Abstract
Little is known about the transcriptional regulators that control the proliferation of multipotent bone marrow progenitors. Understanding the mechanisms that restrict proliferation is of significant interest since the loss of cell cycle integrity can be associated with hematopoietic exhaustion, bone marrow failure, or even oncogenic transformation. Here, we show that multipotent LSKs (lineage−Scahickit+) from E47 deficient mice exhibit a striking hyperproliferation associated with a loss of cell cycle quiescence and increased susceptibility to in vivo challenge with a mitotoxic drug. Total LSKs contain long-term self-renewing hematopoietic stem cells (HSCs) and downstream multipotential progenitors (MPPs) that possess very limited or no self-renewal ability. Within total LSKs, we found specific developmental and functional deficits in the MPP subset. E47 knockout (KO) mice have grossly normal numbers of self-renewing HSCs but a 50−70% reduction in non-renewing MPPs and downstream lineage-restricted populations. The residual MPPs in E47 KO mice fail to fully upregulate flk2 or initiate V(D)J recombination, hallmarks of normal lymphoid lineage progression. Consistent with the loss of normal cell cycle restraints, we show that E47 deficient LSKs have a 50% decrease in p21, a cell cycle inhibitor and known regulator of LSK proliferation. Moreover, enforced expression studies identify p21 as an E47 target gene in primary bone marrow LSKs. Thus, E47 appears to regulate the developmental and functional integrity of early hematopoietic subsets in part through effects on p21-mediated cell cycle quiescence.
Introduction
The mechanisms that regulate hematopoietic self-renewal and multi-lineage differentiation potential are of great importance from both the basic biological and clinical perspectives. Primitive hematopoietic cells that repopulate all blood cell lineages reside in the bone marrow (BM) LSK population that lacks lineage markers while expressing high levels of Sca-1 and c-ki Total LSKs are a heterogeneous population that contain HSCs with long-term (LT-) hematopoietic reconstitution activity (1-3) as well as downstream MPPs that have little or no self-renewal capabilities (4, 5). HSCs continually replenish the immune system in steady-state circumstances and regenerate long-term hematopoietic functioning after stress exposure or myeloablative therapy while MPPs can rapidly give rise to multiple downstream lineages (6). Not only is it of significant interest to understand the mechanisms that confer long-term self-renewal capability to LT-HSCs, but also those that restrict the expansion and mitotic capacity of downstream MPPs.
Mounting evidence indicates that cell cycle quiescence is of vital importance for the functional integrity of both HSCs and MPPs. First, the loss of the normal restraints on LSK cell cycling is associated with stem cell exhaustion and loss of self-renewal potential. For example, genetic ablation of the cell cycle inhibitor p21 (7), the PTEN regulator of PI3 kinase gene (8, 9) or the FOXO family of transcriptional regulators (10), leads to increased cell cycle entry with loss of LT- HSC function, and bone marrow failure. Second, the ectopic acquisition of self-renewal capabilities may serve as a platform for malignant transformation. Triple deletion of the p16, p19, and p53 genes involved in cell cycle regulation and survival was recently shown to confer long-term self-renewal capabilities to MPPs (11). While extracellular environmental cues signaling through the Notch and Wnt pathways are important for HSC activity, less is known about the cell-intrinsic factors that govern the development, maintenance, and function of multipotent subsets (12).
The transcription factor E47 is a member of the E protein family that is encoded by the E2A gene. E47 is essential for multiple aspects of B and T lineage development including V(D)J recombination (13), enforcement of developmental checkpoints (13-15), differentiation (13, 16-19), cell cycle regulation (20, 21) and survival (22-24). Furthermore, repression or absence of E47 or E2A activity has been implicated in cancer development (25). Half of E2A KO mice rapidly display T cell tumors at 3 to 10 months of age as do a proportion of mice deficient in the E47 splice product (26, 27). Translocations in which E2A is fused to PBX1 are detectable in 23% of all pediatric pre B cell acute lymphoblastic leukemia (ALL) patients (28-30), and inhibition of E2A activity by the overexpression of antagonists is mechanistically linked to Hodgkin lymphoma (31). That E2A is linked to cancers of multiple lineages raises the possibility that disruption of E2A in uncommitted hematopoietic progenitors acts as a first lesion that renders cells susceptible to secondary transforming events in a lineage-dependent manner. Indeed, indirect evidence hints at a role for E proteins in the regulation of HSC or MPP integrity. Functional ablation of the E protein inhibitors Id1 or SCL/Tal-1 leads to severe defects in hematopoietic progenitor activity and function (32-34). However, direct evidence for a pivotal role of E47 within the HSC and MPP subsets has been lacking.
In this study, we demonstrate a critical role for E47 in the establishment of a robust MPP population. We found that E47 deficient LSKs exhibit hyperproliferation, a loss of cell cycle quiescence, and increased sensitivity to a cell cycle specific drug. Within total LSKs, we found specific defects in the MPP subset. While HSCs are numerically intact, downstream MPPs are significantly reduced in E47 KO mice as compared to wild type mice. Moreover, the lymphoid differentiation potential of E47 KO MPPs is severely compromised. To establish the molecular mechanisms underlying MPP failure, we used gain of function and loss of function approaches to identify E47 target genes. Our results identify two important stem cell regulators, p21 and Ikaros, as potential E47 targets within the primitive LSK population. Together, our data suggest that E47 is required for the developmental and functional integrity of MPPs through effects on cell cycle quiescence. Since E proteins are not restricted to the bone marrow, knowledge about E47 in multipotent hematopoietic progenitors may provide broader insight into the mechanisms that control multi-lineage differentiation potential in non-hematopoietic tissues.
Materials and Methods
Mice
E47 KO mice and H2-SVEX V(D)J recombination reporter mice (13, 35) were bred in accordance with IACUC policies at the University of Pittsburgh.
