Abstract
Astrocyte swelling and brain edema are major neuropathological findings in the acute form of hepatic encephalopathy (fulminant hepatic failure, FHF), and substantial evidence supports the view that elevated brain ammonia level is an important etiological factor in this condition. Although the mechanism by which ammonia brings about astrocyte swelling remains to be determined, oxidative/nitrosative stress and mitogen-activated protein kinases (MAPKs) have been considered as important elements in this process. One factor known to be activated by both oxidative stress and MAPKs is nuclear factor κB (NFκB), a transcription factor that activates many genes, including inducible nitric oxide synthase (iNOS). Since the product of iNOS, nitric oxide (NO), is known to cause astrocyte swelling, we examined the potential involvement of NFκB in ammonia-induced astrocyte swelling. Western blot analysis of cultured astrocytes showed a significant increase in NFκB nuclear translocation (a measure of NFκB activation) from 12 h to 2 days after treatment with NH4Cl (5 mM). Cultures treated with antioxidants, including superoxide dismutase, catalase and vitamin E, as well as the MAPKs inhibitors SB239063 (an inhibitor of p38-MAPK), and SP600125 (an inhibitor of c-Jun N-terminal kinase, JNK) significantly diminished NFκB activation by ammonia, supporting a role of oxidative stress and MAPKs in NFκB activation. The activation of NFκB was associated with increased iNOS protein expression and NO generation, and these changes were blocked by BAY 11-7082, an inhibitor of NFκB. Additionally, ammonia-induced astrocyte swelling was inhibited by the NFκB inhibitors BAY 11-7082 and SN-50, thereby implicating NFκB in the mechanism of astrocyte swelling. Our studies indicate that cultured astrocytes exposed to ammonia display NFκB activation, which is likely a consequence of oxidative stress and activation of MAPKs. NFκB activation appears to contribute to the mechanism of ammonia-induced astrocyte swelling, apparently through its upregulation of iNOS protein expression and the subsequent generation of nitric oxide.
Keywords: ammonia, astrocyte swelling, hepatic encephalopathy, iNOS, MAPK, NFκB, oxidative stress
INTRODUCTION
Hepatic encephalopathy (HE) is a complex neuropsychiatric syndrome resulting from severe liver failure (Jones and Weissenborn, 1997). Brain edema, increased intracranial pressure and brain herniation are the principal neurological complications in patients with the acute form of HE (fulminant hepatic failure, FHF) that occurs following viral hepatitis, acetaminophen toxicity and other acute liver disorders (Ware et al., 1971; Jalan, 2005; Blei, 2005). It presents with the abrupt onset of delirium, seizures, and coma and it has an extremely poor prognosis with an 80–90% mortality rate (Capocaccia and Angelico, 1991). The only effective treatment for this condition is an emergency liver transplantation (Bismuth et al., 1996).
Astroglial swelling is generally believed to represent a major component of the brain edema in FHF (Norenberg 1977; Traber et al. 1987; Swain et al. 1991). Ammonia has been strongly implicated as a major toxin in liver failure (for review, see Albrecht and Jones, 1999; Hazell and Butterworth, 1999), and ammonia has been shown to induce astrocyte swelling in in vivo models of hyperammonemia (Voorhies et al., 1983; Willard-Mack et al., 1996), brain slices (Ganz et al., 1989) and astrocyte cultures (Norenberg et al., 1991; Zwingman et al., 2000). While the precise mechanism by which ammonia brings about such swelling remains poorly understood, recent studies have invoked oxidative/nitrosative stress and mitogen-activated protein kinases (MAPKs) in the mechanism of ammonia-induced astrocyte swelling (for review, see Norenberg et al., 2007).
Oxidative stress (OS) and the activation of MAPKs are known to stimulate transcription factors, including nuclear factor κB (NFκB) (for reviews, see Bowie and O’Neill, 2000; Kyriakis and Avruch, 2001; Bubici et al., 2006). NFκB is known to activate many genes, including inducible nitric oxide synthase (iNOS), resulting in excessive nitric oxide (NO) generation (for reviews, see Xie et al., 1994; Kleinert et al., 2004). Previous studies have shown that the NO donors, SNAP and SIN-1, caused astrocyte swelling and that treatment of cultures with the non-specific nitric oxide synthase (NOS) inhibitor, L-NAME, significantly diminished ammonia-induced astrocyte swelling (Jayakumar et al., 2006). Collectively, these studies suggest the possibility that NFκB may represent an integral component in the mechanism of astrocyte swelling in FHF.
In this study we demonstrate that astrocytes exposed to ammonia caused activation of NFκB, and the inhibition of such activation attenuated ammonia-induced astrocyte swelling. We also show that NFκB activation is mediated by oxidative stress and MAPKs activation, and that the activation of NFκB results in the upregulation of iNOS protein expression and the subsequent generation of nitric oxide (NO); the latter likely representing the proximate cause of ammonia-induced astrocyte swelling.
MATERIALS AND METHODS
Astrocyte cultures
Primary cultures of cortical astrocytes were prepared as described previously (Ducis et al., 1990). Briefly, brains of 1–2 day old rat pups were seeded onto 35 mm culture dishes in DMEM containing penicillin, streptomycin, and 15% fetal bovine serum (FBS), and the culture plates were incubated at 37°C with 5% CO2 and 95% air. Culture media was changed twice weekly. On day 10 post seeding, FBS was replaced with 10% horse serum. After 14 days, the cultures were treated with 0.5 mM dibutyryl cyclic AMP (Sigma, St. Louis, MO) to enhance cellular differentiation (Juurlink and Hertz, 1985). Cultures consisted of 95–99% astrocytes as determined by glial fibrillary acidic protein (GFAP) immunohistochemistry. All cultures used in the experiments were 25–28 days old. Procedures followed guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals, and were approved by the local animal care committee (IACUC).
Western blots
Samples of astrocytes were analyzed for iNOS and NFκB protein content by Western blots as described previously (Jayakumar et al., 2006). In brief, equal amounts of protein were subjected to gel electrophoresis and immunoblotting. Antibodies against NFκB-p65 (H-286) (cat# sc-7151) and iNOS (1:750 and 1:1000 dilution, respectively; Santa Cruz Biotechnology, Inc, Santa Cruz, CA) were used to detect NFκB and iNOS protein expression. Anti-α-tubulin antibody was obtained from Oncogene (San Diego, CA) and lamin antibody was purchased from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies (Vector Laboratories, Burlingame, CA) were used at 1:1000 dilutions. Membranes were visualized using an enhanced chemiluminescence reagent. The optical density of the bands was measured with the Chemi-Imager digital imaging system (Alpha Innotech Corp, San Leandro, CA), and the results were quantified with the Multi-Analyst software (Sigma Scan Pro 5, Deerfield, IL) as a proportion of the signal of house keeping protein bands (lamin and α-tubulin, nuclear and cyctosolic markers, respectively).
