Abstract
Condensed abstract
In a clinical follow-up study of 74 young adult survivors of childhood acute lymphoblastic leukemia (mean age 30 years), BMD of 1 SD or more below the mean was substantially more prevalent in males than in females and was strongly associated with short height. GH insufficiency, low IGF-I Z-score, and current smoking were also suggestive risk factors for low BMD.
Background
The purpose of this study was to determine the prevalence of low bone mineral density (BMD), i.e. osteopenia, and identify factors associated with low BMD in young adult survivors of childhood acute lymphoblastic leukemia (ALL).
Methods
Dual energy X-ray absorptiometry was used to evaluate BMD in 74 randomly selected long-term childhood ALL survivors initially treated in Minneapolis/St. Paul, USA. Growth hormone (GH) releasing hormone-arginine stimulation testing was conducted to evaluate peak GH level, and insulin-like growth factor I (IGF-I) and other markers of endocrine functioning were also evaluated in relation to BMD.
Results
Mean age at interview was 30 years and mean time since diagnosis was 24 years. Low BMD (Z-score ≤ −1) was present in 24% of subjects, including one with osteoporosis. Low BMD was substantially more prevalent in males than in females and was strongly associated with short height. The mean height Z-score for those with low BMD was −1.44, compared with a height Z-score of −0.39 (p<.01) for those with normal BMD. GH insufficiency, low IGF-I Z-score, and current smoking were also suggestive risk factors for low BMD.
Conclusions
In this long-term follow-up of childhood ALL survivors, low BMD was more prevalent than expected based on population normative data, specifically in males. The health consequences of early onset BMD problems in childhood ALL survivors need to be carefully monitored.
Keywords: Adverse treatment effects, Metabolic bone diseases, Neoplasms, Survivorship
Introduction
There are many known risk factors for low bone mineral density (BMD) and its associated morbidity in adults. These include advancing age, sedentary life style, low calcium intake, vitamin D deficiency, hyperparathyroidism, smoking, white or Asian race, and onset of menopause.1 Childhood acute lymphoblastic leukemia (ALL), the most common pediatric malignancy,2 is also a potential risk factor for low BMD among adults who survive their disease and its treatment.3–7 The ALL disease process may alter bone mineralization, as evidenced by a high proportion of children with ALL found to have low 1,25 (OH)2 D3 and osteocalcin levels or hypercalciuria at diagnosis.4, 8 In addition, high-dose glucocorticoids and intrathecal methotrexate, included in almost all ALL treatment regimens, can affect bone formation, at least temporarily, by altering osteoblastic activity and proliferation.3, 7, 9–13 Many current adult survivors of ALL were treated with cranial radiation, which also affects bone growth and repair. Radiation associated damage to the hypothalamic-pituitary axis can result in GH deficiency and hypogonadotropic/hypergonadotropic hypogonadism.5, 14–19 Long term endocrine abnormalities affect bone growth during childhood and the process of bone turnover and repair necessary for bone maintenance during adult life. Finally, the suboptimal activity and nutrition that these children face during their 2- to 3-year treatment course are likely to reduce optimal bone formation during developmentally critical time periods.12, 20
The possibility that adult growth hormone (GH) status among survivors of childhood ALL may be important to long-term bone health is of particular interest. Both GH and its byproduct, insulin-like growth factor I (IGF-I), are important regulators of the longitudinal growth of bone, and the process by which adult bone is maintained.21, 22 GH and IGF-I appear to directly stimulate the proliferation and function of osteoblasts and chondrocytes.22 Chen et al. performed hypophysectomies in rats, and, even after replacing thyroid and glucocorticoid hormones, found decreased bone volume, trabecular number, and trabecular thickness.22, 23 In another study, the same group of authors added GH to the replacement regimen following the hypophysectomies, preventing loss in cortical and cancellous bone.24
Low BMD is prevalent in individuals with either child- or adult-onset GH deficiency (GHD), and is particularly problematic for those individuals whose GHD onset occurred when they were children.25, 26 Replacement during childhood does not completely alleviate the problem. Even children with GHD who were treated optimally with GH until they reached final adult height had BMD values 0.5 standard deviations below age-matched means as adults.27 This may be because bone mass continues to accrue until the late twenties or early thirties.22 Although the impact of low BMD during early adulthood on long-term morbidity is not yet known, it seems possible that an early uncompensated BMD deficit may increase an individual’s risk for early fracture,27 long before it is expected with natural aging.
In a previous evaluation completed by our group, we reported that 64% of young adult survivors of childhood ALL had either insufficient or deficient responses to GH stimulation testing.28 Because we also measured BMD in these individuals, and because our participants had a mean age of 30 years, the age range at which accrual of bone mass is thought to cease, we had a unique opportunity to also examine the association between GHD and BMD in this cohort. The primary aim of this analysis was to determine the prevalence of reduced BMD in young adult survivors of childhood ALL. The second aim was to establish if BMD deficits were associated with ALL treatment factors, including cranial radiation, or treatment-related factors such as GH deficiency or insufficiency. Since IGF-I is important in bone formation and repair, we also examined if reduced IGF-I levels were associated with lower BMD.
