Abstract
Subsets of nucleus accumbens neurons process information about operant responses for drug as well as natural rewards (food and water) by excitations and inhibitions in firing rate time-locked to the operant response. The degree to which ensembles of neurons exhibit similar firing patterns when encoding cues and operant responses across different reinforcer conditions will provide critical information regarding the functional organization of this nucleus. The present experiment evaluated the relative contribution of subsets of accumbens neurons that encode distinct features of lever press responding for ethanol versus water. Electrophysiological recordings (n = 153 neurons) were made in the accumbens of rats trained on concurrent reinforcement schedules for ethanol and water throughout a self-administration session. During operant responding, 52% of neurons exhibited patterned discharges characterized by significant increases or decreases in firing rate ±1 s relative to lever presses for ethanol and/or water. Of these phasic cells, 85% discriminated between presses for ethanol and water (i.e., exhibited firing patterns unique to one reinforcer type), while 15% exhibited identical firing patterns relative to lever presses for both reinforcers. Notably, the data revealed that both high ethanol preference and spatially distinct lever positions contributed to the reinforcer specificity. Together, these data demonstrate that subsets of nucleus accumbens neurons encode conditioned and instrumental aspects of ethanol versus water reinforcement in well-trained rats, and that reinforcer preference and spatial cues are important components of this differential information processing.
Keywords: electrophysiology, conditioning, rat, self-administration, preference
INTRODUCTION
The nucleus accumbens (NAc) is an important neural substrate of goal-directed behaviors, including drug self-administration. Indeed, electrophysiological recordings reveal characteristic firing patterns in subsets of NAc neurons during operant responding for food and water (e.g., Apicella et al., 1991; Carelli et al., 2000) as well as cocaine (e.g., Carelli et al., 1993; Bowman et al., 1996; Peoples & West, 1996), heroin (Chang et al., 1998) and ethanol (Janak et al., 1999). These firing patterns include significant increases and/or decreases in firing rate that are transient and time-locked to the operant response or to conditioned cues (Carelli, 2002). Recordings made during multiple-schedule operant sessions found that a majority of those NAc cells that were phasically active relative to lever press responding for water reinforcement exhibited similar firing patterns during responding for food (Carelli, 2002) or sucrose (Roop et al., 2002). In contrast, largely separate subsets of NAc cells are selectively activated during operant responding for natural rewards (food, water, or juice) versus cocaine (Bowman et al., 1996; Carelli et al., 2000; Carelli & Wondolowski, 2003; 2006) or cocaine versus heroin (Chang et al., 1998).
The finding that the majority of NAc neurons exhibit similar neuronal firing patterns during responding for different types of natural reinforcers (water, sucrose, food) implies that most NAc neurons do not differentiate between goal-directed behaviors for natural rewards. This is in contrast to the distinct differential firing rates observed when animals respond for a natural reward versus cocaine. The similarity of NAc encoding of goal-directed behaviors for liquid or food rewards may be due to the fact that these are consumed, typically together (Grigson, 2002), and share comparable response requirements (proceeding to the receptacle to obtain and ingest the food or water), that may be similarly encoded in the firing rates of NAc neurons. Finally, in the previous studies, rats were water and/or food deprived, inducing a metabolic drive state to ingest the natural reward that may not exist for a drug reward.
Ethanol is an addictive drug that is consumed in a manner identical to water. Thus, the present study used rats trained in ethanol self-administration to test the hypothesis that distinct subsets of NAc neurons encode information about responding for drug versus natural reinforcers, and that this difference is not simply due to variation in metabolic drive or mode of consumption. Specifically, we compared NAc encoding of operant responding for ethanol versus water (concurrent FR1) in the same rats by using electrophysiological recordings at microwire arrays. Rats were not water-restricted, removing any metabolic drive to consume the fluid. The response requirement and reward consumption was identical between the two reinforcers except for spatial location within the chamber.
MATERIALS AND METHODS
Animals
Adult male Long-Evans rats (n = 24) were purchased from Charles Rivers (Raleigh, NC, USA) and individually housed with food and water ad libitum, except during the initial three days of training, as described below. Rats were maintained on a 12:12 hour light:dark cycle with lights on at 07:00 h. All procedures were conducted in the light phase and in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Ethanol self-administration training
Each self-administration session was conducted in a custom-built Plexiglas chamber positioned in a sound-attenuating chamber (Med Associates, St. Albans, VT, USA). Two retractable levers (Coulbourn Instruments, Allentown, PA, USA) were on either side of one wall, and two fluid dispensing cups were positioned between the levers. A cue light was 4 cm above each lever. A house light was located on the wall opposite the levers. A white noise generator and an exhaust fan were on during each session. At the start of each session, the house light illuminated and 30-s later both levers extended into the cage. When a lever was pressed, four events occurred simultaneously: fluid (0.1 ml) was dispensed into the adjacent cup, the cue light above the lever was illuminated, the house light extinguished, and both levers retracted. The length of time for cue illumination and lever retraction was 5 s during early training and was gradually increased to 12 s for experiments. Sessions were run once per day on Monday –Friday.
Rats were > 60 days of age when training began for concurrent operant access to ethanol and water (Samson, 1986; Rassnick et al., 1992; Hodge et al., 1993). For three days, rats’ access to water was restricted to 1 – 3 hours in the training chamber followed by 1-hour access to a water bottle. During these three initial training sessions, rats obtained 20% sucrose (w/v) on an FR1 schedule when either of two levers were pressed. Thereafter, unrestricted access to water in the home cage was restored and all training sessions were 30-min duration. In the next six training sessions, the reinforcing solution (10% sucrose alone or with increasing concentrations of ethanol) was available on one lever only while the other lever was inactive (responses were recorded, but no cues or fluid were delivered). The active lever alternated daily during these sessions to teach the rat to use both levers. Thereafter, water was available at responses on one lever, while a sucrose and ethanol solution was available on the other lever; the position of these levers alternated daily (Rassnick et al., 1992). The entire sucrose fading period lasted ~ 21 days, during which time the ethanol concentration increased in the solution to 10% (w/v) and the concentration of sucrose decreased to 0%. The rats were maintained on the FR1/FR1 schedule for concurrent water and 10% ethanol reinforcement for an additional 4 – 6 weeks to stabilize operant behavior before surgery.