Preparation of nuclear extract and Western blots for NFκB
The nuclear extract was prepared as described by Calabrese et al. (2005). In brief, astrocytes were washed twice with phosphate-buffered saline (PBS), then harvested in 1 ml PBS and centrifuged at 3,000 rpm for 3 min at 4°C. The cell pellet was resuspended in 200 μl cold buffer A, consisting of 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 μM dithiothreitol (DTT), and a complete protease inhibitor cocktail (Roche, Mannheim, Germany). The pellet was then incubated on ice for 15 min to allow cells to swell, after which 15 μl of 10% NP-40 was added, and the sample was vortexed thoroughly for 40 sec. An aliquot of homogenate was used for protein measurement. Equal amount of protein was then centrifuged at 3,000 rpm for 3 min at 4°C. The resulting nuclear pellet was resuspended in 30 μl cold buffer B consisting of 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 μM DTT, and protease inhibitors. The pellet was then incubated on ice and vortexed for 15 sec every 2 min for up to 15 min. The nuclear extract was then centrifuged at 13,000 rpm for 5 min at 4°C. The supernatant (containing the nuclear extract) was loaded on an SDS-polyacrylamide gel, and Western blot analysis with NFκB antibody was performed as described above. The quality of the nuclear extract was analyzed by propidium iodide staining, which indicated a purity of 92–96%.
Immunofluorescence of NFκB
Immunocytochemistry was performed on cultured astrocytes to directly visualize the translocation of the p65 subunit of NFκB after ammonia treatment. Cultures were fixed with 4% paraformaldehyde for 30 min at RT. To prevent non-specific binding, cells were initially treated with PBST (PBS + 0.1% Triton X-100) containing 10% normal goat serum for 30 min, and then incubated overnight with a rabbit polyclonal antibody against p65 (H-286) (cat# sc-7151). Following washing with PBST, astrocytes were incubated with a goat anti-rabbit fluorescent antibody (Alexa Fluor 488, Molecular Probes, Eugene, OR, USA) for 25 min. Cultures were also stained with propidium iodide (PI; 2 μg/ml) to determine the total number of nuclei and the results were visualized with a Nikon Diaphot inverted fluorescent microscope equipped with multivariant fluorescent filters.
Both cytoplasmic and nuclear NFkB staining was observed in ammonia-treated as well as in control cultures, but intensity of nuclear staining was increased in ammonia-treated cells. However, to more clearly ascertain differences in nuclear fluorescence between these two groups, the intensity threshold value of nuclei was set as low as possible in the control cultures so as to avoid any cytoplasmic staining. The same intensity threshold was then applied to the ammonia-treated cultures. The number of NFkB-positive nuclei was counted from 5 random fields of the PI image (10x objective) using Sigma Scan Pro 5, and the results were expressed as a percent change over control. The data were analyzed using a chi-square test.
Cell volume measurement
Astrocyte cell volume (intracellular water space) was determined using 3-O-methyl-[3H]-glucose (OMG, NEN Life sciences, Boston, MA) by the method of Kletzien et al. (1975), as modified for astrocyte cultures by Kimelberg (1987) and Bender and Norenberg (1998). This method is based on the principle that OMG is transported into the cell by the glucose carrier and equilibrates between the intra- and extracellular compartments. The amount of OMG accumulated in the cell is proportional to the intracellular water space or cell volume. Cultures were incubated with [3H] OMG (1 mM containing 1 μCi of radioactive OMG) for 12 h, and at the end of incubation a small aliquot of medium was saved for specific activity determination. Cultures were quickly washed 5 times with ice-cold buffer containing 290 mM sucrose, 1 mM Tris-nitrate (pH 7.4), 0.5 mM calcium nitrate, and 0.1 mM phloretin. Cells were then lysed in 0.5 ml of 1N sodium hydroxide. Radioactivity was converted to intracellular water space and expressed as μl/mg cell protein. Protein content was determined employing the bicinchoninic acid assay (Pierce, Rockford, IL).
Measurement of nitric oxide (NO) generation
NO production was analyzed in cultured astrocytes using DAF-2DA, a green fluorescence probe specific for intracellular NO (Kojima et al., 1998; Fass et al., 2004). Control and ammonia-treated culture plates were washed three times with serum-free culture medium and incubated with DAF-2DA (10 μM) for 15 min at 37°C. Cultures were then washed two to three times with PBS, scraped into 500 μl of 0.2% Triton X-100 and sonicated for 5 sec. A small aliquot of the cell extract was removed for protein estimation and 250 μl of the extract was transferred to a 96-well microtiter plate (Fluorolite 1000, Dynex Technologies Inc, Chantilly, VA, USA). Fluorescence was measured at an excitation wavelength of 492 nm, and the emission fluorescence at 515 nm. The results were expressed as % fluorescence intensity.
Statistical Analysis
All experiments performed were repeated 4–7 times using cells derived from different batches of astrocyte cultures. The number of individual culture plates in each experimental group was 5 for astrocyte swelling, 2–4 for Western blots, and 2–3 for immunocytochemistry studies. The data of all experiments were subjected to analysis of variance followed by Tukey’s post hoc comparisons. A value of p<0.05 was considered significant. Error bars represent mean ± SEM.
RESULTS
Ammonia activates NFκB in cultured astrocytes
Cultured astrocytes were exposed to a pathophysiological concentration of ammonia (NH4Cl, 5 mM) (Swain et al., 1992) for different time periods (1–48 h), and changes in NFκB nuclear translocation (activation) were determined by Western blots of the nuclear fraction. Astrocytes showed no significant change in nuclear NFκB translocation for up to 6 h following ammonia treatment. A significant increase in nuclear NFκB translocation was first observed at 12 h, which remained elevated at 24 and 48 h (84.2, 137.8, and 161.0%, respectively; p<0.05 vs control) (Fig. 1). This increase was blocked by inhibitors of NFκB, BAY 11-7082 (5 μM) and SN-50 (3 μM) (76.3 and 66.8%, respectively, as compared to control, p<0.05) (Fig. 2).
Figure 1.
