Abstract
Peripheral blood and thymic double-positive (DP) CD4+CD8+ T cells from neonates have been described earlier, but the function and immunophenotypic characteristics of other tissue-derived DP T cells are not clearly understood. Here, we demonstrate the functional and immunophenotypic characteristics of DP cells in 6 different tissues, including thymus from normal neonatal rhesus macaques (Macaca mulatta) between 0 and 21 days of age. In general, intestinal DP T cells of neonates have higher percentages of memory markers (CD28+CD95+CD45RAlowCD62Llow) and proliferation compared with single-positive (SP) CD4+ and CD8+ T cells. In addition, percentages of DP T cells increase and CD62L expression decreases as animals mature, suggesting that DP cells mature and proliferate with maturity and/or antigen exposure. Consistent with this, intestinal DP T cells in neonates express higher levels of CCR5 and are the primary targets in simian immunodeficiency virus (SIV) infection. Finally, DP T cells produce higher levels of cytokine in response to mitogen stimulation compared with SP CD4+ or CD8+ T cells. Collectively, these findings demonstrate that intestinal DP T cells of neonates are proliferating, activated memory cells and are likely involved in regulating immune responses, in contrast to immature DP T cells in the thymus.
Introduction
Depletion of intestinal CD4+CD8+ double-positive (DP) cells occurs faster and more profoundly than that of intestinal CD4+ single-positive (SP) cells in adult macaques with primary simian immunodeficiency virus (SIV).1 We have also recently shown that intestinal and peripheral blood DP T cells in adult monkeys are effector memory cells, which distinguishes them from the immature DP cells found in the thymus.2 DP T cells are perhaps best known in the thymus, where they are T-cell precursors in early states of T-cell maturation. Circulating blood DP T cells have been reported in both healthy3,4 and diseased humans and macaques,5–12 and it has been hypothesized that this rare lymphocyte population results from premature release of immature CD4+CD8+ T cells from the thymus to the periphery, where their maturation into functionally competent SP cells continues.13 However, reports suggest that DP T cells in the peripheral blood (PB) of humans differ from those in the thymus.14 In HIV and Epstein-Barr virus (EBV) infections, the percentage of DP T cells can increase to 20% of circulating lymphocytes, suggesting they are effector cells responding to infection.4,5 Moreover, in humans and animals, DP T cells have also been shown to have antiviral activity, further indicating they are antigen-specific effector cells rather than immature cells released from the thymus.2,14–16
Intestinal effector lymphoid tissues (ie, lamina propria) have been shown to be the major site of viral replication and CD4+ T-cell depletion in primary HIV and SIV infection in both adult and neonates.17–19 However, DP T cells in neonates have not been characterized with respect to memory and/or effector status, activation, homing, chemokine receptor expression, or their capacity to produce cytokines upon stimulation. Conceivably, somatic DP T cells of neonates could differ from those of adults because the thymus is much more active in neonates. The continuous development and maturation of the immune system throughout childhood and adolescence emphasizes the need for age-specific data of DP T-cell populations and their potential importance in regulating or maintaining immune responses.
In this study, we performed an extensive analysis of DP T cells obtained from PB, mesenteric lymph node (MLN), thymus, spleen, and lamina propria of the jejunum and colon during the first 21 days of life of normal rhesus macaques by polychromatic flow cytometry. The distribution, frequency, and immunophenotype in regards to memory, activation, homing, and chemokine receptor expression of these cells were compared with those of SP CD4+ and SP CD8+ T cells. Furthermore, we examined and compared levels of cytokine production by SP CD4+, CD8+ cells and DP T cells in response to mitogen stimulation to assess their potential effector capabilities.
This study shows that in neonates, nonthymic DP T cells are found predominantly in the intestine, where their population increases as the animal ages. Most DP T cells have a memory phenotype and are capable of producing higher levels of cytokines than their SP counterparts. In addition, both naive and memory DP T cells in the intestine and other tissues show greater rates of proliferation compared with SP CD4+ and CD8+ T cells, whereas in the thymus, most of the proliferating cells are naive. Finally, DP T cells appear to be prime targets for SIV infection and are lost earlier in infection, presumably due to their high expression of CCR5 and their levels of activation and memory phenotype. Combined, these studies indicate that most intestinal DP T cells in neonates are central memory cells that are “primed” to respond quickly to their cognate antigen.
Methods
Animals, virus, BrdU, and tissue sampling
A total of 7 male and 3 female Indian rhesus macaques (Macaca mulatta) between 0 and 21 days of age, which were negative for HIV-2, SIV, type D retrovirus, and STLV-1 infection, were examined in this study (Table 1). Macaques were obtained from their dams at birth and hand-reared on formula thereafter. For the 0-day infants, cesarean delivery surgery was performed and the neonate was humanely killed within minutes of birth for sample collection. All other neonates were intraperitoneally inoculated with nucleotide analog bromodeoxyuridine (BrdU; 60 mg/kg in sterile saline) 24 hours before killing and tissue collection. One male and 2 female macaques were infected intravenously with 100 tissue-culture infectious dose at 50% (TCID50) SIVMAC251 within 24 hours of their birth and humanely killed either 12 (n = 2) or 21 (n = 1) days after infection for comparison with age-matched, normal, uninfected macaques (Table 1). EDTA-anticoagulated blood, MLN, thymus, spleen, and intestines (jejunum and colon) were collected at necropsy for functional and/or phenotyping experiments. All animals were housed at the Tulane National Primate Research Center in accordance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and all experiments were reviewed and approved by the Tulane Institutional Animal Care and Use Committee.