Surgery
Rats were anesthetized with either xylazine (20 mg/kg) and ketamine (10 mg/kg, with supplemental doses as needed) or with 2% isoflurane and secured in a stereotaxic frame. Arrays of 8 microwires (stainless steel, Teflon coated, 50 μm diameter; NB Labs, Denison, TX, USA) were implanted bilaterally in the NAc, with one array aimed at the core (centered at 1.7 mm anterior and 1.3 mm lateral from bregma) and the other at the contralateral shell (centered at 1.7 mm anterior and 0.8 mm lateral from bregma). The microwires within each array were aligned in a 1 × 8 or a 2 × 4 configuration, with ~ 0.5 mm tip separation. A ground wire attached to the array was implanted 2 – 3 mm in the cortex posterior to bregma and also wrapped around a skull screw. The array assembly was secured with cranioplastic cement, and the rat recovered for one week before resumption of self-administration training.
Electrophysiological recording
After surgery, rats underwent self-administration sessions in an operant chamber identical to the training chambers except that it was positioned in a Faraday cage. The electrophysiological tether was introduced over 2 – 3 days, and within two weeks self-administration behavior was again at pre-surgery performance levels. On the day of the experiment, the rat was placed in the electrophysiological chamber and the tether/headstage assembly (Plexon, Inc., Dallas, TX, USA) was connected to the electrode arrays. The animal waited in the darkened chamber with the fan and white noise on for 20 min while cells recorded from the electrodes were discriminated (see below). Next, rats had access to 10% ethanol (w/v) and water on concurrent FR1 schedules (as described above) for 30 min, after which the house light, white noise and fan turned off. Behavior was observed via a camera mounted on the top of the operant chamber connected to an external video monitor.
The electrophysiological procedures were conducted as previously described (Carelli et al., 2000). Briefly, the headstage was attached to a commutator (MedAssociates, Inc. or Crist Instrument Co., Inc., Hagerstown, MD, USA) via a flexible tether, which allowed the animal free access to the operant chamber. One electrode from each array which displayed no neuronal spike activity was designated as a differential electrode for other electrodes with neuronal activity in order to remove movement-associated electrical noise. Online isolation and discrimination of neuronal spike activity was conducted by using a multichannel acquisition processor (MAP system; Plexon, Inc.). Waveforms from individual neurons were discriminated with a template analysis procedure and principle component analysis by using Sort Client software (Plexon, Inc.). Cell discrimination was further conducted after data collection with principle component analysis in Offline Sorter software (Plexon, Inc.).
Data analysis
All cell type classifications were based on statistical analysis of firing rates. Changes in firing were evaluated by z-scores calculated as (X – X̄)/s, where X is the mean firing rate of the target window, X̄ is the mean firing rate during the baseline period, and s is the standard deviation of the baseline period. The target windows and baseline periods varied for each cell type, as described below. Changes in firing rates were deemed significant when the calculated z-score had a probability of < 0.05. Data are presented as mean ± SEM.
For evaluation of phasic firing patterns during operant responding, firing rates of individual NAc neurons were averaged across trials around the lever press and represented by perievent histograms using NeuroExplorer software (Nex Technologies, Littleton, MA). The analysis window was ± 6 s around the lever press, which was subdivided into baseline (−6 to −2 s before the lever press), pre-response (−1 to 0 s before the lever press), and reinforcer (0 to 1 s after the lever press) epochs. Based on previous studies (Carelli et al., 2000), cells were classified in the following patterns: type pre-response (PR), firing in the pre-response epoch was significantly higher than baseline firing; type reinforcement-excitation (RFe), firing in the reinforcer epoch was significantly higher than baseline firing; and type reinforcement-inhibition (RFi), average firing in the pre-response and/or reinforcer epochs were significantly lower than baseline firing. Note that firing rates time-locked to drinking were not analyzed in the present study as we did not have a time stamp for drinking in several of the experiments, but future studies will address this event.
Binomial comparisons of the proportion of phasic cells among groups (e.g., core versus shell) were made with binomial logistic regression analyses by using the GENMOD procedure in SAS (SAS Institute Inc., Cary, NC). Specifically, proportions of phasic cells into the three reinforcer-selective categories (ethanol-selective, water-selective or responsive to both reinforcers) were made and Bonferroni-corrected. These nonparametric analyses take into account the within-subject variable of multiple neurons being sampled in each rat.
Histological analysis of electrode placement
Animals were anesthetized with ≥ 1.5 g/kg urethane. A 10 – 15 μA current was passed through each microwire for 5 s. The rat was immediately perfused through the heart with saline followed by 10% formalin in saline. The brain was removed, frozen and sectioned in 40 – 50 nm coronal slices throughout the extent of the NAc. The slices were stained with potassium ferricyanide to mark the iron deposit made by the current at the microwire tip, then counterstained with thionin.
RESULTS
Self-administration behavior
Rats (n = 21) pressed 37 ± 3 times for ethanol, resulting in a self-administered dose of 0.64 ± 0.05 g/kg ethanol. This dose is typical of intake during a 30-min session (e.g., Samson, 1986; Rassnick et al., 1992; Hodge et al., 1997) and are slightly below the doses necessary for ethanol-naïve rats to exhibit ataxia (Drugan et al., 1996) and well below those required to induce loss of righting reflex (Eriksson & Sarviharju, 1984). In the same 30 min session, rats pressed 15 ± 2 times for water. Ethanol preference (responding for ethanol/total responses) was 0.70 ± 0.03, with a range of 0.45 – 0.88. As the reinforcer position switched daily, the rats did not always start pressing on the ethanol lever. The latency to first press a lever was 1.9 ± 0.4 s; 10 rats pressed the ethanol lever and 11 rats pressed the water lever first. During the operant session, rats pressed for ethanol at a faster rate as indicated by the median inter-trial interval (ethanol lever: 18 ± 2 s; water lever: 68 ± 11 s; paired t-test, t = −4.08, p < 0.001). Following each session, any fluid in the dispensing cups was measured: 17/21 ethanol and 13/21 water cups were empty. Of those cups with fluid remaining, the mean volume of ethanol was 0.11 ± 0.04 mL and that of water was 0.20 ± 0.03 mL (note that a single reinforcer delivery was 0.1 mL).