Flow cytometry
Hematopoietic progenitors were isolated and stained for surface markers as we have reported (35, 36). Antibodies to murine surface markers were obtained from eBioscience. Primary anti-mouse Abs included AA4.1 APC (clone AA4.1), B220 APC or biotin (clone RA3−6B2), CD3 biotin (clone 2C11), CD11b biotin (clone M1/70), CD19 biotin or Cy5PE or FITC (clone MB19−1), CD27 PE (clone LG.7F9), CD34 FITC (clone RAM34), CD43 PE (clone S7), CD48 PE (clone HM48−1), CD117 PE or Cy5PE (clone 2B8), CD135 PE (clone A2F10), CD150 APC or FITC (clone 9D1), Gr-1 biotin (clone 8C5), IgM (clone 331) biotin or FITC, IL-7R PE (clone SB/14), Ly6C biotin or FITC (clone HK1.4), NK1.1 biotin (clone PK136), TER-119 biotin (clone TER-119), TCR-γδ biotin (clone UC7−13D5), and Sca-1 FITC or APC or Cy5PE (clone D7). Secondary reagents were streptavidin-Cy7-PE or streptavidin-Pacific Blue (Molecular Probes). E2A (clone G127−32, PharMingen) intracellular staining was performed as described (37). In brief, cells were fixed with Cytofix (BD Bioscience), permeabilized with PBS-0.2% Tween 20 for 10 min at 37°C, and stained for E2A at room temperature for 30 mins. Flow cytometry was performed on a three-laser, nine-detector LSR II (BD Biosciences). Data were analyzed with FlowJo software (Tree Star).
BrdU labeling, cell cycle analysis, and fluorouracil treatment
BrdU incorporation assays were performed as we have previously described (35, 36). Briefly, mice were injected i.p. with 200 μg BrdU in PBS, or PBS alone as a control, at 12-h intervals. Twenty four hours after the first injection, bone marrow was isolated, and cells were stained for surface markers and anti-BrdU FITC with BrdU flow kit (BD Bioscience) according to the manufacturer's instructions. Ki-67 intracellular staining was performed as previously described (38). To determine the G2/M cell cycle status, cells stained with Ki-67 were subsequently washed and incubated with DAPI (5 μg/ml) for a minimum of 30 minutes at room temperature before flow cytometric analysis. For in vivo analysis of the restriction on cell cycle entry, mice were injected weekly with 150 mg/kg of the cell-cycle specific drug 5-fluorouracil (5-FU) or PBS i.p. as described (7). Animals were weighed weekly, and animals displaying a change in body weight of greater than 30%, loss of coat quality, or lethargy were promptly sacrificed accordance with university IACUC policies. For short-term experiments, mice were sacrificed 10−12 hours after 5-FU or PBS administration, and bone marrow cells harvested for surface staining.
EMSA
HSCN1c110 LSK cells are a Notch1-transduced cell line that displays multipotency and self-renewal potential in vitro and in vivo (39). Electrophoretic mobility shift assays with HSCN1c110 nuclear extracts was performed using the μE5 probe as described (40). In brief, cells were resuspended in 10 mM HEPES (pH 7.9), 10 mM KCl, 1.0 mM EDTA, 1 mM DTT, 1.5 mM MgCl2, 1 mM PMSF, protease inhibitor mixture (PIM), and Nonidet P-40 (0.1%) and centrifuged at 8000 rpm for 5 mins. The nuclear containing pellet was solubilized in 20 mM HEPES (pH 7.9), 0.1 mM EDTA, 1 mM DTT, 1.5 mM MgCl2, 2 mM PMSF, PIM, and glycerol (10%) on ice for 20 mins. The lysate was centrifuged and the nuclear containing pellet was collected. The nuclear extracts were preincubated with rabbit anti-mouse polyclonal E47 or E2A antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated with radiolabeled DNA probe with 0.5 μg poly(dI-dC) as a nonspecific competitor. The binding complexes were resolved by electrophoresis in 5% polyacrylamide gel for 3 hrs at room temperature.
Retroviral transduction
Lineage-negative BM cells from E47 heterozygous mice were infected with E47-ER-huCD25 or the control bHLH-ER-huCD25 lacking the transactivation domain (25). Retroviral supernatant were obtained from the Phoenix packaging cell line using the Fugene 6 transfection kit (Roche). BM cells were depleted of lineage positive cells (NK1.1, CD11b, CD19, B220, TER-119 and Gr-1) using streptavidin microbeads (Miltenyi Biotec) according to the manufacturer's recommendation. Lineage-negative cells were pre-stimulated overnight in IMDM (Cellgro) with 20% FCS containing stem cell factor (100 ng/ml), flk2/flt3 ligand (100 ng/ml), IL-11 (10 ng/ml), IL-6 (100 ng/ml) (Peprotech), and 1% penicillin/streptomycin. Retroviral supernatant containing 6 μg/ml polybrene (Sigma) was added to the cells, and two rounds of spin-infection were performed as described (41). After 24 hours of culture, infected cells were incubated with 4-OHT (Sigma) for 5 hours to activate the E47-ER fusion protein. Transduced cells with a huCD25+ LSK phenotype were then sorted for mRNA isolation and quantitative PCR analysis.
Statistics
Multiple comparisons were performed using ANOVA followed by Tukey-Kramer HSD post-hoc analysis. Two sample comparisons were performed using the Students t Test. Differences were regarded as significant at p < 0.05. Analyses were performed using the JMP version 5.1 statistical software package (SAS Institute).
