Abstract
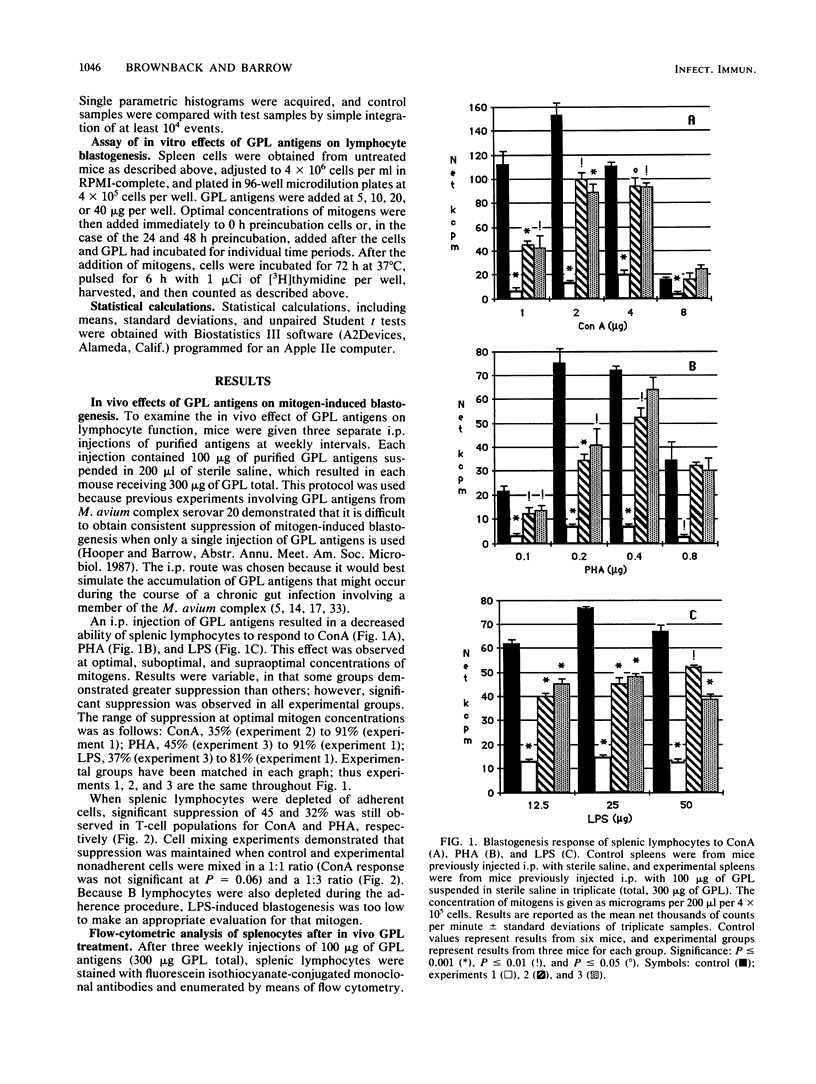
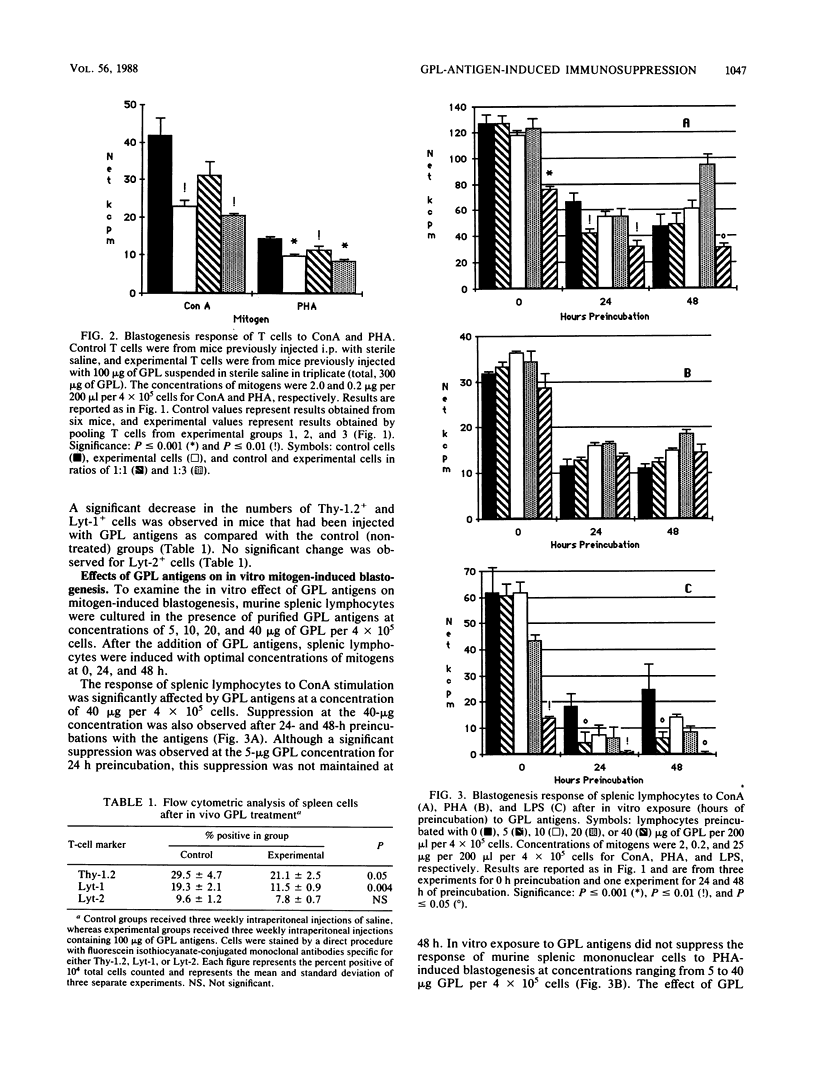
Intraperitoneal injection of glycopeptidolipid (GPL) antigens from Mycobacterium avium complex serovar 4 resulted in the decreased ability of murine splenic lymphocytes to respond to nonspecific-mitogen-induced blastogenesis when exposed to concanavalin A, phytohemagglutinin, and lipopolysaccharide. Adherent cell depletion and cell mixing experiments with T lymphocytes indicated that macrophages were not a major contributor to the immunosuppression observed in this study. Enumeration of splenic lymphocytes by means of flow-cytometry with fluorescein isothiocyanate-conjugated monoclonal antibodies demonstrated that intraperitoneal injection of GPL antigens resulted in a significant decrease in Thy-1+ and Lyt-1+ cells but no change in the numbers of Lyt-2+ cells. Treatment with GPL antigens in vitro affected the ability of splenic mononuclear cells to respond optimally for concanavalin A-induced blastogenesis at 40 micrograms of GPL per 4 X 10(5) cells per 0.2 ml and lipopolysaccharide-induced blastogenesis at concentrations ranging from 5 to 40 micrograms of GPL per 4 X 10(5) cells per 0.2 ml. However, in vitro treatment with GPL antigens did not affect phytohemagglutinin-induced blastogenesis at concentrations ranging from 5 to 40 micrograms of GPL per 4 X 10(5) cells per 0.2 ml. These findings suggest that GPL antigens or their metabolites affect lymphocyte function and may be important cofactors in the overall pathogenesis of M. avium complex infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W., Brennan P. J. Immunogenicity of type-specific C-mycoside glycopeptidolipids of mycobacteria. Infect Immun. 1982 May;36(2):678–684. doi: 10.1128/iai.36.2.678-684.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brennan P. J., Souhrada M., Ullom B., McClatchy J. K., Goren M. B. Identification of atypical mycobacteria by thin-layer chromatography of their surface antigens. J Clin Microbiol. 1978 Oct;8(4):374–379. doi: 10.1128/jcm.8.4.374-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsker B., Bottone E. J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985 Jan;151(1):179–181. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- Davidson P. T., Khanijo V., Goble M., Moulding T. S. Treatment of disease due to Mycobacterium intracellulare. Rev Infect Dis. 1981 Sep-Oct;3(5):1052–1059. doi: 10.1093/clinids/3.5.1052. [DOI] [PubMed] [Google Scholar]
