Abstract
In the CNS, GABA and insulin seem to contribute to similar processes, including neuronal survival; learning and reward; and energy balance and food intake. It is likely then that insulin and GABA may interact, perhaps at the GABAA receptor. One such interaction has already been described [39]; in it a micromolar concentration of insulin causes the insertion of GABAA receptors into the cell membrane, increasing GABA current. I have discovered another effect of insulin on GABAA currents. Using a receptor isoform α1β2γ2s that is the likely main neuronal GABAA isoform expressed recombinantly in Xenopus oocytes, insulin inhibits GABA induced current when applied simultaneously with low concentrations of GABA. Insulin will significantly inhibit currents induced by EC30–50 concentrations of GABA by about 38%. Insulin is potent in this effect; IC50 of insulin was found to be about 4.3 ×10−10 M. The insulin effect on the GABA dose responses looked like that of an antagonist similar to bicuculline or β-carbolines. However, an effect of phosphorylation on the GABAA from the insulin receptor signal transduction pathway cannot yet be dismissed.
Keywords: GABAA, insulin, brain, diabetes, metabolic syndrome, competitive antagonist
The pancreatic hormone insulin can cross the blood-brain barrier and become concentrated in the brain [6, 36]. This neuronal insulin has many potential functions in the brain and individual. Changes in neuronal insulin levels or sensitivity, including in diabetes, can affect many different neurological functions. Many are long term, such as in neuronal survival, including the development of Alzheimer disease [reviewed in 36]. Insulin signaling pathways are involved in glucose regulation, body energy homeostasis [43], and food intake of organisms [33, 9]. Insulin too may block some of the reward pathways in the ventral striatum and prefrontal cortex; the decrease feeling of reward from glucose in these areas may also be part of satiation [4].
Neuronal insulin and the neurotransmitter γ-aminobutyric acid (GABA) may both contribute significant roles in some neural diseases and activities. In many cases these contributions are opposing in nature. These activities include neurodegeneration/neuronal survival [5, 37]; pathology or depressive symptoms associated with Alzheimer's disease [23, 16]; and synaptic plasticity [15].
Since GABA and insulin overlap and usually have opposite effects in many neural activities, it is reasonable to hypothesize that insulin and GABA may intimately interact. One place would be at the GABAA receptor. The GABAA receptor is a reasonable target for insulin-GABA interactions because the GABAA receptor is already a target for many different ligands including hormones; and the GABAA receptor can be phosphorylated by kinases in the insulin receptor signal transduction pathway. The GABAA receptor is a GABA gated chloride channel. Upon binding of GABA, the channel allows Cl- ions to flow into the cell, causing hyperpolarization. Many different ligands can positively or negatively affect the amount of GABA-induced current by binding a site on the receptor. Positive modulators include benzodiazepines (BZs), ethanol, anesthetics, and some pregnane derived steroids. Negative modulators include bicuculline, picrotoxin, and some steroid derivatives. The sites for these ligands are somewhere within the pentamer of the receptor; the pentamer usually consists of 2 α, 2 β and 1 γ subunit drawn from a family of 6 α, 3 β and 3 γ [reviewed in 22, 17]. The subunits expressed in the highest levels in most brain areas as demonstrated by both in situ hybridization and RT-PCR are the α1, β2, and γ2s [30, 22, 28].
Evidence for a GABAA -insulin interaction already exists. Previous research has shown that a 10 minute exposure to 0.5 µM insulin will increase the number of cell surface GABAA receptors [39]. This effect is likely due to phosphorylation of the GABAA receptors by kinases such as phosphoinositide 3-kinase (PI3-K) that are in the insulin receptor signaling pathway [38].
I hypothesized that there could be a quicker, potentially direct, and more potent effect of insulin on GABAA receptors, one that could potentially explain any opposing actions of insulin and GABA. These studies demonstrate such a quicker, more potent inhibitory effect of insulin on GABAA receptors does exist.