Results
E47 is expressed and functionally active in uncommitted hematopoietic progenitors
In examining the differential requirements for E47 activity during the earliest stages of B versus T lineage development, we found a surprising depletion of the earliest B and T lineage precursor subsets in the absence of E47. Specifically, E47 deficient mice had a virtual ablation of BM common lymphoid progenitors (CLPs), efficient progenitors to the B lymphocyte lineage, and a two-fold reduction in the frequency of thymic early T lineage progenitors (ETPs), progenitors to the T lymphocyte lineage. Figure 1 depicts the phenotypic resolution of these subsets that are then quantified in Table I. Across E47 wild type (WT), heterozygous (HET) and knockout (KO mice), BM CLPs were reduced 10-fold, consistent with our previous findings (13). Since young E47 deficient mice frequently develop thymic lymphomas of DN origin (24), we quantified ETPs in two day old animals to avoid leukemia-associated perturbations. Thymic ETPs were reduced four-fold from 485 ± 429 (n=10) to 137 ± 210 (n=6) in E47 HET versus KO mice (Table I). The paucity of both CLPs and ETPs is unexpected since none of the known E47 targets are predicted to recapitulate this defect. Moreover, upstream multipotent LSKs were reduced two-fold in frequency (Figure 1) and three-fold in absolute number (Table I). These data suggest that E47 activity is required earlier in hematopoietic development than was appreciated based on a small sample size (13).
Figure 1.
Disruption of early hematopoietic progenitors in E47 deficient mice. BM from young adult E47 WT/HET or KO mice was stained to resolve total LSKs (Lin−Sca-1hic-kit+) or CLPs (AA4.1+Sca-1loIL7R+lin−) by flow cytometry. Thymocytes from forty eight hour old preleukemic E47 WT/HET or KO mice were resolved for ETPs (c-kit+IL7R−CD44+CD25−Lin−). The data are representative of 6−30 independent animals.
Table 1.
Early hematopoietic defects in mice lacking one or two copies of E47.
| wild type§ | heterozygote§ | knockout§ | ||
|---|---|---|---|---|
| Bone marrow | ||||
| total bone marrow cells × 106 | adult | 31 ± 9 (n=18)A | 29 ± 13 (n=30)A | 20 ± 8 (n=28)B |
| total LSKs | 33,129 ± 13,802 (n=15)A | 24,226 ± 14,648 (n=11)AB | 11,729 ± 7,231 (n=18)B | |
| LT-HSC | ||||
| CD150+CD48− LSK | 868 ± 220 (n=6)ns | 735 ± 326 (n=4)ns | 721 ± 326 (n=8)ns | |
| CD27− LSK | 460 ± 198 (n=8)ns | 340 ± 269 (n=3)ns | 283 ± 159 (n=7)ns | |
| flk2− LSK | 11,156 ± 5,120 (n=7)ns | 9,757 ± 8,060 (n=3)ns | 10,029 ± 3,918 (n=8)ns | |
| MPPs | ||||
| CD150−CD48− LSK | 1,931 ± 1,010 (n=6)A | 1,892 ± 1,133 (n=4)AB | 723 ± 160 (n=7)B | |
| CD27+ LSK | 34,714 ± 14,360 (n=8)A | 16,138 ± 4,852 (n=3)AB | 12,528 ± 12,105 (n=7)B | |
| flk2+ LSK | 36,111 ± 13,625 (n=7)A | 25,396 ± 23,953 (n=3)AB | 14,561 ± 8,376 (n=8)B | |
| flk2bright LSK | 14,463 ± 5,346 (n=5)A | na | 2,468 ± 1,461 (n=6)A | |
| lineage restricted progenitor | ||||
| CD150−CD48+ LSK | 56,338 ± 16,146 (n=3)A | na | 13,917 ± 5,605 (n=4)B | |
| lymphoid specific progenitor | ||||
| CLPs |
|
8,216 ± 4,741 (n=4)A |
7,970 ± 4,574 (n=16)A |
625 ± 354 (n=14)B |
| Thymus | ||||
| total thymocytes × 105 | <12 hrs old | na | 18.0 ± 6.7 (n=8)A | 4.8 ± 2.0 (n=5)B |
| 24−48 hrs old | 20.4 ± 6.8 (n=4)A | 19.5 ± 7.9 (n=8)A | 3.9 ± 1.1 (n=4)B | |
| ETPs | <12 hrs old | na | 342 ± 127 (n=8)A | 126 ± 31 (n=5)B |
| 48 hrs old | na | 485 ± 429 (n=10)A | 137 ± 210 (n=6)B |
Thymus or bone marrow tissue isolated from the indicated mice were examined for the presence of hematopoietic progenitors subsets contained within the LSK subset (lineage−, scahi, kithi). Thymic tissue was analyze from newborn mice to avoid perturbations associated with thymic leukemias apparent in young adult mice. n = number of individuals.
p<0.05, ANOVA followed by Tukey HSD for multiple comparisons or Student's t test for pairwise analysis. Significant differences between groups are indicated by the A or B superscript; ns, not significant; na, data not available.
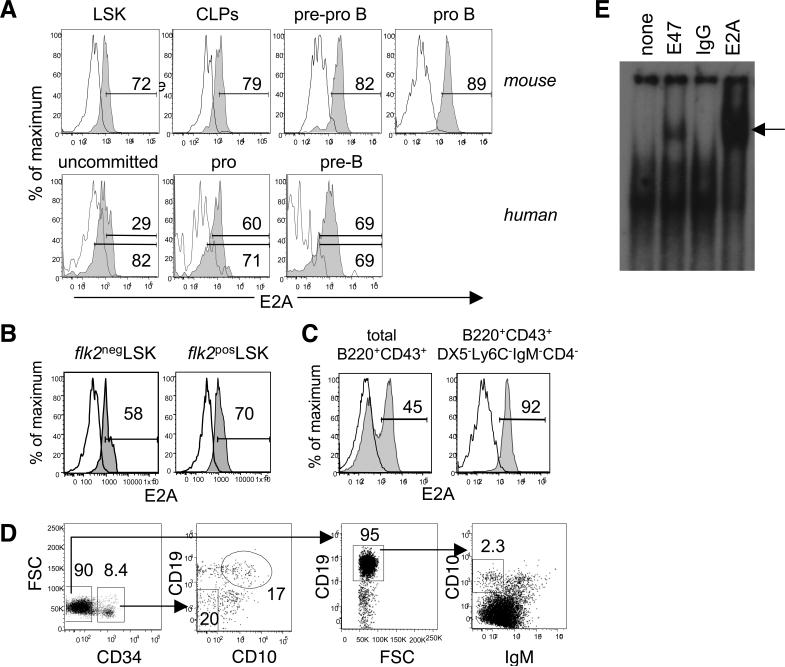
A careful examination throughout the earliest stages of hematopoietic development reveals that E2A protein is detectable in 72% of BM LSKs and 79% of CLPs as assessed by intracellular staining and flow cytometry (Figure 2A). E2A expression further increases during the pre-pro B and pro-B stages of B lineage development in terms of both the frequency of total E2A+ cells and mean fluorescence intensity (Figure 2A), thereby extending previous observations using knockin GFP reporter mice (42, 43). Original studies indicated that the total B220+CD43+ pro B cell subset contains two distinct populations expressing different levels of E2A (44). We initially obtained this result and found that 45% of cells were positive for E2A staining (Figure 2C). However, within the B220+CD43+ population which is fairly heterogeneous, B lineage potential is known to lie in the minor subset that lacks DX5, Ly6C, IgM, and CD4 expression (45). When we re-examined E2A protein levels in the population enriched for pro B potential, we found that 92% of cells are positive for E2A and that expression is uniformly high (Figure 2C). E2A expression is also detectable in human hematopoietic progenitors and is comparably upregulated during B lineage progression, suggesting the generality of our findings across both mouse and man (Figure 2A & D).