Table 1.
List of macaque neonates examined
| Category | Age, d |
||||
|---|---|---|---|---|---|
| 0 | 3 | 12 | 14 | 21 | |
| SIV-uninfected normal macaques | GP72 | GN99 | GK92 | GP43 | GP52 |
| GP44 | GB15 | ||||
| Age-matched SIV-infected macaques | GL48 | GP20 | |||
| GN84 | |||||
Uninfected normal control macaques were killed and necropsied for sample collection at 0, 3, 12, 14, or 21 days old. SIV-infected macaques were killed and necropsied for sample collection at 12 or 21 days after infection. All the infected macaques were infected with SIVmac251 within 24 hours after their birth. Macaques with the prefix G represent individual neonates.
Lymphocyte isolation from tissues
Lymphocytes from the PB, intestine, MLN, thymus, and spleen were isolated as previously described.2,20,21 In brief, intestinal epithelial lymphocytes (IELs) were separated from intestinal pieces by incubating 1-cm2 pieces of tissue in EDTA with rapid shaking at 37°C. Mucus and large debris were removed from the supernatant by filtering through loosely passed glass wool. After epithelial removal, lamina propria lymphocytes (LPLs) were collected by mincing the remaining tissue into 1- to 2-mm pieces, followed by digestion in complete RPMI 1640 medium (RPMI 5) containing 60 U/mL type II collagenase (Sigma-Aldrich, St Louis, MO), again with rapid shaking at 37°C. For enrichment of lymphocytes, supernatants of LPLs were centrifuged over discontinuous Percoll (Sigma-Aldrich) density gradients followed by washing with phosphate-buffered saline (PBS). For lymphocyte isolation from MLN, spleen, and thymus, tissues were simply minced and gently pressed through 70-μm nylon cell strainers. All cells were washed twice and resuspended in complete RPMI-5 medium containing 5% fetal calf serum (FCS) before staining. All lymphocytes were more than 90% viable by the trypan blue dye exclusion method.
Immunofluorescent staining and flow cytometric analysis
For flow cytometric staining, cells were adjusted to 107 cells/mL and 100 μL aliquots or 100 μL of whole blood samples were incubated with appropriately diluted concentrations of antibodies for 30 minutes at 4°C. Whole blood and spleen samples were then lysed and washed using a whole-blood lysis protocol as previously described.2,18 Stained cells were then washed once with PBS and fixed with 1× BD stabilizing fixative buffer (BD Biosciences, San Jose, CA). For BrdU staining, the cells were first surface-stained, then fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences), washed in Perm Buffer (BD Biosciences), incubated for 30 minutes at room temperature with FITC-conjugated anti-BrdU in Perm Buffer, washed, and resuspended in 1× BD stabilizing fixative buffer (BD Biosciences). Cells were kept protected from light at 4°C, and acquisition was performed within 24 hours of staining. Lymphocytes from MLN, intestinal LPLs, and thymus were stained and processed similar to blood tissues with the exception of the whole-blood lysing technique.2 Polychromatic (9- to 11-parameter) flow cytometric acquisition was performed on a FACSAria Becton Dickinson instrument (Franklin Lakes, NJ) with 3 lasers (488-nm blue laser, 633-nm red laser, and 407-violet laser) using FITC, PE, PE–Texas Red, PE-Cy5, PE-Cy7, APC, APC-Cy7, Pacific Blue, and AmCyan as the available fluorochrome parameters. Single-stained controls for each fluorochrome were used for compensation settings. Monoclonal antibodies CD3 Pacific Blue (SP32-2), CD69 APC-Cy7 (FN50), CD28 APC (CD28.2), CCR7 PE or PE-Cy7 (3D12), CD95 PE-Cy5 (DX2), CD62L PE (SK11), CD45RA FITC (5H9), CCR5 PE or APC (3A9), CXCR4 PE-Cy5 (12G5), and BrdU FITC (3D4) were obtained from BD Biosciences. CD8 PE–Texas Red (MHCD0817), CD8β-PE (ST8.5H7), and CD4 AmCyan (L200) were obtained from Caltag Laboratories (Burlingame, CA), Beckman Coulter (Fullerton, CA), and the National Institutes of Health (NIH) Nonhuman Primate Reagent Resource (NPRR; Boston, MA) courtesy of Dr K. Reimann (Harvard University, Cambridge, MA), respectively. At least 20 000 events were collected from each sample by gating on lymphocytes. Data were analyzed using FlowJo software (TreeStar, Ashland, OR) version 8.7.1.
Cytokine flow cytometry assay
To examine cytokine production, a cytokine flow cytometry (CFC) assay was used to detect either CD3+, CD4+, and/or CD8+ T lymphocytes that produced cytokines (IL-2/IFN-γ/TNF-α) in response to mitogen stimulation according to methods described previously.2,22,23 Briefly, peripheral blood mononuclear cells (PBMCs), jejunum LPLs, and splenic lymphocytes were resuspended at 106 cells/mL in complete RPMI 10 with 10% FCS and stimulated with PMA (50 ng/mL) and ionomycin (1 μg/mL). After stimulation, cells were stained for cell-surface markers with directly conjugated mAbs to CD3 PerCP (clone SP34-2), CD4 APC (clone L200), and CD8 FITC or PE (clone SK1) from BD Biosciences for 30 minutes at room temperature and washed with Dulbecco PBS (dPBS)/bovine serum albumin (BSA) wash buffer. Cells were then fixed and permeabilized by using Cytofix/Cytoperm (BD Biosciences), washed twice in Perm Buffer (BD Biosciences), and intracellularly stained with mAbs like IL-2 FITC (MQ1-17H12)/ IFN-γ FITC (4S.B3) and/or TNF-α FITC or PE (Mab11) at room temperature for 30 minutes. Single color and isotype-matched control antibodies were used to confirm staining specificity. After washing in perm buffer, cells were resuspended in 1% paraformaldehyde in PBS and stored in the dark at 4°C. For each sample, at least 50 000 CD3+CD4+ T cells were collected using a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA) using CellQuest software (BD Immunocytometry Systems) and further analyzed with FlowJo software. Positive cytokine responses were determined based on the percentage of cytokine responses obtained above background responses (unstimulated medium control) in each experiment.
