Abstract
BACKGROUND
There are no reliable markers able to identify patients with non-small cell lung cancer (NSCLC) likely to metastasize to the brain. We investigated associations between immunohistochemical markers and development of brain metastases in patients with NSCLC.
METHODS
We performed a hospital-based, case-control study of patients with newly diagnosed NSCLC between 1989 and 2003 that developed brain metastases who had available pathology material from both primary NSCLC and brain metastases. The control patients had NSCLC and no evidence of brain metastases. We examined NSCLC for expression of Ki-67, caspase-3, VEGF-A, VEGF-C, E-cadherin and EGFR in 54 surgical pathology specimens using immunohistochemistry and evaluated associations with development of brain metastases.
RESULTS
Brain metastases developed after a median time of 12.5 months (range 1.7-89.4 months) from the diagnosis of NSCLC. A significantly increased risk of developing brain metastases was associated with patients who had high Ki-67 (adjusted odds ratio 12.2, 95% CI, 2.4 to 70.4, P<0.001), low caspase-3 (adjusted odds ratio 43.0, 95% CI, 5.3 to >100, P<0.001), high VEGF-C (adjusted odds ratio 14.6, 95% CI, 2.0 to >100, P<0.001), and low E-cadherin (adjusted odds ratio 3.6, 95% CI, 0.9 to 16.4, P=0.05), in the primary NSCLC. No significant risk was observed with VEGF-A and EGFR. A high Ki-67 was also associated with a shorter overall survival (P=0.04).
CONCLUSIONS
Patients with NSCLC and high Ki-67, low caspase-3, high VEGF-C, and low E-cadherin in their tumors may benefit from close surveillance since they may have an increased risk of developing brain metastases.
INTRODUCTION
Non-small-cell lung carcinoma (NSCLC) is the leading cause of death from cancer in both men and women.1 Approximately 210,000 cases of NSCLC are diagnosed annually in the United States.1 Lung cancer is the malignancy that most commonly gives rise to brain metastases which are a devastating complication and a major cause of morbidity and mortality.2 Ten percent of patients have brain metastases at the time of diagnosis, and about 40% of all patients with lung cancer will develop brain metastases during the course of the disease.3 Patients with locally advanced NSCLC treated with chemotherapy, chest radiotherapy with or without surgery have a high rate of developing brain metastases.2, 4-6 These patients also have a 15-30% risk of failing first in the brain.4, 7 Brain metastases from NSCLC received increasing attention because combined-modality therapy has lead to improvements in intrathoracic local control and prolonged overall survival.7-9
It is important to identify patients with NSCLC who are at greater risk of developing brain metastases since they may exist in the absence of neurological symptoms.10 Furthermore, recent reports show that prophylactic cranial irradiation may be an effective modality for preventing brain metastases in patients with NSCLC treated with adjuvant chemoradiation.5 Despite advances in diagnosis and therapeutic modalities, and clinical practice guidelines, it remains unclear whether patients with early stage NSCLC should be screened for brain metastases or not.11-13
Studies have shown that the metastatic cascade is rather complex and involves reciprocal interactions between tumor cells and host tissues, including alterations in tumor cell proliferation, adhesion, proteolysis, invasion, and angiogenesis.8 For the morphological assessment of the fraction of proliferating cells, Ki-67 staining with MIB1 is the most commonly used monoclonal antibody, covering late G1, S, G2 and M phases of the cell cycle.14 TUNEL in situ hybridization or caspase-3 immunohistochemistry are suitable for evaluation of apoptosis as these methods cover the mitochondrial as well as the death-receptor pathways.15 Studies that examined the combination of proliferative and pro-apoptotic factors demonstrated that patients with tumors with a high proliferative activity and no expression of pro-apoptotic factors had the shortest survival times and vice versa.16 VEGF family of proteins modulates angiogenesis which is essential for tumor growth and metastasis.17-19 E-cadherin is a calcium-regulated adhesion molecule expressed in most normal epithelial tissues. Loss of cell surface E-cadherin is considered a defining characteristic of epithelial-mesenchymal transition (EMT), a cellular event that occurs during normal embryo development and might also be associated with tumor cell invasion and metastasis.20, 21 EGFR is overexpressed in 40 to 80% of NSCLC and many other epithelial cancers and is frequently correlated with adverse prognosis.22, 23
Molecular factors investigated in our study have been shown previously to play a role in tumor invasion and metastasis formation, but the association of any of those markers with the development of brain metastasis from NSCLC has not been established.
In our study we evaluated patients with NSCLC with and without brain metastasis in a unique and unparalleled series that has tumor material from both the primary lung tumor and metachronous brain metastasis obtained from a single institution. These patients are typically only treated for lung cancer, and brain biopsy is rarely recommended or obtained. This gave us the opportunity to interrogate for expression of Ki-67, caspase-3, VEGF-A, VEGF-C, E-cadherin and EGFR in primary lung tumors using immunohistochemistry and investigate the risk of developing brain metastasis and death. We compared patients that developed brain metastases with control patients with NSCLC that had no evidence of brain metastases that were followed for a median period of 2.5 years.