Figure 2.
The transcription factor E47 is expressed and functionally active in uncommitted hematopoietic progenitors. A) Murine and human BM cells stained to resolve the indicated subsets were fixed and permeabilized to detect intracellular E2A (shaded histograms) or the isotype control (open histograms). Murine LSKs and CLPs were resolved as in Figure 1 while murine pre-pro B and pro B were defined as B220+CD43+DX5−Ly6C−IgM− cells that lacked or expressed CD19, respectively. Human bone marrow cells were resolved as uncommitted BM progenitors (CD34+CD10−CD19−), pro-B cells (CD34+CD10+CD19+), and pre-B cells (CD34-CD19+IgM−). Due to variation in background fluorescence across human B cell precursor subsets, the gating is shown relative to the background staining in each individual subset (upper gate) as well as by applying a uniform gate across all populations (lower gate). B) Total murine LSKs were further resolved based on flk2 expression. C) Murine pro-B cells resolved as total B220+CD43+ were further refined as B220+CD43+DX5−Ly6C−IgM− BM cells. D) Gating strategy to resolve the human B cell subsets depicted in A. E) EMSA analysis of E47 activity in an LSK cell line. Nuclear extracts prepared from HSCN1c110 cells were pre-incubated in the presence of antibodies to E47, E2A (E47 + E12) or control IgG and then incubated with the radiolabeled μE5 DNA probe. The arrow indicates the supershift. The data are representative of 3−5 independent mice or primary human samples (A-D) or 2 independent experiments (E).
Total LSKs are a heterogeneous population that contain HSCs as well as downstream MPPs. The transition from LT-HSC to MPP is associated with the acquisition of the flk2/flt3 cytokine receptor (1). Interestingly, murine E2A expression increased from 53% ± 6.1 in the flk2−LSK LT-HSC population to 71% ± 6.1% in the flk2+LSK MPP subset (n=3 independent experiments; representative data shown in Figure 2B). These data indicate that E2A is expressed in primary HSCs from unmanipulated mice, raising questions about the functional role of this transcription factor at this pivotal stage of development.
We exploited a model stem cell line to examine E47 binding activity in uncommitted hematopoietic progenitors (39). Notch-1 transduced HSCN1c110 is self-renewing clonal line that retains pluripotency, and gives rise to lymphoid and myeloid lineages in vivo (39). HSCN1c110 nuclear extracts bound to the E-box containing μE5 target probe were clearly supershifted by antibodies to E47 and E2A (Figure 2E). No supershifting was seen using the isotype control, demonstrating the specificity of E47 and E2A activity. Thus, E2A protein is expressed and functional in uncommitted hematopoietic progenitors but its role in this compartment remains unknown.
E47 promotes the development of MPPs
Total LSKs contain both long-term self-renewing HSCs and MPPs with very limited or no self-renewal ability. We analyzed the presence of each developmental compartment in E47 WT, HET and KO mice. Within total LSKs, the minority HSC subset can be resolved on the basis of SLAM marker expression (2), CD27 (46), or flk2 (1), phenotypic schemes that enrich HSCs to varying degrees (33). We obtained identical results using all three phenotypic models. E47 WT, HET and KO mice had comparable numbers of phenotypic HSC defined as CD150+CD48−LSKs, CD27−LSKs, or flk2−LSKs (Figure 3A & B and Table I). By contrast, MPPs defined as CD150−CD48−LSK, CD27+LSK or flk2+LSK were uniformly reduced by 50% in E47 KO versus WT mice across all three phenotypic schemes. The early developmental defect was even more pronounced in downstream lineage restricted progenitors (LRP; CD150−CD48+LSKs), cells that can give rise to B or myeloid lineages but have little T cell potential (2) and CLPs (AA4.1+ScaloIL7R+lin−), cells that efficiently give rise to B cells. LRPs and CLPs were reduced 70% and 90%, respectively. Thus, disruption of E47 did not alter the absolute number of HSCs (p > 0.05) but did significantly reduce MPPs and downstream LRPs and CLPs (p < 0.05). That identical results were obtained using all three developmental schemes emphasizes the robustness of the data, and precludes the possibility of an apparent loss of MPPs due to perturbation in any single marker used to characterize this population. Similar results were observed using the CD34 marker to distinguish LT-HSCs and MPPs, again emphasizing the generality of our findings (data not shown).
Figure 3.
E47 is required for the developmental integrity of MPPs. A) BM from young adult E47 WT/HET or KO mice was stained to resolve HSCs and MPPs using three independent phenotypic schemes. HSCs were resolved as flk2/flt3− LSKs, CD150+CD48−LSKs or CD27-LSKs. B) BM LSKs from E47 WT, HET or KO mice were stained to resolve the indicated subsets as described in Table I. The number within or over each bar indicates the number of mice used to calculate mean ± SD. The letters A and B indicate statistical significance as determined in an ANOVA followed by Tukey-Kramer HSD post-hoc analysis, p < 0.05. ns, not significant.