Statistics
Results of experimental groups were compared using either a 2-tailed Student paired t test or nonparametric Mann-Whitney t test using Prism software (GraphPad, SanDiego, CA). P values less than .05 were considered significant.
Results
Neonatal DP T-cell populations in intestine increase over time as the animal ages
For statistical analyses, all uninfected neonates were grouped into either 0 to 3 days (group A) or 12 to 21 days (group B) of age to compare DP T-cell populations between newborn and aged neonates. Frequencies of SP CD4+, SP CD8+, and DP CD4+CD8+ T cells in both groups A and B were compared between blood, MLN, spleen, thymus, jejunum, and colon LPLs by flow cytometry (Figure 1A). In group A, the ratio of SP CD4+ to CD8+ T cells was 3.6:1 in blood, 2.9:1 in jejunum LPLs, 2.0:1 in colon LPLs, 5.1:1 in MLN, 1.6:1 in spleen, and 2.3:1 in thymus. Similarly in group B, the ratio of SP CD4+ to CD8+ T cells was in 2.9:1 in blood, 2.7:1 in jejunum LPLs, 2.9:1 in colon LPLs, 4.3:1 in MLN, 1.4:1 in spleen, and 2.1:1 in thymus. Significantly higher percentages of DP T cells were detected in jejunum LPLs of group B (mean, 8.5% of gated CD3+ T cells; range, 6.8%-11.9%), compared with group A neonates (mean, 1.7%; range, 1.4%-2.4%; P < .05). In addition, significantly (P < .05) higher percentages of DP cells were detected in the jejunum LPLs compared with PB cells of neonates in group B. Similarly, there were significantly higher percentages (P < .05) of DP cells in the thymus of group B (mean, 82.5%) compared with group A (mean, 74.7%) neonates. In other tissues there were also higher percentages of DP T cells in older (group B) neonates, but these data were not significant.
Figure 1.
Distribution of SP CD4+, CD8+ and DP T cells in various tissues and their phenotype from normal uninfected neonates of 2 age groups. (A) Mean percentages of SP and DP CD4+/CD8+ T-cell populations of normal uninfected neonates are shown. Each bar represents the mean plus or minus SEM percentage of T-cell subsets in newborn to 3-day-old (group A) versus 12- to 21-day-old (group B) neonates. Note that DP T cells increase in tissues, particularly the intestine with age. (B) Representative flow cytograms of SP CD4+, CD8+, and DP T cells showing the percentages of naive (CD28+CD95−), central memory (CD28+CD95+), and effector memory (CD28−CD95+) T-cell populations in each polygonal gate as well as other naive/memory markers (CD45RA, CCR7), L-selectin (CD62L), and the early activation marker (CD69) in jejunum LPLs in each quadrant from a normal uninfected 21-day-old neonate examined by 9-color flow cytometry. Note, that in contrast to SP T cells, essentially all DP cells in the jejunum have a “transitional memory” (CD28+CD95+CCR7+) phenotype. Plots were generated by gating first through lymphocytes using forward scatter (FSC) and side scatter (SSC) parameters and then through CD3+ T cells. (C) Mean frequencies of naive and memory cell populations are shown for different tissues from neonates of different ages. Each bar represents mean plus or minus SEM. * indicates significant differences between group A and B neonates for the specified T-cell subsets.
Naive and memory cell differentiation was performed by defining the expression of CD45RA, CD62L, CD95, and CD28 on different T-cell subsets (SP CD4+/CD8+ and DP T cells) in macaques as previously described2,24,25 (Figure 1B,C). Higher percentages of naive (CD28+CD95−) T cells were detected in PB, MLN, thymus, and spleen compared with jejunum LPLs in both groups (Figure 1C). In newborn animals (group A), naive cells were most frequent within SP CD4+ T-cell populations (mean, 95.9%) followed by DP (mean, 90.4%) then SP CD8+ T cells (mean, 82.4%) in PB. Similar percentages of naive cells were observed within SP CD4+ (range, 89.9%-95.3%) and CD8+ (range, 66.2%-94.5%) T cells from MLN, spleen, and thymus (Figure 1C). In both age groups, jejunum LPLs had significantly fewer naive cells in CD3+ T-cell subpopulations compared with other tissues (P < .05). In group A, the mean percentages of naive (CD28+CD95−) and central memory (CD28+CD95+) T-cell populations were 36.4%, 39.1%, and 45.2% and 55.2%, 40.0%, and 52.7% for SP CD4+, CD8+, and DP T cells, respectively. Naive (CD28+CD95−) T-cell populations in intestinal tissues decreased as animals aged, presumably due to exposure to foreign (dietary) antigens. In group B, mean percentages of naive (CD28+CD95−) and central memory (CD28+CD95+) T-cell populations were 40.7%, 36.0%, and 11.8% and 57.0%, 46.4%, and 84.7% for SP CD4+, CD8+, and DP T cells, respectively. In addition, significantly increased percentages of central memory and lower percentages of naive DP T cells were observed in older (group B) neonates (P < .05). In group B, all DP cells in the jejunum have a central memory (CD28+CD95+CD45RAlowCD62Llow) phenotype, whereas in contrast, substantial numbers of SP CD4 and CD8+ cells have a naive phenotype (Figure 1B). In summary, there was an overall increase in memory T-cell subpopulations observed in most tissues (except thymus) as neonates matured.