Placement of microwires
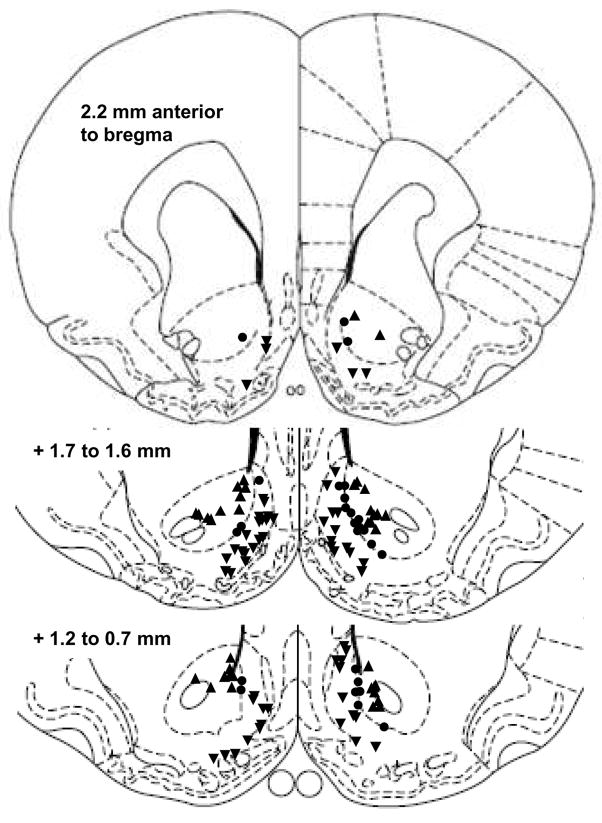
Out of 336 microwires surgically implanted, 251 were confirmed to be in the NAc: 85 in the core, 114 in the shell, and 52 in the border between the core and the shell. Spike activity was recorded on these microwires from 153 NAc neurons, depicted in Figure 1: 47 in the core, 74 in the shell, and 32 in the border between the core and the shell. Neurons with average firing rates across the operant session of ≥15 Hz were excluded (n = 6), as these are above the typical firing rates of NAc medium spiny neurons (Kish et al., 1999).
Figure 1.
Recording sites of electrodes in the NAc core (upward triangles), shell (downward triangles) or in the border between the two regions (circles; adapted from Paxinos & Watson, 1998). Some placements overlap, and two placements in the rostral shell (+2.7 mm anterior to bregma) are not depicted.
Subsets of NAc neurons exhibit characteristic patterned discharges during operant responding for ethanol versus water
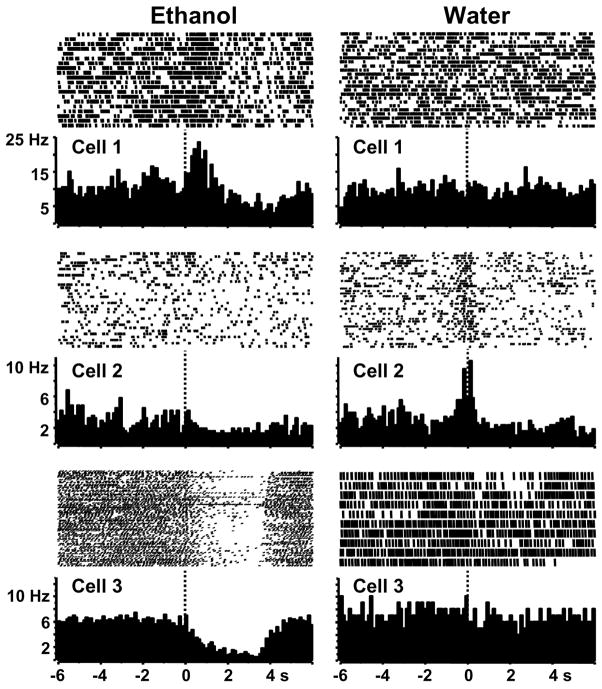
Of the 153 NAc neurons recorded during concurrent operant responding for ethanol and water, 52% (79/153 cells) showed patterned discharges that occurred within ± 1 s around the lever presses that have been previously described for NAc neurons during operant responding for other reinforcers (Carelli et al., 2000): type PR, RFe and RFi patterns. The major finding of this study is that while the same PR, RFe and RFi firing patterns were observed for both ethanol and water presses, the majority of neurons responded differently for the two reinforcers. Examples of neurons that exhibited phasic firing patterns selectively at lever presses for ethanol or water are shown in Figure 2. Phasic-firing cells were categorized into one of three groups (described in detail below): ethanol-selective (47%, 37/79), water-selective (28%, 22/79) or responsive to both reinforcers (25%, 20/79).
Figure 2.
Rasters and perievent histograms of individual cells that selectively exhibit phasic firing at lever presses for ethanol (left) or water (right). The firing rate of Cell 1 (top) significantly increased immediately after the lever presses for ethanol (type RFe), but was nonphasic after those for water. The firing rate of Cell 2 (middle) significantly increased immediately before the lever presses for water (type PR), but was nonphasic after those for ethanol. The firing rate of Cell 3 (bottom) significantly decreased immediately after the lever presses for ethanol (type RFi), but was nonphasic after those for water. Lever press responses occurred at time 0 (dotted line) here an in subsequent figures. Each perievent histogram is comprised of 100 ms bins here and in subsequent figures.