Not only are MPPs reduced in number in E47 KO mice but this population appears to be functionally comprised. The flk2 brightest subset of MPPs contains the early lymphoid progenitor (ELP) population that first initiates rag expression, a key step in B lineage specification (47). We found that MPPs from E47 KO mice fail to fully upregulate the flk2 cytokine receptor. The 25% of flk2bright LSKs associated with lymphoid potential (48) is markedly reduced in E47 KO LSKs (Figure 4A). Specifically, this subset is reduced from 25.1% ± 2.3 (n=5) to 9.4% ± 3.9% (n=6); p < 0.05. This is an important observation because cells with the potential to undergo V(D)J recombination are exclusively contained within the flk2bright LSK population in WT mice. While 1.2% of WT LSKs express V(D)J recombinase activity as visualized using a fluorescent recombination reporter, this flk2bright recombination+ subset is completely absent in E47 KO LSKs (Figure 4B). In an analysis across multiple independent mice, the frequency of recombination+ LSKs was reduced from 1.3%±0.42 (n=4) to 0.05%±0.05 (n=5) in E47 WT versus KO mice, respectively; p < 0.05. Thus, E47 activity is required for the development and/or maintenance of a robust flk2bright MPP compartment that is competent to perform V(D)J recombination.
Figure 4.
Lymphoid differentiation potential is compromised in E47 deficient MPPs. A) BM LSKs from E47 WT or KO mice were analyzed for the presence of the brightest flk2+ cells enriched for lymphoid potential. B) E47 KO mice were crossed to the H2-SVEX V(D)J recombination reporter strain in which the VEX variant of green fluorescent protein indicates V(D)J recombinase activity in live cells. non-tg, non-transgenic. The data are representative of five (A) or four (B) independent experiments.
E47 regulates LSK quiescence
A key component of hematopoietic integrity is cell cycle quiescence. To examine the role of E47 in multipotent progenitors, we first examined the in vivo requirement of E47 for the proliferation and survival of total LSKs. After two days of administration of the thymidine analogue BrdU, 40% ± 6.4 of WT/HET LSKs are BrdU+ versus 66% ± 12 of KO LSKs (Figure 5A & D; average ± SD of three independent experiments). Elevated levels of BrdU incorporation in KO versus WT LSKs may reflect enhanced survival of labeled cells within a particular compartment or increased rates of proliferation. We found uniformly low levels of apoptosis of WT and KO LSKs directly ex vivo (<5% apoptosis; Figure 5B) as well as after overnight in vitro culture of rigorously purified LSKs that had been depleted of phagocytes that might otherwise clear dying progenitors (data not shown). Identical results were observed using both the mitotracker and annexin-V methods for detecting apoptotic cells (data not shown). Thus, increased BrdU incorporation is unlikely to reflect enhanced survival of E47 KO LSKs. Rather, increased BrdU incorporation likely reflects entry into the cell cycle. Direct analysis of the cell cycle status of E47 WT versus KO LSKs reveals interesting differences. While the proportion of cells in the active phases of the cell cycle (S + G2/M) is comparable between WT and KO mice, the latter mice display an increased proportion of LSKs that have exited G0. The frequency of LSKs in S + G2/M is 19.4% ± 3.4 (n=4) versus 17.4% ± 3.2 (n=5) in E47 WT versus KO mice, respectively (Figure 5C & D). The proliferation antigen Ki-67 is expressed in all stages of the cell cycle except for G0, rendering the absence of this protein a sensitive marker of quiescence. The frequency of Ki-67NEG cells was reduced from 32.3% ± 3.3 (n=6) to 23% ± 3.1 (n=6) in WT versus KO LSKs indicating a loss of quiescence and, by consequence, increased entry into the cell cycle (Figure 5C & D). This cell cycle perturbation appeared to be restricted to the Sca-1+c-kit+ subset of lineage-negative cells as the frequency of Ki-67NEG Sca-1−c-kit+ progenitors was similar between WT versus KO mice, 6.2% ± 1.2 (N=6) versus 4.9% ± 0.9 (N=6), p > 0.05, respectively. Together, these data indicate that E47 acts to restrain LSK cell cycle entry.
Figure 5.
Disruption of LSK quiescence in the absence of E47. A) BM from E47 WT or KO mice treated with BrdU for 48 hours was stained to identify LSKs followed by intracellular staining with anti-BrdU antibodies. Background fluorescence was determined by injecting E47 HET mice with PBS followed by the identical staining procedures. The data are representative of 3 independent experiments. B & C) Total BM LSKs from E47 WT or KO mice were fixed and stained with antibodies to Ki-67, mitotracker, or DAPI. The percent of cells in the gates is indicated. D) Cumulative data from A-C (3−4 independent mice per group) were analyzed by the Wilcoxon Rank Sum test. **p < 0.05; ns, not significant. E) The cell cycle specific drug 5-FU was administered weekly to E47 WT (n=5), HET (n=5) or KO (n=3) mice, and survival outcome examined. The data are depicted as Kaplan-Meier Survival curves. The data are representative of 2 independent experiments. F) BM from E47 WT and KO mice treated with 5-FU or PBS for 10 to 12 hours were stained to identify flk2/flt3− LSKs and flk2/flt3+ LSKs. The data are representative of 3−4 pairs of age-matched mice per group.
