Abstract
Identified for its role in development, β-catenin has been implicated in neuronal synapse regulation and remodeling. We examined β-catenin expression in the adult mouse brain and its role in amygdala-dependent learning and memory. We found alterations in β-catenin mRNA and protein phosphorylation during fear memory consolidation. Such alterations correlated with a change in the association of β-catenin with cadherin. Pharmacologically, this consolidation was enhanced with lithium-mediated facilitation of β-catenin. Genetically, the role of β-catenin was confirmed with site-specific deletions of floxed β-catenin in the amygdala. Baseline locomotor, anxiety-related behaviors, and the acquisition or expression of conditioned fear were normal. However, amygdala-specific deletion prevented the normal transfer of newly formed fear learning into long-term memory. Thus, β-catenin within the amygdala may be required for the normal consolidation, but not acquisition, of fear memory. This suggests a general role for β-catenin in synaptic remodeling and stabilization underlying long-term memory in adults.
Introduction
Structural changes at synapses are thought to underpin long-term memory formation. Dendritic spines, where most excitatory synapses terminate1,2, show alterations in motility and morphology following a learning event3-5. The processes governing dendritic morphogenesis are many and varied, but recent work has focused on the role of cell adhesion molecules in mediating activity-dependent changes at synapses.
β-catenin is a candidate molecule that may function in mediating the structural changes associated with long-term memory formation. It associates with the cytoplasmic domain of cadherin and directly links to the actin cytoskeleton via α-catenin6. This cadherin-catenin complex is localized in synaptic junctions, and their alterations are thought to influence synaptic size and strength7. Recent work has suggested that the cadherin-catenin complex is not only involved in synapse development, but also in modulation of synaptic connectivity and activity8,9.
In addition to the role of β-catenin in cadherin-mediated cell-cell adhesion, it also plays an important role in the Wnt signal transduction pathway. In the resting state, β-catenin is phosphorylated by glycogen synthase kinase 3β (GSK-3β) and rapidly degraded by the proteasome pathway. Upon activation of Wnt signaling, β-catenin is stabilized through the inhibition of GSK-3β, and translocates to the nucleus, where it binds the TCF/LEF family of transcription factors, to regulate the expression of Wnt target genes10,11. The Wnt/β-catenin signaling pathway has recently been shown to be involved in the regulation of synaptic plasticity in a hippocampal slice preparation12.
Thus β-catenin appears to be a very important ‘hub’ protein in synaptic plasticity, with involvement in regulating both activity-dependent synaptic remodeling as well as gene transcription. Taken together, there is tremendous face validity to the hypothesis that β-catenin is directly involved in critical events that mediate learning and memory. However, since knockouts of β-catenin are embryonic lethal13, it has not been possible to examine the potentially critical role of this protein in learning and memory assays in animals. Also, since no specific pharmacological agents that target β-catenin have yet been identified, no pharmacological studies have directly examined learning and memory modulation by β-catenin.
These experiments outline a role for β-catenin regulation along with its interaction with cadherin during the consolidation phase of fear memory formation. We demonstrate that acute administration of lithium, which acts, in part, by stabilizing β-catenin through the inhibition of GSK-3β, enhances memory formation. We then use an inducible genetic approach to examine whether β-catenin is required for the consolidation of fear memories in vivo. When examining the effects of temporal- and region-specific deletion, we find that β-catenin within the amygdala is required for the consolidation, but not acquisition or expression, of fear memory.
Results
β-catenin mRNA expression in the adult mouse brain
Due to the heavy emphasis on the role of β-catenin in development, there are sparse data on the expression of β-catenin in adult animals. Thus we examined β-catenin expression in the brains of wild-type adult (8-10 week old) C57Bl/6J mice. Using an antisense probe spanning exons 2 through 6 of β-catenin, in situ hybridization analyses revealed very dense expression of this gene throughout the adult brain, particularly in regions associated with synaptic plasticity (Fig. 1). A sense probe spanning the same region was used as a negative control, which demonstrated no significant labeling above background (data not shown). These data demonstrate that β-catenin is present in the adult brain and may be required for normal neuronal functioning in adults.
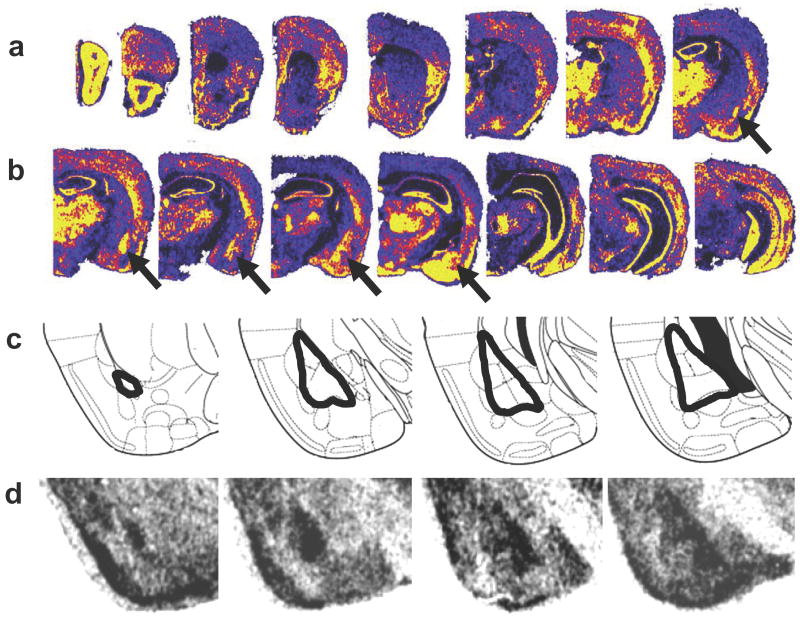
Figure 1. β-catenin expression in the adult mouse brain.
(a-b) Pseudocolored in situ hybridization photomicrographs, illustrating mRNA expression showing high levels of β-catenin throughout the brain, particularly within the amygdala, some cortical regions, thalamus, and hippocampus. The arrows point to the basolateral amygdala (BLA). Yellow represents highest level of expression; blue-black lowest levels of expression. (c) Schematic diagram from Paxinos and Watson32 demonstrating the location of the amygdala within the temporal lobe and its subunits (note that the BLA is outlined). (d) β-catenin mRNA is present at high levels spanning the basolateral nuclei of the amygdala as outlined in (c).
