Abstract
Calcitriol (3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-1α,3β,25-triol) is a powerful oncostatic form of vitamin D3 that is of limited clinical utility due to hypercalcemic (toxic) effects. Since the removal of the side chain reduces or eliminates the calcemic activity of vitamin D3, secosteroidal compounds lacking or with a shortened side chain are good candidates for anti-cancer drugs. In addition, 5,7-steroidal dienes without a side chain can be generated in vivo under pathological conditions. A series of androsta- and pregna-5,7-dienes was efficiently synthesized from their respective 3-acetylated 5-en precursors by bromination-dehydrobromination and deacetylation reactions. Ultraviolet B (UVB) irradiation was used to generate corresponding 9,10-secosteroids with vitamin D-like structures. Additional products with tachysterol-like (T-like) structures or 5,7-dienes with inverted configuration at C-9 and C-10 (lumisterol, L-like) were also detected. Different doses of UVB resulted in formation of various products. At low doses, previtamin D-, T- or L-like compounds were formed as the main products, while higher doses induced further isomerization, with formation of potentially oxidized derivatives. In summary, we describe dynamic UVB induced conversion of androsta- and pregna-5,7-dienes into vitamin D-like compounds and their rearranged analogues; additionally novel T-like and L-like structures were also produced and characterized. Further biological evaluation of newly synthesized compounds should help to select the best candidate(s) for potential treatment of hyperproliferative diseases including cancer.
Introduction
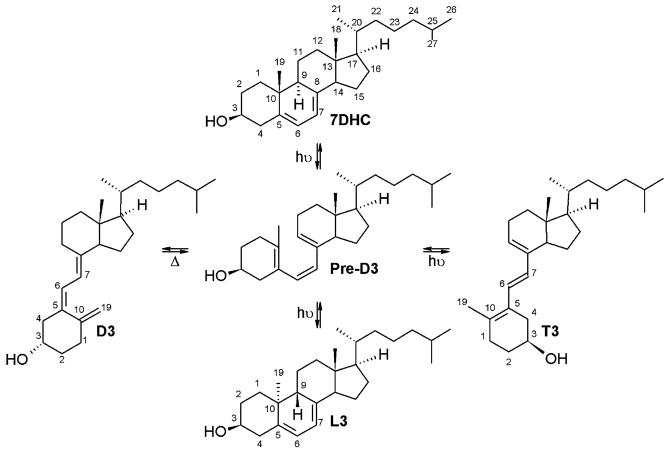
The UVB-driven photolysis of the steroidal B ring of cholesta-5,7-diene-3β-ol (7-dehydrocholesterol, 7DHC) is one of most fundamental reactions in photobiology, leading to synthesis of the powerful hormone - vitamin D3 ((3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol, cholecalciferol, D3). The reaction takes place in the epidermal layer of the epidermis and the rearrangement of the photo-activated molecule (pre-D3) generates not only vitamin D3, but also tachysterol3 (6E-9,10-secocholesta-5(10),6,8-trien-3β-ol, T3) and lumisterol3 (9β,10α-cholesta-5,7-diene-3β-ol, L3).1-3 Vitamin D3 (D3), the main product of the process, plays a fundamental role in biology, serving as a precursor for the hormone 1,25-dihydroxyvitamin D3 ((1α,3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-1,3,25-triol, 1,25(OH)2D3) with its most fundamental role in the regulation of body calcium homeostasis.2-5 This conversion of 7DHC has been demonstrated by the Holick group as a two-step process (Scheme 1).
Scheme 1.
Photolysis of cholesta-5,7-diene-3β-ol.