Materials and Methods
Oocytes (Stage IV–V) from Xenopus laevis were isolated and defolliculated by mechanical separation and incubation in 0.05% collagenase. Oocytes were washed extensively in OR-3 media (70% Leibovitz’ L15/Gibco). All animal care, use and surgeries are standard protocols and were approved by the WSSU IACUC committee. Insulin was the bovine form (cat I-5500) from Sigma (St. Louis, MO). Insulin was dissolved in 0.1% acetic acid and diluted in perfusion buffer. No change in pH was detected in the dilutions (not shown). All other chemicals are from commercial sources.
Rat GABAA subunit cDNAs are cloned into the pGEMHE vector. Wild type α1, β2, and γ2S subunits were transcribed in vitro using T7 kits from Ambion/Applied Biosciences and diluted to 200 ng/µl using nuclease free water. RNAs were injected into the oocytes at a 1:1:1 ratio of subunits in 50 nL total volume. Oocytes incubated at 18°C for 2–3 days in OR-3 media to allow for surface expression of the receptors. By using the 1:1:1 ratio for the subunits, we assume the surface receptors will be the typical α1β2γ2s in a 2α:2β:1γ ratio [41]. Though the insulin is bovine, and the receptor subunits from rat, insulin is well conserved. Between bovine and rat forms of insulin, there are only 4 amino acid differences, 2 on each the α and β chains, out of a total of 54 residues (NCBI data base).
Electrophysiology was performed by the two-electrode voltage clamp technique. Oocytes were perfused with Calcium Free Frog Ringer’s (CFFR) (115 mM NaCl, 2.5 mM KCl, 1.8 mM Mg2Cl, 10 mM HEPES, pH 7.5) at a rate of 5 ml/min and clamped at −60 mV at room temperature. Electrodes filled with 3 M KCl had a resistance between 0.5–2.5 mOhms. Currents were collected using the Warner TEV700 workstation/oocyte clamp with the HAI118 data acquisition systems using LabScribe Software, sampled at 100 samples/sec. Stable GABA-induced currents were established before continuing experiments. Currents were defined as stable if the peak amount of current induced in 20–30 sec was within 5%. If GABA induced currents were stable, then GABA and a certain concentration of insulin were added simultaneously for 20–30 sec and that peak recorded. The GABA-insulin co-application was repeated. GABA was then applied alone to be sure insulin washed out, or had no other slightly longer effects on subsequent currents. To do the insulin dose response curve a constant concentration of GABA (1 µM, approximate EC30) was applied in the presence of varying amounts of insulin. To do the GABA dose response curves various concentrations of GABA were applied in the absence or presence of a constant concentration of insulin, 100 nM. The large dose of insulin was used to ensure a significant effect. Percent changes from control currents were calculated as [I+insulin\Icontrol]x100. Significance between control (no insulin) and experimental (with insulin) GABA induced currents for a single concentration was determined by t-test. In the dose responses, any significance between concentrations was determined by one-way ANOVA (Instat, GraphPad, San Diego, CA).