DP T cells lose CD45RA and increase activation marker expression in aged neonates
In both groups, very low levels of activated (CD69+) T cells were detected in PB, whereas other tissues demonstrated a wide range of CD69 expression (mean range, 12.9%-73.7% in group A and 10.1%-81.4% in group B) in all T-cell subsets (Figure 2). Overall, higher levels of CD69 were detected in intestinal tissues (mean range, 40.3%-65.3% and 40.0%-81.4% in groups A and B macaques, respectively) and SP CD4+ and CD8+ thymocytes (mean value, 50.4%-73.7% and 45.6%-78.5% in groups A and B macaques, respectively). Interestingly, as animals aged, CD69 expression increased significantly on DP T cells in both jejunum and colon LPLs (P < .05) compared with other tissues. No significant differences were observed in CD69 expression in thymus between these groups in any of the T-cell subsets.
Figure 2.
Relative expression of activation, trafficking, and naive/memory markers expression in different subpopulations of CD3+ T cells. Mean percentages of CD69, CD62L, CD45RA, and CCR7 are compared between different T-cell subsets (SP, DP) and between age groups. Bars represent mean values of 3 to 4 macaques plus or minus SEM. * indicates significant differences between group A (0- to 3-day-old) and B (12- to 21-day-old) neonates for the specified T-cell subsets.
High expression of CD62L,26 a lymph node homing molecule, was detected in all 3 T-cell subsets (SP CD4+, SP CD8+, and DP) in PB, MLN, and spleen in group A neonates. In group B neonates, the expression of CD62L in blood, MLN, and spleen was decreased, and there were significant differences between groups A and B neonates among SP CD8+ and DP T cells. In intestinal tissues, high expression of CD62L was detected on DP T cells in group A, yet CD62L was markedly reduced in group B neonates (67.0% vs 8.3% and 42.7% vs 10.8% in jejunum and colon LPLs, respectively; P < .05). Expression of CD62L in thymus did not differ significantly in any of these T-cell compartments (Figure 2).
Distribution of CD45RA, predominately expressed on naive/resting T cells and medullary thymocytes, was examined and compared in all tissues (Figure 2). High expression of CD45RA was detected on SP CD4+, SP CD8+, and DP T cells in the PB (mean range, 78.5%-94.7% and 82.4%-85.8% in groups A and B, respectively), MLN (mean range, 82.3%-94.5% and 73.6%-95.2% in groups A and B, respectively), and spleen (mean range, 85.4%-96.8% and 68.8%-77.6% in groups A and B, respectively). However, CD45RA expression was comparatively lower in all intestinal tissues and even lower for DP T cells in group B neonates compared with group A neonates (P < .05). In contrast, CD45RA expression was very low in all 3 populations of thymic CD3+ T cells (mean value range, 1.2%-9.5%).
CCR7, an important chemokine receptor with a role in trafficking of B and T lymphocytes and dendritic cells,27 was also studied. High expression of CCR7 was detected in PB (mean range, 80.4%-91.8% and 70.9%-93.7% in groups A and B, respectively), MLN (mean range, 80.2%-86.9% and 74.8%-87.9% in groups A and B, respectively) and spleen (mean range, 66.7%-75% and 61.8%-77.9% in groups A and B, respectively). Lower expression of CCR7 was detected in both jejunum and colon LPLs (mean range, 44.5%-66.7% and 50.8%-68.3% in groups A and B, respectively). As neonates aged, CCR7 expression in jejunum LPLs decreased on DP T cells (P < .05). CCR7 expression was also lower on colon LPLs in older (group B) neonates, but these results were not significant. All 3 populations of CD3+ T cells in the thymus showed low to moderate CCR7 expression (mean range, 12.7%-33.5% and 14.2%-39.6% in group A and B neonates, respectively).
To further characterize these DP T cells, they were examined for both CD8αα (CDαβnegative) and CD8αβ expression. Interestingly, increased percentages of CD8αα expression was detected in PB (51.3%), MLN (52.7%), and LPL (92.4%) DP T cells, whereas the majority of DP T cells in thymus were CD8αβ cells (98.7%; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, the majority of the SP CD8+ T cells were CD8αβ+.