NAc neurons selective for ethanol lever presses
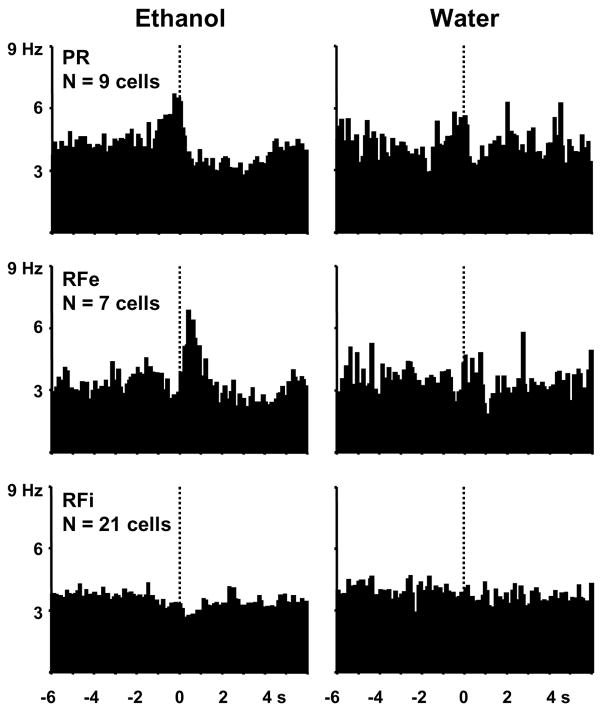
Twenty-four percent (37/153 cells) of NAc cells exhibited statistically significant changes in firing ± 1 s around the lever presses for ethanol but not for water, as determined by z scores. These ethanol-exclusive cells accounted for almost half of the phasically active cells and are shown in population perievent histograms in Figure 3. Of these cells, 24% (9/37) showed excitations in the second leading up to the lever press response for ethanol (type PR), with an average firing rate of 148 ± 13% of baseline. Nineteen percent (7/37) of the phasic neurons increased firing rates immediately after the ethanol reinforced lever press (type RFe), with an average firing rate of 170 ± 19% of baseline. The remaining 57% (21/37) displayed decreased firing rates in the pre- and/or post-response epochs (type RFi) specific to ethanol responding, with an average decrease of 28 ± 3% below baseline firing.
Figure 3.
Composite perievent histograms of all NAc neurons exhibiting patterned discharges selectively at the lever press for ethanol (left). The same neurons (right) displayed no statistically significant change in firing rate relative to water-reinforced responses.
NAc neurons selective for water lever presses
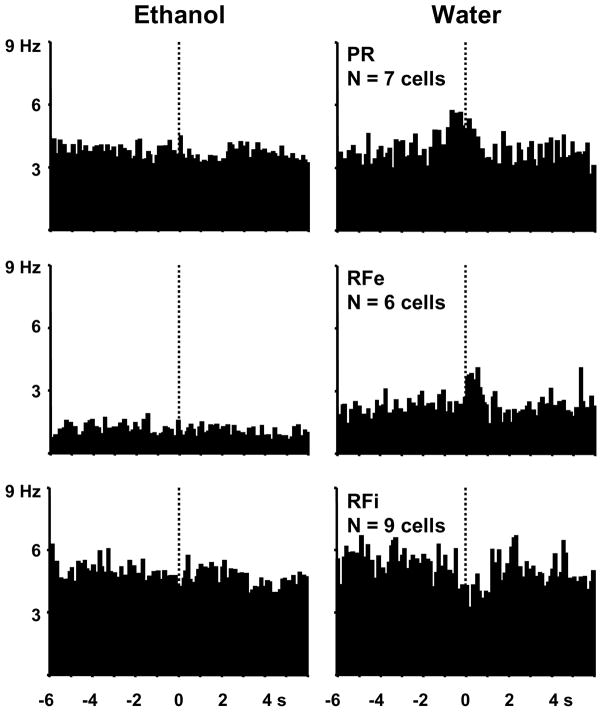
Although fewer cells showed phasic firing patterns exclusively around the lever presses for water reinforcement as compared to ethanol, the same firing patterns were observed. Fourteen percent (22/153 cells) of NAc cells showed phasic changes in firing ± 1 s around the lever presses for water but not for ethanol, as determined by significant z scores. These water-exclusive cells accounted for 28% of the phasically active cells and are shown in the population perievent histograms in Figure 4. Of these cells, 32% (7/22) showed excitations in the second leading up to the lever press (type PR), with an average firing rate of 226 ± 67% of baseline. Twenty-seven percent (6/22) increased firing rates immediately after the lever press (type RFe), with an average firing rate of 206 ± 35% of baseline. The remaining 41% (9/22) displayed decreased firing rates in the pre- and/or post-response epochs (type RFi), with an average decrease of 35 ± 7% below baseline.
Figure 4.
Composite perievent histograms of all NAc neurons exhibiting patterned discharges selectively at the lever press for water (right). The same neurons (left) displayed no statistically significant change in firing rate relative to ethanol-reinforced responses.