β-catenin mRNA increases in the amygdala following fear conditioning
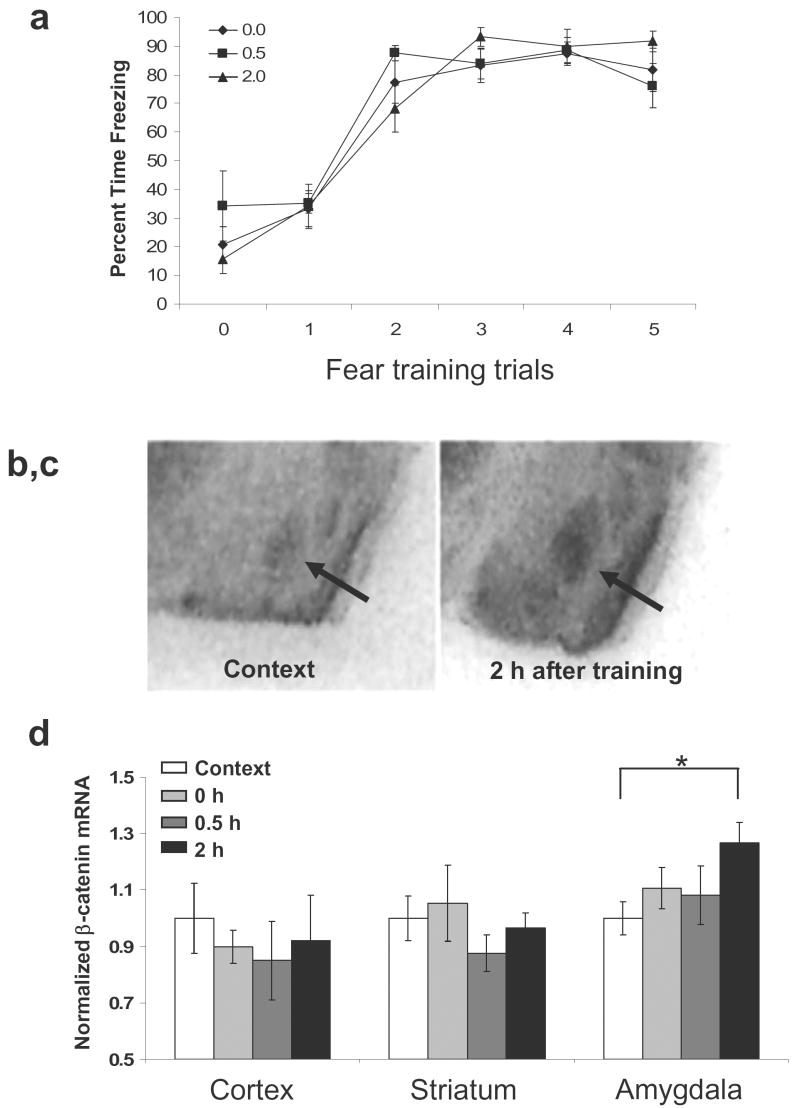
We next began to examine the hypothesis that β-catenin is involved in the synaptic plasticity underlying learning and memory in adults, specifically fear conditioning within the amygdala. Following three days of habituation to the conditioning chambers, mice received five tone-shock pairings. A context control group was placed in the conditioning chambers for the same amount of time, but no stimuli were presented, and brains were collected immediately. Trained animals were sacrificed at 0, 0.5, or 2 hr after conditioning. Animals that had received the five tone-shock pairings were able to acquire and express fear, as shown by increased freezing throughout the training (Fig. 2a). β-catenin mRNA levels were then measured in various brain regions at different time points following fear conditioning. We found that β-catenin mRNA in the basolateral amygdala (BLA) was altered with fear conditioning, with a significant increase at 2 hr following training (control, 1.00 ± 0.06 vs. 2 hr, 1.27 ± 0.08) (Fig. 2b-d; t(14) = 2.764, P < 0.05). We did not find any significant differences in β-catenin mRNA within the somatosensory cortex or striatum (Fig. 2d; P > 0.05).
Figure 2. β-catenin gene expression in the amygdala is increased following fear conditioning.
Animals were exposed to five tone-shock pairings and then sacrificed 0, 0.5, or 2 hr after training. (a) Acquisition curve showing the % freezing during each tone prior to the presentation of the footshock. Animals in all groups displayed similar levels of freezing before the presentation of any tones (0) and then showed increased freezing during the tones throughout training (tones 1-5). Arrows represent the presentation of footshock. (b-c) Qualitative in situ hybridization analysis of β-catenin mRNA in the amygdala in context exposed animals (b) and animals sacrificed 2 hr after training (c). (d) Relative levels of β-catenin mRNA expression in the somatosensory cortex, striatum, and amygdala, normalized to context exposed animals. Only β-catenin mRNA levels within the amygdala are significantly increased at 2 hr after fear conditioning. (n = 8 for context, 0 hr, and 2 hr, n = 7 for 0.5 hr; Bars indicate mean ±s.e.m; * denotes P < 0.05)
β-catenin is post-translationally regulated following fear conditioning
We then examined whether β-catenin is altered in its level of expression or post-translational modification with fear learning. Western blot analyses were used to examine β-catenin expression in mice exposed to the context alone, mice exposed to unpaired tone and shock presentations, and mice trained and sacrificed 0, 0.5, 2, 4, 12, or 24 hr following fear conditioning (5 tone-shock trials) (Fig. 3a). In contrast to the observed increase in its mRNA expression, total levels of β-catenin protein in the amygdala did not change with training (ANOVA, P > 0.05; Fig. 3b), suggesting that protein modification or degradation may be occurring in concert with the increases in gene transcription.
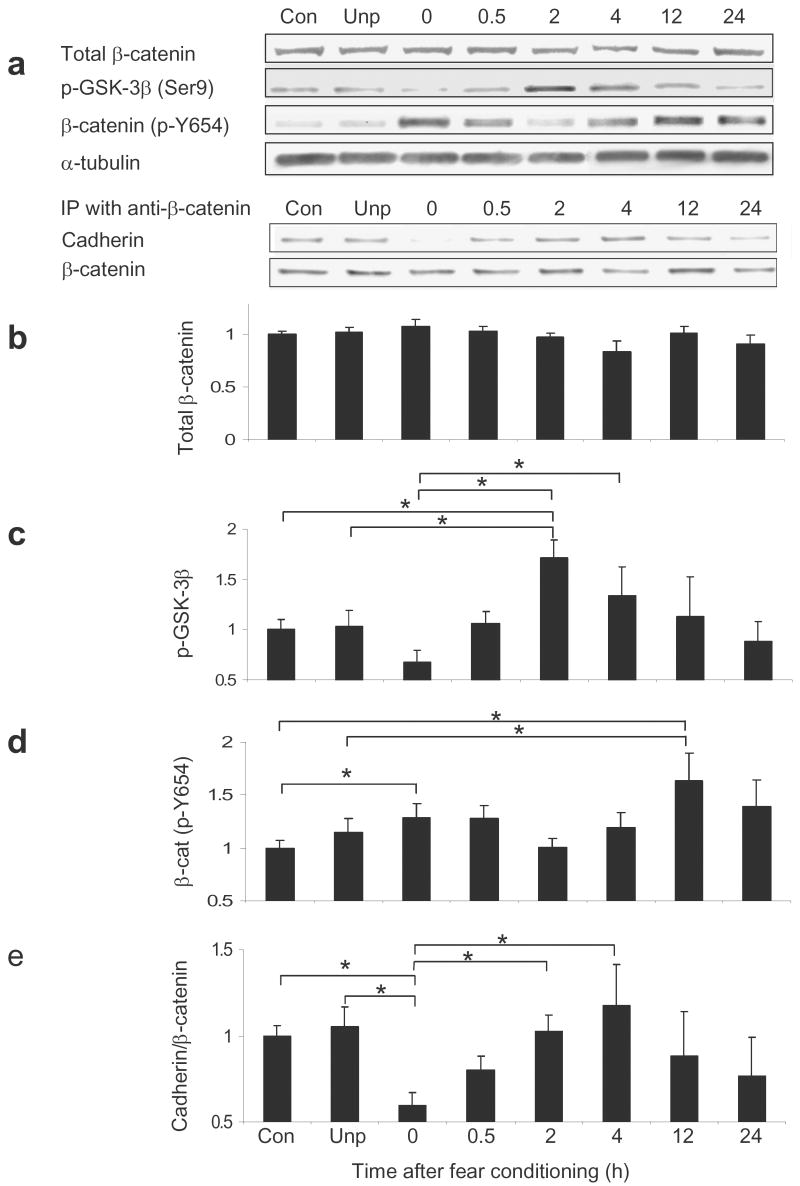
Figure 3. Phosphorylation state of β-catenin and GSK-3β are altered following fear conditioning.