- Dimitrijevich S. D., Johnson M. M., Barrow W. W. One-step column chromatographic procedure for purification of mycobacterial glycopeptidolipid antigens. J Chromatogr. 1986 Apr 25;377:345–349. doi: 10.1016/s0378-4347(00)80791-4. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Fainstein V., Bolivar R., Mavligit G., Rios A., Luna M. Disseminated infection due to Mycobacterium avium-intracellulare in a homosexual man with Kaposi's sarcoma. J Infect Dis. 1982 Apr;145(4):586–586. doi: 10.1093/infdis/145.4.586. [DOI] [PubMed] [Google Scholar]
- Fukunishi Y., Okada S., Nishiura M., Kohsaka K. Ultrastructural features of the multiplication of human and murine leprosy bacilli in macrophages of nude mice. Int J Lepr Other Mycobact Dis. 1982 Mar;50(1):68–75. [PubMed] [Google Scholar]
- Greene J. B., Sidhu G. S., Lewin S., Levine J. F., Masur H., Simberkoff M. S., Nicholas P., Good R. C., Zolla-Pazner S. B., Pollock A. A. Mycobacterium avium-intracellulare: a cause of disseminated life-threatening infection in homosexuals and drug abusers. Ann Intern Med. 1982 Oct;97(4):539–546. doi: 10.7326/0003-4819-97-4-539. [DOI] [PubMed] [Google Scholar]
- Hast R., Bernell P., Befrits R., Dowding C., Sjögren A. M. Decrease in helper (T4+) lymphocytes following cimetidine treatment for duodenal ulcer. Clin Exp Immunol. 1986 Apr;64(1):114–118. [PMC free article] [PubMed] [Google Scholar]
- Hooper L. C., Johnson M. M., Khera V. R., Barrow W. W. Macrophage uptake and retention of radiolabeled glycopeptidolipid antigens associated with the superficial L1 layer of Mycobacterium intracellulare serovar 20. Infect Immun. 1986 Oct;54(1):133–141. doi: 10.1128/iai.54.1.133-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Brannon P., Tsang A. Y., Maio M., Gold J. W., Whimbey E., Wong B., McClatchy J. K., Armstrong D. Infections caused by Mycobacterium avium complex in immunocompromised patients: diagnosis by blood culture and fecal examination, antimicrobial susceptibility tests, and morphological and seroagglutination characteristics. J Clin Microbiol. 