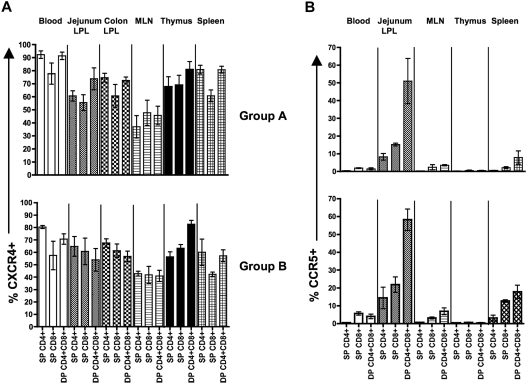
Increased CCR5 expression and cytokine production by intestinal DP T cells
The major chemokine coreceptors (CCR5 and CXCR4) responsible for HIV/SIV entry were compared in different tissues of neonates (Figure 3A,B). In blood, higher CXCR4 expression was observed on SP CD4+ T cells (mean, 92.4% and 80.4% in groups A and B, respectively) compared with DP T cells (mean value, 91.4% and 70.8% in groups A and B, respectively). CXCR4 expression on MLN was significantly lower than blood for all T-cell subsets (P < .05) in group A neonates. In addition, all T-cell subsets in the jejunum LPLs with the exception of DP T cells expressed significantly lower levels of CXCR4 compared with PB T cells (P < .05) in group A neonates. As animals aged, CXCR4 expression increased on SP CD4+/CD8+ T cells, yet decreased on DP T cells (54.0%-56.7% vs 64.8%-67.6%) in intestinal LPLs, but these differences were not significant. However, DP T cells in jejunum LPLs did have significantly higher levels of CCR5 compared with SP CD4+ T cells (51.0% vs 8.2% and 58.3% vs 14.4% in groups A and B neonates, respectively; P < .05; Figure 3B). In addition, aged neonates had increased expression of CCR5 on DP T cells compared with group A neonates, again suggesting this molecule increases in the gut in response to antigen exposure. Collectively, these results indicate that jejunum LPL DP T cells express higher levels of CCR5 compared with SP CD4+ and CD8+ T cells.
Figure 3.
Increased CCR5 expression by DP CD4+CD8+ T cells subsets in neonates. Mean percentages of cells expressing CXCR4 (A) and CCR5 (B) are shown for all subpopulation of T cells as indicated. Percentages represent means of 3 to 4 macaques plus or minus SEM.
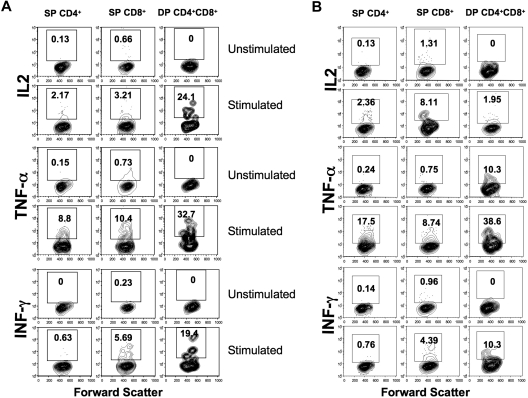
To compare effector functions of DP cells to those of SP CD4+ and CD8+ T cells, lymphocytes from group B neonates were stimulated with PMA/ionomycin and examined for cytokine production. DP T cells had increased production of cytokines (IL2, IFN-γ, and TNF-α mean range from 19.0%-33%) compared with SP CD4+ (mean range, 0.15%-2.61%) and CD8+ T cells (mean range, 0.73%-11.2%) in PBMCs, jejunum LPLs, and spleen. However, IL-2 production by splenic CD8+ T cells was higher than both SP CD4 and DP T cells (Figure 4A,B). Overall, these results indicate that DP T cells in the intestine are capable of producing more cytokines compared with SP CD8+ and SP CD4+ T cells.
Figure 4.
Increased cytokine production by DP CD4+CD8+ T-cell subsets in neonates. Cytokine production by mitogen-stimulated or unstimulated jejunum lamina propria (A) and spleen (B) T-cell subsets in a normal, uninfected neonate at 12 days old. Cells were unstimulated or stimulated with PMA/ionomycin, and cytokine production was detected by intracellular cytokine staining. The percentage of cytokine-producing cells is indicated in the top box of each panel. Note that DP T cells had higher IL-2, TNF-α, and IFN-γ responses compared with SP CD4+/CD8+ T cells in jejunum LPLs, whereas SP CD8+ T cells had higher IL-2 responses in spleen lymphocytes.
Increased rate of lymphocyte turnover in DP CD4+CD8+ T cells
Because more DP cells in neonates had a memory phenotype compared with SP CD4+/CD8+ T cells, we hypothesized that DP cells would have higher rates of proliferation than their SP counterparts. To test this, group B neonates with BrdU inoculation were selected, where only cells in S-phase (DNA synthesis) cell division would incorporate BrdU. Overall, DP T cells demonstrated the highest level of BrdU incorporation in all tissues (mean, 12.8%-30.5%), followed by SP CD8+ T cells (mean, 2.8%-9.7%) and SP CD4+ T cells (mean, 1.4%-7.2%; Figure 5A). Moreover, intestinal T cells (particularly SP CD4+ and CD8+ T cells) had higher percentages of BrdU incorporation compared with all other tissues examined. We also examined BrdU+ cells for naive/memory phenotypes in older neonates (group B), and showed that memory cells (CD95+) in most tissues had markedly increased turnover rates compared with naive (CD95−) T-cell subpopulations (mean value ranged from 0.4% to 14% in all T-cell subsets). However, there were markedly higher percentages of naive BrdU+ cells in the thymus, and substantial percentages of naive BrdU+ cells were also detected in the blood and spleen (Figure 5B-D).
Figure 5.