MATERIALS AND METHODS
Patient Characteristics
Subjects were identified as patients with newly diagnosed NSCLC between 1989 and 2003 that developed brain metastases and had available pathology material from both primary NSCLC and brain metastases (Table 1). The group was identified through a combined search of the registry databases from the Departments of Pathology and Neuropathology maintained by the Department of Pathology and included patients with both confirmed primary NSCLC and metastatic NSCLC to brain who had available pathology material. The cases were reviewed by two pathologists and histologically classified using the WHO criteria for tumors of the lung.24 In each case, the pathologic and immunophenotypic features of brain metastases were compared with the corresponding NSCLC and the diagnosis of NSCLC metastatic to brain was confirmed.
TABLE 1.
Clinical and Pathologic Characteristics of the Study Group and Control Group*
| Characteristic | Study Group | Control Group | P Value |
|---|---|---|---|
| (N=21) | (N=33) | ||
| Sex - no. (%) | 1.00 | ||
| Male | 10(48) | 15(45) | |
| Female | 11(52) | 18(55) | |
| Age - yr | 0.05 | ||
| Mean | |||
| Median (range) | 60(42-75) | 65(39-84) | |
| Histology - no. (%) | 0.57 | ||
| Adenocarcinoma | 12(57) | 22(67) | |
| Squamous cell carcinoma | 9(43) | 11(33) | |
| Primary location - no. (%) | 1.00 | ||
| Right lung | 12(57) | 18(55) | |
| Left lung | 9(43) | 15(45) | |
| Tumor size - cm | 0.63 | ||
| Median (range) | 2.2(0.3-9.5) | 2.6(0.6-7.3) | |
| Tumor stage - no. (%) | 0.45 | ||
| T1 | 13(62) | 18(55) | |
| T2 | 5(24) | 13(39) | |
| T3 | 2(10) | 2(6) | |
| T4 | 1(5) | 0(0) | |
| Node stage - no. (%) | 0.54 | ||
| N0 | 14(67) | 25(76) | |
| N1 | 7(33) | 8(24) | |
| Pathologic pTNM stage - no. (%)† | 0.19 | ||
| I | 12(57) | 23(70) | |
| II | 7(33) | 10(30) | |
| III | 2(10) | 0(0) |
Because of rounding, not all percentages total 100.
pTNM denotes pathologic tumor-node-metastasis stage.
The inclusion criteria for the study were a diagnosis NSCLC (either lung adenocarcinoma, squamous cell carcinoma or NSCLC not otherwise specified), initial treatment by surgery alone, with or without postoperative adjuvant treatment; no other previous or synchronous malignant tumors; histologically confirmed NSCLC metastatic to brain; and no deaths in the perioperative period less than 30 days after surgery, resulting in 21 evaluable cases.
The control group consisted of patients that were seen for follow-up of their NSCLC treated in the same period between 1989 and 2003, in the same clinic from which the case patients were enrolled that had either no metastases or developed metastases to distant sites other than brain (Table 1). Only patients with negative neurologic examination and/or negative brain imaging studies were included in the control group, resulting in 33 evaluable cases.
The clinicopathologic features of the two subgroups were similar, except that patients in the control group were slightly older than the cases at the diagnosis of primary NSCLC (P=0.05, Table 1). The study protocol was approved by the institutional review board of the Brigham and Women's Hospital.
Immunohistochemistry
Immunoperoxidase studies were performed on paraffin embedded sections from 54 NSCLC specimens following deparaffinization and heat induced epitope retrieval with 0.01 M citrate, pH 6.0 or 0.001 M EDTA, pH 8.0 buffers and quenching of endogenous peroxidase with 3% aqueous hydrogen peroxide. Tris buffer, pH 7.5, supplemented with 3% porcine serum, and Tris buffer alone were used for soaking and rinsing tissues, respectively, during processing. Details of the methodology and sources of the antibodies used in this study are summarized in Table 2.
Table 2.