Results
Establishing an effect
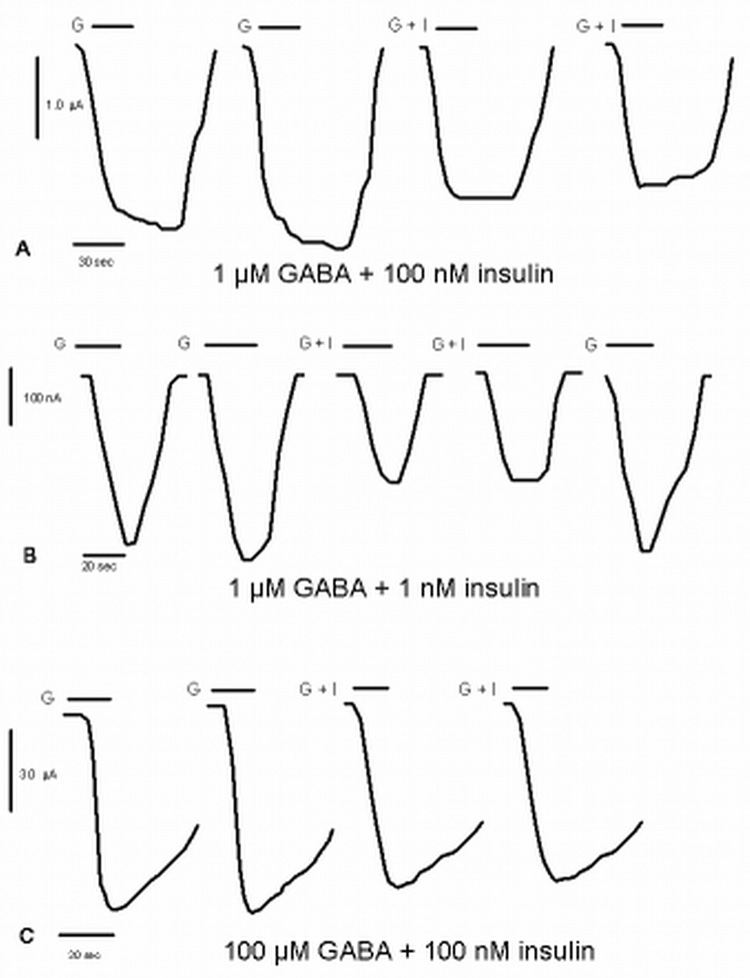
After stable GABA induced currents were established 100 nM insulin was added simultaneously with a submaximal concentration of GABA (EC30; 1 µM). A significant decrease in GABA induced current was seen at 1 µM GABA (−38 ± 8.3 % n= 7; p< 0.01) (fig. 1a). At 1 µM GABA, a reduction of about −22 ± 4.0 % (n =6; p < 0.01) occurs when only 1 nM insulin is co-applied (fig. 1b). Near saturating GABA (100 µM) currents were not significantly affected by simultaneous application of 100 nM insulin (−0.33 ± 2.0 %) (fig. 1c). The high dose of insulin, 100 nM, did not cause any changes in current when added alone (data not shown.)
Figure 1. Effect of insulin on GABA-mediated currents at α1β2γ2S receptors.
All currents are from α1β2γ2s GABAA receptors expressed in Xenopus oocytes clamped at −60 mV.
A. The first series of tracings shows that 100 nM insulin inhibits currents elicited by 1 µM GABA. The first two tracings are controls; the bar corresponds to the application of 1 µM GABA only (G). The next two are tracings of 1 µM GABA co-applied with 100 nM insulin with the bar corresponding to the time of co-application (G + I).
B. The second series of tracings shows that 1 nM insulin inhibits currents elicited by 1 µM GABA. The first two tracings are the controls; the bar corresponds to the application of 1 µM GABA only (G). The third and fourth tracings are the currents of GABA co-applied with 1 nM insulin with the bar corresponding to the time of co-application (G + I). The last trace shows that the inhibitory effect of insulin is reversible with the bar corresponding to the application of 1 µM GABA only (G).
C. The third series of tracings shows no significant effect of 100 nM insulin in the presence of 100 µM GABA. The first two tracings the controls; the bar corresponds to the application of 1 µM GABA only (G). The last two tracings are the GABA currents in the presence of insulin with the bar corresponding to the time of co-application (G + I).