Methods
As described in greater detail previously,28 we recruited 75 active participants from the Childhood Cancer Survivor Study29 who were treated for ALL at either the University of Minnesota Children’s Hospital or Children’s Hospitals and Clinics of Minneapolis/St. Paul, USA and diagnosed before age 21 years. From 207 eligible ALL survivors, potential subjects were stratified by past cranial radiation treatment (no cranial radiation, < 24 Gy, ≥ 24 Gy) and 25 subjects from each treatment group were recruited using a block randomization sampling scheme designed to minimize the potential for favoring inclusion of ‘fast responders’. Twenty-nine subjects (14%) actively or passively refused, 22 (10.6%) were lost to follow-up, 10 (4.8%) agreed to participate but were never scheduled because accrual in their block was met, and 71 (34.3%) were never contacted because their random number was not reached in our sampling scheme before the final recruitment sample size of 75 was reached. Written informed consent was obtained for each subject as approved by the Human Subjects Review Committees at the participating hospitals.
Biochemical tests were conducted in the fasting state in the General Clinical Research Center at the University of Minnesota. The GH-releasing hormone with arginine stimulation test (GHRH/ARG) for measuring peak GH secretion30, 31 was completed for 72 of the 75 participants. GH-releasing hormone was administered at one mcg/kg as an intravenous bolus followed immediately by an intravenous infusion of arginine HCL (0.5 grams/kg, maximum 30 grams) given over 30 minutes. GH levels were obtained at 30, 40, 60, 90 and 120 minutes after completion of the arginine infusion. Consistent with previous studies using the GHRH/ARG stimulation test,31, 32 peak GH level was categorized into three qualitative categories: normal GH, > 16.5 μg/L; GH insufficient, 9–16.5 μg/L; and GH deficient, < 9 μg/L. Insulin, IGF-I, thyroxine and thyroid stimulating hormone were measured with chemiluminescent immunoassays.33 Three subjects had missing data for IGF-I (not the same three subjects with a missing GH study). The chemiluminescent immunoassays for IGF-I were performed at ARUP Laboratories. Standardized IGF-I Z-scores were calculated based on the participant’s age and sex according to the method described by Brabant34 and used by ARUP.35 This method uses a cutoff of < 2 SD below the predicted mean based on a mathematical modeling method to define abnormally low IGF-I.34 The cutoff values for low IGF-I using this method are considerably lower than other published standards, such as Esoterix.36
BMD was measured by dual energy X-ray absorptiometry (DXA) (GE Lunar, Madison, WI, Prodigy software version 6.7), which provided Z-scores standardized to sex, age, weight, and race/ethnicity for femoral neck, lumbar spine (L2-L4) and total body. Leukemia treatment data were abstracted from medical records as part of the Childhood Cancer Survivor Study.29 The official position of the International Society for Clinical Densitometry states that the World Health Organization classifications (T-scores) for osteoporosis and osteopenia are not applicable to premenopausal women or to men, and recommended instead that Z-scores be used.37 Therefore, using the lowest Z-score of the femoral neck, lumbar region, or total body, we defined low BMD as a Z-score ≤ −1, and osteoporosis as a Z-score ≤ −2.5, compared to individuals of the same sex, age, weight, and race, at one or more of the three measures.
Published data from the National Health and Nutrition Examination Study (NHANES) were used to define age- and sex-specific normative values for height in non-Hispanic white Americans;38 from these data a height Z-score was calculated for each study participant. The frequency and proportions of low BMD were calculated across a number of categorical variables and compared using the χ2-test. Unadjusted odds ratios and corresponding 95% confidence intervals were estimated from logistic regression models. Differences in continuous variables were analyzed using Student’s t-test. Pearson correlation analyses were used to estimate linear associations between variables.
Results
We previously showed that there were no statistically significant differences between the 75 subjects in the study and the remaining cohort of 132 ALL survivors presumed eligible to participate, according to sex, age at diagnosis or interview, body mass index (BMI), years of survival, or chemotherapies received.28
One male subject was too large to fit in the DXA machine and was not included in this analysis. Table 1 shows mean values, stratified by sex and low BMD status, for clinical variables among the 74 subjects who underwent a DXA scan. The mean age at interview was 30 years and the mean time since diagnosis was 24 years. Neither time factor differed by sex. Male ALL survivors in the study were considerably shorter on average than same-age white males based on NHANES population norms (mean height Z-score = −1.06), with only a minor difference in average height among female subjects relative to female population norms (mean Z-score = −0.35). Male subjects also had a lower mean peak GH level (18.5 μg/L) than did female subjects (34.8 μg/L; p=0.06). Overall, 18 subjects (24%) had a BMD that was 1 SD or more below the mean, including one whose BMD was ≤ −2.5 SD.
TABLE 1.