We examined the biological consequence of the hyperproliferation in E47 KO LSKs by challenging the ability of these progenitors to recover in response to mitotoxic challenge. Repeated exposure to the cell cycle-specific drug 5-fluorouracil (5-FU) depletes proliferating hematopoietic progenitors (49, 50), thereby challenging the restriction on cell cycle entry of stem cells in intact animals (7). Consistent with altered patterns of cell cycling, E47 KO mice are preferentially sensitive to in vivo challenge with 5-FU. While all E47 KO mice died within 15 days of 5-FU administration, 90% of WT and HET mice survived beyond this point (Figure 5E). Moreover, our data indicate a specific loss of cell cycle integrity in specific phenotypic subsets contained within total LSKs. For this analysis, we quantified each the flk2− and flk2+ LSK subsets after short-term (10−12 hours) in vivo challenge with 5-FU. While short-term exposure to 5-FU has little effect on the number of flk2− and flk2+ LSK subsets in WT mice, these subsets are reduced two-fold and four-fold, respectively, in E47 KO mice (Figure 5F). These data provide clear evidence that E47 is required for the proliferative integrity of MPPs in vivo. Together, these data indicate that E47 KO LSKs have increased proliferation together with a loss of quiescence, an interpretation consistent with the role of E47 in restraining B and T cell precursor proliferation (20, 21).
E47 is known to restrict the proliferation of primary B and T cell progenitors (20, 21) as well as some non-hematopoietic cell lines (51). E47 has also been shown to bind to the p21 promoter, and activate gene expression (25, 51, 52). The CDK inhibitor p21 is of particular interest because its genetic ablation leads to a loss of quiescence (7), and consequent bone marrow failure. Quantitative PCR analysis of sorted LSKs reveals a 50% decrease in p21 expression in KO versus WT LSKs (Figure 6A), suggesting that p21 expression may be regulated either directly or indirectly by E47. To determine the capacity of E47 to directly activate p21 expression in primary LSKs, we performed gain of function experiments in which we infected LSKs with a tamoxifen-inducible form of E47 (E47-ER) that allows the selective induction of E47 following tamoxifen exposure. E47-ER activation by 4-OHT induced p21 transcript abundance suggesting E47 regulates p21 in primary LSKs (Figure 6B). Enforced expression of E47 also induced Ikaros, a key regulator of early hematopoietic differentiation (Figure 6B). By contrast, no changes were detectable in the other cell cycle regulators gfi1, cdk6 or c-myb, indicating the specificity of the p21 and Ikaros expression alterations. Indeed, the hyperproliferation of E47 deficient LSKs is strikingly reminiscent of p21 knockout LSKs which exhibit loss of quiescence associated with severe hematopoietic reconstitution deficits (7).
Figure 6.
The transcription factor E47 regulates the expression of cell cycle regulator p21 in LSKs. A) LSKs sorted from E47 WT or KO mice were examined for the expression of p21, cdk6 or gfi1 by real time RT-PCR. The data are normalized to β-actin. Levels of gene expression are presented as E47 KO/WT ratio. The data represent the mean of four independent analyses from three different sorts (p21), three independent analyses from two different sorts (cdk6), or two independent analyses from two sorts (gfi1); standard deviation is not depicted for gfi1 since only two data points are available. B) Lin− BM from E47 HET mice transduced with E47-ER-huCD25 or the control vector bHLH-ER-huCD25 were incubated with 4-OHT to activate E47. Cells were harvested, huCD25+ LSKs were sorted by FACS, and mRNA was isolated for QPCR. The β-actin expression ratio in E47-ER/bHLH-ER was set as 1, and the expression of the indicated genes was then normalized based on actin. The data are representative of 2−4 independent sorts (A) and 3 independent experiments (B).
Discussion
To date, knowledge about the transcription factors that regulate the integrity of the individual HSC and MPP compartment within the multipotent LSK population has been limited. Here, we define a critical role for the transcriptional factor E47 in the developmental and functional integrity of BM LSKs, with specific requirement in the MPP subset. Not only is the number of MPPs significantly reduced in E47 KO mice as shown by multiple independent phenotypic schemes but the lymphoid differentiation potential of E47 KO MPPs is severely compromised. In addition, E47 KO MPPs have reduced expression of the essential cytokine receptor flk2/flt3 and an absence of V(D)J recombinase activity, defects that are associated with a profound reduction in the earliest B and T lineage progenitors in these deficient mice. Moreover, we show that total LSKs from E47 KO animals exhibit hyperproliferation and a loss of G0 quiescence. Reciprocal gain of function and loss of function studies identify the cell cycle inhibitor p21, a known regulator of hematopoietic integrity, as an E47 target gene.
Our data highlight an important role for E47 in the balance between proliferation and differentiation of hematopoietic progenitors toward the lymphoid lineages. We show that E47 KO MPPs exhibit hyperproliferation and loss of quiescence at the expense of lymphoid differentiation. Inappropriate entry into the cell cycle has been shown to inhibit lineage-specific differentiation events (53). The cell cycle regulator CDK6 that is specifically expressed in proliferating cells appears to block differentiation to the myeloid lineage (53). Ectopic expression of CDK6 enhances proliferation but inhibits differentiation of primary murine myeloid progenitors. As another example, the orphan nuclear receptor Nurr1 promotes dopamine cell differentiation through cell cycle arrest (54). Established literature also shows that expression of the cell cycle regulator p21 is upregulated during the differentiation of myeloid cells (55) and non-hematopoietic oligodendrocytes (56), suggesting that p21 might regulate cell differentiation through its cell cycle regulatory function. Furthermore, both loss of function and gain of function experiments performed here suggest that the key cell cycle regulator p21 is an E47 target in primary LSK progenitors. Thus, E47 appears to promote the differentiation of MPPs towards lymphoid lineage while controlling cell cycle quiescence.
Our data identify the key regulator of early hematopoietic differentiation Ikaros as a potential E47 target. Disruption of Ikaros activity in E47 deficient LSKs may contribute to the severe lymphoid differentiation defects in E47 KO MPPs. Like E47, Ikaros is essential for robust B and T lymphocyte development (57). The B cell arrest in E47 KO mice and Ikaros KO mice occurs at similar stages, with severe defects in the CLP compartment and reduced flk2 expression in MPPs. Also, in accordance with our finding of E47 KO MPP deficits, previous studies showed that Ikaros null mice have severe defects in HSC function as well as MPP differentiation deficits (58, 59). Here, we show E47 deficient mice exhibit developmental and differentiation perturbations in both of these compartments. Therefore, our finding of an interaction between E47 and Ikaros warrants further investigation.
