The differences in reactivity were detectable by a cytokine release assay and, to a lesser extent, by a cytotoxicity assay. Although the T-cell phenotype of the cultures was non-predictive of specificity, differences in the CD4+:CD8+ ratio could have accounted for variability in the detection of CTL reactivity,11,19 particularly in patient #2, in whom only 9% of cultured cells were CD8+. Testing the CD8+ subpopulation may have resulted in stronger reactivity but would not have explained the reason for the relatively poor proliferation of CTL effectors (in this patient, the same results were obtained in three separate attempts).
The data obtained in the first eight patients suggested that serial vaccine administration could boost the detectability of cytotoxicity in vitro, as observed by Vitiello et al in five individuals immunized with HBVc (500-μg dose).7 Analysis of PBMC obtained at a longer interval from the completion of the vaccination course was not possible because the patients were subsequently enrolled in other antimelanoma protocols. Therefore, no data are provided by this study regarding the long-term detectability of T-cell sensitization in the periphery. Murine models predict that the effects of peptide vaccines subside quickly in the periphery; however, enhancement of CTL reactivity can be detected for years in splenocyte cultures.7
No correlation was noted between CTL activation and vaccine doses of 0.1 to 10 mg of peptide. This is only apparently in contrast with the dose–effect relationship noted with HBVc vaccination by others.7 For the HBVc lipopeptide to function, it requires complex interactions with its host: the peptide needs to be incorporated and cleaved in vivo by antigen-presenting cells for it to associate with the HLA class I molecules. This process may not be very efficient and on a molar basis may require a larger amount of immunogen to reach a clinically relevant threshold. Vitiello et al7 did not detect CTL activation at vaccine doses lower than 50 μg. Detectable T-cell activation was noted at doses of 50 to 500 μg, and five out of five patients responded to the latter dose. In the MART-127–35 protocol, the lowest dose of peptide used was 100 μg, and four of four patients showed sensitization at that dose by cytokine release assay. This dose is comparable, on a molar basis, to the highest dose in the HBVc protocol, considering that the molecular weight of the HBVc lipopeptide is manyfold larger than that of the MART-127–35 nonamer. Lack of further enhancement of in vivo T-cell activation with higher doses of peptide could be a result of biochemical variables affecting its tissue distribution, solubility, and absorption rate or of a dose-independent refractoriness of individual patients to sensitization against a “self” epitope. Individual heterogeneity in CTL reactivity in response to vaccination is consistent with previous human7,11,16 and simian20 studies. Multiple phenomena could account for these differences, including variability of expression of HLA21 or MAA22 by tumor cells or individual exposure to MART-1 analogs expressed by environmental or endogenous sources (unpublished observations).
The data presented here are, to the best of our knowledge, the first demonstration that in vivo administration to humans of an MAA peptide emulsified in IFA is sufficient to stimulate CTL reactivity in the peripheral circulation. The finding that the parenteral administration of a T-cell epitope to a host chronically exposed to the same epitope expressed by metastatic lesions can incrementally enhance peripheral immunity suggests that the immunogenicity of tumor lesions may be limited qualitatively or quantitatively and underscores the legitimacy of immunization to enhance tumor reactivity in vivo. The enhancement of CTL reactivity did not achieve tumor regression. Since MAA are self-proteins, peripheral tolerance may account for the discrepancy observed between in vitro and in vivo CTL reactivity. Furthermore, heterogeneity in HLA and MAA expression by tumors could have allowed escape from CTL recognition.21 It is possible, however, that the CTL reactivity stimulated by the vaccine was not quantitatively sufficient to cause tumor regression. Murine data suggest that administration of peptide alone is weakly immunogenic compared with approaches in which helper T-cell epitopes and fatty acids7 or signal sequences23 are included in fusion peptides or viral vectors.24 Preclinical data have also shown that intravenous or intramuscular administration is more effective than the subcutaneous route and that the concomitant stimulation of the host with IL-2 or other cytokines during vaccination significantly enhances the immunogenicity of the peptide in animal models.24 It is possible that the use of recombinant immunogens with increased immunomodulatory capabilities in future clinical trials could reach the threshold of CTL activation necessary to cause tumor regression.