Dose response of insulin at 1 µM GABA
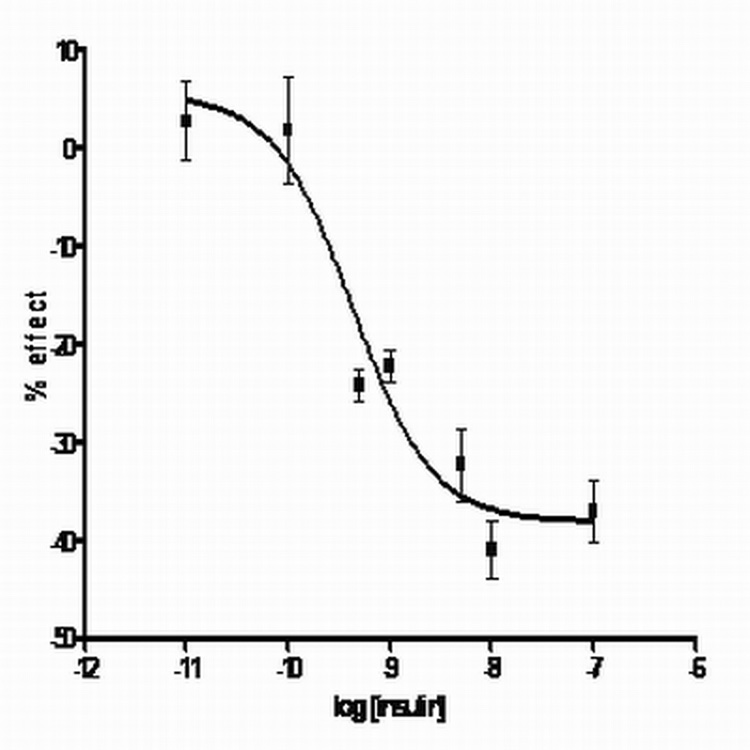
Using 1 µM GABA, where the largest percent decrease in current seemed to occur, a dose response curve for the inhibitory effect of insulin was done (fig. 2). Insulin in ranges from 0.01 nM to 100 nM was added in the presence of 1 µM GABA. The data was plotted percent change in current v. the concentration of insulin. The best fit curve is a one site model with variable slope using the equation Y= Bottom + (Top-Bottom)/1 + 10(logEC50-X)HillSlope [26]; the IC50 was 0.43 nM and the Hill number was 0.2 (Graphpad; r = 0.95). The maximal effect was calculated to be −38 ± 1.5%.
Figure 2. Insulin dose response at 1 µM GABA for α1β2γ2s receptors.
Various concentrations of insulin (100 nM to 0.01 nM) were added with 1 µM GABA. The percent effect is the change in current from control currents (no insulin). Points are the average and standard deviations of 3–6 experiments. The curve was fit using a two site model on GraphPad. Insulin effects on currents are statistically significant at 100 nM and 10 nM (p <0.05). The maximal effect of insulin is approximately −38%, with an IC50 in the 4.3 × 10−10 range.
Effect of insulin on GABA dose response
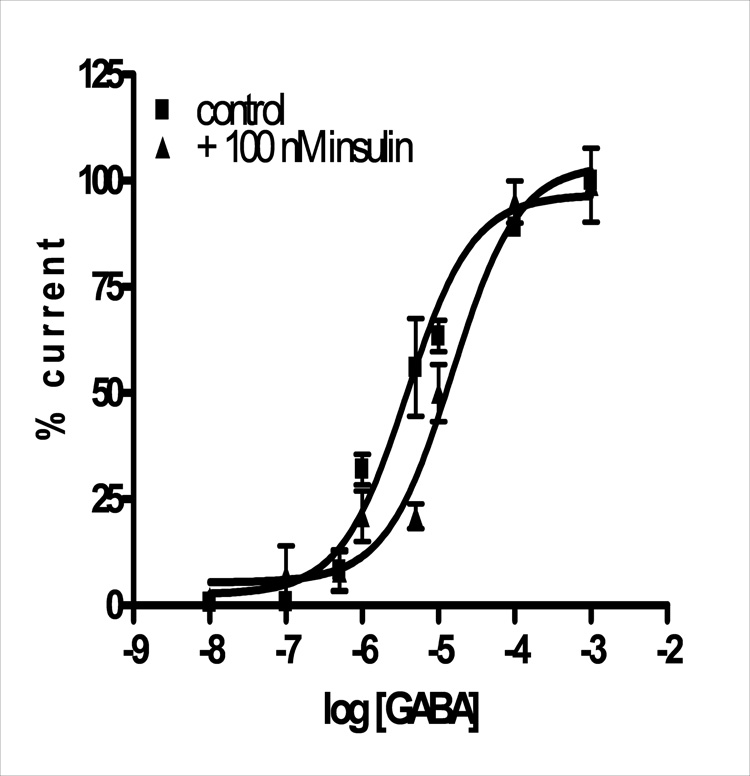
Using 100 nM insulin, changes in the GABA dose response were investigated. Various concentrations of GABA ranging from 0.1 µM to 1 mM were added alone and then simultaneously with 100 nM insulin. The dose responses show a significant shift in the mean EC50 of GABA from 3.8 ± 1.1 µM (control) to 15 ± 1.1 µM (with insulin) (p = 0.0003) with no effect on maximal current (99 ± 13% of maximal) (fig. 3). Significant decreases in GABA induced current occurred at submaximal concentrations of GABA ranging from 5 µM to 1 µM (p = 0.03).