Demographics and Study Variables by Gender and Low BMD Status
| Total
(n=74) |
Males
(n=31) |
Females
(n=43) |
Normal BMD* (n=56) |
Low BMD* (n=18) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | p | Mean | (SD) | Mean | (SD) | p | |
| Age at interview (years) | 30.04 | (7.23) | 30.84 | (7.39) | 29.45 | (7.14) | 0.42 | 29.48 | (7.07) | 31.75 | (7.65) | 0.25 |
| Age at diagnosis (years) | 5.64 | (4.34) | 6.05 | (5.07) | 5.34 | (3.77) | 0.49 | 5.37 | (4.39) | 6.47 | (4.21) | 0.35 |
| Survival time (years) | 24.4 | (4.84) | 24.79 | (4.86) | 24.11 | (4.87) | 0.56 | 24.11 | (4.75) | 25.28 | (5.17) | 0.38 |
| Height (cm) | 166.01 | (9.06) | 171.84 | (7.68) | 161.8 | (7.57) | <0.01 | 166.77 | (8.77) | 163.62 | (9.77) | 0.20 |
| Height Z-score** | −0.65 | (1.22) | −1.06 | (1.33) | −0.35 | (1.04) | 0.01 | −0.39 | (1.13) | −1.44 | (1.15) | <0.01 |
| Weight (kg) | 74.18 | (17.27) | 78.72 | (17.60) | 70.90 | (16.45) | 0.05 | 74.72 | (16.41) | 72.47 | (20.13) | 0.63 |
| Body mass index (kg/m2) | 26.87 | (5.85) | 26.50 | (5.00) | 27.14 | (6.43) | 0.64 | 26.90 | (5.80) | 26.79 | (6.18) | 0.95 |
| Percent body fat | 33.22 | (11.07) | 26.08 | (8.15) | 38.36 | (10.04) | <0.01 | 33.53 | (10.96) | 32.24 | (11.67) | 0.67 |
| Fat mass (kg) | 25.12 | (12.17) | 21.36 | (10.3) | 27.82 | (12.8) | 0.02 | 25.42 | (11.99) | 24.19 | (13.03) | 0.71 |
| Lean mass (kg) | 45.36 | (10.60) | 53.74 | (9.14) | 39.32 | (6.81) | <0.01 | 45.4 | (10.38) | 45.23 | (11.55) | 0.95 |
| Bone mass (kg) | 2.71 | (0.57) | 3.03 | (0.57) | 2.47 | (0.45) | <0.01 | 2.79 | (0.55) | 2.43 | (0.54) | 0.02 |
| Thyroxine (ng/dL) | 1.07 | (0.17) | 1.05 | (0.15) | 1.07 | (0.18) | 0.60 | 1.06 | (0.18) | 1.10 | (0.13) | 0.38 |
| TSH (mU/L)† | 2.58 | (3.96) | 2.08 | (0.97) | 2.95 | (5.15) | 0.29 | 2.71 | (4.53) | 2.18 | (1.05) | 0.42 |
| Insulin (mU/L) | 8.11 | (7.92) | 6.68 | (4.35) | 9.14 | (9.64) | 0.14 | 8.21 | (8.11) | 7.78 | (7.53) | 0.84 |
| Peak growth hormone (μg/L) | 28.13 | (35.13) | 18.53 | (29.93) | 34.76 | (37.21) | 0.06 | 31.07 | (36.4) | 18.79 | (29.78) | 0.21 |
| IGF-I Z-score (ARUP) | −0.75 | (0.88) | −0.59 | (0.88) | −0.88 | (0.87) | 0.17 | −0.66 | (0.81) | −1.04 | (1.05) | 0.12 |
| IGF-I Z-score (Esoterix) | −1.23 | (0.76) | −1.19 | (0.77) | −1.27 | (0.76) | 0.67 | −1.14 | (0.74) | −1.51 | (0.77) | 0.08 |
| Bone mineral density Z-scores | ||||||||||||
| Femoral neck | 0.18 | (1.07) | 0.14 | (1.26) | 0.21 | (0.93) | 0.77 | 0.55 | (0.90) | −0.97 | (0.70) | <0.01 |
| Lumbar (L2-L4) | 0.13 | (1.14) | 0.13 | (1.38) | 0.14 | (0.96) | 0.96 | 0.54 | (0.97) | −1.12 | (0.60) | <0.01 |
| Total body | 0.34 | (1.05) | 0.46 | (1.16) | 0.25 | (0.97) | 0.41 | 0.65 | (0.95) | −0.63 | (0.70) | <0.01 |
| Lowest Z-score (any site) | −0.25 | (1.02) | −0.26 | (1.21) | −0.24 | (0.86) | 0.96 | 0.14 | (0.83) | −1.44 | (0.48) | <0.01 |
SD indicates standard deviation; TSH, thyroid stimulating hormone; IGF-I, insulin-like growth factor 1
Low bone mineral density defined as a Z-score ≤ −1; normal bone mineral density defined as a Z-score of > −1
Calculated with age- and sex-specific NHANES norms for non-Hispanic whites
Mean height Z-score was the only characteristic or biological value that statistically differed at a type I error probability of 5% between those with vs. without low BMD. The mean height Z-score was −1.44 for subjects with low BMD compared with −0.39 (p<.01) for those without low BMD. The Z-scores for BMD by femoral neck, L2-L4 lumbar spine, and total body are also shown in Table 1. Matched by sex, age, weight, and race/ethnicity, the lowest mean BMD Z-score for those with low BMD was in the lumbar spine (−1.12), and the mean Z-score for the lowest of the three site scores was −1.44.
The distribution of selected demographic and clinical characteristics is presented in Table 2, focusing on the relative proportion with low BMD. Low BMD was present in 40% of current smokers compared with 20% of non-smokers (p=0.17). A substantially higher proportion of low BMD was observed in males (36%) than in females (16%; p=.06), and a strong differential was seen between those with a height Z-score of ≤ −1 (48% with low BMD) compared with a height Z-score of > −1 (11% with low BMD; p<.001). Current smokers tended to have a higher likelihood of a low BMD, as did subjects with an abnormally low peak GH level or an abnormally low IGF-I level, however random variability could not be ruled out as an explanation for these latter differences. There was some indication, although not statistically precise, that cranial radiation therapy of 24 Gy may be a risk factor for low BMD. BMI category did not appear to be associated with prevalence of low BMD, nor did fasting insulin level.
TABLE 2.