Increased proliferative capacity in DP CD4+CD8+ T cells. (A) Bar charts showing the mean BrdU+ proliferative responses in different T-cell subsets from different tissues. (B) Mean BrdU expression in naive (CD95−) and memory (CD95+) T-cell subsets are shown from different tissues in normal, uninfected neonates 12 to 21 days old. Each bar represents mean plus or minus SEM. Representative flow cytograms show increased proliferative capacity in DP T cells in whole blood (C) and jejunum LPLs (D). Note that memory (CD95+) cells have higher proliferative responses compared with naive (CD95−) cells. Percentages of CD95+ and BrdU+ cells are shown in each quadrant (C and D).
Combined, these data indicate that in all tissues, DP T cells have much higher rates of proliferation than SP cells. In addition, DP T cells increase in most tissues (intestines, spleen) predominantly due to expansion of preexisting memory cells, yet proliferating DP cells in the thymus are essentially all naive, again emphasizing these differ from DP cells in other tissues.
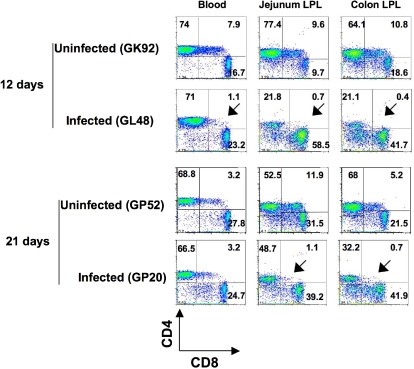
Early loss of DP T cells during SIV infection
To assess whether DP T cells in neonates were major targets of SIV infection, we compared DP T cells in tissues of SIV-infected and normal age-matched uninfected neonates. There was a marked reduction of DP T cells in intestinal tissues on both day 12 and day 21 after SIV infection (Figure 6). Furthermore, the ratio of DP T-cell loss in the intestine was much higher than for SP CD4+ T cells in this tissue compared with age-matched uninfected neonates (1:7.4 to 1:27 for DP T cells and 1:1 to 1:3.5 for SP CD4+ T cells). Mild reductions in DP T cells were also detected in other tissues, but these were not significant, probably because these tissues had lower basal levels of DP T cells in general.
Figure 6.
Early loss of DP T cells in intestinal tissues in SIV infection. Dot plots comparing T-cell subsets from intestinal (jejunum and colon) tissues and peripheral blood of 2 normal, uninfected neonatal macaques (GK92 and GP52) to age-matched SIVmac251-infected (GL48 and GP20) neonates. Plots were generated by gating on lymphocytes and then through CD3+ T cells. Note that a more dramatic loss of DP CD4+CD8+ T cells was detected in intestinal tissues in infected macaques very early in infection (as indicated by the arrows) compared with other tissues. The percentages of CD4+ and CD8+ cells are shown in each quadrant.
Discussion
Immature DP T cells in the thymus have been well characterized.28 T-cell maturation begins in the thymus as triple-negative (CD3−CD4−CD8−) T-cell precursors originating in the bone marrow, populate the thymus, and begin to express CD3, becoming double-negative (DN) T (CD4−CD8−) T cells. These cells then become DP cells and, through positive selection, develop into SP cells. Thymic DP cells that bind with major histocompatibility complex (MHC) class II antigens become SP CD4+ T cells, whereas DP cells that bind MHC class I antigen complexes become SP CD8+ T cells, which are both released into the circulation where they may migrate to secondary lymphoid tissues.5 Eventually, antigenic stimulation induces proliferation and differentiation of SP CD4+ and CD8+ T cells into effector cells with the ability to participate in immune responses against pathogens.
The current study demonstrates that DP T cells are present in all the neonatal tissues examined, particularly in the thymus, intestine, and blood. However, percentages of central memory DP T cells increase in the intestine, blood, lymph node, and spleen as neonates age, whereas DP cells in the thymus maintain a naive phenotype (Figure 1C). In addition, differences in phenotypic and functional characteristics between thymus and extrathymic DP T cells were detected in neonates. Importantly, DP T cells demonstrated higher rates of proliferation compared with their SP counterparts. The mechanism by which the DP T cells are lost during the acute phase of SIV infection remains unclear. DP T cells were rapidly depleted in intestinal tissues, presumably due to their higher expression of CCR5 and increased levels of cellular activation that support SIV infection and replication, respectively. Because these DP T cells are activated cells, the immune activation after SIV infection may also predispose these cells to activation-induced apoptosis.