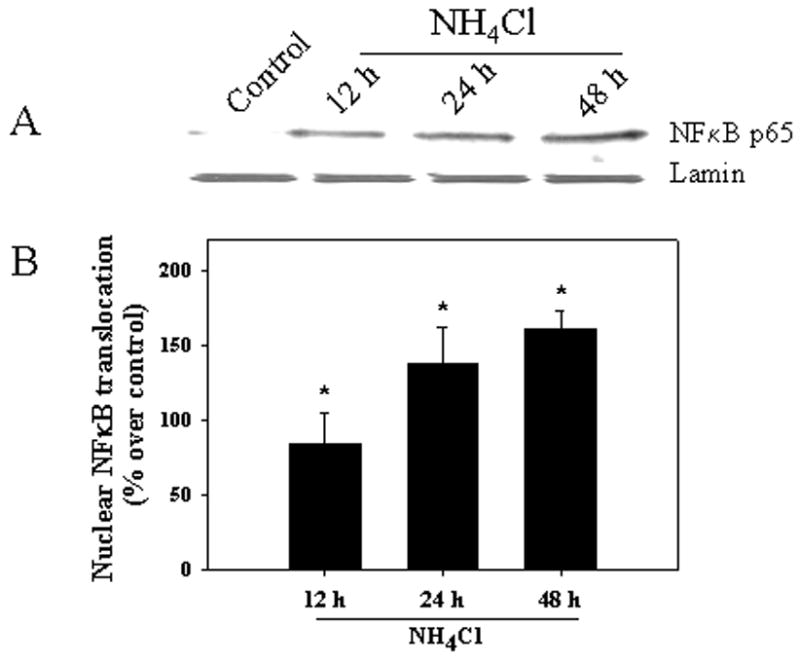
Increased nuclear NFκB was identified in cultured astrocytes treated with 5 mM NH4Cl at 12–48 h. A. Western blots of nuclear fraction; B. Quantification of Western blots. No significant increase in nuclear NFκB was detected at 1, 3 and 6 h (data not shown). NFκB content was normalized against lamin. ANOVA, n=5. *p<0.05 vs. control.
Figure 2.
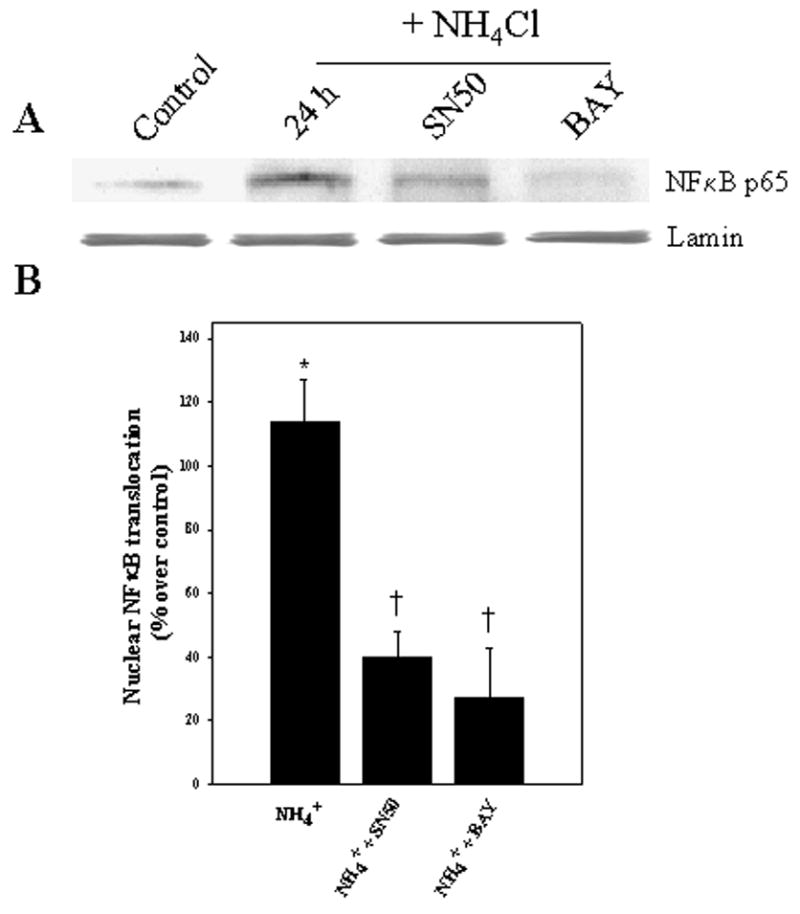
NFκB inhibitors diminish ammonia-induced translocation of NFκB to nuclei in cultured astrocytes as detected by immunoblots. A. Cultures exposed to 5 mM NH4Cl by 1 day significantly increased NFκB translocation, while co-treatment with BAY 11-7082 (BAY, 5 μM) and SN50 (3.5 μM) significantly diminished such translocation. B. Quantification of ammonia-induced NFκB content after treatment with BAY 11-7082. NFκB content was normalized against lamin. ANOVA, n=5. *p<0.05 vs. control; † p<0.05 vs. NH4Cl.
NFκB translocation to the nucleus was also assessed by immunocytochemistry by measuring the number of NFκB positive nuclei in control and ammonia-treated astrocyte cultures. The percent of NFκB-positive nuclei was greater in ammonia-treated cultures when compared to controls at 24 h (4-fold increase as compared to controls; p<0.05) (Fig. 3).
Figure 3.
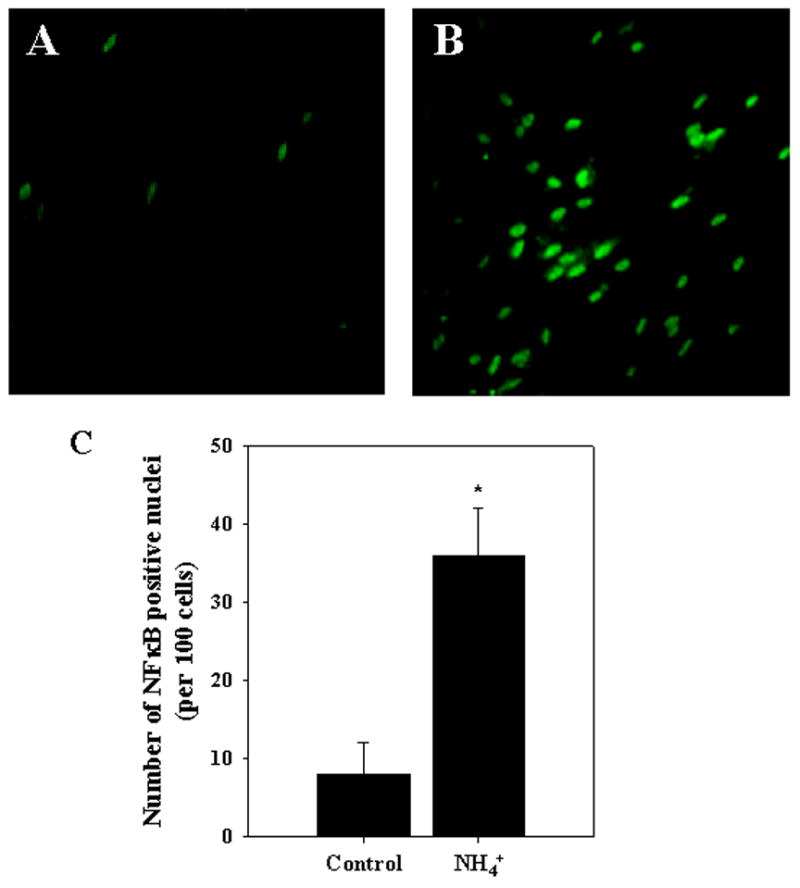
Immunocytochemical staining for NFκB in ammonia-treated cultured astrocytes. A. Control. B. After 24 h of ammonia treatment. C. Quantification of immunocytochemical staining of ammonia-treated cultured astrocytes. Ammonia progressively increased the percentage of NFκB-possitive nuclei. n=4. *p<0.05 vs. control.