Demographics and Clinical Characteristics in Participants with Low BMD
| Total No. | Low BMD* | |||||
|---|---|---|---|---|---|---|
| No. | (%) | p | OR** | (95% CI) | ||
| Sex | 0.06 | |||||
| Female | 43 | 7 | (16.3) | Ref | ||
| Male | 31 | 11 | (35.5) | 2.83 | (0.95–8.45) | |
| Age group at interview | 0.53 † | |||||
| 19–29 | 39 | 8 | (20.5) | Ref | ||
| 30–39 | 27 | 7 | (25.9) | 1.36 | (0.43–4.33) | |
| 40–45 | 8 | 3 | (37.5) | 2.33 | (0.46–11.85) | |
| Year diagnosed | 0.60 | |||||
| 1970–1979 | 33 | 9 | (27.3) | 1.33 | (0.46–3.87) | |
| 1980–1986 | 41 | 9 | (22.0) | Ref | ||
| Cranial radiation treatment | 0.24 | |||||
| None | 25 | 5 | (20.0) | Ref | ||
| <24 Gy | 24 | 4 | (16.7) | 0.80 | (0.19–3.42) | |
| 24+ Gy | 25 | 9 | (36.0) | 2.25 | (0.63–8.06) | |
| Cranial radiation treatment | 0.09 | |||||
| 0–23 Gy | 49 | 9 | (18.4) | Ref | ||
| 24+ Gy | 25 | 9 | (36.0) | 2.50 | (0.84–7.44) | |
| Current smoker | 0.17 † | |||||
| No | 59 | 12 | (20.3) | Ref | ||
| Yes | 15 | 6 | (40.0) | 2.61 | (0.78–8.77) | |
| Oral contraceptives (female) | 0.39 † | |||||
| No | 27 | 3 | (11.1) | Ref | ||
| Yes | 16 | 4 | (25.0) | 2.67 | (0.51–13.88) | |
| Height Z-score†† | <0.01 | |||||
| Z-score ≤ −1 | 27 | 13 | (48.1) | 7.80 | (2.36–25.79) | |
| Z-score > −1 | 47 | 5 | (10.6) | Ref | ||
| Height Z-score†† | 0.03 † | |||||
| Z-score ≤ −2.25 | 6 | 4 | (66.7) | 7.71 | (1.28–46.50) | |
| Z-score > −2.25 | 68 | 14 | (20.6) | Ref | ||
| Body mass index | 0.74 † | |||||
| Normal (<25 kg/m2) | 34 | 9 | (26.5) | Ref | ||
| Overweight (25–29.9 kg/m2) | 18 | 3 | (16.7) | 0.56 | (0.13 – 2.38) | |
| Obese (≥30 kg/m2) | 22 | 6 | (27.3) | 1.04 | (0.31 – 3.49) | |
| Peak growth hormone | 0.47 † | |||||
| Normal (>16.5 μg/L) | 26 | 4 | (15.4) | Ref | ||
| Insufficient (9–16.5 μg/L) | 13 | 4 | (30.8) | 2.44 | (0.50–11.97) | |
| Deficient (<9 μg/L) | 32 | 9 | (28.1) | 2.15 | (0.58–8.02) | |
| Peak growth hormone‡ | 0.20 | |||||
| Normal (>16.5 μg/L) | 26 | 4 | (15.4) | Ref | ||
| Low (≤16.5 μg/L) | 45 | 13 | (28.9) | 2.23 | (0.64–7.76) | |
| IGF-I: ARUP reference range | 0.17 † | |||||
| Normal | 65 | 15 | (23.1) | Ref | ||
| Low | 6 | 3 | (50.0) | 3.33 | (0.61–18.27) | |
| IGF-I: Esoterix reference range | 0.01 | |||||
| Normal | 47 | 7 | (14.9) | Ref | ||
| Low | 24 | 11 | (45.8) | 4.84 | (1.55–15.05) | |
OR indicates odds ratio; Gy, grays; IGF-I, insulin-like growth factor 1
Low bone mineral density defined as a Z-score ≤ −1
Relative odds of having low BMD
Fisher’s exact test
Calculated with age- and sex-specific NHANES norms for non-Hispanic whites
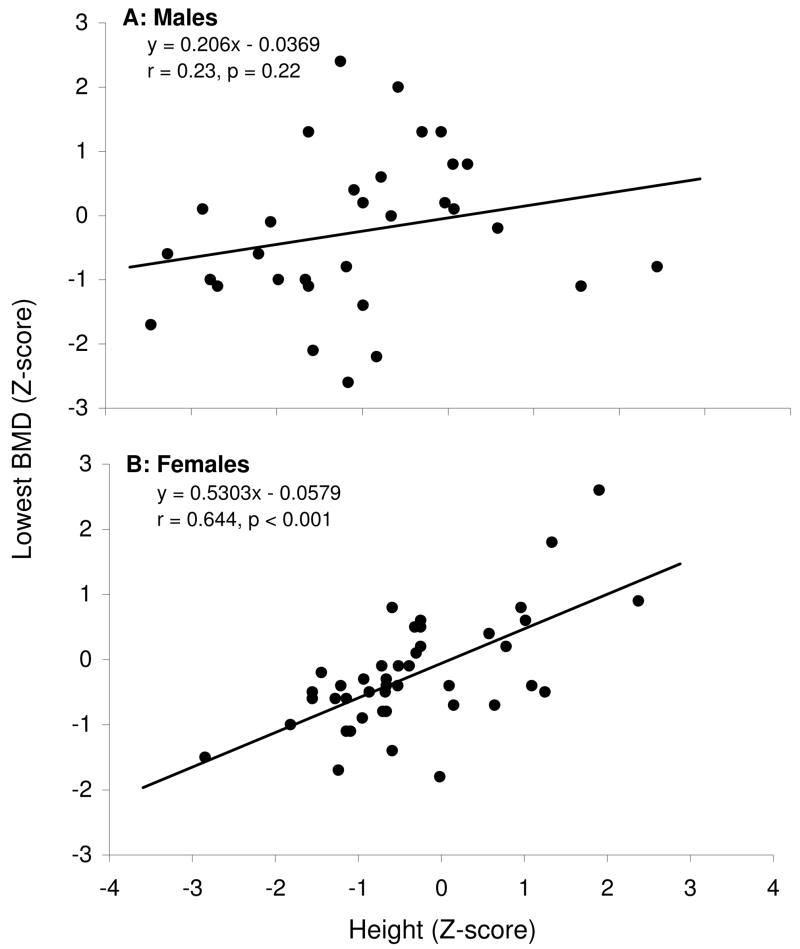
Because prevalence of low BMD appeared to differ substantially by sex and by height, we evaluated the interactive effect of sex and height Z-score on low BMD. Although males were more likely than females to be shorter than expected based on population norms (≤ −1 SD for height), the odds of having prevalent low BMD among short females relative to non-short females (OR 12.50, 95% CI 1.95–80.25) was considerably stronger than that of short males relative to non-short males (OR 4.00, 95% CI 0.81–19.82). The male-female difference in BMD (lowest Z-score of any site evaluated) by height Z-score is illustrated in the two panels of Figure 1. For females, a fairly strong linear correlation (r=0.64, R2=0.42, p<.001) between BMD and height Z-score is observed, while in men the evidence for a linear correlation was substantially weaker (r=0.23, R2=0.051, p=0.22).