Figure 3. GABA current changes in the presence of 100 nM insulin at α1β2γ2s receptors.
The change in GABA-induced current due to the presence of 100 nM insulin is plotted as a function of GABA concentration. GABA EC50 is approximately 4 µM and shifts to 15 µM in the presence of insulin. Points are mean and standard deviations of 3–5 experiments.
Discussion
The data presented suggest that insulin has a rapid inhibitory effect on GABAA receptor current. The term rapid is used to differentiate the effect from the decreases of GABA current seen by Wan et al. [39], in which insulin is incubated with GABAA receptors for 10 minutes, not seconds. The effect seems potent with an IC50 around 0.43 nM. Serum insulin concentrations are approximately 49 pmol/L for a population of fasting men [21], and 50 pmol/L for women [1]. Insulin can cross the blood-brain barrier [6] and become concentrated in the brain; brain levels are reported to be 10–100 times higher than that of serum, depending on the brain area [18]. This higher neuronal insulin concentration compares favorably with the IC50 of insulin for the GABAA inhibitory effect. The IC50 for insulin at the α1β2γ2s receptor (0.43 nM) also compares favorably with the EC50 for insulin for the insulin receptor (about 0.05 nM to 3 nM depending on the tissues) [13, 17, 21]. The effect of these concentrations of insulin, when co-applied with low concentrations of GABA, is to inhibit GABA induced current at neuronal type α1β2γ2s receptor isoforms by approximately 38%.
This rapid inhibitory effect of insulin is different from the described effect of an increase in current due to receptor insertion into the plasma membrane. Both this inhibitory effect and the previously described potentiating effect occur at α1β2γ2s isoforms. This inhibitory effect is more rapid; it occurs simultaneously with a 20–30 sec application of GABA. It is more potent: the IC50 is in the 10−10 M range with 100 nM insulin at or near saturating. The potentiating effect described by Wan et al., [39] is much different; it requires 500 nM insulin and incubation times of at least 10 minutes. Therefore the simultaneous, rapid inhibition of GABA-induced currents by nanomolar amounts of insulin represents a novel, separate effect of insulin on GABAA receptors. This effect may be important in some of the roles of insulin in brain.
The effect of insulin on the α1β2γ2s isoform of GABAA receptors is clearly antagonistic. The type of antagonism, whether competitive or non-competitive is less clear. The effect of insulin: a rightward shift in the GABA EC50 and no significant effect on maximal current looks like a typical competitive inhibitor, bicuculline [2]. Other known competitive inhibitors of the GABAA receptor, such as pitrazepin [14] and thiocolchicoside [11] result in similar effects and shifts in GABA dose response curves [2, 11, 14]. However, β-carboline non-competitive (inverse agonist) inhibitors sometimes have similar effects on GABA dose response curves, reducing the affinity of GABA [10], so an antagonistic effect similar to β-carbolines by insulin cannot be totally eliminated. More typically though, β-carbolines show a mixed type inhibition, with significant changes in GABA EC50 and maximal response [34, 35], which is not seen for GABA inhibition by insulin at this α1β2γ2s isoform (fig 3). Also, activation of the receptor is not seen by large amounts of insulin as can occur with the β-carbolines [34]. With significant effects only on GABA EC50 and no induced current by high concentrations of insulin, a competitive nature for the insulin inhibition cannot be dismissed for α1β2γ2s GABAA receptors. Another isoform (α4β3γ1) shows the mixed type inhibition [40] similar to β-carbolines, so as an overall mechanism of inhibition at GABAA receptors, insulin may be a non-competitive inhibitor; the effects of insulin would be dependent on the subunit composition of the GABAA receptor.