Animals were exposed to five tone-shock pairings and then sacrificed 0, 0.5, 2, 4, 12, or 24 hr after training. (a) Qualitative representation of western blot data. In all bar graphs quantitative levels of proteins determined with western blots are expressed relative to the α-tubulin loading control. (b) Total levels of β-catenin, determined by western blot, do not change with fear conditioning. (c) Phospho-GSK-3β levels are significantly changed across timepoints. (d) β-catenin (phospho-Y654) levels are significantly changed across timepoints. (e) Immunoprecipitation results from a cadherin immunoblot after β-catenin immunoprecipitation. Cadherin interaction with β-catenin is significantly changed across timepoints. (n = 31 for context; n =13 for unpaired, n = 14 for 0 hr, n = 27 for 0.5 and 2 hr, n = 8 for 4 hr, n = 6 for 12 hr, and n = 7 for 24 hr; ‘con’ = context control group, ‘unp’=unpaired shock control group; Bars indicate mean ± s.e.m.; * denotes P ≤ 0.05 for the different comparisons identified).
Thus, we wanted to determine if there were post-translational modifications of β-catenin with learning. First, we measured changes in the stabilization of β-catenin by GSK-3β inactivation. GSK-3β destabilizes β-catenin by phosphorylating it at Ser33/37/Thr41. However, when the GSK-3β protein is phosphorylated, it is unable to destabilize β-catenin. Therefore, we examined levels of phospho-GSK-3β following learning with ANOVA and found a significant main effect for time, F(7, 132) = 3.943, P ≤ 0.001. Using posthoc LSD analyses, levels of phospho-GSK-3β were significantly increased at 2hr (1.71 ± 0.18) compared to unpaired control animals (1.03 ± 0.16; P ≤ 0.005), context exposed animals (1.00 ± 0.10; P ≤ 0.001), and the immediately sacrificed animals (0 hr) (0.68 ± 0.11; P ≤ 0.001) (Fig. 3c). Notably the 0 hr time point was also significantly different from the 4 hr timepoint (1.34 ± 0.28; P ≤ 0.001). This significant increase in phospho-GSK-3β following fear conditioning is consistent with enhanced stabilization of β-catenin during fear consolidation.
We then examined levels of β-catenin, phosphorylated at residue Y654. When β-catenin is phosphorylated at this site, it results in a decreased affinity for cadherin14. Overall, with ANOVA we found a significant main effect for time, F(7,132) = 2.107, P ≤ 0.05. Posthoc tests revealed that β-catenin (phospho-Y654) levels significantly increased at 12 hr (1.63 ± 0.26) after conditioning as compared to unpaired control animals (1.14 ± 0.14; P ≤ 0.05) and context exposed animals (1.00 ± 0.08; P ≤ 0.01) (Fig. 3d). Notably, compared to the context exposed group, β-catenin (phospho-Y654) was also significantly increased at 0.5 hr (1.28 ± 0.12; P ≤ 0.05) following conditioning. Thus the affinity of β-catenin for cadherin within the amygdala appears to be dynamically regulated during fear consolidation.
Based on these results, we wanted to determine if these phospho-Y654 changes in β-catenin significantly affected the association of β-catenin with cadherin. β-catenin was immunoprecipitated from the amygdala of the above mice, and then probed with an anti-pan-cadherin antibody. Using ANOVA, we found a significant main effect for time, F(7,132) = 2.320, P ≤ 0.05, (Fig. 3e). Posthoc analyses revealed that the amount of cadherin co-immunoprecipitated with β-catenin was significantly decreased immediately (0 hr) (0.60 ± 0.08) following training as compared to unpaired (1.05 ± 0.12; P ≤ 0.01) and context control mice (1.00 ± 0.06; P ≤ 0.01). Notably, this immediate decrease is followed by a significant increase in binding at 2 hr (1.02 ± 0.10; P ≤ 0.01) and 4 hr (1.17 ± 0.24; P ≤ 0.01), returning cadherin binding to normal. Furthermore, we found a significant negative correlation between the amount of cadherin co-immunoprecipitated with β-catenin and the levels of p-Y654 β-catenin, r(133) = -0.184, P ≤ 0.05, confirming a significant relationship between these measures.
Note that in all Western blot analyses, there were no significant differences between context-exposed mice and those mice receiving unpaired tones and shocks, P > 0.05. Thus, the time-dependent differences we are observing in β-catenin modulation are likely due to associative learning, and not due to the stress of shock alone.
Increasing β-catenin functional stability results in an enhancement in learning
Since β-catenin regulation within BLA is correlated with fear conditioning, we examined whether manipulating β-catenin function would affect this learning process. Unfortunately no specific pharmacological agents that target β-catenin have yet been identified, making it difficult to directly examine the effect of β-catenin function on learning. However, lithium chloride (LiCl), despite not being as specific as we would like, is one compound that has received wide acceptance as a modulator of β-catenin. LiCl inhibits GSK-3β, decreasing its ability to phosphorylate β-catenin at its Ser33/37/Thr41 sites. As a consequence, the unphosphorylated β-catenin is more stable and less prone to degradation15-17. The temporal changes seen with phospho-GSK-3β suggested that this might be a good target for pharmacologically manipulating β-catenin with learning.