Figure 1.
FIGS. 1A AND 1B: Correlation between bone mineral density and height by gender. (A) Males (B) Females
Discussion
In this long-term follow-up study of young adult survivors of childhood ALL we found that 24% of subjects had an abnormally low BMD based on DXA scan results. Several studies have been published both supporting and refuting the premise that low BMD is an important negative outcome for childhood ALL survivors because of their disease, treatment or associated effects.3, 5, 7, 19, 39, 40 Arikoski et al found decreased BMD in both the lumbar and femoral regions among 29 survivors of ALL who had received cranial irradiation who were tested approximately eight years after completion of therapy.3 Brennan et al found low BMD values among 31 adults who were examined approximately 17.8 years after ALL diagnosis.39 In contrast, Kadan-Lottick et al, even though they demonstrated decreased BMD during maintenance therapy in children treated for ALL, reported BMD recovery in the same children shortly after completion of therapy.6 Both Van der Sluis and Lequin et al also found that, even in children treated for ALL with high-dose dexamethasone and methotrexate but not cranial irradiation, they did not have any significant long-term effects on height, BMD or lean body mass and fat percentage at ten years after treatment.7, 41 Our study was one of the larger studies that included a long time period from diagnosis, and provides some support for the hypothesis that a potential serious effect of childhood ALL and its treatment is low BMD in early adulthood.
According to the World Health Organization, 11.2% of 30-year-old men and 18.8% of 30-year-old females have low BMD.42 Although manufacturer norms differ across DXA machines, thus making direct comparisons tenuous, we found an opposite gender effect: low BMD was present in a higher percentage of males compared with females (36% of males and 16% of females). Low BMD more often afflicts women than men,37, 43 although the gender difference we observed in ALL survivors has been noted previously.3, 20, 44, 45 Male subjects in our sample were not as heavy on average (based on BMI) as the female subjects, but BMI did not appear to be a risk factor for low BMD in this study. A heavier weight has arguably been considered to be a protective measure against low BMD,46 however, heavy weight may interfere with the accuracy of a DXA scan, so this phenomenon must be viewed cautiously.47
One third of our female survivors were on oral contraceptives (OCPs) for birth control. Even though it had been debated that the estrogen in the OCPs is protective for bone density,48, 49 the recent decrease in doses and variety of estrogen products may no longer be a guarantee that they are protective.50, 51 One of our male subjects (without low BMD) was on testosterone replacement. Although estrogen is considered the more important hormone in regulating bone mass in men, testosterone may have some independent effects on bone formation and resorption and provide the substrate needed for aromatization to estrogen.52 Androgen replacement is more difficult in males and usually is preceded by an extensive endocrine investigation before commencement of therapy. It is possible that some of the males who had low BMD may have an unrecognized testosterone deficiency.