Insulin is a peptide hormone, which makes it unusual when compared to the many other ligands that interact at GABAA receptors. Most other GABA-acting ligands are small organic molecules, like propofol or BZs, or steroid hormones [22]. However at least one peptide is known to interact at GABAA receptors though its mechanism of action seems different from the inhibitory effect of insulin illustrated in this study. Diazepam Binding Inhibitor (DBI) is an endogenous inhibitor that binds the extracellular benzodiazepine site directly blocking BZ binding and allosterically inhibiting the GABAA receptor [8]. Though DBI unknown if the inhibitory mechanisms of insulin and DBI are similar, DBI provides corraborating evidence that a peptide, like insulin, could interact in the extracellular binding sites of the receptor.
The oocyte contains insulin receptors and is sensitive to insulin [31, 12]. Therefore, a component of the insulin receptor signaling pathway could phosphorylate GABAA receptors when the oocyte is exposed to these nanomolar concentrations of insulin. Though the inhibitory effect of insulin on GABA-induced current seems to be a direct antagonism, the possibility that the effect could be from phosphorylation cannot be dismissed. Exposure of the oocyte to insulin would activate the insulin receptor signaling pathway. The insulin receptor signaling pathway consists of many kinases. The PI3K/Akt kinase pathway seems to be the one activated for the insertion of GABAA receptors into the membrane [38]. Other kinases in the pathway could cause inhibition. Some studies indicate that activation of kinases in the "opposite" arm of the insulin receptor signal transduction pathway could have opposite effects on targets [rev in 36]. For example, in neuronal survival, the activation of the Akt branch increases neuronal survival (perhaps partly by the increase in cell surface GABAA receptors [27]) while activation of the ERK pathway contributes to neuronal death [36]. Therefore the other branch of the insulin receptor signal transduction pathway with ERK kinases could phosphorylate GABAA receptors and cause the inhibition in GABA-mediated current. Recent evidence indicates that ERK kinases do in fact inhibit GABA mediated current [7]. Phosphorylation acts on an intracellular site, so any immediate modifications there would be more allosteric in nature; longer term changes involve receptor insertion or degradation [3]. The relatively rapid nature of the inhibitory effect of insulin suggest a direct interaction on the receptor; the fact that insulin has a nanomolar affinity for the GABA receptor approximately equal to the EC50 of insulin for the insulin receptor suggests an interaction potentially involving the insulin receptor and phosphorylation. Further experiments will be necessary to determine which of these interactions is more important in the inhibitory effect of insulin on GABAA receptors.
Because at this putative neuronal isoform α1β2γ2s of GABAA receptors, insulin only affects currents induced by lower amounts of GABA, the main effect of insulin could be on tonic GABA currents, especially if the inhibitory effect is extended to α4 or α6 containing receptors (there is some evidence for an effect at α4 containing receptors [40]). At the α1β2γ2s isoform studied here, the lower affinity for GABA caused by insulin could increase the deactivation or unbinding rate of GABA, reducing synaptic currents. Such an effect would be difficult to detect in the oocyte system used in this study due to slow solution exchange rates [42]. GABA currents are important in the overall excitability of the brain, and play a role in synaptic plasticity [32, 22, 28]. Insulin, by inhibiting GABA currents, could therefore affect activities associated with GABA-mediated inhibition, especially dysfunctions associated with diabetes or improper insulin amounts. The GABAA isoform of this study, α1β2γ2s, is the most likely isoform expressed in most brain regions including cerebral cortex, hypothalamus, olfactory bulb and hippocampus, as well as many others [30, 19]. These regions are ones where insulin could have opposing roles to GABA in different behaviors, including memory (hippocampus and cortex [43]); food intake and appetite (cortex [4], hypothalamus [9] and olfactory bulb [25]); and control of glucose concentrations (hypothalamus [20]). The premetabolic syndrome is characterized by increased levels of insulin [24]. Possible then, insulin inhibition of GABAA currents could contribute to the development metabolic syndrome including loss of control of glucose concentrations [20], and improper food intake [4, 9, 25] associated with the disorder [24].