1985 Feb;21(2):168–173. doi: 10.1128/jcm.21.2.168-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Gelmann E. P., Longo D. L., Steis R. G., Chused T., Whalen G., Edgar L. C., Fauci A. S. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985 Mar;78(3):417–422. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Masur H. Mycobacterium avium-intracellulare: another scourge for individuals with the acquired immunodeficiency syndrome. JAMA. 1982 Dec 10;248(22):3013–3013. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung N. D., Davidson P. T. Increased suppressor cell activity in a patient with Mycobacterium avium-intracellulare pulmonary disease and hypogammaglobulinemia. Ann Allergy. 1981 Apr;46(4):204–207. [PubMed] [Google Scholar]
- Rose L. M., Ginsberg A. H., Rothstein T. L., Ledbetter J. A., Clark E. A. Selective loss of a subset of T helper cells in active multiple sclerosis. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7389–7393. doi: 10.1073/pnas.82.21.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J., Rikiishi H., Nagahashi M., Ohuchi E., Kumagai K. Mitogen responsiveness of various immune tissues: heterogeneity of accessory cells and susceptibility to suppression by macrophages. Cell Immunol. 1980 Nov;56(1):1–15. doi: 10.1016/0008-8749(80)90076-3. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Respir Dis. 1965 Dec;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- Schot J. D., Elferink D., Hooijkaas H., Neijens H. J., Schuurman R. K. Deficiency of immunity to Mycobacterium avium that can be restored by allogeneic lymphocytes. Clin Exp Immunol. 1987 Apr;68(1):48–57. [PMC free article] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Wolke A., Meyers S., Adelsberg B. R., Bottone E. J., Damsker B., Schwartz I. S., Janowitz H. D. Mycobacterium avium-intracellulare-associated colitis in a patient with the acquired immunodeficiency syndrome. J Clin Gastroenterol. 1984 Jun;6(3):225–229. [PubMed] [Google Scholar]
- Yeager H., Jr, Raleigh J. W. Pulmonary disease due to Mycobacterium intracellulare. Am Rev Respir Dis. 1973 Sep;108(3):547–552. doi: 10.1164/arrd.1973.108.3.547. [DOI] [PubMed] [Google Scholar]