Antioxidants attenuate NFκB activation by ammonia
To determine whether oxidative stress is involved in the mechanism of ammonia-induced NFκB activation, we examined the effect of various antioxidants, including superoxide dismutase (SOD; 25 units/ml), catalase (250 units/ml), α-tocopherol (vitamin E; 100 μM), desferioxamine (DFX) and N-tert-butyl-α-phenylnitrone (PBN; 50 μM) on NFκB activation by ammonia. SOD, vitamin E and catalase all significantly diminished ammonia-induced NFκB activation (69.7, 59.8 and 55.0% respectively, p<0.05), whereas DFX and PBN attenuated the ammonia-induced NFκB activation, but this decrease was not statistically significant (Fig. 4).
Figure 4.
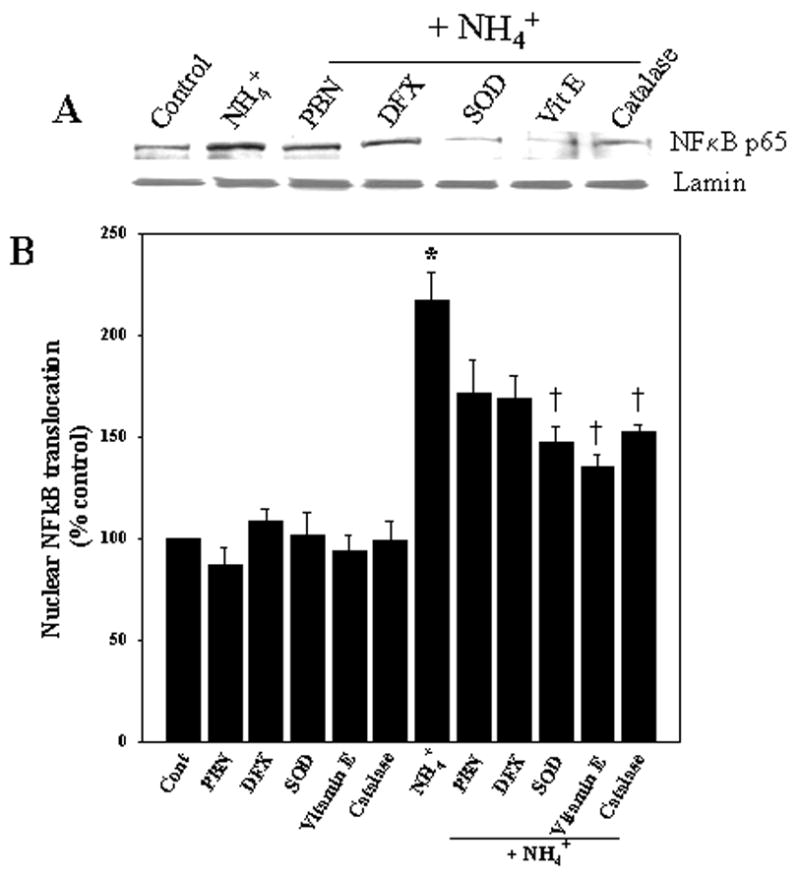
Antioxidants attenuate the ammonia-induced NFκB nuclear translocation. A. Cultured astrocytes exposed to 5 mM NH4Cl significantly increased nuclear NFκB content at 24 h. Co-treatment with SOD (25 units/ml), vitamin E (100 μM) and catalase (250 units/ml) significantly diminished ammonia-induced NFκB translocation, while desferioxamine (DFX) and PBN showed minimal effect. B. Quantification of ammonia-induced NFκB translocation after treatment with antioxidants. NFκB content was normalized against lamin. ANOVA, n=4. *p<0.05 vs. control; †p<0.05 vs. NH4Cl.
MAPK inhibitors attenuate NFκB activation by ammonia
Ammonia-induced OS was recently shown to activate MAPKs in cultured astrocytes (Jayakumar et al., 2006). Since MAPKs are known to activate NFκB, we examined whether inhibition of MAPK activation could diminish the ammonia-induced activation of NFκB. Concentrations of inhibitors that prevented ammonia-induced MAPK phosphorylation (Jayakumar et al., 2006) were chosen for the present studies. Astrocyte cultures were treated with 10 μM U0126 (1, 4-diamino-2, 3-dicyano-1, 4-bis (2-aminophenylthio) butadiene), an inhibitor of MEK, the upstream kinase of ERK1/2; 10 μM SB239063 (an inhibitor of p38-MAPK) and 1 μM SP600125 (an inhibitor of JNK), with and without ammonia. Cultures treated with SB239063 and SP600125 significantly dimished ammonia-induced NFκB activation (at 24 h by 79.5% and 46.6%, respectively) (Fig. 5). UO126 showed no significant inhibitory effect suggesting a lack of involvement of ERK1/2 in the ammonia-induced NFκB activation (Fig. 5). Cultures treated with drug alone had no effect on NFκB activation.
Figure 5.
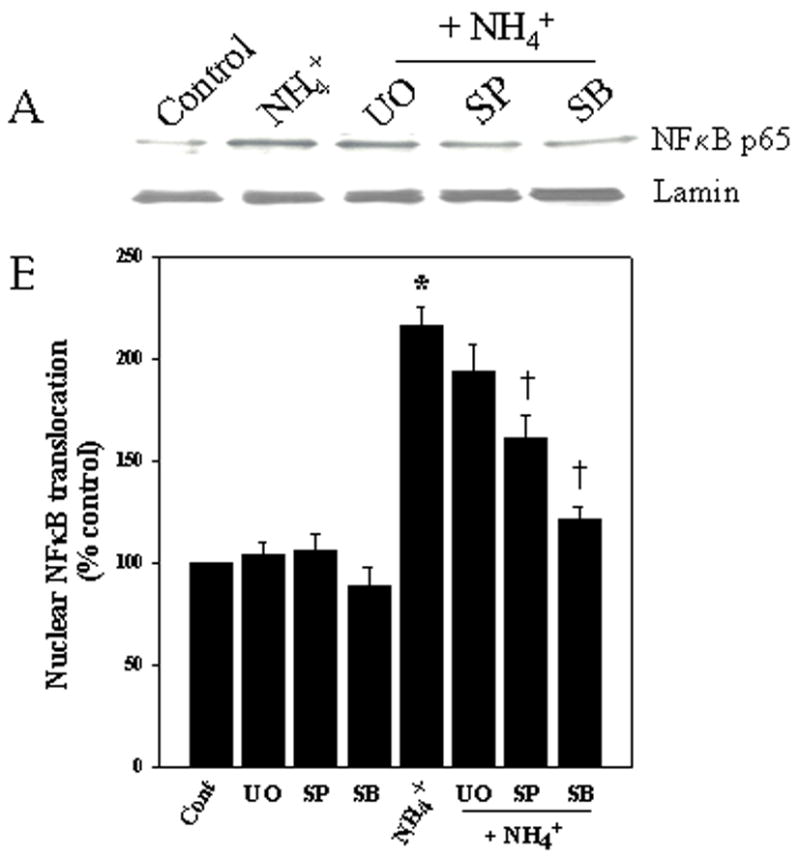
MAPK inhibitors prevent ammonia-induced NFκB translocation. A. Western blots reveal a slight, but not significant, reduction in NFκB activation at 1 day when cultures were exposed to the ERK1/2 inhibitor UO126 (10 μM). However, a significant inhibition in NFκB activation was observed when cultures were co-treated with inhibitors of p38-MAPK, SB239063 (10 μM) and JNK, SP600125 (1 μM). B. Quantification of ammonia-induced NFκB translocation after treatment with MAPK inhibitors. NFκB content was normalized against lamin. ANOVA, n=4. *p<0.05 vs. control; †p<0.05 vs. NH4Cl. SB, SB239063; UO, UO126; SP, SP600125.