Although these data provided some indication for our hypothesis that chronic GH insufficiency would be associated with low BMD (28.9% of those with a peak GH of ≤ 16.5 μg/dL, versus 15.4% of subjects with peak GH >16.5, had low BMD), we could not reliably rule out the possibility that this difference occurred due to random variability (p=.20). Because some of these survivors likely developed GH deficiency during childhood, it is possible they were unable to achieve optimal bone mass, leaving them with an inadequate skeletal mineral foundation for adult life.53
Our finding that low BMD was associated with lower mean IGF-I Z-score is similar to that reported by Reed et al, who demonstrated lower IGF-I levels in a group of adults (age 44 ± 12 years) who had idiopathic osteoporosis.54 Even though IGF-I can be influenced by many factors, including poor nutrition and acute and chronic illness,55 lower IGF-I levels could represent a problem with bone mineralization among ALL survivors, since it directly affects the function of osteoblasts.22
The results of our study should be interpreted with consideration for potential study limitations. BMD measurements from DXA are dependent on area (length and size), but do not take into account bone depth (volumetric density).56, 57 In individuals who are very short, measurements may not be reliable.56, 57 Some have advocated calculating volumetric BMD in these situations,58, 59 but since 89% of our adult survivors had a height within 2 SD of the NHANES age-, race- and sex-specific normative means38 we chose not to adjust their BMDs using bone mineral apparent density calculations. An alternative suggestion could be to use quantitative computed tomography to further assess volume and bone geometry, but this requires a significant increase in exposure to ionizing radiation and is not broadly available.57 Furthermore, even though the results may be in the osteopenic or osteoporotic range, this does not necessarily equate to bone strength.57 It is recommended by the International Society for Clinical Densitometry that the diagnosis of low BMD in younger individuals should not be decided solely on densitometric criteria since the this categorization was designed initially to classify postmenopausal white women and their risk for fracture.37 Z-scores were identified to help decipher the values for younger individuals, but the fracture risk and clinical significance has not yet been well-determined.37
Interpreting IGF-I values in our study was difficult because of the wide variability in the normative values set by individual laboratories. Our assay was completed through ARUP laboratories, whose reference values were much lower than another widely used reference laboratory, Esoterix. Based on the recommendation from Granada et al, we decided to present our IGF-I values as standard deviations which allowed a more uniform interpretation of the age- and gender-related data.34, 60 It may be helpful for clinicians who care for cancer survivors to test for IGF-I levels along with their normal screening laboratories. If IGF-I is on the lower side of normal or frankly abnormal, it may be advisable to obtain a baseline DXA scan to document BMD and start treatment with calcium and vitamin D or other therapies as necessary.
Our data were consistent with previous evidence suggesting that smoking may increase risk for low BMD.61, 62 Additionally, our results arguably provide some indication that cranial radiation exposure of ≥ 24 Gy, but not lower doses, was associated with decreased BMD (OR = 2.50 for a BMD Z-score of ≤ −1 among those who received ≥ 24 Gy vs < 24 Gy or none; 95% CI 0.84–7.44). Although this finding may be important to the bone health of current adult survivors of ALL who were treated with high doses of cranial radiation, modern ALL treatment for standard and low risk ALL no longer includes cranial irradiation63 and thus this potential risk factor is of limited relevance for most children treated today for ALL.
In conclusion, we found that 24% of our participants had a BMD of 1 SD or more below the mean, which is a higher percentage than expected based on the World Health Organization’s report.42 This could not fully be explained by their GH status or IGF-I Z-score. Shorter females appeared to have a higher risk of developing low BMD than shorter males, even though males overall had a substantially higher prevalence of low BMD than females. Further studies will need to be conducted to define the etiology of low BMD and predict risk factors for any related medical consequence for these ALL survivors as they age.
Acknowledgments
Support: NIH funding: R21-CA106778; M01-RR00400; U24-CA55727.
Contributor Information
Inas H. Thomas, Division of Pediatric Endocrinology, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
Janet E. Donohue, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
Kirsten K. Ness, St. Jude Children’s Research Hospital, Memphis, TN.
Donald R. Dengel, School of Kinesiology, University of Minnesota, Minneapolis, MN.
K. Scott Baker, Division of Hematology/Oncology/Bone Marrow Transplantation, Department of Pediatrics, University of Minnesota, Minneapolis, MN.
James G. Gurney, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
References
- 1.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(2 Suppl):S3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Gurney JG, Davis S, Severson RK, Fang JY, Ross JA, Robison LL. Trends in cancer incidence among children in the U.S. Cancer. 1996;78:532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Arikoski P, Komulainen J, Voutilainen R, et al. Reduced bone mineral density in long-term survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1998;20:234–240. doi: 10.1097/00043426-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr. 1995;126:557–564. doi: 10.1016/s0022-3476(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 5.Hoorweg-Nijman JJG, Kardos G, Roos JC, et al. Bone mineral density and markers of bone turnover in young adult survivors of childhood lymphoblastic leukaemia. Clin Endocrinol (Oxf) 1999;50:237–244. doi: 10.1046/j.1365-2265.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 6.Kadan-Lottick N, Marshall JA, Baron AE, Krebs NF, Hambidge KM, Albano E. Normal bone mineral density after treatment for childhood acute lymphoblastic leukemia diagnosed between 1991 and 1998. J Pediatr. 2001;138:898–904. doi: 10.1067/mpd.2001.113102. [DOI] [PubMed] [Google Scholar]