The first and rapid step is photolysis of the unsaturated B ring of 7DHC and formation of pre-D3 product. After irradiation, pre-D3 undergoes slow time-dependent isomerization to three main products: D3, T3 and L3. T3 has shifted double bonds when compared with D3, and L3 is formed by recyclization of the B ring, with reversed configuration at C-9 and C-10 (Scheme 1). The efficiency of chemical conversion, diversity and ratio of products depends on the strength and length of UV irradiation absorbed by the chromophore system.6-9 The optimal wavelength facilitating formation of vitamin D3 was found to be at the UVB range (295 and 300 nm),8 while irradiation with UVC (254 nm) resulted in a higher yield of T3.6 In addition, high doses of UVB or the presence of trace amount of hydrochloric acid stimulates isomerization of T3 to isotachysterol products (isoT-like). IsoT3s are very reactive and easily undergo autoxidation.10 An additional pathway of vitamin D3 transformation has been shown in the skin with the production of 5,6-transvitamin D3, suprasterols I and II in response to high doses of UVB.9 Moreover, the ratio and type of products generated strongly depend on the solvent and experimental model used for irradiation (biological membranes, cells, tissue, or organism); the presence of biological membranes generally accelerates photolysis.3,8,9,11,12 Significantly, there is a paucity of information on the photolytic transformation of steroidal 5,7-dienes to the corresponding D-, L- or T-like compounds. This is surprising taking into consideration the fact androsta- and pregna-5,7-dienes are readily produced in humans under pathologic conditions (Smith-Lemli-Optiz syndrome (SLOS)13-15 and under physiological conditions (horse gonads).16-18
In addition to its fundamental role in calcium metabolism 1,25(OH)2D3 exerts powerful anticarcinogenic properties affecting proliferation, differentiation and apoptosis in cells of different lineages, as well as functioning as an immunomodulator and hormonal modifier.2,19,20 Unfortunately, the use of vitamin D3 or its hydroxylated derivatives in the treatment of cancer or hyperproliferative disorders is limited, because of hypercalcemic toxicity when used at pharmacological concentrations. Interestingly, the calcemic effect can be strongly reduced by shortening of the side chain.21,22 Thus, vitamin D-like compounds with androstane and pregnane side chains may serve as good candidates for the treatment of cancer or other pathologies. To define photochemical production and the nature of novel vitamin D-like compounds, we synthesized a series of androsta- and pregna-5,7-dienes from their 3-acetylated 5-diene precursors. UVB (280–320 nm) irradiation was used to generate corresponding 9,10-secosteroids. Furthermore, using different doses of UVB, we characterized other products with L-like and T-like structures and studied the dynamics of their formation.
Experimental
Chemical synthesis
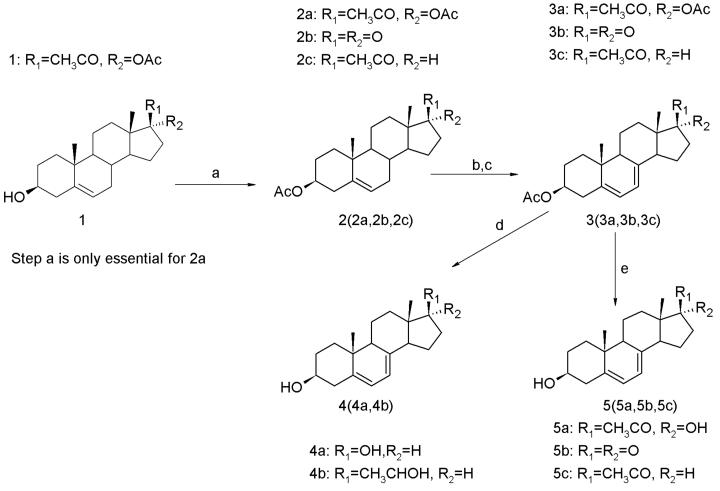
The synthesis of compounds 4(4a, 4b) and compounds 5(5a, 5b, 5c) is shown in Scheme 2.
Scheme 2.
Synthesis of androsta- and pregna-5,7-dienes. Reagents and conditions: (a) Ac2O, microwave, p-toluenesulfonic acid monohydrate; (b) dibromantin, 2,2′-azobisisobutyronitrile, benzene–hexane (1 : 1), 100°C, reflux; (c) Bu4NBr, Bu4NF, THF, room temperature; (d) LiAlH4,THF,0°C; (e) K2CO3, MeOH–THF, room temperature.
Synthesis of 2a
The acetylation of 17α-acetoxy-pregnenolone 1 was carried out following a known procedure.23 Yield: 95%. 1H NMR (500 MHz, CDCl3) for compound 2a: δ 5.39 (d, J = 5 Hz, 1H), 4.61 (m, 1H), 2.94 (m, 1H), 2.30–2.36 (m, 2H), 2.12 (s, 3H), 2.04 (s, 3H), 2.05 (s, 3H), 1.98–2.02 (m, 2H), 1.86–1.90 (m, 2H), 1.46–1.80 (m, 9H), 1.29 (m, 1H), 1.16 (m, 1H), 1.07 (m, 1H), 1.03 (s, 3H), 0.64 (s, 3H). ESI-MS: calculated for C25H36O5, 416.3, found 439.3 [M + Na]+.