NFκB inhibitors diminish ammonia-induced astrocyte swelling
To investigate the role of NFκB in ammonia-induced astrocyte swelling, we examined the effect of NFκB inhibitors on cell swelling. Astrocytes were treated with different concentrations of NFκB inhibitors BAY 11-7082 and SN-50, as mentioned above, with or without ammonia for 24 h. Ammonia significantly increased astrocyte swelling by 47.2% (p<0.05, compared to control). Treatment with BAY 11-7082 (at 5 μM) and SN-50 (at 3.5 μM) significantly diminished ammonia-induced astrocyte swelling by 85.2 and 78.6%, respectively (p<0.05) (Fig. 6).
Figure 6.
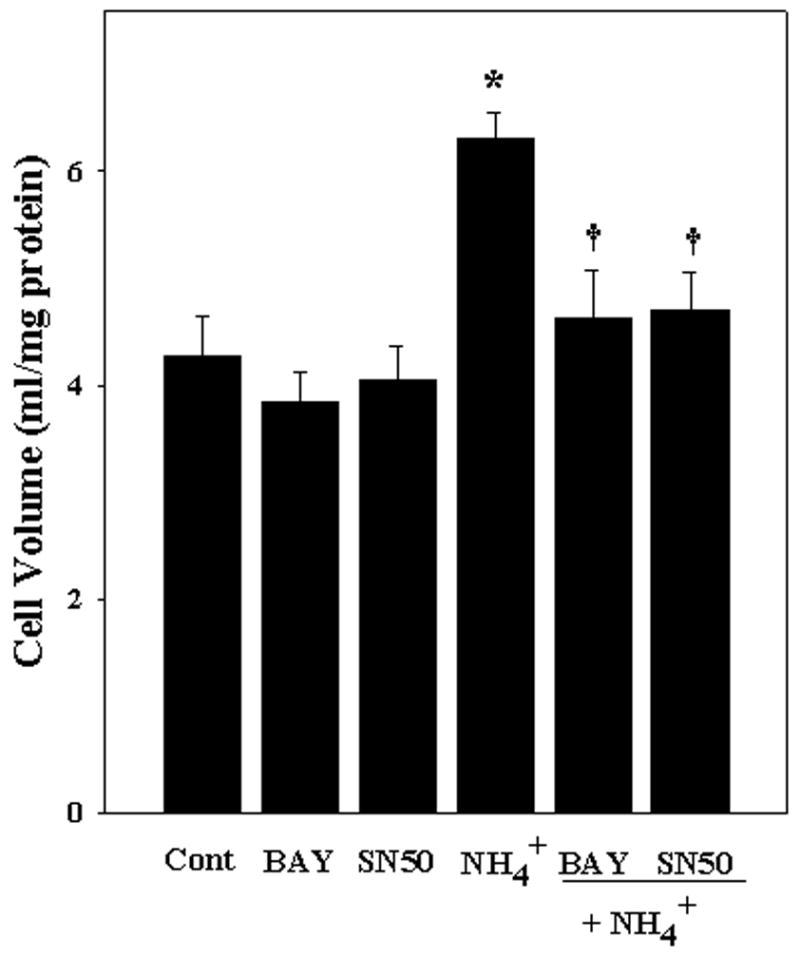
NFκB inhibitors prevent ammonia-induced astrocyte swelling. Cultured astrocytes exposed to 5 mM NH4Cl significantly increased cell swelling (47.19% by 24 h). Co-treatment with BAY 11-7082 (5 μM) and SN50 (3.5 μM) significantly diminished the extent of astrocyte swelling. ANOVA, n=7. *p<0.05 vs. control; † p<0.05 vs. NH4Cl.
Inhibition of NFκB reduces ammonia-induced iNOS protein expression and NO generation
We have recently shown that inhibition of NOS by L-NAME diminished astrocyte swelling in cultures following ammonia treatment (Jayakumar et al., 2006). Since NFκB is a major transcriptional factor responsible for iNOS gene induction, we determined whether NFκB-mediated iNOS protein expression and subsequent increase in NOS activity and NO generation might explain the mechanism by which NFκB activation causes astrocyte swelling. Astrocyte cultures exposed to ammonia (5 mM) for 12 and 24 h showed an increase in iNOS protein expression (Fig. 7A), in agreement with a previous report by Schliess et al. (2002). Ammonia also increased NO production (Fig. 7B). These changes were significantly attenuated by the NFκB inhibitor BAY 11-7082 (5 μM).
Figure 7.
