Abstract
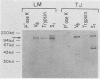
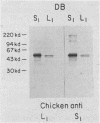
To evaluate the variation in the expression of cell wall antigens between Staphylococcus aureus grown in liquid medium and solid support, bacteria were harvested from liquid chemically defined medium and chemically defined medium in a 1% agar base. Cell wall proteins were then extracted by lysostaphin in a protoplast-stabilizing medium (30% raffinose). After separation of the cell wall antigens by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blots, they were probed with chicken antiserum to an S. aureus strain grown on a solid support. For each of the 15 clinical strains analyzed, high-molecular-size bands (molecular size range, 120 to 220 kilodaltons) were either enhanced or distinctly present when compared with those from the cell wall extract of the same strain grown in liquid medium. Results of enzymatic treatment of whole staphylococci grown on solid medium suggested the proteinaceous nature and the surface location of these antigens. Limited passage studies demonstrated the ability of the staphylococci to alter these surface proteins when passaged alternately on liquid and solid media. These observations suggested the importance of the microenvironment to the expression of cell wall proteins in S. aureus. Correlations with observations in vivo may help identify the determinants of microbial pathogenicity in S. aureus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkas T., Watson C. M. Induction of an Fc conformational change by binding of antigen: the generation of protein A-reactive sites in chicken immunoglobulin. Immunology. 1979 Mar;36(3):557–561. [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brock J. H., Reiter B. Chemical and biological properties of extracellular slime produced by Staphylococcus aureus grown in high-carbohydrate, high-salt medium. Infect Immun. 1976 Mar;13(3):653–660. doi: 10.1128/iai.13.3.653-660.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Bayer A. S., Peters J., Ward J. I. Analysis by gel electrophoresis, Western blot, and peptide mapping of protein A heterogeneity in Staphylococcus aureus strains. Infect Immun. 1987 Apr;55(4):843–847. doi: 10.1128/iai.55.4.843-847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975 Feb 1;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt R. D. Immune response to surface antigens of Staphylococcus aureus and their role in resistance to staphylococcal disease. Ann N Y Acad Sci. 1974 Jul 31;236(0):203–220. doi: 10.1111/j.1749-6632.1974.tb41492.x. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukelekian H., Heller A. Relation between Food Concentration and Surface for Bacterial Growth. J Bacteriol. 1940 Oct;40(4):547–558. doi: 10.1128/jb.40.4.547-558.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J. Kinetic investigation of the staphylococcal protease-catalyzed hydrolysis of synthetic substrates. Eur J Biochem. 1976 Sep 15;68(2):621–627. doi: 10.1111/j.1432-1033.1976.tb10850.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (second of two parts). N Engl J Med. 1984 May 31;310(22):1437–1442. doi: 10.1056/NEJM198405313102206. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]