Characteristics of the Antibodies Used in the Study
| Antibody to | Clone | Source | Dilution | Retrieval | Detection |
|---|---|---|---|---|---|
| Ki-67 | MIB-1 | Dakoa | 1:150 | Steamer/EDTA buffer | Envisiona |
| Caspase-3 (cleaved, ASP-175 rabbit antibody) | Cell Signalingb | 1:250 | Microwave/citrate buffer | ABCc | |
| VEGF-A (A20) (rabbit antibody) | Santa Cruzd | 1:150 (overnight incubation) | Pressure cooker/citrate buffer | Envision+ | |
| VEGF-C (N-19) (goat antibody) | Santa Cruz | 1:250 (overnight incubation) | Steamer/EDTA buffer | LSAB+a | |
| E-Cadherin | 36B5 | Novocastrae | 1:50 | Pressure cooker/citrate buffer | Envision+ |
| EGFR | EGFR.25 | Novocastra | 1:50 | Pressure cooker/citrate buffer | Envision+ |
Dako Corp., Carpinteria, CA
Cell Signaling Technologies, Inc., Beverly, MA
Vector Laboratories, Burlingame, CA
Santa Cruz Biotechnology, Inc., Santa Cruz, CA
Vision-Biosystems, Norwell, MA
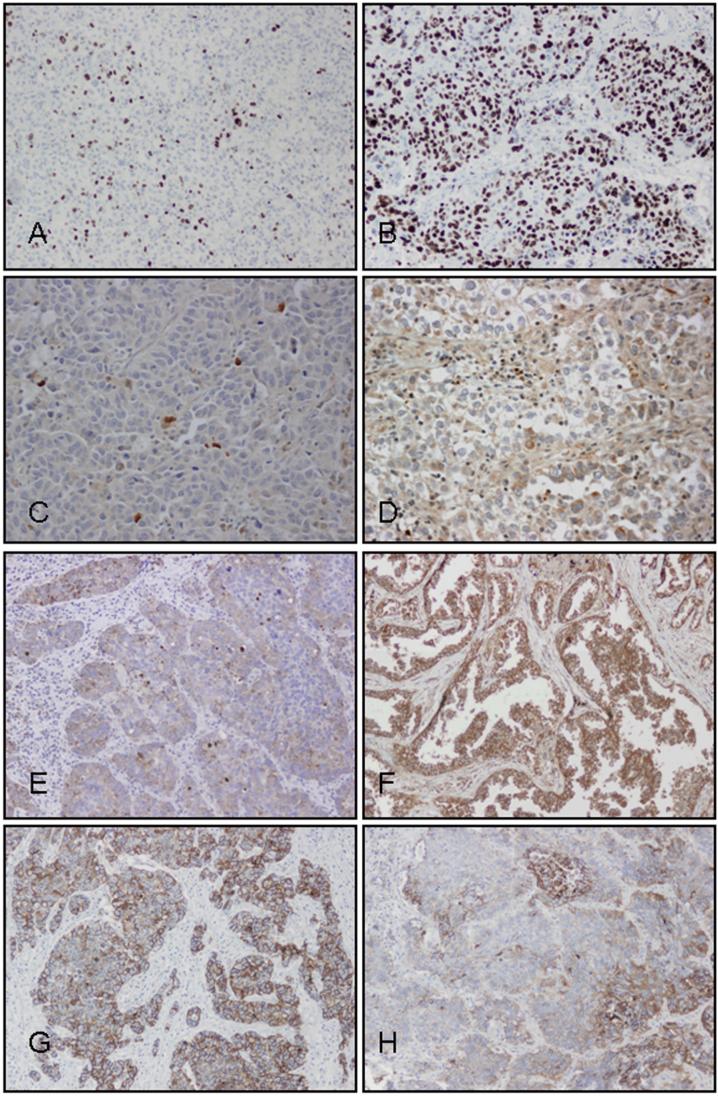
The immunostained slides were examined by light microscopy. Ki-67 and caspase-3 were recorded as labeling indices (Fig. 1). The labeling index (LI) is defined as the number of Ki-67 or active caspase-3-positive tumor cells per 1000 tumor cells. We selected and evaluated five representative high-power fields (1 mm2, Olympus BX41 microscope, 400x magnification) that have the greatest number of tumor cells staining for Ki-67 or caspase-3 (hot spots). For each patient we recorded Ki-67 LI, caspase-3 LI and the ratio of Ki-67 LI to caspase-3 LI as a marker of tumor survival (Fig. 1). VEGF-A, VEGF-C, and membranous E-cadherin were recorded as percentages of positive tumor cells in the entire tumor using five-percent increments on a representative slide. EGFR was recorded according to published criteria25 as: 0 if tumor cells had complete absence of staining or any staining intensity in less than 10% of tumor cells; 1+ if tumor cells had faint or barely perceptible membrane staining in more than 10% of tumor cells or weak heterogeneous staining in more than 50% of positive cells; 2+ if tumor cells had moderate membrane staining in more than 10% of cells or moderate heterogeneous staining in more than 50% of positive cells; 3+ if tumor cells had strong membrane staining in more than 10% of cells or strong heterogeneous staining in more than 50% of positive cells. Tumors with 1+, 2+, and 3+ expression were interpreted as positive for EGFR expression, and tumors with no expression (0 score) were interpreted as negative for EGFR expression.25
Figure 1.
Immunohistochemistry with antibodies against Ki-67 (Panels A and B), caspase-3 (Panels C and D), VEGF-C (Panels E and F), and E-cadherin (Panels G and H), in a patient who did not develop brain metastasis (left column) and a patient who developed brain metastasis (right column).
Statistical analysis
The primary end point of this study was the occurrence of brain metastasis. Brain metastasis (present v absent) was considered a binary response variable.
The distribution of patient characteristics was compared between study and control groups using the Wilcoxon rank-sum test and Fisher's exact test for continuous and discrete variables, respectively. The distribution of protein expression was compared between study and control groups also using the Wilcoxon rank-sum test. In addition, the test was stratified according to <60, 61-70 and >70 years of age in order to adjust for the age difference between the two groups. The association between development of brain metastases and each molecular marker was based on the age-adjusted Mantel-Haenszel estimate of the odds ratio.