NAc neurons responsive to both ethanol and water reinforcement
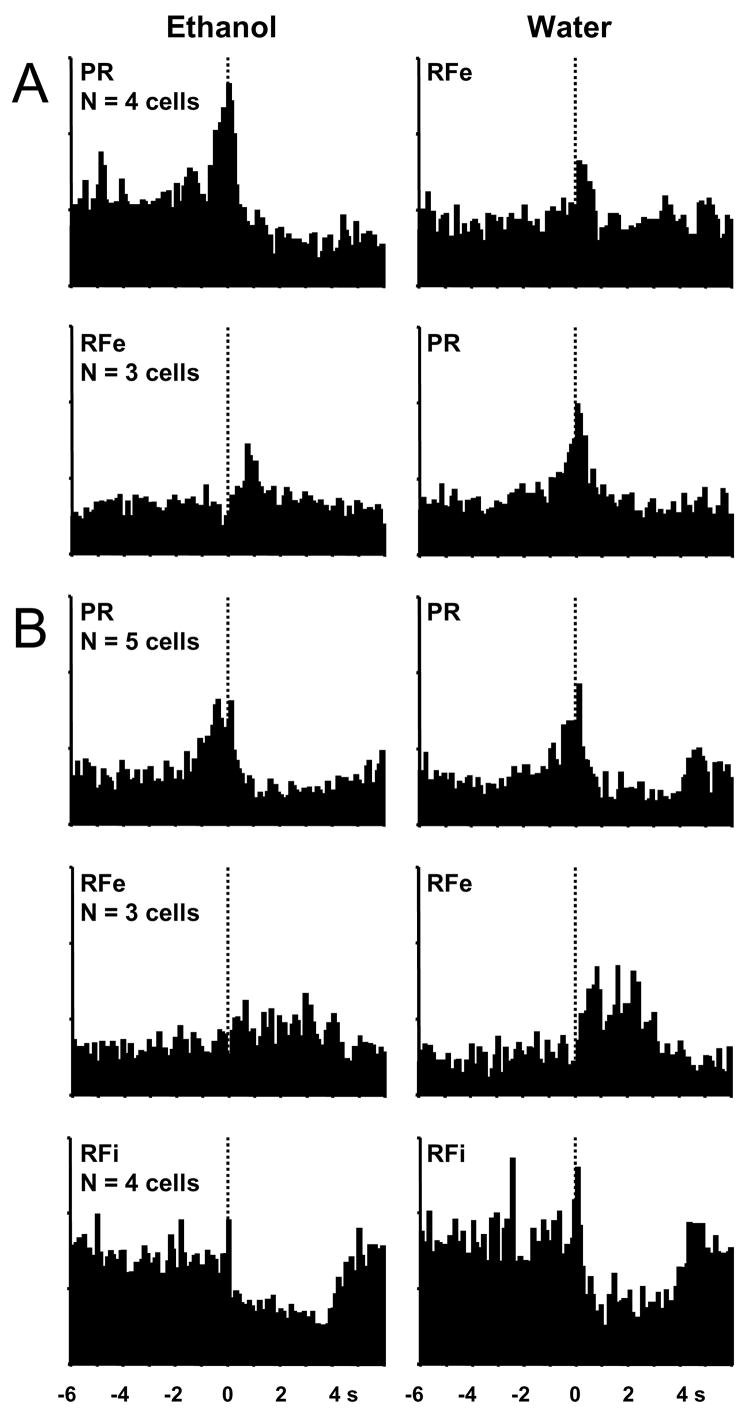
Of the NAc cells recorded, 13% (20/153 cells) exhibited phasic firing patterns to both ethanol and water lever presses, accounting for one quarter of the phasically active cells (Figure 5). Eight of these neurons distinguished the two reinforcers with different types of phasic firing patterns at lever presses for ethanol versus water. The firing patterns exhibited by these cells were as follows: 4 cells showed ethanol PR and water RFe types (Figure 5A, top); 3 cells exhibited ethanol RFe and water PR types (Figure 5A, bottom); 1 cell showed ethanol PR and water RFi types (not shown). The remaining 12 neurons displayed similar discharge patterns to both reinforcers, as shown in Figure 5B. This group consisted of 5 type PR (Figure 5B, top), 3 type RFe (Figure 5B, middle) and 4 type RFi neurons (Figure 5B, bottom). Thus, of all the cells showing patterned discharges to lever presses for ethanol and/or water, 15% (12/79) encoded both reinforcers in an identical manner and an additional 10% (8/79) encoded both reinforcers but in distinguishing patterns.
Figure 5.
Composite perievent histograms of NAc neurons exhibiting patterned discharges relative to lever press responding for both ethanol (left) and water (right). A: Patterned discharges in cells that exhibit type PR firing patterns for one reinforcer and type RFe patterns for the other. B: Patterned discharges in cells that exhibit type PR, RFe, or RFi firing patterns for both reinforcers.
Control for number of lever presses
As most of the rats (18/21) responded more for ethanol than water, it is possible that more phasic activity was detected at lever presses for ethanol simply because there were more data to analyze and, thus, more statistical power. To control for this, additional comparisons were made in which the number of trials analyzed was equal between the two reinforcers. Specifically, X trials (random selection) of the preferred reinforcer were analyzed, with X = number of lever presses for the non-preferred reinforcer. This analysis yielded significantly fewer phasically active neurons than when all trials were analyzed; restricting the preferred reinforcer to X trials reduced the proportion of phasic cells from 51% (all trials) to 42% (restricted trials; z = −3.04, p < 0.005). While there was a trend for fewer ethanol-selective cells (z = −1.9) and fewer cells responsive to both ethanol and water (z = −2.3), these decreases were not significant after Bonferroni correction. Specifically, of those NAc cells exhibiting patterned discharges (64/153), 40.5% (26/64) were exclusively activated at lever presses for ethanol, 36% (23/64) were exclusively activated at those for water, 11% (7/64) were activated at those for both reinforcers but with different firing patterns, and 12.5% (8/64) were activated at those for both reinforcers with similar patterns. Thus, controlling for the number of lever presses decreased the number of phasically active cells, but did not significantly affect the distribution of reinforcer selectivity of those cells.
Effect of ethanol preference
To test whether ethanol preference (responding for ethanol/total responses) was associated with the number of ethanol- or water-selective cells, the proportion of ethanol and/or water responsive cells were correlated with the ethanol preference displayed by each rat. Ethanol preference was significantly correlated with the proportion of ethanol-selective neurons (Spearman r = 0.72, p < 0.005) and neurons responsive to both ethanol and water (r = −0.62, p < 0.01), but not with water-selective neurons (r = −0.1, p > 0.05).
To further investigate the contribution of preference on NAc neuronal encoding of ethanol reinforcement, we compared data from rats with the highest ethanol preferences (n = 6, 0.85 ± 0.01 ethanol preference) to those with the lowest (n = 6, 0.52 ± 0.03 ethanol preference). During the operant session, the proportion of phasically active cells was similar in the high-(25/51 neurons, 49%) versus low-preference rats (24/48 neurons, 50%). However, the distribution of activity between the reinforcers significantly differed (Table 1). Phasically active cells were three times more likely to be ethanol-selective in the high-preference rats than in the low-preference rats (z = 4.37, p < 0.05). In contrast, water-selective neurons were nonsignificantly more prevalent in the low-preferring rats than the high-preferring ones (p > 0.05). Finally, neurons exhibiting phasic discharge patterns at both reinforcers made up half of the phasically active cells in the low-preferring rats but only 12% of those in the high-preferring rats (z = −4.24, p < 0.05). Similar results were obtained when the number of lever presses were restricted to X trials, as described above (data not shown). Together, these data demonstrate that the selectivity of phasic discharges of NAc neurons to ethanol versus water is strongly influenced by the degree to which the two reinforcers are preferred.