Comparing newborn animals (group A) to older neonates (group B) demonstrated a marked expansion of memory cells at the expense of the naive T-cell pool and an increased expression of activation markers (CD69) on DP cells as the animal ages in all tissues examined except the thymus. This suggests that in contrast to thymic DP cells, DP cells in extrathymic tissues develop and mature in response to antigenic stimulation. Although CD69 was also expressed at high levels on thymocytes in both groups of neonates, CD69 expression in the thymus is known to be associated with T-cell development and is expressed in the early stages of positive selection.29 Presence of CD69 in DP T-cell population in thymus suggest that those cells are ready for the positive selection.29 Although indirect indication of cell activation is the expression of CD62L (L-selection), a peripheral homing receptor expressed on naive CD4+ and CD8+ T cells in various tissues and is involved in “tethering” and subsequent rolling of lymphocytes along endothelial cells of secondary lymphoid tissues and sites of inflammation.30 However, lymphocyte activation leads to rapid proteolytic cleavage and release of L-selectin, leading to loss of CD62L expression on “activated” T-cell subsets. Thus, the fact that we found markedly decreased expression of CD62L on DP cells in all tissues except for the thymus in older neonates (Figure 2) also supports the hypothesis that these cells become activated as animals age and increase their exposure to environmental antigens. Similarly, decreased CD45RA (a marker for naive T cells) was detected on all DP T cells except for those in the thymus, again indicating extrathymic DP cells were transitioning to a memory phenotype. Increased naive markers on DP T-cell populations of 0- to 3-day-old neonates may represent recent thymic emigrants that have left the thymus before down-regulating CD4 or CD8. Alternatively, the increased expression of CD8αα in intestinal DP T cells compared with thymic DP T cells suggests that those cells might have differentiated outside the thymus within the intestinal mucosa.31–36 We have also shown that intestinal DP T cells had higher expression of both CCR5 and CXCR4. However, CXCR4 was highly expressed on thymic DP T cells compared with SP CD4/8 T cells. These observations support an earlier report showing that immature T cells had higher CXCR4 expression compared with mature CD3+ thymocytes.37,38 Importantly, the majority of the DP T cells in intestine are memory cells with high CCR5 expression, suggesting they are prime targets for SIV/HIV infection and replication. Memory CD4+ T cells have been shown to be the major target for both SIV and HIV infection.1,18,39–41 These data demonstrate that DP T cells in the intestine are rapidly eliminated at a faster rate than SP CD4+ T cells, presumably due to their phenotype and state of activation and are consistent with previous studies in adult macaques.1
Few studies have described the function of DP cells in humans5,14,42,43 and in monkeys.2 Antigen-specific cytotoxic and proliferation responses of DP cells have been examined in response to CMV and HIV-1 viral antigens that demonstrated they are effector cells.42 The data presented here also support the role of intestinal DP T cells in neonates in having effector functions against pathogens or in regulating intestinal homeostatic immune responses.2,14,16 In comparison to SP cells, DP cells of neonates have much greater capacity to produce cytokines in response to mitogens (Figure 4). By modeling the rate of BrdU uptake in vivo in normal neonatal macaques without SIV, we were able to study the differences in the lymphocyte turnover rates in different T-cell subsets. Despite the presence of higher CD4 count in these neonates, the SP CD8+ T-cell turnover was higher than SP CD4+ T cells. Again DP T cells had the highest rate of proliferation in the tissues examined. Similarly, CD8+ T-cell turnover in SIV− rhesus macaques appeared to be equal to or higher than CD4+ T lymphocyte turnover in adult rhesus macaques.44 From this present study, it can be presumed that these highly proliferating, activated, and memory DP T cells are the early target cells for SIV/HIV infection. This paper is also reporting on the cycling properties of naive and memory T-cell subsets in the normal SIV− neonates. The turnover rate in CD95+ memory cells was higher than the CD95− naive T lymphocytes that has been reported earlier in adult SIV− monkeys.44 In future studies, it will be important to define the antigen specificity of these DP T cells by generating antigen-specific DP T-cell clones and characterizing their MHC restriction.
To our knowledge, this is the first report comparing the phenotype and function of DP and SP T cells in tissues of neonatal primates. The DP T cells also produced high levels of cytokines (IL2, IFN-γ, and TNF-α) in response to mitogen, suggesting that these cells may have cytotoxic function and capabilities similar to or even greater than those of SP CD8+ T cells. In summary, this study demonstrates that the intestinal DP T cells are highly proliferative, activated memory T cells rather than immature DP T cells found in thymus, and their population increases as the animal ages and plays an important role in cytotoxic function.
Supplementary Material
Acknowledgments
We thank Maryjane Dodd, Janell LeBlanc, Linda Green, Kelsi Rasmussen, Maury Duplantis, Nancy Parr, Melinda Martin, and all animal care staff of the Department of Veterinary Medicine for their technical assistance and Calvin Lanclos, Edmund Benes, and Julie Bruhn for their excellent help in operating the FACSAria and FACSCalibur instruments, respectively.
This work was supported by National Institutes of Health grants AI062410, AI49080, RR00164, RR016930, and RR018397.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.W., B.P., and R.S.V. performed all experiments; B.P. and R.S.V. designed this experiment and wrote the manuscript; X.W., A.D., and B.P. performed all flow cytometry staining and its analysis; A.A.L. and R.S.V. provided instrumentation, data interpretation, sample support, and assisted in writing the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bapi Pahar, Division of Immunology, Tulane National Primate Research Center, 18703 Three Rivers Road, Covington, LA 70433; e-mail: bpahar@tulane.edu.