In conclusion, I have found a novel action of insulin on neuronal type α1β2γ2s GABAA receptors. This action is inhibitory and occurs simultaneously with the application of low concentrations of GABA; this action acts competitive in nature. The inhibitory effect of insulin on low concentration GABA-induced current could be important in the progress of metabolic syndrome to diabetes, and in some of the neurological side effects of diabetes.
Acknowledgements
I thank Dr. Myles Akabas (Albert Einstein College of Medicine) for the pGEMHE constructs; Drs. David Kump (WSSU), Joseph V. Martin (Rutgers Univ.-Camden, NJ), for helpful comments on the manuscript; and the large number of WSSU students who provided technical assistance. The WSSU RIP program, and the NIH (NIMH) 1R15MH076896-01 provided research support for work in my lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important fort postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 2.Akaike N, Yakushiji T, Tokutomi N, Carpenter DO. Multiple mechanisms of antagonism of gamma-aminobutyric acid (GABA) responses. Cell Mol. Neurobiol. 1987;7:97–103. doi: 10.1007/BF00734993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akk G, Covery DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of steroid interactions with GABAA receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony KA, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 5.Azimi-Zoonoz A, Shuttleworth CW, Connor JA. GABAergic protection of hippocampal neurons against glutamate insult: deficit in young animals compared to adults. J Neurophysiol. 2006;96:299–308. doi: 10.1152/jn.01082.2005. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 7.Bell-Horner CL, Dohi A, Nguyen Q, Dillon GH, Singh M. ERK/MAPK pathway regulates GABAA receptors. J Neurobiol. 2006;66:1467–1474. doi: 10.1002/neu.20327. [DOI] [PubMed] [Google Scholar]
- 8.Bormann J. Electrophysiological characterization of diazepam binding inhibitor (DBI) on GABAA receptors. Neuropharmacology. 1991;30:1387–1389. doi: 10.1016/s0028-3908(11)80006-7. [DOI] [PubMed] [Google Scholar]
- 9.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behavior. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Capogna M, Berretta N, Berton F, Bianchi R, Brunelli M, Franscesconi W. The beta-carboline derivative DMCM decreases gamma-aminobutyric acid responses and Ca2+ mediated K+ conductance in rat neocortical neurons in vitro. Neuropharmacology. 1994;33:875–883. doi: 10.1016/0028-3908(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 11.Carta M, Murru L, Botta P, Talani G, Sechi G, de Riu P, Sanna E, Biggio G. The muscle relaxant thiocolchicoside is an antagonist of GABAA receptor function in the central nervous system. Neuropharmacology. 2006;51:805–815. doi: 10.1016/j.neuropharm.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Chuang L-M, Myers G, Jr, Seidner GA, Birnbaum MJ, White MF, Kahn CR. Insulin receptor substrate 1 mediates insulin and insulin-like growth factor I-stimulated maturation of Xenopus oocytes. Proc Natl Acad Sci USA. 1993;90:5172–5175. doi: 10.1073/pnas.90.11.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuatrecasas P. Insulin-receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci USA. 1971;68:1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuro A, Martinez-Torres A, Fransesconi W, Miledi R. Antagonistic action of pitrazen on human and rat GABAA receptors. Br. J. Pharmacol. 1999;127:57–64. doi: 10.1038/sj.bjp.0702504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaisrsa J-L, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends in Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Alloza M, Tsang SW, Gil-Bea FJ, Francis PT, Lai MK, Marcos B, Chen CP, Ramirez MJ. Involvement of the GABAergic system in depressive symptoms of Alzheimer’s Disease. Neurobiol Aging. 2006;27:1110–1117. doi: 10.1016/j.neurobiolaging.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Gliemann J, Gammeltoft S, Vinten J. Time course of insulin-receptor binding and insulin-induced lipogenesis in isolated rat fat cells. J Biol Chem. 1975;250:3368–3374. [PubMed] [Google Scholar]