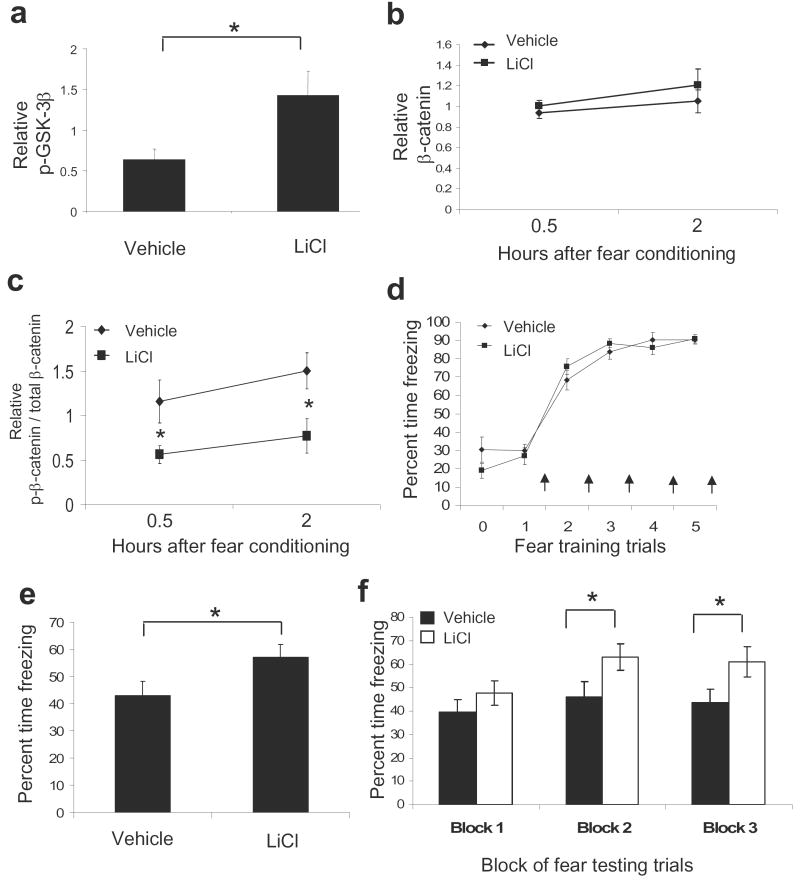
To examine the effects of acute LiCl administration on learning, we first confirmed that systemic administration of LiCl inhibits GSK-3β in the amygdala. We injected mice intraperitoneally (i.p.) with either saline or LiCl (100 mg kg-1) and sacrificed the animals 30 min following treatment. As expected, we found that acute administration of LiCl significantly increased phospho-GSK-3β levels in the amygdala (1.43 ± 0.30) as compared to controls (0.64 ± 0.13), t(17) = 2.344, P ≤ 0.05 (Fig. 4a). We then examined whether LiCl alters GSK-3β-dependent phosphorylation of β-catenin following fear conditioning. Mice were injected with either saline or LiCl 30 min prior to training, and then sacrificed at 0.5 or 2 hr post fear conditioning. We found that total β-catenin levels in the amygdala were increased, although not significantly, in LiCl treated animals (Fig. 4b). Notably, in agreement with the model of LiCl inhibiting GSK-3β, we found that levels of β-catenin phosphorylated at the GSK-3β-dependent sites were significantly decreased at the 0.5 and 2 hr time points (Fig 4c, Normalized for total β-catenin levels, F(1, 28) = 11.931, P < 0.01). Together, these results suggest that acute LiCl administration inhibits GSK-3β-mediated phosphorylation of β-catenin, potentially enhancing its overall stability, during the consolidation period following fear conditioning.
Figure 4. Lithium chloride decreases GSK-3β-mediated β-catenin phosphorylation in the amygdala and enhances learning.
(a) Animals were sacrificed from their home-cage 30 min after systemic injections of either vehicle or LiCl (100 mg kg-1). Phospho-GSK-3β levels in the amygdala were significantly increased in LiCl compared to vehicle treated animals (n = 9 for vehicle group, n = 10 for vehicle group). (b-c) A separate group of animals was injected with either vehicle or LiCl 30 min prior to fear conditioning, and then sacrificed 0.5 hr or 2 hr following training (n = 8 per group). (b) Total β-catenin levels in the amygdala were increased, although not significantly, at the 0.5 and 2 hr time points in LiCl treated animals. (c) The ratios of β-catenin phosphorylated at the GSK-3β-dependent sites (Ser33/37, Thr41) to total β-catenin levels were significantly decreased in LiCl treated animals at the 0.5 and 2 hr time points following fear conditioning. (d-f) An additional group of animals was injected with either vehicle or LiCl 30 min prior to fear conditioning and then tested 48 hr later (n = 19 per group). (d) Acquisition curve showing the % freezing during each tone prior to the presentation of the footshock. All animals were able to acquire and express equal levels of fear. Arrows represent the presentation of footshock. (e) Animals that had received LiCl prior to fear training showed significantly higher levels of fear than animals that had received saline upon retesting 48 hrs later in the absence of drug. (f) Examining the freezing data in panel E by blocks of 5 suggests that this difference in fear retention is maintained across the testing session (Bars indicate mean ± s.e.m.; * denotes P < 0.05)
Next we wanted to determine if decreasing GSK-3β-mediated phosphorylation of β-catenin through acute LiCl administration could affect learning. Mice were injected with either saline or LiCl and then fear conditioned 30 min later. The intensity of the US was lowered to 0.6 mA to prevent ceiling effects on fear expression. Throughout this training paradigm, we were able to measure freezing behavior during each tone (Conditioned stimulus, CS) presentation (prior to the presentation of footshock) (Fig. 4d). We found a significant main effect of time across all animals (F(5,180) = 111.495, P ≤ 0.01); however, there was no main effect of LiCl treatment (F(1,36) = .167, P = .685).
Forty-eight hours after fear conditioning, in the absence of drug, mice were placed in a novel chamber and presented with 15 CS tones. Average percent time spent freezing during these tones was obtained and used as a measure for conditioned fear (Fig. 4e). We found that mice receiving LiCl prior to fear training 2 days earlier now showed significantly higher levels of fear (57.00 ± 4.64) than animals that had received saline (43.07 ± 4.99), t(38) = 2.044, P < 0.05. Note that this increase in retention of fear was present throughout the testing session, but most notable towards the middle and end of the session (Fig. 4f, grouped blocks of 5 trials each: Block 1, Vehicle = 39.5 ± 5.5; Lithium = 47.6 ± 5.3; t(38) = 1.06, ns; Block 2, Vehicle = 46.2 ± 6.4; Lithium = 63.0 ± 5.7; t(38) = 1.95, P ≤ 0.05; Block 3, Vehicle = 43.7 ± 5.6; Lithium = 61.2 ± 6.5; t(38) = 2.05, P ≤ 0.05). These data suggest that the difference in retention of fear memory was not due to differences in extinction within testing. The enhancement of fear memory also did not appear to be due to effects on locomotor behavior, since mice did not show any significant differences across groups in activity level or freezing behavior prior to the first CS. Similarly, previous reports have shown that this specific dose of LiCl does not produce locomotor effects18.
Together these data suggest that a single, although non-specific, pharmacological manipulation that increases the level of functional β-catenin during or soon after fear conditioning leads to relatively specific increases in the expression of fear behavior 48 hr later. This is consistent with the hypothesis that enhancing functional β-catenin serves to enhance consolidation of new memories.