E47 has been suggested to control the cell cycle progression of hematopoietic as well as nonhematopoietic cells. Mice lacking one or both alleles of E47 or the E2A parent gene exhibit hyperproliferation in primary B (21) and T lineage progenitors (20) as well as CLPs (13). Consistent with these findings, we demonstrate that E47 acts to restrain proliferation by controlling the cell cycle quiescence of LSKs (Figure 5). Thus, in primary lymphocytes, E47 uniformly restrains cell cycle proliferation. Observations in cell lines are more heterogeneous, suggesting that E47 activates or inhibits proliferation in a cell type specific manner (51, 60). Several key cell cycle regulators have been identified as E47 targets including p21 and p16. E47 has been found to physically interact with the p21 promoter and induce p21 expression the HeLa cell line (51). Our data provide convincing evidence that p21 is a potential E47 target in primary LSKs and multipotent hematopoietic progenitors. Since p21 is of vital importance in the differentiation and self-renewal of hematopoietic as well as non-hematopoietic tissues, we propose that E47 may control early hematopoietic development and differentiation through interaction with its immediate downstream target p21.
Together, our results define a role for the transcription factor E47 in the developmental integrity of bone marrow MPPs. We show that E47 is required for the formation of a robust MPP subset that is capable of lymphoid lineage progression. We also show that that E47 regulates the proliferative integrity of multipotent progenitor subsets through effects on p21. Recent studies have found that MPPs with oncogenic mutations display hyperproliferation and increased self-renewal ability (11), indicating that loss of quiescence in MPPs might be associated with tumorigenesis. As mentioned above, MPPs from mice mutant for three tumor suppressor genes (p53, p16 and p19) showed hyperproliferation accompanied by the abnormal acquisition of long-term renewal capabilities, suggesting the potential for transformation (11). Furthermore, MLLGAS7 oncoprotein transformed MPPs displayed significantly heightened proliferation as well as induced leukemias of multiple lineages in lethally irradiated mice, indicating that loss of constraints on MPP proliferation might offer the opportunity for malignancy (61). Consistent with the hyperproliferation and loss of quiescence of E47 KO MPPs, T cell leukemia is frequently seen in E47 and E2A KO mice (27). In humans, disruption of E2A activity is associated with the cancers of B and T lineage (31, 62, 63). The link between cell cycle restraint defects in E47 deficient MPPs and transformation of the lymphoid lineages remains to be investigated.
Acknowledgements
We sincerely thank Bonnnie Blomberg, John Choi, Dewayne Falkner, Daniela Frasca, Barbara Kee, Kees Murre, Xiao-Hong Sun and Will Walker for reagents and technical advice. We greatly appreciate critical input from Binfeng Lu, Kay Medina, and Richard Steinman.
Footnotes
This work is supported by NIH R03 AR054529, the Elsa U. Pardee Foundation, US Immune Deficiency Network and the Winters Foundation (Borghesi); NIH R01 CA086433 (Milcarek); NIH P01 HL084205 (Bernstein). I.D.B. is an American Cancer Society Clinical Research Professor.
References
- 1.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 2.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 4.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 6.Pawliuk R, Eaves C, Humphries RK. Evidence of both ontogeny and transplant dose-regulated expansion of hematopoietic stem cells in vivo. Blood. 1996;88:2852–2858. [PubMed] [Google Scholar]
- 7.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 10.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 12.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghesi L, Aites J, Nelson S, Lefterov P, James P, Gerstein R. E47 is required for V(D)J recombinase activity in common lymphoid progenitors. J Exp Med. 2005;202:1669–1677. doi: 10.1084/jem.20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agata Y, Tamaki N, Sakamoto S, Ikawa T, Masuda K, Kawamoto H, Murre C. Regulation of T cell receptor Beta gene rearrangements and allelic exclusion by the helix-loop-helix protein, e47. Immunity. 2007;27:871–884. doi: 10.1016/j.immuni.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Bain G, Romanow WJ, Albers K, Havran WL, Murre C. Positive and negative regulation of V(D)J recombination by the E2A proteins. J Exp Med. 1999;189:289–300. doi: 10.1084/jem.189.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 17.Bain G, Robanus Maandag EC, te Riele HP, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 18.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2−2, and HEB. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. Embo J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herblot S, Aplan PD, Hoang T. Gradient of E2A activity in B-cell development. Mol Cell Biol. 2002;22:886–900. doi: 10.1128/MCB.22.3.886-900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kee BL, Rivera RR, Murre C. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-beta. Nat Immunol. 2001;2:242–247. doi: 10.1038/85303. [DOI] [PubMed] [Google Scholar]
- 23.Lazorchak AS, Wojciechowski J, Dai M, Zhuang Y. E2A promotes the survival of precursor and mature B lymphocytes. J Immunol. 2006;177:2495–2504. doi: 10.4049/jimmunol.177.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz R, Engel I, Fallahi-Sichani M, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci U S A. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bain G, Engel I, Robanus Maandag EC, te Riele HP, Voland JR, Sharp LL, Chun J, Huey B, Pinkel D, Murre C. E2A deficiency leads to abnormalities in alphabeta T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan W, Young AZ, Soares VC, Kelley R, Benezra R, Zhuang Y. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol Cell Biol. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aspland SE, Bendall HH, Murre C. The role of E2A-PBX1 in leukemogenesis. Oncogene. 2001;20:5708–5717. doi: 10.1038/sj.onc.1204592. [DOI] [PubMed] [Google Scholar]
- 29.Carroll AJ, Crist WM, Parmley RT, Roper M, Cooper MD, Finley WH. Pre-B cell leukemia associated with chromosome translocation 1;19. Blood. 1984;63:721–724. [PubMed] [Google Scholar]