Table 1.
Neurons exhibiting phasic discharges ± 1 s around the lever press for ethanol and water in rats with high versus low ethanol preferences.
| High Preference (n = 6 rats) | Low Preference (n = 6 rats) | |
|---|---|---|
| Ethanol preference | 0.85 ± 0.01 | 0.52 ± 0.03 |
| Range 0.82 – 0.88 | Range 0.45 – 0.61 | |
|
| ||
| Total Nac cells recorded | 51 | 48 |
|
| ||
| Phasic activity (% of phasically active cells) | ||
| Ethanol-selective cells | 19 (76%) | 5 (21%)a |
|
| ||
| Water-selective | 3 (12%) | 7 (29%) |
|
| ||
| Ethanol and water | ||
| Different discharge patterns | 1 (4%) | 7 (29%)a |
|
| ||
| Same discharge patterns | 2 (8%) | 5 (21%) |
statistically different from neurons in high-preference rats, p < 0.05
Contribution of lever position: Ethanol reinforcement at both levers
While hippocampal neurons (e.g., place cells) can encode static aspects of space, NAc neurons have been shown to encode dynamic spatial aspects of reward (Shibata et al., 2001) which may result from hippocampal inputs (Trullier et al., 1999). In the present study the location of the lever (with associated cue light and dispensing cup) for each reinforcer were spatially distinct and alternate daily, rendering lever position an important dynamic cue of reward that may be encoded by NAc neurons. To begin to address this issue, we tested the contribution of lever position to phasic encoding of reinforcer selectivity in the present study by making additional electrophysiological recordings in six rats (three that are included in the main experiment and an additional three rats that were identically trained). In a single operant session, ethanol was available on both levers on a concurrent FR1/FR1 schedule; this was the only session in which water was not the alternative reinforcer. These rats pressed 23 ± 3 times on the left lever and 24 ± 6 times on the right, with a range of 10 – 43 presses on any given lever.
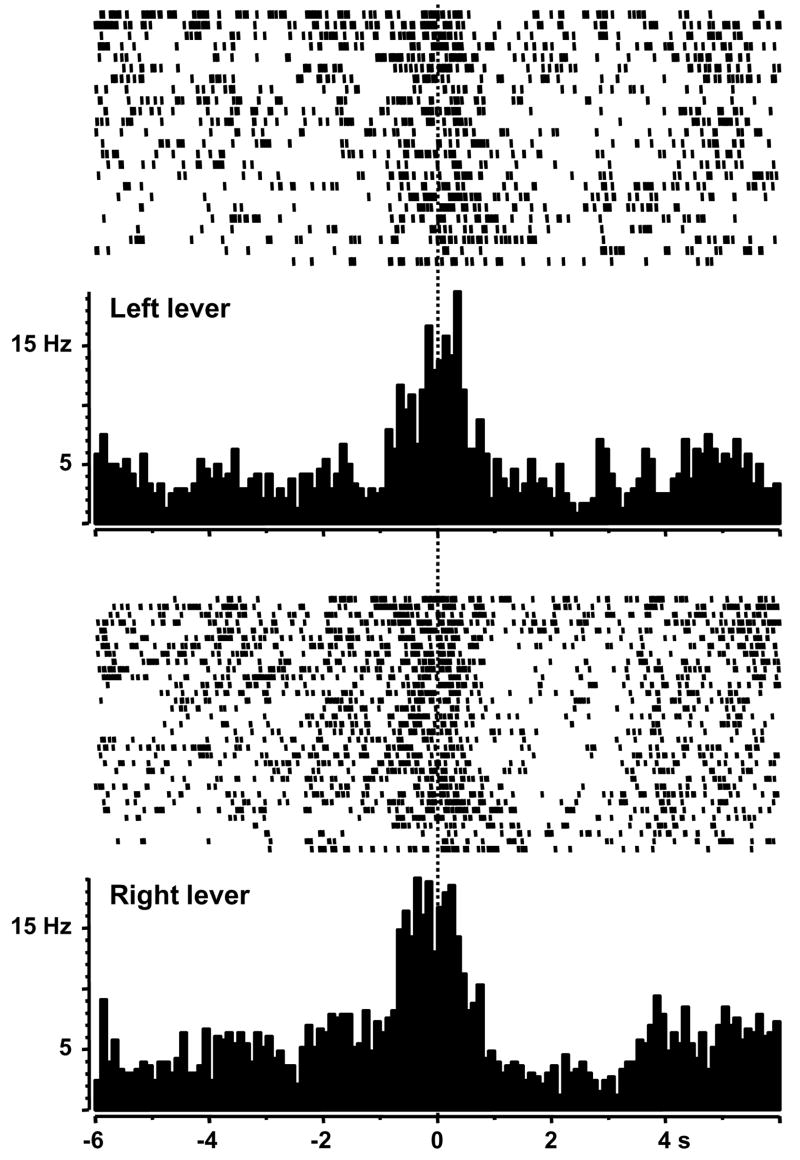
We compared NAc neuronal activity at presses for the left lever versus those for the right, both resulting in ethanol delivery. Nineteen out of 31 of the recorded neurons showed significant phasic activity during the operant session. Ten of the phasically active neurons (53%) exhibited identical discharge patterns at both the left and right lever presses, which is more than three times the proportion of similar firing patterns for ethanol versus water reinforcement. Figure 6 shows a type PR neuron that exhibited firing immediately before presses for ethanol at both levers. Importantly, the remainder of the phasically active cells (9/19, 47%) were exclusively activated at either the right or left lever. Similar results were obtained when the number of lever presses were restricted to X trials, as described above (data not shown). Thus, these data suggest that the spatial position of the lever and associated cues likely contribute to the differential firing at ethanol versus water reinforcement in this study.