Region specific deletion of β-catenin in the adult brain
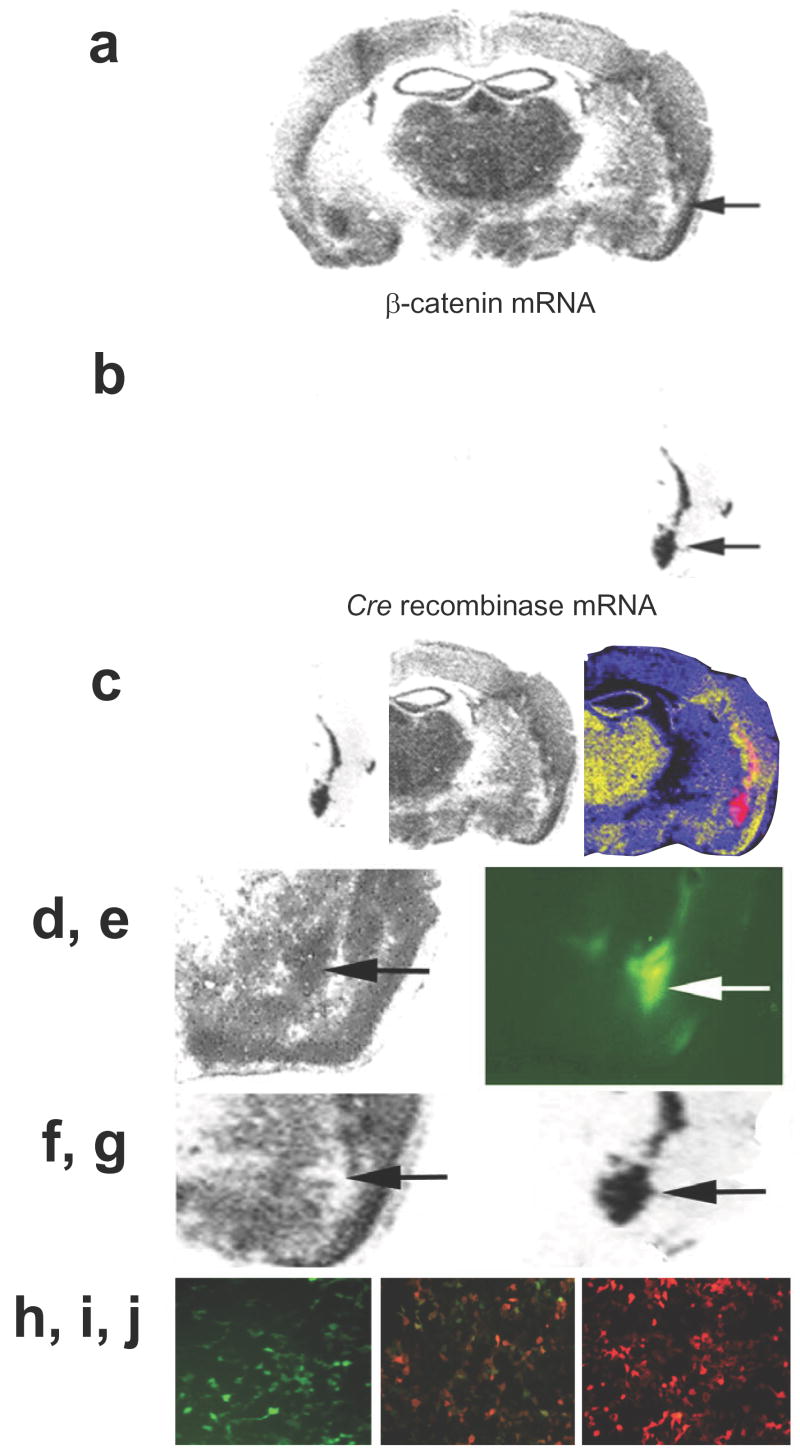
As stated above, LiCl is somewhat non-specific19. We sought to examine the effect of β-catenin on learning and memory through a more direct genetic mechanism using the floxed β-catenin mice (generated by Kemler et al20), which possess loxP sites located in introns 1 and 6 of β-catenin. When these mice were injected with the Cre recombinase-expressing lentivirus (LV-Cre), we observed region-specific deletion of the β-catenin allele (Fig. 5a). 10 days following unilateral infection with LV-Cre virus, adjacent brain sections were probed with antisense β-catenin radiolabeled mRNA (Fig. 5a,f) or Cre Recombinase (Fig. 5b,g). This demonstrates the relatively specific deletion of the β-catenin gene within the amygdala that can be obtained with LV-Cre injection. Note that when animals are injected with a control lentivirus, such as LV-GFP, the levels of β-catenin remain as in wild-type animals (Fig. 5d,e). Notably, there were no effects of Cre-mediated β-catenin gene deletion or LV-GFP injection on amygdala cellular or anatomical structure as examined with a Nissl stain (Supplemental Fig. 1).
Figure 5. Region specific deletion of β-catenin in the adult brain.
β-catenin floxed animals were injected with either the LV-Cre virus or a control lentivirus, LV-GFP, and sacrificed 10 d later. Unilateral injection of LV-Cre demonstrates site-specific loss of expression. Adjacent sections were probed with antisense radiolabeled mRNA specific for β-catenin (a) or Cre Recombinase (b) A pseudocolor overlay of these two adjacent sections is also shown (c) to demonstrate the regional specificity. Animals that that had received LV-GFP injections into the amygdala had normal levels of β-catenin expression (d), where the LV-GFP was injected (e). This is in contrast to β-catenin mRNA expression (f) in the amygdala of an LV-Cre injected mouse (g). (h-j) In vitro functional assay of lentivirally expressed Cre recombinase. HEK293T cells were transiently transfected with a vector containing a floxed green fluorescent reporter protein, pLoxpGFP-DsRed in the absence (h) or presence (i) of LV-Cre and visualized using a green filter. (j) HEK293T cells transfected with pLoxpGFP-DsRed in the presence of LV-Cre, visualized using a red filter.
Amygdala-specific β-catenin deletions do not affect baseline anxiety or activity measures
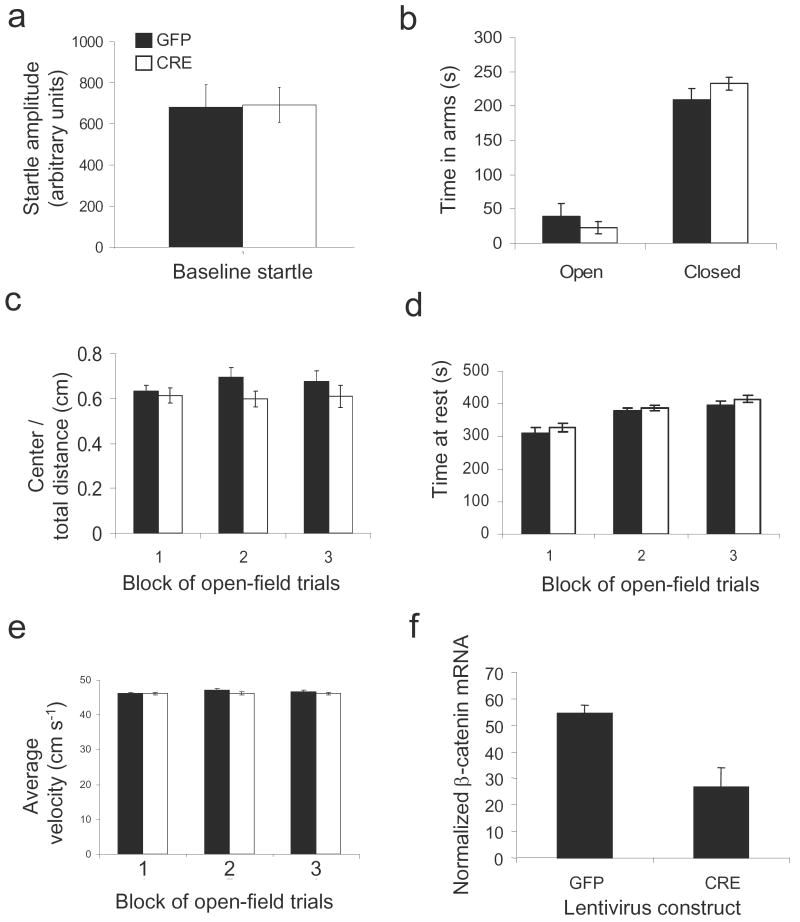
We first examined if deleting β-catenin within the amygdala would affect baseline anxiety or activity measures. Mice were injected with either LV-GFP or LV-Cre bilaterally within the amygdala at approximately 6-8 weeks of age. Ten days following injection, animals were examined in a series of basic behavioral tasks. In these baseline measures, we found no difference (P > 0.1) in level of anxiety as measured by baseline startle, elevated plus maze (time in open arms or time in closed arms) and open field (distance traveled in center, time at rest, average velocity) (Fig. 6 a-e). These data suggest that amygdala-specific deletions of the β-catenin gene do not alter motor activity or anxiety levels.