- 30.Williams DL, Look AT, Melvin SL, Roberson PK, Dahl G, Flake T, Stass S. New chromosomal translocations correlate with specific immunophenotypes of childhood acute lymphoblastic leukemia. Cell. 1984;36:101–109. doi: 10.1016/0092-8674(84)90078-3. [DOI] [PubMed] [Google Scholar]
- 31.Mathas S, Janz M, Hummel F, Hummel M, Wollert-Wulf B, Lusatis S, Anagnostopoulos I, Lietz A, Sigvardsson M, Jundt F, Johrens K, Bommert K, Stein H, Dorken B. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol. 2006;7:207–215. doi: 10.1038/ni1285. [DOI] [PubMed] [Google Scholar]
- 32.Jankovic V, Ciarrocchi A, Boccuni P, DeBlasio T, Benezra R, Nimer SD. Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc Natl Acad Sci U S A. 2007;104:1260–1265. doi: 10.1073/pnas.0607894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry SS, Zhao Y, Nie L, Cochrane SW, Huang Z, Sun XH. Id1, but not Id3, directs long-term repopulating hematopoietic stem-cell maintenance. Blood. 2007;110:2351–2360. doi: 10.1182/blood-2007-01-069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 35.Borghesi L, Hsu LY, Miller JP, Anderson M, Herzenberg L, Schlissel MS, Allman D, Gerstein RM. B lineage-specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J Exp Med. 2004;199:491–502. doi: 10.1084/jem.20031800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghesi L, Gerstein RM. Developmental separation of V(D)J recombinase expression and initiation of IgH recombination in B lineage progenitors in vivo. J Exp Med. 2004;199:483–489. doi: 10.1084/jem.20031802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King AM, Van der Put E, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- 38.Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, Antonchuk J, Jacobsen SE. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 39.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 40.Frasca D, Nguyen D, Riley RL, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Zhang Y, Joe GJ, Pompetti R, Emerson SG. NF-Ya activates multiple hematopoietic stem cell (HSC) regulatory genes and promotes HSC self-renewal. Proc Natl Acad Sci U S A. 2005;102:11728–11733. doi: 10.1073/pnas.0503405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon K, Hutter C, Sun Q, Bilic I, Cobaleda C, Malin S, Busslinger M. Instructive Role of the Transcription Factor E2A in Early B Lymphopoiesis and Germinal Center B Cell Development. Immunity. 2008 doi: 10.1016/j.immuni.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Zhuang Y, Jackson A, Pan L, Shen K, Dai M. Regulation of E2A gene expression in B-lymphocyte development. Mol Immunol. 2004;40:1165–1177. doi: 10.1016/j.molimm.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 44.Quong MW, Martensson A, Langerak AW, Rivera RR, Nemazee D, Murre C. Receptor editing and marginal zone B cell development are regulated by the helix-loop-helix protein, E2A. J Exp Med. 2004;199:1101–1112. doi: 10.1084/jem.20031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tudor KS, Payne KJ, Yamashita Y, Kincade PW. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity. 2000;12:335–345. doi: 10.1016/s1074-7613(00)80186-7. [DOI] [PubMed] [Google Scholar]
- 46.Wiesmann A, Phillips RL, Mojica M, Pierce LJ, Searles AE, Spangrude GJ, Lemischka I. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 47.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 48.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Berardi AC, Wang A, Levine JD, Lopez P, Scadden DT. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 50.Lerner C, Harrison DE. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990;18:114–118. [PubMed] [Google Scholar]
- 51.Prabhu S, Ignatova A, Park ST, Sun XH. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol Cell Biol. 1997;17:5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim W, Kook S, Kim DJ, Teodorof C, Song WK. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J Biol Chem. 2004;279:8333–8342. doi: 10.1074/jbc.M312026200. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto T, Anderson K, Jacobsen SE, Nishikawa SI, Nerlov C. Cdk6 blocks myeloid differentiation by interfering with Runx1 DNA binding and Runx1-C/EBPalpha interaction. Embo J. 2007;26:2361–2370. doi: 10.1038/sj.emboj.7601675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro DS, Hermanson E, Joseph B, Wallen A, Aarnisalo P, Heller A, Perlmann T. Induction of cell cycle arrest and morphological differentiation by Nurr1 and retinoids in dopamine MN9D cells. J Biol Chem. 2001;276:43277–43284. doi: 10.1074/jbc.M107013200. [DOI] [PubMed] [Google Scholar]
- 55.Steinman RA, Huang J, Yaroslavskiy B, Goff JP, Ball ED, Nguyen A. Regulation of p21(WAF1) expression during normal myeloid differentiation. Blood. 1998;91:4531–4542. [PubMed] [Google Scholar]
- 56.Zezula J, Casaccia-Bonnefil P, Ezhevsky SA, Osterhout DJ, Levine JM, Dowdy SF, Chao MV, Koff A. p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep. 2001;2:27–34. doi: 10.1093/embo-reports/kve008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirstetter P, Thomas M, Dierich A, Kastner P, Chan S. Ikaros is critical for B cell differentiation and function. Eur J Immunol. 2002;32:720–730. doi: 10.1002/1521-4141(200203)32:3<720::AID-IMMU720>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 58.Nichogiannopoulou A, Trevisan M, Neben S, Friedrich C, Georgopoulos K. Defects in hemopoietic stem cell activity in Ikaros mutant mice. J Exp Med. 1999;190:1201–1214. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida T, Yao-Ming Ng S, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7:382–391. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao F, Vilardi A, Neely RJ, Choi JK. Promotion of cell cycle progression by basic helix-loop-helix E2A. Mol Cell Biol. 2001;21:6346–6357. doi: 10.1128/MCB.21.18.6346-6357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.So CW, Karsunky H, Passegue E, Cozzio A, Weissman IL, Cleary ML. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 2003;3:161–171. doi: 10.1016/s1535-6108(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 62.Kamps MP, Murre C, Sun XH, Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990;60:547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- 63.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, Su X, Pui CH, Relling MV, Evans WE, Shurtleff SA, Downing JR. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]