Figure 6.
Rasters and perievent histograms of a NAc neuron that exhibited phasic firing at presses for ethanol on both the left and right levers. The firing rate of this cell significantly increased in the second before the lever presses (type PR).
Comparison of NAc cell firing in core versus shell
To test whether neuronal firing at lever presses varied within accumbens subregions, we compared data from the core (n = 47; upward triangles in Figure 1) versus the shell (n = 74; downward triangles in Figure 1) by using binomial logistic regression; cells that were not clearly located within the core or shell (circles in Figure 1) were not included in this analysis. Both core and shell neurons exhibited characteristic firing patterns time-locked to the lever presses for ethanol and/or water: PR, RFe and RFi patterns. The proportion of phasic cells was nonsignificantly higher in the core (28/47 cells, 60%) than the shell (36/74 cells, 49%). The reinforcer selectivity of the neurons across ethanol and water reinforcement was similar between the two NAc subregions (all p’s > 0.05 after Bonferroni correction). Specifically, 46% (13/28 cells) in the core and 42% (15/36) in the shell were specific to ethanol presses, while 39% (11/28) in the core and 25% (9/36) in the shell were specific to water presses. In the core, no cells encoded lever pressing of both reinforcers with different firing patterns, while that group represented 11% (4/36) of phasic neurons in the shell. Finally, 14% (4/28) of phasic neurons in the core versus 22% (8/36) of those in the shell exhibited similar patterns to both ethanol and water lever presses. Thus, this study measured no significant differences in phasic firing activity of NAc neurons between the core and shell.
DISCUSSION
The major finding of this study is that largely distinct subsets of NAc neurons encode information related to operant responding for ethanol versus water in a concurrent FR1/FR1 reinforcement schedule, despite identical modes of consumption and metabolic drive. Importantly, the data reveal that differences in phasic firing patterns at lever presses for ethanol versus water are driven in part by reinforcer preference and spatial location of levers and cues. Although these results are consistent with the hypothesis that distinct subsets of NAc neurons encode information about goal-directed behaviors for drug versus natural reinforcers (Carelli et al., 2000; Carelli, 2002; Carelli & Wightman, 2004), further studies are required to dissociate the contributions of preference and spatial cues to differential NAc encoding.
While previous studies have reported phasic changes in firing rates of NAc neurons that were time-locked to the operant response for ethanol (Janak et al., 1999) and water (e.g., Carelli & Deadwyler, 1994; Roop et al., 2002), this is the first study to compare NAc firing during goal-directed behaviors for both ethanol and water reinforcement in the same session. The present data revealed that while subsets of NAc neurons exhibited the same characteristic discharge patterns at lever presses for the two reinforcers (type PR, RFe or RFi), these cells were largely selective – that is, they displayed phasic discharges specific to one reinforcer only. Of the NAc neurons that showed phasic activity during lever pressing, a minority (25%) responded to presses for both ethanol and water, and almost half of these distinguished between them with different response patterns for the two reinforcers. Thus, the proportion of cells that did not differentiate between ethanol and water (15% of phasically active cells) falls within that reported for cocaine versus water (8%, Carelli et al., 2000; Carelli & Wondolowski, 2006) and cocaine versus heroin reinforcement (25%, Chang et al., 1998), and much lower than the proportion reported for water versus food or sucrose (65 – 68%, Carelli et al., 2000; Roop et al., 2002). These findings support theories of NAc organization in which collections of neurons (i.e., neuronal ensembles) have different functional properties and are activated in specific circumstances, including goal-directed behaviors for different reinforcers (Pennartz et al., 1994; Carelli, 2004; Carelli & Wightman, 2004). However, the present study also revealed previously unknown factors that appear to contribute to the degree of overlap between subsets of NAc cells that encode ethanol and water reinforcement: reinforcer preference and spatial cues.
By comparing free-choice self-administration of two concurrent reinforcers (as opposed to sequential), we obtained an operant measurement of preference for one reinforcer over the other. Indeed, rats with high ethanol preferences had significantly more cells exclusively activated at lever presses for ethanol and fewer cells activated at presses for both ethanol and water than rats with lower ethanol preferences. This association is consistent with the hypothesis that a stronger preference or motivation for a particular reward induces greater recruitment of NAc neurons to encode that reinforcer. Previous experiments did not address the potential contribution of preference due to the sequential availability of the reinforcers (e.g., Carelli et al., 2000). However, future experiments can test this hypothesis directly by measuring NAc neuronal activity in rats under different concentrations of ethanol versus sucrose that results in a range of behavioral preferences. A slightly different interpretation is that rats are more motivated to self-administer ethanol than water, and the stronger motivation recruits more NAc phasic activity. This hypothesis can be tested by assessing motivation with progressive ratio reinforcement schedules, i.e., incrementally increasing the response requirement for each reinforcer to determine the break points.
A consequence of the range of preference observed in this study was that most rats pressed fewer times for water than ethanol. Thus, it was important to limit the number of trials analyzed of the preferred reinforcer in order to control for differences in trial number (and, thus, statistical power to detect changes in firing rate). Limiting the analysis trials of the preferred reinforcer resulted in fewer detected phasically active cells, but importantly the proportion of cells showing reinforcer selectivity did not significantly change. These findings further support our conclusion that ethanol and water reinforcement are distinctly encoded in the NAc of rats.