Figure 6. Amygdala specific β-catenin deletions do not affect baseline anxiety or activity measures.
Animals received bilateral injections of LV-GFP or LV-Cre into the amygdala and were allowed 10 days to recover. (a) Baseline startle for animals injected with LV-GFP and LV-Cre. (b) Duration of time spent in the open and closed arms in the Elevated Plus Maze. (c-e) All animals were placed in an open field apparatus for 30 min and activity measures were collected in 3 blocks of 10 minutes. There were no differences between the LV-GFP and LV-Cre injected animals in terms of distance traveled in the center compared to total distance (c), time at rest (d), or average velocity (e). Following testing, animals were sacrificed and β-catenin mRNA levels were assessed in both LV-GFP and LV-Cre animals. (f) Normalized β-catenin mRNA levels of LV-GFP and LV-Cre animals. (n=7 for LV-GFP group, n = 6 for LV-Cre group; Bars indicate mean ± s.e.m.)
We then wanted to quantitatively confirm that injections of LV-Cre into the amygdala decreased β-catenin mRNA. Brains were processed for in situ hybridization, and β-catenin mRNA levels were measured. Animals receiving LV-Cre injections showed significantly lower levels of β-catenin mRNA (26.73 ± 7.20) than animals receiving injections of LV-GFP (54.60 ± 2.96), t(6.88) = 3.580, P ≤ 0.01 (Fig. 6f).
B-catenin is required in the amygdala for normal fear memory consolidation
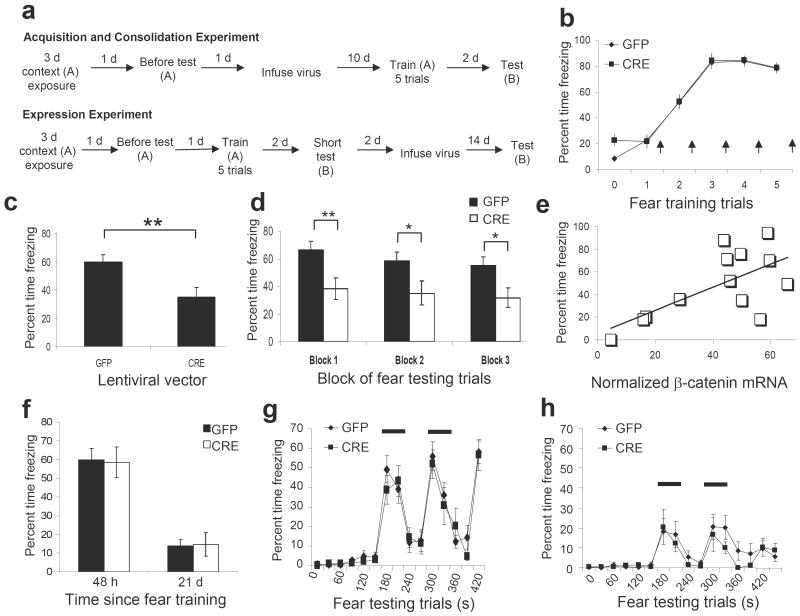
We then examined whether amygdala specific deletion of β-catenin affects amygdala-dependent learning (outlined in Fig. 7a). We fear-conditioned the mice and obtained freezing measures during fear acquisition (Fig. 7b). Similar to acute LiCl administration, there was a significant main effect of trial (F(5,125) = 104.698, P ≤ 0.01), but no effect for virus (F(1,25) = 1.964, P = .173). The similar acquisition curves for LV-GFP and LV-Cre animals suggest that mice with β-catenin deletions are able to initially encode and express fear memories normally.
Figure 7. Amygdala specific β-catenin deletions prevent the consolidation, but not expression, of conditioned fear.
(a) Timeline for acquisition, consolidation, and expression experiments. ‘A’ and ‘B’ below each training/testing period represent the context used. (b) Acquisition curve for LV-GFP and LV-Cre injected animals during training. Arrows represent the presentation of footshock. (c) Percent freezing during the 48hr post-training test in LV-GFP and LV-Cre animals in response to the tone presented in a novel context. (d) Examination of the freezing data in panel C by blocks of 5 (n = 15 for LV-GFP group, n = 12 for LV-Cre group). (e) Correlation between β-catenin mRNA expression and freezing behavior (n = 7 for LV-GFP group, n = 6 for LV-Cre group). (f) Percent freezing in LV-GFP and LV-Cre injected animals at 48 hr (pre-infusion) and 21 d (post-infusion) following training. (g) Freezing behavior 48 hr post-training, graphed in 30 s intervals. (h) Freezing behavior 21 d post-training, graphed in 30 s intervals. The bars illustrate the periods in which the CS was present (n = 10 for LV-GFP group, n = 9 for LV-Cre group). (Bars indicate mean ± s.e.m; ** denotes p ≤ 0.01)
Forty-eight hours later, animals were tested for cue fear conditioning in a novel context (Fig. 7c). In contrast to the acquisition data above, animals infected with LV-Cre, and thus those with the β-catenin deletions showed over 40% less freezing averaged across all sessions (35.00 ± 7.26) as compared to those infected with LV-GFP (60.00 ± 4.94), t(25) = 2.935, P < 0.01. Notably, even in the first freezing trial of the test, LV-Cre infected animals were freezing less than those that received LV-GFP, which were freezing at rates near their acquisition levels. The data of within-session freezing across the full testing session are shown in Fig. 7d in grouped blocks of 5 trials each (Block 1, GFP = 66.33 ± 6.24; CRE = 38.33 ± 7.67; t(25) = 2.863, P < 0.01; Block 2, GFP = 58.67 ± 6.35; CRE = 35.00 ± 8.77; t(25) = 2.239, P < 0.05; Block 3, GFP = 55.00 ± 6.45; CRE = 31.67 ± 7.11; t(25) = 2.426, P < 0.05). These data confirm that the decrease in fear is most likely a function of decreased consolidation at, or soon after, the time of the initial learning event, since animals were able to acquire and express fear normally (Fig. 7b) and did not show decreased average fear due to increased within-session extinction (Fig. 7d). In addition, there was a positive correlation between the level of β-catenin mRNA and freezing as a measure of fear (Fig. 7e), r(13) = 0.752, P < 0.01.
Together, these data suggest that β-catenin expression in amygdala is not required for normal anxiety-related behaviors or for the acquisition of fear, an amygdala-dependent task. However, consistent with the dynamic regulation of β-catenin levels during the consolidation period following fear acquisition, these data suggest that β-catenin is required for the normal consolidation of fear memory. In the absence of β-catenin, newly formed memories do not appear to be stabilized and are thus not able to be expressed 48 hrs later.