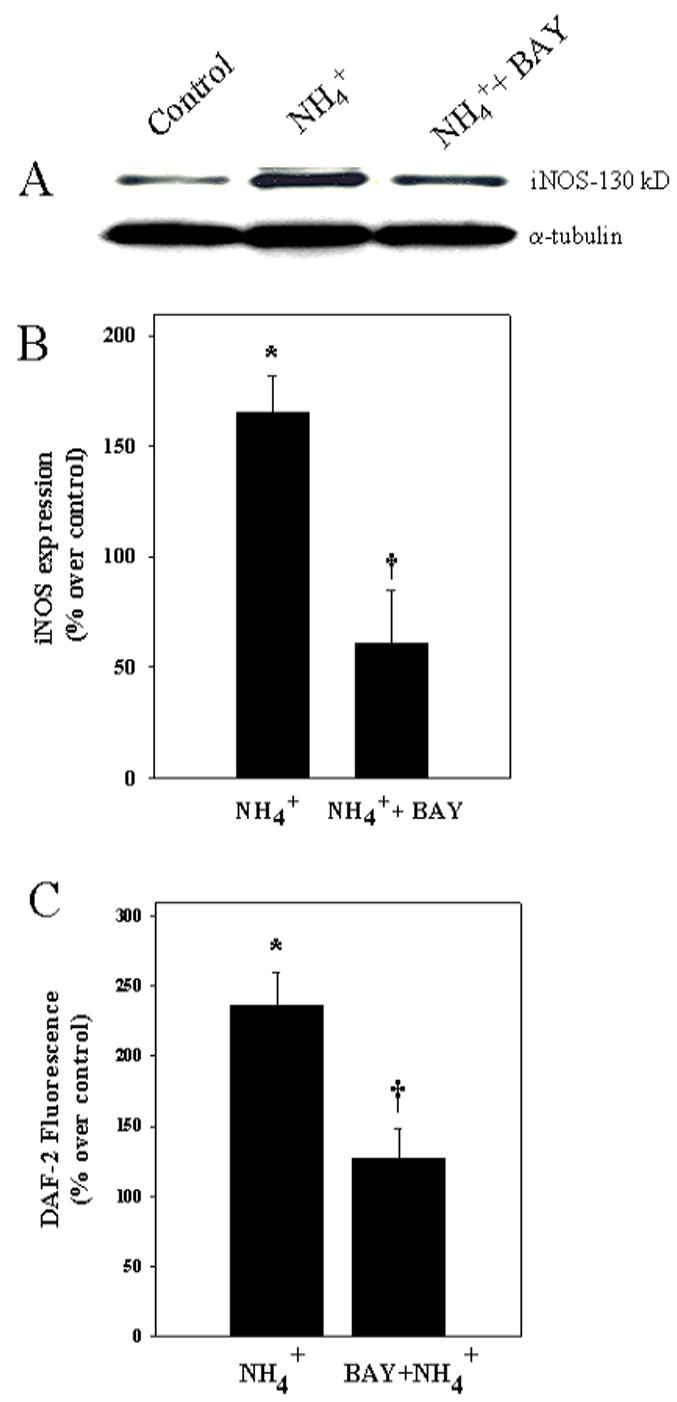
NFκB inhibitors prevent ammonia-induced iNOS protein expression and NO generation. A. Cultured astrocytes exposed to 5 mM NH4Cl for 24 h increased iNOS protein expression and co-treatment with BAY 11-7082 (5 μM) significantly diminished this effect. B. Quantification of ammonia-induced iNOS protein expression. C. Cultures exposed to 5 mM NH4Cl for 24 h increased nitric oxide generation, and such effect was significantly diminished by co-treatment with BAY 11-7082 (5 μM). iNOS expression was normalized against α-tubulin ANOVA, n=5. *p<0.05 vs. control; † p<0.05 vs. NH4Cl.
Inhibition of iNOS prevent ammonia-induced astrocyte swelling
To investigate the role of iNOS in ammonia-induced astrocyte swelling, we examined the effect of the specific iNOS inhibitor, aminoguanidine, on cell swelling. Astrocytes were treated with different concentrations of aminoguanidine (0.1, 0.3, 0.5 and 1 mM) with or without ammonia for 24 h. Ammonia significantly increased astrocyte swelling by 40.2% (p<0.05, compared to control). Treatment with 500 μM aminoguanidine partially prevented ammonia-induced astrocyte swelling, while 1 mM completely blocked it (Fig. 8).
Figure 8.
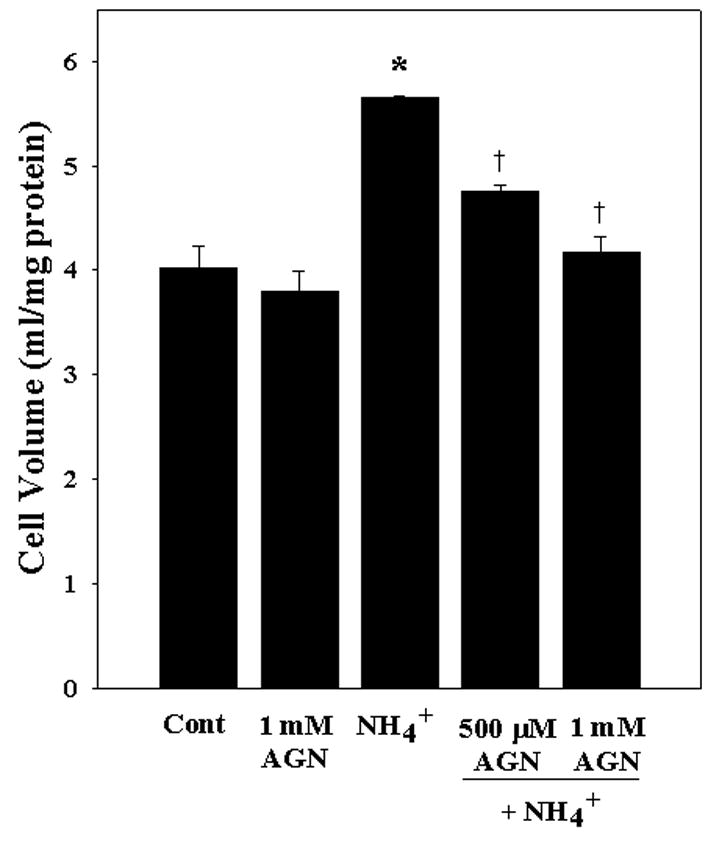
Inhibition of iNOS prevents ammonia-induced astrocyte swelling. Cultured astrocytes exposed to 5 mM NH4Cl significantly increased cell swelling (40.2% by 24 h). Co-treatment with the iNOS inhibitor aminoguanidine (AGN) blocked such swelling. ANOVA, n=3. *p<0.05 vs. control; † p<0.05 vs. NH4Cl.
DISCUSSION
This study demonstrates that astrocyte cultures exposed to ammonia increases NFκB nuclear translocation (a measure of NFκB activation) as shown by Western blots and immunocytochemical detection of its translocation to the nucleus. These findings are consistent with studies by Schliess et al. (2002), who showed IkB degradation following ammonia treatment in cultured astrocytes. NFκB activation was significantly diminished by antioxidants and by inhibitors of MAPKs, indicating a role of oxidative stress and MAPKs in the ammonia-induced activation of NFκB. Blockade of NFκB activation reduced the extent of ammonia-induced astrocyte swelling. Such blockade was associated with a reduction in the ammonia-induced increase in iNOS protein expression and NO production. These studies suggest that oxidative stress and related MAPKs contribute to the ammonia-induced activation of NFκB, and that such activation leads to iNOS upregulation and increased NO formation; the latter likely represents a major factor in the mechanism of ammonia-induced astrocyte swelling.
NFκB is an important element in the biological response to stress (Bowie and O’Neill, 2000). NFκB is kept in an inactive form in the cytoplasm where it is bound to a member of the IκB family of inhibitory proteins (Baeuerle and Baltimore, 1988; Israel, 1995). Normally, NFκB is activated by physiological as well as pathological stimuli. Upon cell activation, the IκB inhibitors are modified through site-specific phosphorylation which targets them for subsequent ubiquitination and proteolytic degradation by the proteasome. Removal of the inhibitor unmasks the nuclear localization signals on subunits of NFκB allowing it to translocate to the nucleus where it binds to target DNA elements and activates the transcription of many genes, especially those involved in immune responses, inflammation and cell proliferation (for review, see Baldwin, 1996).