Overall survival was calculated from the time of NSCLC diagnosis to the time of death from any cause or to the time of the last follow-up, at which point the survival time was censored. Overall survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to evaluate the survival difference between patient groups. The statistical analysis was computed using SAS 9.1 (SAS institute, Cary, NC), while the plots were produced using S-Plus 6.2 for Windows (Insightful Corp, Seattle, WA). All significance tests were based on a two-sided hypothesis.
RESULTS
Patient and Tumor Characteristics
The patient characteristics are summarized in Table 1. In patients with newly diagnosed NSCLC included in our study, 21 (39%) developed brain metastases after a median time of 12.5 months (range 1.7-89.4 months), and 33 (61%) had no evidence of brain metastases by negative neurologic examination and/or negative brain imaging. The follow-up period of the control group (median 3.5 years; range 0.8-6.6 years) was at least as long as the study period of the patient group (median 2.6 years; range 0.7-8.4 years, P=0.54).
Expression of Molecular Markers and the Risk of Developing Brain Metastases
Table 3 shows the distribution of case patients and control subjects according to the expression of molecular markers and cutoff values used in the study. The primary analysis in Table 3 is focused on the results for differential expression that is meaningful from a pathology perspective. For each marker, we reported the cutoff point associated with the highest odds ratio, in terms of quantifying the risk of developing brain metastases. Nevertheless, the strong association between biomarker expression and development of brain metastases was maintained generally over a broad range of different cutoff values of the semiquantitative score. In general, we explored the range between the 25th and 75th percentiles of marker expression in the combined study and control groups to ensure reasonable patient numbers in the comparison subgroups for reasons of statistical power and clinical utility.
Table 3.
Risk of Developing Brain Metastases According to the Expression of Molecular Markers in NSCLC
| Study | Control | ||||||
|---|---|---|---|---|---|---|---|
| Patients | Patients | ||||||
| Characteristic | No. | (%) | No. | (%) | Adjusted Odds Ratio* | (95% CI)† | P Value |
| Ki-67 LI | |||||||
| <30 | 6 | (29) | 26 | (79) | 1.0 | ||
| ≥30 | 15 | (71) | 7 | (21) | 12.2 | (2.4 to 70.4) | <0.001 |
| Caspase-3 LI | |||||||
| ≥ 2 | 7 | (33) | 32 | (97) | 1.0 | ||
| <2 | 14 | (67) | 1 | (3) | 43.0 | (5.3 to >100) | <0.001 |
| VEGF-A (%) | |||||||
| 0-90 | 8 | (38) | 16 | (48) | 1.0 | ||
| 91-100 | 13 | (62) | 17 | (52) | 1.3 | (0.4 to 5.1) | 0.77 |
| VEGF-C (%) | |||||||
| 0 | 2 | (10) | 18 | (55) | 1.0 | ||
| 1-100 | 19 | (90) | 15 | (45) | 14.6 | (2.0 to >100) | 0.001 |
| E-Cadherin (%) | |||||||
| 91-100 | 5 | (24) | 17 | (52) | 1.0 | ||
| 0-90 | 16 | (76) | 16 | (48) | 3.6 | (0.9 to 16.4 ) | 0.05 |
| EGFR | |||||||
| Negative | 2 | (10) | 5 | (15) | 1.0 | ||
| Positive | 19 | (90) | 28 | (85) | 0.7 | (<0.1 to 10.4) | 1.00 |
Odds Ratios were adjusted for age.
CI denotes confidence interval.
The study patient group had 15 tumors (71%) with a high Ki-67, whereas the control group had 7 tumors (21%, Table 3). A high caspase-3 LI was present in seven (33%) tumors from the study group whereas almost all the tumors (97%) in the control group had a high caspase-3 (21%, Table 3).
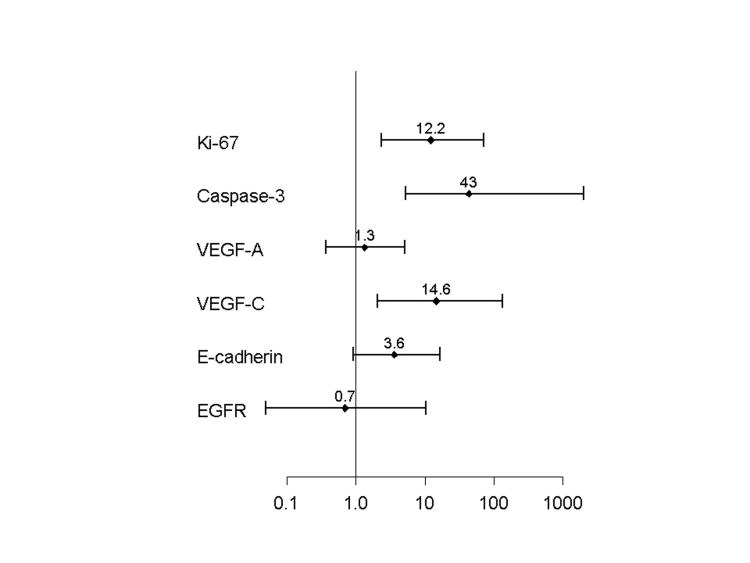
An increased risk of developing brain metastases was associated with a Ki-67 LI of 30 or higher (adjusted odds ratio 12.2, 95% CI, 2.4 to 70.4, P<0.001). Caspase-3 LI was also significantly associated with development of brain metastases (P<0.001), but the correlation was negative. Therefore, lower staining percentages of caspase-3 were observed in NSCLC from patients who developed brain metastases (adjusted odds ratio 43.0, 95% CI, 5.3 to >100, P<0.001). The ratio of Ki-67 LI to caspase-3 LI was also significantly associated with development of brain metastases (odds ratio 68.0, 95% CI, 7.7 to >100, P<0.001). Staining percentages of VEGF-C and E-cadherin were also significantly associated with development of brain metastases (P values of 0.001, and 0.05, respectively, Table 3, Fig. 2). No significant risk of developing brain metastases was observed with VEGF-A and EGFR expression (Table 3, Fig. 2).