β-catenin is not required for the expression of fear memory
Thus far, our pharmacological and genetic manipulations of β-catenin have shown that it is not involved in the acquisition of fear, but instead in the stabilization of the memory. We then examined if deleting β-catenin after the consolidation of the memory would affect further expression of conditioned fear (Outlined in Fig. 7a-‘Expression Experiment’). To examine the effect of β-catenin deletion on expression, all animals were trained, and then presented with a 3 trial ‘short-test’ for freezing 48 hr later to confirm that they had acquired and consolidated the fear memory (Figs. 7f (48 hr) and 7g). We did not administer the full 15 trials in order to reduce the likelihood of extinction processes. The mice were then injected with either LV-GFP or LV-Cre bilaterally into the amygdala and allowed 14 d to recover. They were then tested again for the fear memory after this delay (2-3 weeks post-training, Figs. 7f (21 d) and 7h). Although the levels of freezing in this 21-day fear expression test were lower than freezing levels in the 48 hr experiments, both groups of mice showed significantly more freezing during the tone than prior to the CS during this expression test, F(1,17) = 14.786, P ≤ 0.01. Nissl staining of infected amygdala showed that the observed decrease in freezing within both groups is not due to damage to the amygdala. Thus, it is more likely that the decrease in freezing is due to the passage of time between training and testing. Notably, upon testing the animals after this delay, we found that mice receiving LV-Cre had similar levels of freezing (14.58 ± 6.34) compared to mice receiving LV-GFP (13.91 ± 3.45), t(17) =0.095, P > 0.05. Since both LV-Cre and LV-GFP groups demonstrated comparable, and statistically significant, levels of freezing when tested for fear expression while Cre recombinase and GFP protein are being expressed within the amygdala, these data suggest that β-catenin in the amygdala is not required for fear expression after the memory has been consolidated.
Discussion
In summary, our data suggest that β-catenin plays a role in long term memory formation in adults. We have shown that β-catenin is highly expressed in the adult mouse amygdala and is dynamically regulated at both the transcriptional and post-translational levels with fear learning. Pharmacologically enhancing the stability of β-catenin with lithium chloride resulted in an enhancement in learning, while genetic deletion of β-catenin within the amygdala resulted in deficits in learning. By studying the effects of β-catenin deletion in adults, we have been able to identify a novel role for β-catenin in learning and memory, distinct from its role in development.
Our data suggest that β-catenin is required for the consolidation, but not acquisition, of fear memory. However, once the memory has been consolidated, we found that β-catenin is no longer required to express the memory. During this consolidation period, we found that the interaction between β-catenin and cadherin is dynamically regulated. This suggests that β-catenin may be involved in the structural conversion of short-term labile to long-term stable memory traces.
We found that β-catenin mRNA expression was increased in the BLA, but not the somatosensory cortex and striatum, following fear training. Although this is the first study to examine β-catenin in vivo with learning, this result is consistent with previous in vitro studies of hippocampal slices showing an increase in nuclear β-catenin with tetanic stimulation12. In addition, Wnt target genes have also been shown to be up-regulated with long-term potentiation in hippocampal slices, anywhere from 15 min to 120 min following stimulation12.
Interestingly, when we measured total β-catenin protein levels, we did not see any alterations with training. It has been shown that depolarization of hippocampal neurons with KCl does not change the total levels of β-catenin at the synapse, but instead, causes a redistribution from dendritic shafts to spines 7. Thus, it is possible that rapid dynamic changes in breakdown, redistribution, and replacement lead to no apparent change in total protein visualized with immunoblots.
We reported biochemical changes that suggest that both β-catenin's role in cell-cell adhesion and Wnt signaling are affected by fear conditioning. Phosphorylation of β-catenin on tyrosine residue, Y654, has been shown to decrease the affinity of β-catenin for cadherin14,21. In addition, inhibiting the phosphorylation of Y654 with a point mutation redistributes β-catenin from dendritic shafts to spines, thereby increasing the β-catenin-cadherin interaction7. In our study, we found that p-Y654 levels were dynamically regulated following training. Our co-immunoprecipitation experiments show a very rapid period of β-catenin-cadherin destabilization, followed by a period of stabilization during consolidation. Overall, these findings suggest that the affinity of β-catenin for cadherin may initially weaken to allow for modifications of the synapse, and then strengthen to stabilize the synapse, which we hypothesize to be a molecular and cellular correlate of memory consolidation.
Such dynamic regulation of p-Y654 on β-catenin has previously been proposed. Brain-derived neurotrophic factor (BDNF) treatment has been shown to induce synaptic vesicle dispersion in hippocampal cultures, which is associated with an increase in β-catenin tyrosine phosphorylation and decrease in β-catenin-cadherin interactions. Soon after this dispersion, the phosphorylation levels decrease, and the β-catenin-cadherin interaction is restored22. Notably, we have previously shown that BDNF activation of the TrkB receptor is required within the amygdala for consolidation of fear memories23. Thus, a similar mechanism may be taking place in this in vivo learning paradigm, such that when new memories are formed, pre-existing synapses must become de-stabilized transiently prior to stabilization of synapses involved in memory formation.
We have also provided both biochemical and behavioral evidence suggesting that increased stabilization of β-catenin, through the inhibition of GSK-3β, may be important for learning and memory. Normally, GSK-3β phosphorylates β-catenin at Ser33/37/Thr41, marking the protein for degradation. However, when GSK-3β is inactivated by phosphorylation at Ser9, β-catenin becomes stabilized10. In our study, we reported an increase in phosphorylated GSK-3β in the amygdala at 2 hr following fear conditioning. In addition, when we pharmacologically increased the inhibition of GSK-3β with LiCl, we observed decreased β-catenin phosphorylation. Furthermore, acute administration of LiCl 30 min before training resulted in an enhancement in learning measured 48 hr later, without any effect on acquisition. Although administration of LiCl has previously been shown to produce behaviors similar to those displayed by overexpression of β-catenin in the mouse brain24, LiCl's actions are not necessarily specific to β-catenin.
To more definitively identify the role of β-catenin in long-term memory formation, we used genetic manipulations to delete β-catenin from the adult amygdala. We found that deletion of β-catenin before training does not affect the acquisition or immediate expression of fear, but does produce deficits in learning when measured 48 hr after training. In addition, deletion of β-catenin after consolidation has occurred does not affect the expression of learned fear. These findings provide further support that normal β-catenin expression is necessary to consolidate the newly acquired memory.
One limitation of this study is our inability to specifically inhibit or delete β-catenin immediately after training. Previous work has elegantly demonstrated, using consolidation of inhibitory avoidance, that post-training manipulations are the gold-standard for demonstrating disruption of fear consolidation33,34. Although the data on consolidation of amygdala-dependent classical conditioning paradigms has been less clear, this is an important manipulation for interpretation of consolidation effects. Unfortunately, there are no drugs currently available that selectively inhibit β-catenin. Additionally, since a minimum of 7-10 days is required for optimal lentiviral gene expression, we are unable to delete β-catenin shortly after training. However, we feel that our current powerful method of genetic manipulation is an important approach to specifically examine the role of β-catenin within the amygdala during learning. Furthermore, we feel that the lack of an effect of β-catenin deletion on acquisition of fear and expression of fear makes a strong case for its role during the consolidation period.
Based on the results obtained thus far, we propose that during the acquisition of fear and immediately afterwards, synapses may weaken, as demonstrated with decreased β-catenin-cadherin association at 0 and 0.5 hr post-training, thereby alleviating the requirement for β-catenin. Then, once the synapses have been modified during the consolidation process, β-catenin is required to convert that memory trace into long-term memory. Examination of such proposed changes in synaptic strength will need to be further explored. Additional studies are also needed to determine if it is β-catenin's role in cell-cell adhesion, Wnt signaling, or both, that are contributing to its observed effects on learning and memory.