- 7.Lequin MH, van der Sluis IM, van Rijn RR, et al. Bone mineral assessment with tibial ultrasonometry and dual-energy x-ray absorptiometry in long-term survivors of acute lymphoblastic leukemia in childhood. J Clin Densitom. 2002;5:167–173. doi: 10.1385/jcd:5:2:167. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson SA, Halton JM, Bradley C, Wu B, Barr RD. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: Influence of disease, drugs and nutrition. Int J Cancer. 1998;Supp 11:35–39. [PubMed] [Google Scholar]
- 9.Barr RD, Halton J, Willan A, Cockshott WP, Gill G, Atkinson S. Impact of age and cranial irradiation on radiographic skeletal pathology in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30:347–350. doi: 10.1002/(sici)1096-911x(199806)30:6<347::aid-mpo8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: Implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81:3441–3447. doi: 10.1210/jcem.81.10.8855781. [DOI] [PubMed] [Google Scholar]
- 11.Robson H, Anderson E, Eden OB, Isaksson O, Shalet S. Chemotherapeutic agents used in the treatment of childhood malignancies have direct effects on growth plate chondrocyte proliferation. J Endocrinol. 1998;157:225–235. doi: 10.1677/joe.0.1570225. [DOI] [PubMed] [Google Scholar]
- 12.Sala A, Barr RD. Osteopenia and cancer in children and adolescents - the fragility of success. Cancer. 2007;109:1420–1431. doi: 10.1002/cncr.22546. [DOI] [PubMed] [Google Scholar]
- 13.Scheven BAA, Vanderveen MJ, Damen CA, et al. Effects of methotrexate on human osteoblasts in-vitro - modulation by 1,25-dihydroxyvitamin D-3. J Bone Miner Res. 1995;10:874–880. doi: 10.1002/jbmr.5650100608. [DOI] [PubMed] [Google Scholar]
- 14.Brennan BM, Rahim A, Mackie EM, Eden OB, Shalet SM. Growth hormone status in adults treated for acute lymphoblastic leukaemia in childhood. Clin Endocrinol (Oxf) 1998;48:777–783. doi: 10.1046/j.1365-2265.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 15.Gilsanz V, Carlson ME, Roe TF, Ortega JA. Osteoporosis after cranial irradiation for acute lymphoblastic-leukemia. J Pediatr. 1990;117:238–244. doi: 10.1016/s0022-3476(05)80536-0. [DOI] [PubMed] [Google Scholar]
- 16.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41:208–211. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 17.Melin AE, Adan L, Leverger G, Souberbielle JC, Schaison G, Brauner R. Growth hormone secretion, puberty and adult height after cranial irradiation with 18 Gy for leukaemia. Eur J Pediatr. 1998;157:703–707. doi: 10.1007/s004310050918. [DOI] [PubMed] [Google Scholar]
- 18.Spoudeas HA. Growth and endocrine function after chemotherapy and radiotherapy in childhood. Eur J Cancer. 2002;38:1748–1759. doi: 10.1016/s0959-8049(02)00169-7. [DOI] [PubMed] [Google Scholar]
- 19.Vassilopoulou-Sellin R, Brosnan P, Delpassand A, Zietz H, Klein MJ, Jaffe N. Osteopenia in young adult survivors of childhood cancer. Med Pediatr Oncol. 1999;32:272–278. doi: 10.1002/(sici)1096-911x(199904)32:4<272::aid-mpo6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Kaste SC, Chesney RW, Hudson MM, Lustig RH, Rose SR, Carbone LD. Bone mineral status during and after therapy of childhood cancer: An increasing population with multiple risk factors for impaired bone health. J Bone Miner Res. 1999;14:2010–2014. doi: 10.1359/jbmr.1999.14.12.2010. [DOI] [PubMed] [Google Scholar]
- 21.Hulthen L, Bengtsson BA, Sunnerhagen KS, Hallberg L, Grimby G, Johannsson G. GH is needed for the maturation of muscle mass and strength in adolescents. J Clin Endocrinol Metab. 2001;86:4765–4770. doi: 10.1210/jcem.86.10.7897. [DOI] [PubMed] [Google Scholar]
- 22.Ohlsson C, Bengtsson B-A, Isaksson OGP, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 23.Chen MM, Yeh JK, Aloia JF. Skeletal alterations in hypophysectomized rats II: A histomophometric study on tibial cortical bone. Anat Rec. 1995;241:513–518. doi: 10.1002/ar.1092410409. [DOI] [PubMed] [Google Scholar]
- 24.Chen MM, Yeh JK, Aloia JF. Histologic evidence: Growth hormone completely prevents reduction in cortical bone gain and partially prevents cancellous osteopenia in the tibia of hypophysectomized rats. Anat Rec. 1997;249:163–172. doi: 10.1002/(SICI)1097-0185(199710)249:2<163::AID-AR2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.de Boer H, Blok GJ, van Lingen A, Teule GJ, Lips P, van der Veen EA. Consequences of childhood-onset growth hormone deficiency for adult bone mass. J Bone Miner Res. 1994;9:1319–1326. doi: 10.1002/jbmr.5650090822. [DOI] [PubMed] [Google Scholar]
- 26.Holmes SJ, Economou G, Whitehouse RW, Adams JE, Shalet SM. Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab. 1994;78:669–674. doi: 10.1210/jcem.78.3.8126140. [DOI] [PubMed] [Google Scholar]
- 27.Baroncelli GI, Bertelloni S, Sodini F, Saggese G. Lumbar bone mineral density at final height and prevalence of fractures in treated children with GH deficiency. J Clin Endocrinol Metab. 2002;87:3624–3631. doi: 10.1210/jcem.87.8.8754. [DOI] [PubMed] [Google Scholar]
- 28.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 29.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 30.Biller BMK, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079. doi: 10.1210/jcem.87.5.8509. [DOI] [PubMed] [Google Scholar]
- 31.Ghigo E, Aimaretti G, Arvat E, Camanni F. Growth hormone-releasing hormone combined with arginine or growth hormone secretagogues for the diagnosis of growth hormone deficiency in adults. Endocrine. 2001;15:29–38. doi: 10.1385/ENDO:15:1:029. [DOI] [PubMed] [Google Scholar]
- 32.Link K, Moell C, Garwicz S, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004;89:5003–5012. doi: 10.1210/jc.2004-0126. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Sagrado M, Martin-Gil FJ. Population-specific reference values for thyroid hormones on the Abbott ARCHITECT i2000 analyzer. Clin Chem Lab Med. 2004;42:540–542. doi: 10.1515/CCLM.2004.091. [DOI] [PubMed] [Google Scholar]
- 34.Brabant G, von zur Muhlen A, Wuster C, et al. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: Results from a multicenter study. Horm Res. 2003;60:53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 35.ARUP Laboratories. National reference laboratory test directory [monograph online] [accessed April 30, 2008]; Available from URL: http://www.aruplab.com/guides/ug/tests/0070125.jsp.