References
- 1.Veazey RS, Mansfield KG, Tham IC, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 3.Sala P, Tonutti E, Feruglio C, Florian F, Colombatti A. Persistent expansions of CD4+ CD8+ peripheral blood T cells. Blood. 1993;82:1546–1552. [PubMed] [Google Scholar]
- 4.Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem Biophys Res Commun. 1993;191:601–609. doi: 10.1006/bbrc.1993.1260. [DOI] [PubMed] [Google Scholar]
- 5.Weiss L, Roux A, Garcia S, et al. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis. 1998;178:1158–1162. doi: 10.1086/515674. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M, Ido E, Uesaka H, et al. Comparison of susceptibility to SIVmac239 infection between CD4(+) and CD4(+)8(+) T cells. Arch Virol. 2005;150:1517–1528. doi: 10.1007/s00705-005-0536-7. [DOI] [PubMed] [Google Scholar]
- 7.Akari H, Nam KH, Mori K, et al. Effects of SIVmac infection on peripheral blood CD4+CD8+ T lymphocytes in cynomolgus macaques. Clin Immunol. 1999;91:321–329. doi: 10.1006/clim.1999.4700. [DOI] [PubMed] [Google Scholar]
- 8.Scott CS, Wheeler R, Ford P, Bynoe AG, Roberts BE. T lymphocyte subpopulations in idiopathic thrombocytopenic purpura (ITP). Scand J Haematol. 1983;30:401–406. doi: 10.1111/j.1600-0609.1983.tb02525.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsui M, Fukuyama H, Akiguchi I, Kameyama M. Circulating CD4+CD8+ cells in myasthenia gravis: supplementary immunological parameter for long-term prognosis. J Neurol. 1989;236:329–335. doi: 10.1007/BF00314374. [DOI] [PubMed] [Google Scholar]
- 10.Airo P, Rossi G, Facchetti F, et al. Monoclonal expansion of large granular lymphocytes with a CD4+ CD8dim+/− phenotype associated with hairy cell leukemia. Haematologica. 1995;80:146–149. [PubMed] [Google Scholar]
- 11.Bagot M, Echchakir H, Mami-Chouaib F, et al. Isolation of tumor-specific cytotoxic CD4+ and CD4+CD8dim+ T-cell clones infiltrating a cutaneous T-cell lymphoma. Blood. 1998;91:4331–4341. [PubMed] [Google Scholar]
- 12.Parel Y, Aurrand-Lions M, Scheja A, Dayer JM, Roosnek E, Chizzolini C. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum. 2007;56:3459–3467. doi: 10.1002/art.22927. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez E, Sacedon R, Vicente A, Hernandez-Lopez C, Zapata AG, Varas A. Rat peripheral CD4+CD8+ T lymphocytes are partially immunocompetent thymus-derived cells that undergo postthymic maturation to become functionally mature CD4+ T lymphocytes. J Immunol. 2002;168:5005–5013. doi: 10.4049/jimmunol.168.10.5005. [DOI] [PubMed] [Google Scholar]
- 14.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 15.Periwal SB, Cebra JJ. Respiratory mucosal immunization with reovirus serotype 1/L stimulates virus-specific humoral and cellular immune responses, including double-positive (CD4+/CD8+) T cells. J Virol. 1999;73:7633–7640. doi: 10.1128/jvi.73.9.7633-7640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500–512. [PMC free article] [PubMed] [Google Scholar]
- 17.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Rasmussen T, Pahar B, et al. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veazey RS, Lifson JD, Pandrea I, Purcell J, Piatak M, Jr., Lackner AA. Simian immunodeficiency virus infection in neonatal macaques. J Virol. 2003;77:8783–8792. doi: 10.1128/JVI.77.16.8783-8792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veazey RS, Rosenzweig M, Shvetz DE, et al. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 22.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J Immunol Methods. 2003;282:103–115. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okoye A, Meier-Schellersheim M, Brenchley JM, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitcher CJ, Hagen SI, Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Ivetic A, Ridley AJ. The telling tail of L-selectin. Biochem Soc Trans. 2004;32:1118–1121. doi: 10.1042/BST0321118. [DOI] [PubMed] [Google Scholar]
- 27.Gosling J, Dairaghi DJ, Wang Y, et al. Cutting edge: identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J Immunol. 2000;164:2851–2856. doi: 10.4049/jimmunol.164.6.2851. [DOI] [PubMed] [Google Scholar]
- 28.Pier GB, Geri H, Mody C, Preston M. T-cell maturation and activation. Washington, DC: ASM Press; 2004. [Google Scholar]
- 29.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–1872. [PubMed] [Google Scholar]
- 30.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 31.Naito T, Shiohara T, Hibi T, Suematsu M, Ishikawa H. RORyt is dispensable for the development of intestinal mucosal T cells. Mucosal Immunol. 2008;1:198–207. doi: 10.1038/mi.2008.4. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Oida T, Hamada H, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13:691–702. doi: 10.1016/s1074-7613(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 33.Saito H, Kanamori Y, Takemori T, et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 34.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 35.Howie D, Spencer J, DeLord D, et al. Extrathymic T cell differentiation in the human intestine early in life. J Immunol. 1998;161:5862–5872. [PubMed] [Google Scholar]
- 36.Spencer J, MacDonald TT, Finn T, Isaacson PG. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin Exp Immunol. 1986;64:536–543. [PMC free article] [PubMed] [Google Scholar]
- 37.Zaitseva MB, Lee S, Rabin RL, et al. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 38.Pedroza-Martins L, Boscardin WJ, Anisman-Posner DJ, Schols D, Bryson YJ, Uittenbogaart CH. Impact of cytokines on replication in the thymus of primary human immunodeficiency virus type 1 isolates from infants. J Virol. 2002;76:6929–6943. doi: 10.1128/JVI.76.14.6929-6943.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 40.Veazey RS, Marx PA, Lackner AA. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 2001;22:626–633. doi: 10.1016/s1471-4906(01)02039-7. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 42.Suni MA, Ghanekar SA, Houck DW, et al. CD4(+)CD8(dim) T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31:2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 43.Zloza A, Sullivan YB, Connick E, Landay AL, Al-Harthi L. CD8+ T cells that express CD4 on their surface (CD4dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood. 2003;102:2156–2164. doi: 10.1182/blood-2002-07-1972. [DOI] [PubMed] [Google Scholar]
- 44.De Boer RJ, Mohri H, Ho DD, Perelson AS. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J Immunol. 2003;170:2479–2487. doi: 10.4049/jimmunol.170.5.2479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.