Oxidative stress (OS) was previously shown to be involved in ammonia-induced astrocyte swelling (Jayakumar et al., 2006). While the mechanism by which oxidative stress induces swelling remains to be elucidated, OS is known to activate NFκB (Bowie and O’Neill, 2000), and such activation was blocked by antioxidants (Li et al., 2006; Caccamo et al., 2005; Suzuki, et al., 1992; Schreck et al., 1992; Ho et al., 1999). Consistent with these results, our study shows that the ammonia-induced oxidative stress may be responsible for the activation of NFκB, as various antioxidants were able to inhibit this activation.
One mechanism by which ammonia-induced oxidative stress may lead to NFκB activation is through the stimulation of intracellular signaling kinases, in particular, the mitogen-activated protein kinases (MAPKs) (Kyriakis and Avruch, 2001; Bubici et al., 2006). Ammonia has been shown to activate MAPKs in cultured astrocytes (Schliess et al., 2002; Jayakumar et al., 2006) and such activation was blocked by antioxidants (Jayakumar et al., 2006). The stimulation of MAPKs was likely responsible for the NFκB activation produced by ammonia, since MAPK inhibitors SB239063 (p38-MAPK inhibitor) and SP600125 (JNK inhibitor) significantly diminished it.
While ammonia was shown to activate all three MPAKs in cultured astrocytes, the activation of NFκB was effectively prevented by inhibitors of JNK and p38-MAPK, but not by an inhibitor of ERK. Such differential effects of MAPK inhibitors have been reported by us and others (Xia et al., 1995; Mori et al., 2002; Xie et al., 2004; Jayakumar et al., 2006). These findings may be a reflection of the well known specificity of the MAPK system, in that ERK1/2 is generally activated by growth factors, whereas p38-MAPK and JNK are generally activated by stress (Davis, 1994; Cobb and Goldsmith, 1995).
One possible mechanism by which NFκB leads to astrocyte swelling may be through the upregulation of iNOS leading to the generation of NO, an agent known to cause astrocyte swelling (Jayakumar et al., 2006). Increased brain NO production was shown in portacaval-shunted rats given ammonia infusions (Master et al., 1999). Treatment of brain slices with the non-specific NOS inhibitor, L-NAME, prevented tissue swelling induced by ammonia (Zielinska et al., 2003). L-NAME also markedly diminished ammonia-induced cell swelling in cultured astrocytes (Jayakumar et al., 2006). iNOS activity has been shown to be elevated in experimental models of hepatic encephalopathy (HE) (Rao et al., 1997). The present study shows elevated iNOS protein expression in cultured astrocytes after ammonia treatment, in agreement with a report by Schliess et al., 2002. Additionally, we found that inhibition of NFκB with BAY 11-7082 blocked the elevation in iNOS protein expression and NO generation, and that treatment of astrocyte cultures with the specific iNOS inhibitor, aminoguanidine, completely blocked ammonia-induced cell swelling (Figure 8). Collectively, these studies suggest that ammonia-induced NFκB activation and subsequent induction of iNOS protein expression and NO generation represent important signaling pathways in the mechanism of ammonia-induced cell swelling in cultured astrocytes.
While, NFκB activation is generally considered as a major transcriptional factor responsible for iNOS induction, iNOS protein expression and subsequent increase in NO generation has also been shown to activate NFκB (Janssen-Heininger et al., 1999; Simpson and Morris, 1999; von Knethen et al., 1999). Such activation of NFκB by NO may result in a positive feedback system (Nam et al., 2004). A similar positive feedback system may also occur with ammonia-induced free radical production (Rama Rao et al., 2003). ROS is known to activate NFκB which can lead to increases in intracellular ROS (for review, see Bowie and O’Neill, 2000).
The mechanism by which NO leads to cell swelling is not known. Recent studies have shown increased protein tyrosine nitration in ammonia-treated astrocyte cultures (Schliess et al., 2002; Häussinger and Schliess, 2005). One may therefore speculate that some membrane proteins that are critically involved in cell volume regulation may become impaired due to nitrosylation which might result in cell swelling.
The findings of the present study highlighting an important role of NFκB in astrocyte swelling, may be relevant to an evolving role for inflammation and cytokines in FHF-associated brain edema (Detry et al., 2006; O’Beirne et al., 2006; Wright et al., 2007). As cytokines are well known to contribute to NFκB activation (Stancovski and Baltimore, 1997; Verma and Stevenson. 1997), it is conceivable that ammonia, by activating NFκB, may sensitize astrocytes to the effects of cytokines and endotoxins, thereby resulting in enhanced astrocyte swelling and brain edema in the setting of FHF.
In summary, our study demonstrates that ammonia activates NFκB in cultured astrocytes and that such activation is a consequence of an ammonia-induced activation of MAPK and oxidative stress. NFκB activation was associated with astrocyte swelling, likely through the induction of iNOS gene expression and subsequent NO production. These findings help define the molecular basis for cell swelling following ammonia exposure, and identifies potential targets for novel therapies in the treatment of the brain edema associated with fulminant hepatic failure.
Acknowledgments
This work was supported by a Merit Review from the Department of Veterans Affairs and by National Institutes of Health Grant DK063311. A.R.J is supported by the American Association for the Study of Liver Disease/American Liver Foundation Grant. We thank Alina Fernandez-Revuelta for the preparation of cell cultures.