Figure 2.
Adjusted odds ratios (OR) with 95% confidence intervals (CI) of developing brain metastasis according to expression of Ki-67, caspase-3, VEGF-A, VEGF-C, E-Cadherin, and EGFR in the primary NSCLC.
Outcome of Patients with NSCLC
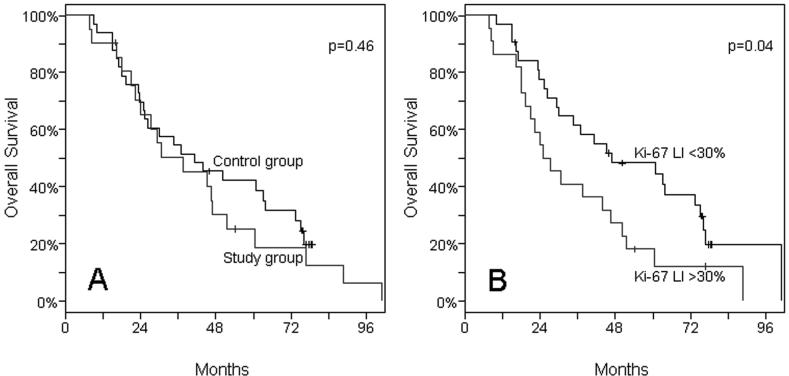
We found no significant differences in overall survival between patients in the study group and those in the control group (P=0.46, Fig. 3).
Figure 3.
Kaplan-Meier Estimates of Overall Survival among Patients with NSCLC. Panel A. There is no difference in overall survival between the patient and control groups. Panel B. Overall survival is significantly shorter in patients with high Ki-67 LI (P=0.04).
As in previous studies,2, 4 brain metastases developed after a median time of 12.5 months (range 53 days to 89.4 months) from the primary diagnosis of NSCLC. When the study and control groups were analyzed together, a high Ki-67 LI was significantly associated with poor prognosis (P=0.04). Patients who had a Ki-67 LI of 30 or higher had a shorter overall survival than those with a Ki-67 LI of less than 30 (median overall survival times 26 and 47 months, respectively, Fig. 3). The other molecular markers did not show any association with overall survival.
DISCUSSION
In this study we evaluated patients with NSCLC with and without brain metastasis in a unique series that has tumor material from both the primary lung tumor and matched metachronous brain metastasis obtained from a single institution. These patients are typically only treated for lung cancer, and brain biopsy is rarely recommended or obtained. This gave us the opportunity to confirm that the brain tumors are metastases from lung primary NSCLC and to interrogate for expression of a broad panel of biomarkers reported to be involved in the pathogenesis of brain metastasis.8, 9, 14-16, 18, 19, 22, 26 Our goal was to determine if any of these markers or a combination of markers could identify the primary NSCLC tumors that are more likely to metastasize to the brain. We have found that patients with high Ki-67, low caspase-3, high VEGF-C, and low E-cadherin in their primary NSCLC tumors have a higher risk of developing brain metastases when compared with a control group of NSCLC diagnosed during the same period of time (Fig. 2). These biomarkers are promising for predicting the development of brain metastases, and additional studies on larger series of patients are needed to validate the findings from our study.
Recent studies on the molecular biology in human cancers have shown that numerous factors affect the behavior of malignant neoplasms.27 Tumors with a high Ki-67 LI are frequently more aggressive than those with low Ki-67 LI.28 In addition, factors involved in angiogenesis such as VEGF are associated with tumor growth and metastasis.17-19 Furthermore, reduced expression of molecules involved in epithelial mesenchymal transformation (EMT) such as E-cadherin, could induce tumor cells with high metastatic potential.21 Although studies have shown association between expression of biomarkers and metastatic potential of other cancers,18, 28-30 the value of these molecular markers in predicting brain metastasis in patients with NSCLC has not been explored yet.