Our data indicate that spatial location of the lever and associated cues likely influenced the degree of overlap between subsets of NAc cells that encoded ethanol and water reinforcement. The levers, cue lights and cups for ethanol and water were identical except for placement in the operant chamber, and that alternated daily (Rassnick et al., 1992). This alternation results in initial sampling behavior to determine which lever supports which reinforcer, and in our hands results in higher self-administration of water across the session than when positions are fixed (unpublished observations, D.L. Robinson). Thus, lever position is likely to be an important cue for these rats and, combined with previous reports of NAc neurons encoding reward-related spatial cues in their firing rates (Shibata et al., 2001; Mulder et al., 2005), is a probable contribution to the data reported here. Previous comparisons of NAc neuronal activity during operant responding for multiple reinforcers also placed the reinforcers on different levers (e.g., Carelli et al., 2000). While the levers did not alternate daily in these previous studies, it is nevertheless possible that lever and cue position contributed to the differential encoding by NAc neurons, which ranged from 92% (cocaine versus food or water) to 35% of phasically active cells (water versus sucrose or food; Carelli et al., 2000; Roop et al., 2002). In contrast, Miyazaki et al. (1998) reported that NAc neurons exhibiting phasic activation to the presentation of food or water were insensitive to spatial location. To begin to address this issue, we measured NAc activity in a single session with ethanol reinforcement following lever presses at both the right and left levers. Although we found that the overlap in discharge patterns at each lever tripled as compared to when water was the alternate reinforcer, almost half of the phasically active neurons were still selective for one or the other lever. While these data highlight the real contribution of lever position to NAc cell firing patterns, they leave open multiple interpretations. For example, a shortcoming of this control is that when rats have trained for months with lever position as a differentiating cue and then run a single session in which it is not (i.e., ethanol suddenly on both levers), neuronal activity may still encode this previously important cue. Thus, the contribution of spatial position of levers and cues must be further explored by systematic manipulation of reinforcer and position during both training and testing.
The roles of the NAc core and shell in reinforcement processing differ in that the core appears more involved in conditioned learning while the shell appears more involved in responses to unconditional stimuli (Kelley, 1999; Cardinal et al., 2002). Nevertheless, similar proportions of NAc neurons in both the core and the shell encode lever presses for cocaine and water reinforcement (Uzwiak et al., 1997; Carelli & Wondolowski, 2006). Likewise, the present study found no differences between the NAc core and shell in patterned discharges at lever presses for ethanol and/or water. Thus, the data suggest that, in this operant context, functional variation between core and shell arises by way of differential afferent and efferent connections rather than differences in the physiological integration of afferent inputs.
In summary, we present the first examination of short-term firing patterns of NAc neurons during concurrent operant responding for ethanol and water, and report that information about the two reinforcers is differentially encoded in the firing rates of largely separate neuronal ensembles in the NAc. These data highlight the role of NAc neurons in processing information about goal-directed behavior toward distinct rewards and reveal important contributions of reinforcer preference and spatial cues.
Acknowledgments
The authors gratefully acknowledge technical support from Dawnya Bohager, Matthew Fecteau, Amber Kinard, Shin-Yi Lao, Scott McConnell and especially Wilbur Williams III. Dr. Clyde Hodge and Dr. Jason Schroeder provided advice on ethanol self-administration training. This research was funded by NIH (AA014591 to D.L.R. and DA14339 to R.M.C.).
ABBREVIATIONS
- FR1
one reinforcer delivery per lever press
- NAc
nucleus accumbens
- PR
pre-response excitation
- RFe
reinforcer excitation
- RFi
pre-response or reinforcer inhibition
References
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. Experimentelle Hirnforschung. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacol. 2004;47(Suppl 1):180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, King VC, Hampson RE, Deadwyler SA. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain research. 1993;626:14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opinion Neurobio. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Anatomic distribution of reinforcer selective cell firing in the core and shell of the nucleus accumbens. Synapse (New York, NY) 2006;59:69–73. doi: 10.1002/syn.20217. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC, Coyle TS, Healy DJ, Chen S. Stress controllability influences the ataxic properties of both ethanol and midazolam in the rat. Beh Neurosci. 1996;110:360–367. doi: 10.1037//0735-7044.110.2.360. [DOI] [PubMed] [Google Scholar]
- Eriksson CJ, Sarviharju M. Motor impairment, narcosis and hypothermia by ethanol: separate genetic mechanisms. Alcohol. 1984;1:59–62. doi: 10.1016/0741-8329(84)90038-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Beh. 2002;76:389–395. doi: 10.1016/s0031-9384(02)00758-8. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: Further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Lewis RS, Erickson HL. Specific decreases in ethanol-but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205-930. Alcohol (Fayetteville, N Y) 1993;10:191–196. doi: 10.1016/0741-8329(93)90034-l. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Kish LJ, Palmer MR, Gerhardt GA. Multiple single-unit recordings in the striatum of freely moving animals: effects of apomorphine and D-amphetamine in normal and unilateral 6-hydroxydopamine-lesioned rats. Brain Res. 1999;833:58–70. doi: 10.1016/s0006-8993(99)01496-1. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Mogi E, Araki N, Matsumoto G. Reward-quality dependent anticipation in rat nucleus accumbens. Neuroreport. 1998;9:3943–3948. doi: 10.1097/00001756-199812010-00032. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Shibata R, Trullier O, Wiener SI. Spatially selective reward site responses in tonically active neurons of the nucleus accumbens in behaving rats. Experimental brain research. Experimentelle Hirnforschung. 2005;163:32–43. doi: 10.1007/s00221-004-2135-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci. 1996;16:3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacol. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse (New York, NY) 2002;43:223–226. doi: 10.1002/syn.10041. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Shibata R, Mulder AB, Trullier O, Wiener SI. Position sensitivity in phasically discharging nucleus accumbens neurons of rats alternating between tasks requiring complementary types of spatial cues. Neurosci. 2001;108:391–411. doi: 10.1016/s0306-4522(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Trullier O, Shibata R, Mulder AB, Wiener SI. Hippocampal neuronal position selectivity remains fixed to room cues only in rats alternating between place navigation and beacon approach tasks. Eur J Neurosci. 1999;11:4381–4388. doi: 10.1046/j.1460-9568.1999.00839.x. [DOI] [PubMed] [Google Scholar]
- Uzwiak AJ, Guyette FX, West MO, Peoples LL. Neurons in accumbens subterritories of the rat: phasic firing time-locked within seconds of intravenous cocaine self-infusion. Brain Res. 1997;767:363–369. doi: 10.1016/s0006-8993(97)00752-x. [DOI] [PubMed] [Google Scholar]