In summary, our results suggest that β-catenin, a ‘hub protein’ involved in both transcriptional regulation and maintaining stability of cell-cell contacts and synaptogenesis, is required for normal consolidation of new memories in adult mice. This finding adds to the body of knowledge describing the role of β-catenin in normal cell functioning, tumor regulation, and development. Although it has been previously implicated with in vitro approaches in synaptogenesis and synaptic plasticity, our results provide definitive support for its function in learning and memory processes. Further understanding its role may provide important insights into the nature of the molecular mechanisms underlying memory consolidation. Additionally in humans, the development of new small molecule specific inhibitors of β-catenin function may eventually provide for a powerful clinical approach to transiently inhibit the consolidation of newly formed trauma memories in the prevention of fear-related disorders, such as post-traumatic stress disorder. Similarly, enhancing β-catenin function may be helpful in disorders of memory such as Alzheimer's disease.
Method
Animals
Adult male C57BL/6J mice (Jackson Labs) were used for immunoblotting and drug treatment experiments. All other experiments were performed using homozygous β-catenin floxed mice (Jackson Labs; B6.129-Ctnnb1tm2Kem/KnwJ20). Mice were housed 4 per cage in a temperature-controlled (24 °C) animal colony, with ad libitum access to food and water, on a 12 hr light/dark cycle, with all behavioral procedures being performed during the light cycle.
Immunoblotting, Immunoprecipitation, and In Situ hybridization
(See Supplemental Methods for full details) Following behavioral procedures, brains were blocked rapidly and kept frozen at -80 °C. Bilateral amygdala punches were obtained and homogenized. Twenty micrograms of protein per animal were electrophoretically separated on SDS-PAGE, transferred onto nitrocellulose, blocked for 1 hr, and incubated in primary antibody overnight at 4 °C [1:100, β-catenin (phospho-Y654); 1:500, β-catenin; 1:1000, phospho-β-catenin; 1:1000, phospho-GSK-3β; 1:000, Pan-Cadherin]. Membranes were washed and incubated with an HRP-labeled secondary antibody then detected by SuperSignal West Chemiluminescence (Pierce). Total blotted protein levels were normalized to levels of α-tubulin, and relative values are expressed as the protein of interest divided by the loading control.
For immunoprecipitation, solubilized proteins were incubated with Protein A/G PLUS-Agarose beads and centrifuged. β-catenin antibody and Protein A/G PLUS-Agarose beads were then added to the supernatant of each protein sample, incubated overnight at 4 °C, washed, and examined with Western blot analyses.
In situ hybridization was performed as previously described20 (see Supplemental Methods). The full-length clone for β-catenin (GI Accession #31419847) was used to amplify and subclone the regions between exons 2 and 6 of β-catenin, the area flanked by loxP sites in the mutant mouse. This loxP-flanked subclone was then linearized and both antisense and sense 35S-riboprobes were generated using the appropriate RNA polymerase and [35S]-UTP in the reaction. Following autoradiography, density of β-catenin mRNA in the BLA, somatosensory cortex, and striatum were assessed using the mean luminosity function of Adobe Photoshop.
Lentiviral vectors and viral injections
(See Supplemental Methods for full details) Viral vectors were produced and concentrated as previously described25-31. Briefly, a Cre-recombinase expressing vector (referred to as ‘LV-Cre’) or a green-fluorescent protein -expressing control vector (referred to as ‘LV-GFP’) with a final infectious unit titer of 109 IU ml-1 was used for stereotaxic injections into the amygdala. Following anesthesia, small holes were drilled in the skull above the injection site; BLA coordinates were as follows: AP = -1.8, DV = -4.9, ML = ± 3.2 relative to bregma. A 10 μl BSA pre-coated Hamilton microsyringe was used to deliver bilateral intra-amygdala injections of lentiviral vectors. 0.2 μl of virus per side were injected at a rate of 0.025 μl min-1. The needle was left in place for 10 minutes following the injection, and animals were allowed to recover for 10-14 days before testing.
Behavioral Studies
Elevated plus maze and Open Field maze were performed as per standard protocol (behavioral techniques and materials described fully in Supplemental Methods). Mice were fear-conditioned in eight identical startle response systems. After 3 d of exposure to the conditioning cambers, mice were given a pre-training test to examine baseline levels of startle in the presence of the tone conditioned stimulus (CS). Twenty-four hours after the pre-test, mice were placed in the conditioning chamber, and after 5 min presented with 5 tone-shock pairings at an inter-trial interval (ITI) of 5 min. Each pairing consisted of a 30 s tone (6 kHz, 85 db) CS, which co-terminated with a 0.5 s footshock US (1.0 mA, except where noted). Freezing in startle reflex chambers during fear acquisition was assessed as described previously35,36. 48 hrs after training, mice were tested for freezing in rodent modular test chambers with an inside area of 30.5 cm × 24.1 cm × 21.0 cm. Three minutes later, 15 CS tones (6 kHz, 85 db) with an ITI of 1.5 min were delivered through a high-frequency speaker attached to the side of each chamber. Percentage time spent freezing during the CS presentations was calculated for each mouse using FreezeFrame (Coulbourn Instruments, #ACT-100).
Data analysis
Statistically significant differences were determined by Student's t test, or ANOVA, with posthoc least squares difference (LSD) tests for multiple comparisons. The results are presented as mean ± s.e.m.
Supplementary Material
Acknowledgments
Support was provided by NIH (MH069884; DA019624; and AG025688), NSF (GRFP DGE-0234618), the Center for Behavioral Neuroscience (NSF agreement IBN-987675), Burroughs Wellcome Fund, and by an NIH/NCRR base grant (P51RR000165) to Yerkes National Primate Research Center.
References
- 1.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–71. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 2.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–67. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–89. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 6.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 7.Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 8.Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol. 2005;15:216–21. doi: 10.1016/j.tcb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 11.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–6. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 13.Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–43. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- 14.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 15.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 16.Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–56. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- 17.Yost C, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 18.Gould TD, O'Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–33. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- 19.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 21.Piedra J, et al. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem. 2001;276:20436–43. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- 22.Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–99. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould TD, et al. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–83. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 25.Chhatwal JP, Hammack SE, Jasnow AM, Rainnie DG, Ressler KJ. Identification of cell-type-specific promoters within the brain using lentiviral vectors. Gene Ther. 2007;14:575–83. doi: 10.1038/sj.gt.3302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–2. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. PNAS. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 30.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–5. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 31.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element Enhances Expression of Transgenes Delivered by Retroviral Vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos G, Watston C. The mouse brain in stereotaxic coordinates. Academic Press; New York: 2003. [Google Scholar]
- 33.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosc. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 34.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12(2):205–10. doi: 10.1016/s0959-4388(02)00306-9. pr. [DOI] [PubMed] [Google Scholar]
- 35.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12(7):656–70. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SV, Heldt SA, Davis M, Ressler KJ. Olfactory-mediated fear conditioning in mice: simultaneous measurements of fear-potentiated startle and freezing. Behav Neurosci. 2005;119(1):329–35. doi: 10.1037/0735-7044.119.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.