- 18.Havrankova J, Schmechel D, Rith J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang RQ, Dillon GH. Functional characterization of GABA(A) receptors in neonatal hypothalamic brain slice. J. Neurophysiol. 2002;88:1655–1663. doi: 10.1152/jn.2002.88.4.1655. [DOI] [PubMed] [Google Scholar]
- 20.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Nakatani K, Yano Y, Adachi Y. Increased oxidative stress is associated with serum levels of triglyceride, insulin resistance and hyperinsulinemia in Japanese metabolically obese, normal weight men. Diabetes Care. 2004;27:631–632. doi: 10.2337/diacare.27.2.631. [DOI] [PubMed] [Google Scholar]
- 22.Korpi ER, Grunder G, Luddens H H. Drug interactions at the GABAA receptor. Progress Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 23.Lanctot KL, Herrmann N, Rothenburg L, Eryavev G. Behavioral correlates of GABAergic disruption in Alzheimer’s disease. Inter Psychoger. 2006;19:151–158. doi: 10.1017/S1041610206003899. [DOI] [PubMed] [Google Scholar]
- 24.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic Syndrome. Med Clin N Am. 2007;91:1063–1077. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Marks JL, Eastman CJ. Effects of starvation on insulin receptors in rat brain. Neuroscience. 1989;30:551–556. doi: 10.1016/0306-4522(89)90272-8. [DOI] [PubMed] [Google Scholar]
- 26.Motulsky HJ, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. San Diego, CA: Graph Pad Software Inc; 2003. [Google Scholar]
- 27.Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell surface GABAA receptors following oxygen-glucose deprivation in cultured cortical neurons. J Neurochem. 2005;92:103–113. doi: 10.1111/j.1471-4159.2004.02841.x. [DOI] [PubMed] [Google Scholar]
- 28.Mohler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 29.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 30.Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 31.Scavo L, Shuldiner AR, Serrano J, Dashner R, Roth J, dePablo F. Genes encoding receptors for insulin and insulin-like growth factor I are expressed in Xenopus oocytes and embryos. Proc Natl Acad Sci USA. 1991;88:6214–6218. doi: 10.1073/pnas.88.14.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends in Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocrine Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 34.Sigel E, Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus ooocytes. J. Neurosci. 1988;8:289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skovgaard Jensen M, Lambert JDC. Electrophysiological studies in cultured mouse CNS neurons of the actions of an agonist and an inverse agonist at the benzodiazepine receptor. Br. J. Pharmacol. 1986;88:717–731. doi: 10.1111/j.1476-5381.1986.tb16244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Heide LP, Ramakers GMJ, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Progress Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Velasco I, Tapia R. High extracellular gamma-aminobutyric acid protects cultured neurons against damage induced by the accumulation of endogenous extracellular glutamate. J Neurosci Res. 2002;67:406–410. doi: 10.1002/jnr.10114. [DOI] [PubMed] [Google Scholar]
- 38.Vetiska SM, Ahmadian G, Ju W, Liu L, Wymann MP, Wang YT. GABAA receptor associated phoshoinositide 3-kinase is required for insulin-induced recruitment of postsynaptic GABAA receptors. Neuropharmacology. 2007;52:146–153. doi: 10.1016/j.neuropharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 40.Williams DB. Comparing an effect of insulin on GABA-mediated current at a neuronal and a pancreatic isoform of the GABAA receptor. FASEB J. 2007;21:721.2. [Google Scholar]
- 41.Williams DB, Akabas MH. Residues in the M3 membrane spanning segment of the γ- aminobutyric acid type A receptor are on the water accessible surface of the protein. Biophys J. 1999;77:2563–2574. doi: 10.1016/s0006-3495(99)77091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Chen Q, Takahashi A, Goubaeva F. Kinetic properties of GABA rho1 homoeric receptors expressed in HEK293 cells. Biophys J. 2006;91:2155–2162. doi: 10.1529/biophysj.106.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao W-Q, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in the experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]