To date, it remains unclear whether patients with early stage NSCLC should be screened for brain metastases according to published clinical guidelines.11-13 In its latest joint statement, the American Thoracic Society and the European Respiratory Society recommend no preoperative imaging of the brain in patients with NSCLC.11 In fact, it is believed that the routine screening is cost-ineffective due to the low incidence of brain metastasis in asymptomatic patients.31-34 Some reports argue that early detection of occult brain metastases will avoid increased morbidity either by allowing earlier treatment of the brain or by avoiding futile thoracotomies.10, 35-38 However, evidence suggests an increased incidence of cerebral metastases after preoperative radiotherapy and chemotherapy in patients with locally advanced NSCLC.39 In addition, studies have shown contrasting results regarding the correlation between tumor histology and the risk of developing brain metastases.4, 12 Therefore, there is a need to identify biologic markers to help identify patients with NSCLC who are at increased risk of developing brain metastases.
Patients with NSCLC who develop brain metastases are most likely to benefit from therapy addressing the brain that would substantially improve the quality of life.7 Analysis of subgroups of patients with NSCLC shows that certain populations are at particularly high risk of brain metastasis.7
Studies have investigated predictors of survival in patients with NSCLC and brain metastases. Similar to our results, Gaspar et al.2 found an increased risk of brain metastases in patients younger than 50 years of age. It is also known that performance status, extent of extracranial disease, control of primary cancer, and age are predictors of overall survival in patients with NSCLC.
Immunostaining with the Ki-67 antibody is a widely accepted method for evaluating proliferative activity in a variety of human tumors. Ki-67 LI correlates well with the predicted growth fraction despite that it has been consistently shown to overestimate the proliferative activity of tumors and is therefore not an exact reflection of tumor growth.40 Sarbia et al.41 found a significant association between Ki-67 LI and the mitotic activity in tumor tissue. Numerous studies have shown that Ki-67 LI correlates with other markers of cell proliferation.42, 43 Our data showed a strong correlation between high Ki-67 LI and development of brain metastases (Fig. 2). Patients with Ki-67 LI of ≥30% had a 12 times increased risk of developing brain metastases (P<0.001, Table 3) compared to those with lower proliferative activity. Furthermore, in our study, a high Ki-67 LI was associated with poor prognosis (Fig. 3).
Abnormalities in the molecular mechanisms of apoptosis are important in the pathogenesis of malignant processes because they increase the longevity of neoplastic cells, develop resistance to generally harmful stresses, and increase invasiveness resulting in disease progression and metastasis.29 This process is the result of the interaction between several proteins involved in either inhibition or activation of the apoptotic cascade. Several studies have shown that activation of caspase system is involved in gefitinib-induced apoptosis in patients with NSCLC.44, 45 Our results showed that a low caspase-3 LI was associated with a significant increase in the risk of developing brain metastases (Table 3, Fig. 2).
VEGF family of proteins modulates angiogenesis which is essential for tumor growth and metastasis.46 Since its identification around 15 years ago, this family has expanded considerably to include VEGF-A, -B, -C and -D.17, 47, 48 Although VEGF has been linked to angiogenesis in various tumors,49, 50 its role in the metastatic ability of NSCLC has been poorly characterized. VEGF-C production by a tumor is associated with increased incidence of lymphatic metastasis, but the mechanism remains unclear.51 It is believed that tumors that overexpressed VEGF-C were surrounded by lymphatic vessels that have abnormal lymphatic flow when compared to controls.52, 53 Our results showed a strong correlation between high expression of VEGF-C and increased risk of developing brain metastases in patients with NSCLC (Table 3). When more than 5% of tumor cells express VEGF-C, the risk of developing brain metastases increases 15 times (P<0.001, Table 3). There was no association between expression of VEGF-A and the risk of developing brain metastases (Fig. 2).
E-cadherin is a calcium-regulated adhesion molecule expressed in most normal epithelial tissues that plays an important role in cell-sorting mechanisms by offering adhesion specificities that govern tissue integrity. It has been demonstrated that this molecule may regulate the invasiveness of neoplastic cells.54 Our results showed that loss of E-cadherin is associated with an increased risk of developing brain metastases in patients with NSCLC (P=0.05, Table 3).
EGFR is overexpressed in 40 to 80% of NSCLC and many other epithelial cancers.22, 23 Despite the fact that anti-EGFR agents are being used in clinical trials for patients with advanced disseminated disease, the value of EGFR in predicting brain metastases is unknown. In this study we found that EGFR was overexpressed in 90% of patients in the study group and 85% of patients in the control group, but the difference was not statistically significant (Table 3, Fig. 2).
Our study shows that patients with NSCLC and high Ki-67, high VEGF-C, low E-cadherin and low caspase-3 in their tumors have a higher risk of developing brain metastases (Table 3, Fig. 2). Our study suggests that patients with NSCLC and a specific biomarker expression may benefit from an individualized surveillance regimen and preventive therapeutic intervention designed to prevent or delay the development of brain metastases.
Acknowledgments
This study was supported by CA90578, CA074386, and CA092824 from the National Institutes of Health and International Mesothelioma Program from Brigham and Women's Hospital.