- 36.Esoterix Laboratory Services Inc. Expected values and S.I. Unit conversion pocket book [monograph online] [accessed April 29, 2008]; Available from URL: http://www.esoterix.com/files/expected_values.pdf.
- 37.Lewiecki EM, Watts NB, McClung MR, et al. Official positions of the International Society for Clinical Densitometry. J Clin Endocrinol Metab. 2004;89:3651–3655. doi: 10.1210/jc.2004-0124. [DOI] [PubMed] [Google Scholar]
- 38.McDowell M, Fryar C, Hirsch R, Ogden C. Anthropometric reference data for children and adults: U.S. Population, 1999–2002. Hyattsville, MD: 2005. [PubMed] [Google Scholar]
- 39.Brennan BM, Rahim A, Adams JA, Eden OB, Shalet SM. Reduced bone mineral density in young adults following cure of acute lymphoblastic leukaemia in childhood. Br J Cancer. 1999;79:1859–1863. doi: 10.1038/sj.bjc.6690296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nysom K, Molgaard C, Holm K, Hertz H, Michaelsen KF. Bone mass and body composition after cessation of therapy for childhood cancer. Int J Cancer. 1998:40–43. [PubMed] [Google Scholar]
- 41.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, Keizer-Schrama S. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 2000;35:415–420. doi: 10.1002/1096-911x(20001001)35:4<415::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone. 2000;27:585–590. doi: 10.1016/s8756-3282(00)00381-1. [DOI] [PubMed] [Google Scholar]
- 43.Melton LJ., 3rd The prevalence of osteoporosis. J Bone Miner Res. 1997;12:1769–1771. doi: 10.1359/jbmr.1997.12.11.1769. [DOI] [PubMed] [Google Scholar]
- 44.Tillmann V. Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res. 2002;17:1073–1080. doi: 10.1359/jbmr.2002.17.6.1073. [DOI] [PubMed] [Google Scholar]
- 45.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: Frequency of occurrence and risk factors for their development. Leukemia. 2001;15:728–734. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 46.Frost HM. Obesity, and bone strength and “mass”: A tutorial based on insights from a new paradigm. Bone. 1997;21:211–214. doi: 10.1016/s8756-3282(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 47.Bolotin HH. DXA in vivo BMD methodology: An erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41:138–154. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Cromer BA. Effects of hormonal contraceptives on bone mineral density. Drug Saf. 1999;20:213–222. doi: 10.2165/00002018-199920030-00002. [DOI] [PubMed] [Google Scholar]
- 49.Martins SL, Curtis KM, Glasier AF. Combined hormonal contraception and bone health: A systematic review. Contraception. 2006;73:445–469. doi: 10.1016/j.contraception.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221–224. doi: 10.1016/0010-7824(95)00036-a. [DOI] [PubMed] [Google Scholar]
- 51.Reed SD, Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Longitudinal changes in bone density in relation to oral contraceptive use. Contraception. 2003;68:177–182. doi: 10.1016/s0010-7824(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 52.Khosla S, Melton LJ, Riggs BL. Clinical review 144 - estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87:1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 53.Nussey SS, Hyer S, Brada M, Leiper A. Bone mineralization after treatment of growth-hormone deficiency in survivors of childhood malignancy. Acta Paediatr. 1994;83:9–15. doi: 10.1111/j.1651-2227.1994.tb13276.x. [DOI] [PubMed] [Google Scholar]
- 54.Reed BY, Zerwekh JE, Sakhaee K, Breslau NA, Gottschalk F, Pak CY. Serum IGF 1 is low and correlated with osteoblastic surface in idiopathic osteoporosis. J Bone Miner Res. 1995;10:1218–1224. doi: 10.1002/jbmr.5650100812. [DOI] [PubMed] [Google Scholar]
- 55.Rosenbloom A, Connor EL. Hypopituitarism and other disorders of the growth hormone-insulin-like growth factor-I axis. In: Lifshitz F, editor. Pediatric endocrinology. 5. Vol. 2. New York: Informa Healthcare USA; 2007. pp. 65–100. [Google Scholar]
- 56.Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI. Discrepancies in pediatric bone mineral density reference data: Potential for misdiagnosis of osteopenia. J Pediatr. 1999;135:182–188. doi: 10.1016/s0022-3476(99)70020-x. [DOI] [PubMed] [Google Scholar]
- 57.Cowell CT, Wuster C. The effects of growth hormone deficiency and growth hormone replacement therapy on bone - a meeting report. Horm Res. 2000;54:68–74. doi: 10.1159/000063451. [DOI] [PubMed] [Google Scholar]
- 58.Binkovitz LA, Henwood MJ, Sparke P. Pediatric dual-energy x-ray absorptiometry: Technique, interpretation, and clinical applications. Semin Nucl Med. 2007;37:303–313. doi: 10.1053/j.semnuclmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 60.Granada ML, Ulied A, Casanueva FF, et al. Serum IGF-I measured by four different immunoassays in patients with adult GH deficiency or acromegaly and in a control population. Clin Endocrinol (Oxf) 2007 doi: 10.1111/j.1365-2265.2007.03120.x. [DOI] [PubMed] [Google Scholar]
- 61.Krall EA, Dawson-Hughes B. Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res. 1999;14:215–220. doi: 10.1359/jbmr.1999.14.2.215. [DOI] [PubMed] [Google Scholar]
- 62.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: Recognition of a major effect. BMJ. 1997;315:841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]