References
- Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170:138–146. doi: 10.1016/s0022-510x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Bender AS, Norenberg MD. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J Neurosci Res. 1998;54:673–680. doi: 10.1002/(SICI)1097-4547(19981201)54:5<673::AID-JNR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Samuel D, Castaing D, Williams R, Pereira SP. Liver transplantation in Europe for patients with acute liver failure. Semin Liver Dis. 1996;16:415–425. doi: 10.1055/s-2007-1007254. [DOI] [PubMed] [Google Scholar]
- Blei AT. The pathophysiology of brain edema in acute liver failure. Neurochem Int. 2005;47:71–77. doi: 10.1016/j.neuint.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- Caccamo D, Campisi A, Currò M, Bramanti V, Tringali M, Li Volti G, Vanella A, Ientile R. Antioxidant treatment inhibited glutamate-evoked NF-kappaB activation in primary astroglial cell cultures. Neurotoxicology. 2005;26:915–921. doi: 10.1016/j.neuro.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- Capocaccia L, Angelico M. Fulminant hepatic failure: clinical features, etiology, epidemiology and current management. Dig Dis Sci. 1991;36:775–779. doi: 10.1007/BF01311236. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Detry O, De Roover A, Honore P, Meurisse M. Brain edema and intracranial hypertension in fulminant hepatic failure: pathophysiology and management. World J Gastroenterol. 2006;12:7405–7412. doi: 10.3748/wjg.v12.i46.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducis I, Norenberg LO, Norenberg MD. The benzodiazepine receptor in cultured astrocytes from genetically epilepsy-prone rats. Brain Res. 1990;531:318–321. doi: 10.1016/0006-8993(90)90793-b. [DOI] [PubMed] [Google Scholar]
- Fass U, Panickar K, Williams K, Nevels K, Personett D, McKinney M. The role of glutathione in nitric oxide donor toxicity to SN56 cholinergic neuron-like cells. Brain Res. 2004;1005:90–100. doi: 10.1016/j.brainres.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Ganz R, Swain M, Traber P, DalCanto M, Butterworth RF, Blei AT. Ammonia-induced swelling of rat cerebral cortical slices: implications for the pathogenesis of brain edema in acute hepatic failure. Metab Brain Dis. 1989;4:213–223. doi: 10.1007/BF01000297. [DOI] [PubMed] [Google Scholar]
- Häussinger D, Schliess F. Astrocyte swelling and protein tyrosine nitration in hepatic encephalopathy. Neurochem Int. 2005;47:64–70. doi: 10.1016/j.neuint.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J. 1999;13:1845–1854. [PubMed] [Google Scholar]
- Israel A. A role for phosphorylation and degradation in the control of NF-kappa B activity. Trends Genet. 1995;11:203–205. doi: 10.1016/s0168-9525(00)89045-9. [DOI] [PubMed] [Google Scholar]
- Jalan R. Acute liver failure: current management and future prospects. J Hepatol. 2005;42(Suppl(1)):S115–123. doi: 10.1016/j.jhep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Macara I, Mossman BT. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol. 1999;20:942–52. doi: 10.1165/ajrcmb.20.5.3452. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Murthy ChR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Weissenborn K. Neurology and the liver failure. J Neurol Neurosurg Psychiat. 1997;63:279–293. doi: 10.1136/jnnp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Anisotonic media and glutamate-induced ion transport and volume responses in primary astrocyte cultures. J Physiol (Paris) 1987;82:294–303. [PubMed] [Google Scholar]
- Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Kletzein RF, Pariza MW, Becker JE, Potter VR. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Ann Biochem. 1975;68:537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Tanaka J, Kudo Y, Nagano T. Direct of NO production in rat hippocampus and cortex using a new fluorescent indicator: DAF-2 DA. NeuroReport. 1998;9:3345–3348. doi: 10.1097/00001756-199810260-00001. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Li YQ, Zhang ZX, Xu YJ, Ni W, Chen SX, Yang Z, Ma D. N-Acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibited nuclear factor-kappaB activation in alveolar macrophages by different mechanisms. Acta Pharmacol Sin. 2006;27:339–346. doi: 10.1111/j.1745-7254.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Master S, Gottstein J, Blei AT. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology. 1999;30:876–880. doi: 10.1002/hep.510300428. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Jung JC, Sumii T, Singhal AB, Fini ME, Dixon CE, Alessandrini A, Lo EH. Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J Cereb Blood Flow Metab. 2002;22:444–452. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Nam HY, Choi BH, Lee JY, Lee SG, Kim YH, Lee KH, Yoon HK, Song JS, Kim HJ, Lim Y. The role of nitric oxide in the particulate matter (PM2.5)-induced NFkappaB activation in lung epithelial cells. Toxicol Lett. 2004;148:95–102. doi: 10.1016/j.toxlet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis. 2007;22:219–234. doi: 10.1007/s11011-007-9062-5. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab Invest. 1977;36:618–627. [PubMed] [Google Scholar]
- Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorious JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991;16:833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- O’Beirne JP, Chouhan M, Hughes RD. The role of infection and inflammation in the pathogenesis of hepatic encephalopathy and cerebral edema in acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2006;3:118–119. doi: 10.1038/ncpgasthep0417. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg DM. Ammonia neurotoxicity: role of the mitochondrial permeability transition. Metab Brain Dis. 2003;18:113–127. doi: 10.1023/a:1023858902184. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Audet M, Butterworth RF. Increased neuronal nitric oxide synthase expression in brain following portacaval anastomosis. Brain Res. 1997;765:169–172. doi: 10.1016/s0006-8993(97)00652-5. [DOI] [PubMed] [Google Scholar]
- Schliess F, Gorg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Häussinger D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002;16:739–741. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CS, Morris BJ. Activation of nuclear factor kappaB by nitric oxide in rat striatal neurones: differential inhibition of the p50 and p65 subunits by dexamethasone. J Neurochem. 1999;73:353–61. doi: 10.1046/j.1471-4159.1999.0730353.x. [DOI] [PubMed] [Google Scholar]
- Stancovski ID, Baltimore D. NF- B activation: the I B kinase revealed? Cell. 1997;91:299. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Aggarwal BB, Packer L. Alpha-lipoic acid is a potent inhibitor of NF-kappa B activation in human T cells. Biochem Biophys Res Commun. 1992;189:1709–1715. doi: 10.1016/0006-291x(92)90275-p. [DOI] [PubMed] [Google Scholar]
- Swain MS, Blei AT, Butterworth RF, Kraig RP. Intracellular pH rises and astrocytes swell after portacaval anastomosis in rats. Am J Physiol. 1991;261:R1491–1496. doi: 10.1152/ajpregu.1991.261.6.R1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber PG, Dal Canto M, Ganger DR, Blei AT. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology. 1987;7:1272–1277. doi: 10.1002/hep.1840070616. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson J. I B kinase, beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Knethen A, Callsen D, Brüne B. NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol Biol Cell. 1999;10:361–72. doi: 10.1091/mbc.10.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhies TM, Ehrlich ME, Duffy TE, Petito CK, Plum F. Acute hyperammonemia in the young primate: physiologic and neuropathologic correlates. Pediatr Res. 1983;17:970–975. doi: 10.1203/00006450-198312000-00009. [DOI] [PubMed] [Google Scholar]
- Ware AJ, D’Agostino AN, Combes B. Cerebral edema: a major complication of massive hepatic necrosis. Gastroenterology. 1971;61:877–884. [PubMed] [Google Scholar]
- Willard-Mack CL, Koehler RC, Hirata T, Cork LC, Takahashi H, Traystman RJ, Brusilow SW. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience. 1996;71:589–599. doi: 10.1016/0306-4522(95)00462-9. [DOI] [PubMed] [Google Scholar]
- Wright G, Shawcross D, Olde Damink SW, Jalan R. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis. 2007;22:375–388. doi: 10.1007/s11011-007-9071-4. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45:170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Lee FS. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J Biol Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- Zielinska M, Law RO, Albrecht J. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem Int. 2003;43:299–303. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Flogel U, Pfeuffer J, Leibfritz D. Effects of ammonia exposition on glioma cells: Changes in cell volume and organic osmolytes studied by diffusion-weighted and high-resolution NMR spectroscopy. Dev Neurosci. 2000;22:463–471. doi: 10.1159/000017476. [DOI] [PubMed] [Google Scholar]