Footnotes
Part of this study has been presented at the 2007 United States and Canadian Academy of Pathology Annual Meeting (Pulmonary Pathology Society Trainee Award), March 26, 2007 San Diego, California. We are indebted to Ms. Laura Kwan for secretarial support.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar LE, Chansky K, Albain KS, Vallieres E, Rusch V, Crowley JJ, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol. 2005;23(13):2955–61. doi: 10.1200/JCO.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer. 2004;45(suppl 2):S253–7. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- 4.Mamon HJ, Yeap BY, Janne PA, Reblando J, Shrager S, Jaklitsch MT, et al. High Risk of Brain Metastases in Surgically Staged IIIA Non-Small-Cell Lung Cancer Patients Treated With Surgery, Chemotherapy, and Radiation. J Clin Oncol. 2005;23(7):1530–37. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- 5.Pottgen C, Eberhardt W, Grannass A, Korfee S, Stuben G, Teschler H, et al. Prophylactic Cranial Irradiation in Operable Stage IIIA Non Small-Cell Lung Cancer Treated With Neoadjuvant Chemoradiotherapy: Results From a German Multicenter Randomized Trial. J Clin Oncol. 2007;25(31):4987–92. doi: 10.1200/JCO.2007.12.5468. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JD, Paulus R, Graham MV, Ettinger DS, Johnstone DW, Pilepich MV, et al. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: promising long-term results of the Radiation Therapy Oncology Group--RTOG 9705. J Clin Oncol. 2005;23(15):3480–7. doi: 10.1200/JCO.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 7.Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. 2001;19(5):1344–9. doi: 10.1200/JCO.2001.19.5.1344. [DOI] [PubMed] [Google Scholar]
- 8.Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13(6):1656–62. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 9.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13(6):1644–7. doi: 10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 10.Ferrigno D, Buccheri G. Cranial computed tomography as a part of the initial staging procedures for patients with non-small-cell lung cancer. Chest. 1994;106(4):1025–9. doi: 10.1378/chest.106.4.1025. [DOI] [PubMed] [Google Scholar]
- 11.Shen KR, Meyers BF, Larner JM, Jones DR. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):290S–305S. doi: 10.1378/chest.07-1382. [DOI] [PubMed] [Google Scholar]
- 12.Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol. 2006;1(3):205–10. doi: 10.1016/s1556-0864(15)31569-0. [DOI] [PubMed] [Google Scholar]
- 13.Ettinger DS, Bepler G, Bueno R, Chang A, Chang JY, Chirieac LR, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4(6):548–82. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 14.Thunnissen FB, Schuurbiers OC, den Bakker MA. A critical appraisal of prognostic and predictive factors for common lung cancers. Histopathology. 2006;48(7):779–86. doi: 10.1111/j.1365-2559.2006.02386.x. [DOI] [PubMed] [Google Scholar]
- 15.Koomagi R, Volm M. Relationship between the expression of caspase-3 and the clinical outcome of patients with non-small cell lung cancer. Anticancer Res. 2000;20(1B):493–6. [PubMed] [Google Scholar]
- 16.Volm M, Koomagi R. Relevance of proliferative and pro-apoptotic factors in non-small-cell lung cancer for patient survival. Br J Cancer. 2000;82(10):1747–54. doi: 10.1054/bjoc.1999.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 18.Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis. 2004;21(2):107–18. doi: 10.1023/b:clin.0000024761.00373.55. [DOI] [PubMed] [Google Scholar]
- 19.Donnem T, Al-Saad S, Al-Shibli K, Delghandi MP, Persson M, Nilsen MN, et al. Inverse Prognostic Impact of Angiogenic Marker Expression in Tumor Cells versus Stromal Cells in Non Small Cell Lung Cancer. Clin Cancer Res. 2007;13(22):6649–57. doi: 10.1158/1078-0432.CCR-07-0414. [DOI] [PubMed] [Google Scholar]
- 20.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 21.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67(23):11254–62. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 24.Travis WDBE, Muller-Hermelink HK, et al. World Health Organization Classification of Tumours. IARC Press; Lyon: 2004. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus, and Heart. [Google Scholar]
- 25.Grupka NL, Lear-Kaul KC, Kleinschmidt-DeMasters BK, Singh M. Epidermal growth factor receptor status in breast cancer metastases to the central nervous system. Comparison with HER-2/neu status. Arch Pathol Lab Med. 2004;128(9):974–9. doi: 10.5858/2004-128-974-EGFRSI. [DOI] [PubMed] [Google Scholar]
- 26.Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19(53):6122–9. doi: 10.1038/sj.onc.1203969. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Liu D, Masuya D, Nakashima T, Kameyama K, Ishikawa S, et al. Clinical application of biological markers for treatments of resectable non-small-cell lung cancers. Br J Cancer. 2005;92(7):1231–9. doi: 10.1038/sj.bjc.6602481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank I, Cheville J, Blute M. Prognostic value of p53 and MIB-1 in transitional cell carcinoma of the urinary bladder with regional lymph node involvement. Cancer. 2004;101:1803–08. doi: 10.1002/cncr.20567. [DOI] [PubMed] [Google Scholar]
- 29.Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, Roehrborn CG, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8(2):128–36. doi: 10.1016/S1470-2045(07)70002-5. [DOI] [PubMed] [Google Scholar]