Footnotes
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
For related commentary, see page 4.
References
- 1.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Nad Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas [see comments] J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brichard V, Van Pel A, Wolfel T, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Nad Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Bruggen P, Bastin J, Gajewski T, et al. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 6.Kast WM, Roux L, Curren J, et al. Protection against letfial Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Nad Acad Sci USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello A, Ishioka G, Grey HM, et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I Induction of a primary cytotoxic T lymphocyte response in humans. J Clin Invest. 1995;95:341–349. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noguchi Y, Richards EC, Chen YT, et al. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Nad Acad Sci USA. 1995;92:2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami Y, Eliyahu S, Sakaguchi K, et al. Identification of me immunodominant peptides of me MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens EJ, Jacknin L, Robbins PF, et al. Generation of tumor-specific CTLs from melanoma patients by using peripheral blood stimulated with allogeneic melanoma tumor cell lines. Fine specificity and MART-1 melanoma antigen recognition. J Immunol. 1995;154:762–771. [PubMed] [Google Scholar]
- 11.Rivoltini L, Kawakami Y, Sakaguchi K, et al. Induction of tumor reactive CTL from peripheral blood and tumor infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J Immunol. 1995;154:2257–2265. [PubMed] [Google Scholar]
- 12.Salgaller ML, Afshar A, Marincola FM, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by peripheral blood lymphocytes stimulated in vitro with synthetic peptides. Cancer Res. 1995;55:4972–4979. [PubMed] [Google Scholar]
- 13.Marincola FM, Shamamian P, Alexander RB, et al. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J Immunol. 1994;153:1225–1237. [PubMed] [Google Scholar]
- 14.Rivoltini L, Loffus DJ, Barracchini KC, et al. Binding and presentation of peptides derived from melanoma antigens MART-1 and gp100 by HLA-A2 subtypes: implications for peptide-based immunotherapy. J Immunol. 1996;156:3882–3891. [PubMed] [Google Scholar]
- 15.Wei ML, Cresswell P. HLA-A2 molecules in an antigen processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 16.Marincola FM, Rivoltini L, Salgaller ML, Player M, Rosenberg SA. Differential anti-MART-1/MelanA CTL activity in peripheral blood of HLA-A2 melanoma patients in comparison to healthy donors: evidence for in vivo priming by tumor cells. J Immunother. 1996;19:266–277. doi: 10.1097/00002371-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Rivoltini L, Barracchini KC, Viggiano V, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149–3157. [PMC free article] [PubMed] [Google Scholar]
- 18.Coulie PG, Somville M, Lehmann F, et al. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int J Cancer. 1992;50:289–297. doi: 10.1002/ijc.2910500220. [DOI] [PubMed] [Google Scholar]
- 19.Schwartzentruber DJ, Topalian SL, Mancini M, Rosenberg SA. Specific release of granulocyte-macrophage colony stimulating factor, tumor necrosis factor-alpha and interferon gamma by human tumor infiltrating lymphocytes after autologous tumor stimulation. J Immunol. 1991;146:3674–3681. [PubMed] [Google Scholar]
- 20.Miller MD, Gould-Fogerite S, Shen L, et al. Vaccination of rhesus monkeys with synthetic peptide in a fusogenic proteoliposome elicits simian immunodeficiency virus-specific CD8+ cytotoxic T lymphocytes. J Exp Med. 1992;176:1739–1744. doi: 10.1084/jem.176.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 22.Marincola FM, Hijazi YM, Fetsch P, et al. Analysis of expression of tfie melanoma associated antigens MART-1 and gplOO in meta-static melanoma cell lines and in situ lesions. J Immunother. 1996;19:192–205. doi: 10.1097/00002371-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Minev BR, McFarland BJ, Spiess PJ, Rosenberg SA, Restifo NP. Insertion signal sequence fused to minimal peptides elicits specific CD8+ T-cell responses and prolongs survival of thymoma-bearing mice. Cancer Res. 1994;54:4155–4161. [PMC free article] [PubMed] [Google Scholar]
- 24.Restifo NP. Recombinant anti-cancer vaccines. Cancer J Sci Am. 1996;2:16–18. [PubMed] [Google Scholar]