- 30.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30(9):1097–104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 31.Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S, Jr., et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22(2):330–53. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 32.Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest. 2003;123(1 Suppl):147S–56S. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Kubota K, Kodama T, Nagai K, Nishiwaki Y. Extrathoracic staging is not necessary for non-small-cell lung cancer with clinical stage T1-2 N0. Ann Thorac Surg. 1999;68(3):1039–42. doi: 10.1016/s0003-4975(99)00694-3. [DOI] [PubMed] [Google Scholar]
- 34.Yohena T, Yoshino I, Kitajima M, Uehara T, Kanematsu T, Teruya T, et al. Necessity of preoperative screening for brain metastasis in non-small cell lung cancer patients without lymph node metastasis. Ann Thorac Cardiovasc Surg. 2004;10(6):347–9. [PubMed] [Google Scholar]
- 35.Grant D, Edwards D, Goldstraw P. Computed tomography of the brain, chest, and abdomen in the preoperative assessment of non-small cell lung cancer. Thorax. 1988;43(11):883–6. doi: 10.1136/thx.43.11.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvatierra A, Baamonde C, Llamas JM, Cruz F, Lopez-Pujol J. Extrathoracic staging of bronchogenic carcinoma. Chest. 1990;97(5):1052–8. doi: 10.1378/chest.97.5.1052. [DOI] [PubMed] [Google Scholar]
- 37.The Canadian Lung Oncology Group Investigating extrathoracic metastatic disease in patients with apparently operable lung cancer. Ann Thorac Surg. 2001;71(2):425–33. discussion 33-4. [PubMed] [Google Scholar]
- 38.Hillers TK, Sauve MD, Guyatt GH. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non-small cell lung cancer. Thorax. 1994;49(1):14–9. doi: 10.1136/thx.49.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Law A, Karp D, Dipetrillo T, Daly B. Emergence of increased cerebral metastases after high-dose preoperative radiotherapy with chemotherapy in patients with locally advanced nonsmall cell lung carcinoma. Cancer. 2001;92:160–4. doi: 10.1002/1097-0142(20010701)92:1<160::aid-cncr1304>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Scott RJ, Hall PA, Haldane JS, van Noorden S, Price Y, Lane DP, et al. A comparison of immunohistochemical markers of cell proliferation with experimentally determined growth fraction. J Pathol. 1991;165(2):173–8. doi: 10.1002/path.1711650213. [DOI] [PubMed] [Google Scholar]
- 41.Sarbia M, Bittinger F, Porschen R, Dutkowski P, Torzewski M, Willers R, et al. The prognostic significance of tumour cell proliferation in squamous cell carcinomas of the oesophagus. Br J Cancer. 1996;74(7):1012–6. doi: 10.1038/bjc.1996.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson MS, Anderson E, Bell JC, Pearson JM, Haboubi NY, James RD, et al. An evaluation of five different methods for estimating proliferation in human colorectal adenocarcinomas. Surg Oncol. 1994;3(5):263–73. doi: 10.1016/0960-7404(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 43.Gerdes J, Becker MH, Key G, Cattoretti G. Immunohistological detection of tumour growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J Pathol. 1992;168(1):85–6. doi: 10.1002/path.1711680114. [DOI] [PubMed] [Google Scholar]
- 44.Hopfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherubl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41(6):1008–16. doi: 10.1016/j.jhep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Teraishi F, Kagawa S, Watanabe T, Tango Y, Kawashima T, Umeoka T, et al. ZD1839 (Gefitinib, ‘Iressa’), an epidermal growth factor receptor-tyrosine kinase inhibitor, enhances the anti-cancer effects of TRAIL in human esophageal squamous cell carcinoma. FEBS Lett. 2005;579(19):4069–75. doi: 10.1016/j.febslet.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 47.Olofsson B, Jeltsch M, Eriksson U, Alitalo K. Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol. 1999;10(6):528–35. doi: 10.1016/s0958-1669(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–8. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 49.Lee A, Dublin E, Bobrow L, Poulsom R. Invasive lobular and invasive ductal carcinoma of the breast show distinct patterns of vascular endothelial growth factor expression and angiogenesis. J Pathol. 1998;185:394–401. doi: 10.1002/(SICI)1096-9896(199808)185:4<394::AID-PATH117>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Kim H, Jung J. Expression of vascular endothelial growth factor in renal cell carcinoma and the relation to angiogenesis and p53 protein expression. J Surg Oncol. 2001;77:55–60. doi: 10.1002/jso.1066. [DOI] [PubMed] [Google Scholar]
- 51.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98(2):413–23. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 52.Hoon DS, Kitago M, Kim J, Mori T, Piris A, Szyfelbein K, et al. Molecular mechanisms of metastasis. Cancer Metastasis Rev. 2006;25(2):203–20. doi: 10.1007/s10555-006-8500-x. [DOI] [PubMed] [Google Scholar]
- 53.Isaka N, Padera TP, Hagendoorn J, Fukumura D, Jain RK. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 2004;64(13):4400–4. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 54.Behrens J, Mareel M, Van Roy F, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–47. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]