General procedure for the synthesis of 3 (3a, 3b, 3c)
Compounds 3 (3a, 3b, 3c) were synthesized according to a known procedure.24 Yield: 40–50%. 1H NMR (500 MHz, CDCl3) for compound 3a: δ 5.58 (dd, J = 10 Hz, 3.0 Hz, 1H), 5.45 (m, 1H), 4.71 (m, 1H), 2.98 (m, 1H), 2.61 (m, 1H), 2.52 (m, 1H), 2.36 (t, J = 15 Hz, 1H), 2.11 (s, 3H), 2.08 (s, 3H), 2.05 (s, 3H), 2.03 (m, 1H), 1.82–1.94 (m, 4H), 1.56–1.73 (m, 6H), 1.38 (dt, J = 15 Hz, 5 Hz, 1H), 0.95 (s, 3H), 0.57 (s, 3H). ESI-MS: calculated for C25H34O5, 414.2, found 437.3 [M + Na]+. 1H NMR (300 MHz, CDCl3) for compound 3b: δ 5.51–5.53 (dd, J = 10 Hz, 3.6 Hz, 2H), 4.54 (m, 1H), 1.34–2.60 (m, 16H), 2.08 (s, 3H), 0.99 (s, 3H), 0.83 (s, 3H). ESI-MS: calculated for C21H28O3, 328.2, found 351.3 [M + Na]+. 1H NMR (500 MHz, CDCl3) for compound 3c: δ 5.60 (dd, J = 12 Hz, 4.0 Hz, 1H), 5.45 (m, 1H), 4.74 (m, 1H), 2.66 (t, J = 10 Hz, 1H), 2.54 (m, 1H), 2.39 (m, 1H), 2.24 (m, 1H), 2.17 (s, 3H), 2.15 (m, 1H), 2.06 (s, 3H), 1.72–1.96 (m, 8H), 1.52–1.62 (m, 3H), 1.40 (dt, J = 30 Hz, 5 Hz, 1H), 0.97 (s, 3H), 0.60 (s, 3H). ESI-MS: calculated for C23H32O3, 356.2, found 379.3 [M + Na]+.
General procedure for the synthesis of 4 (androsta-5,7-dien-3β,17β-diol - 4a, pregna-5,7-dien-3β,20-diol - 4b)
Compounds 4 (4a, 4b) were synthesized according to a known procedure.24 Yield: 45–55%. 1H NMR (500 MHz, CD3OD) for compound 4a: δ 5.55 (dd, J = 10 Hz, 6 Hz, 1H), 5.37 (m, 1H), 3.69 (m, 1H), 3.51 (m, 1H), 2.41 (m, 1H), 2.28 (t, J = 10 Hz, 1H), 2.10 (m, 1H), 1.86–2.00 (m, 6H), 1.68–1.76 (m, 3H), 1.46–1.60 (m, 4H), 1.28 (dt, J = 30 Hz, 6 Hz, 1H), 1.18 (dt, J = 25 Hz, 10 Hz, 1H), 0.96 (s, 3H), 0.68 (s, 3H). ESI-MS: calculated for C19H28O2, 288.2, found 311.3 [M + Na]+. 1H NMR (500 MHz, CD3OD) for compound 4b: δ 5.58 (dd, J = 13.5 Hz, 4 Hz, 1H), 5.42 (m, 1H), 3.75 (m, 1H), 3.64 (m, 1H), 2.47 (dq, J = 32 Hz, 12.5 Hz, 4 Hz, 1H), 2.29 (t, J = 19.5 Hz, 3.0 Hz, 1H), 2.18 (m, 1H), 1.22–2.08 (m, 16H), 1.15–1.17 (d, J = 10 Hz, 3H), 0.77 (s, 3H), 0.71 (s, 3H). ESI-MS: calculated for C21H32O2, 332.2, found 355.3 [M + Na]+.
General procedure for the synthesis of 5 (3β,17β-dihydroxypregna-5,7-diene-20-one - 5a, 3β-hydroxyandrosta-5,7-dien-17-one - 5b, 3β-hydroxypregna-5,7-diene-20-one - 5c)
Compounds 5 (5a, 5b, 5c) were synthesized according to a known procedure.24 Yield: 50–60%. 1H NMR (300 MHz, CDCl3) for compound 5a: δ 5.58 (dd, J = 10 Hz, 5.0 Hz, 1H), 5.45 (m, 1H), 3.64 (m, 1H), 2.62–2.75 (m, 2H), 2.48 (m, 1H), 2.29 (s, 3H), 1.26–2.25 (m, 15H), 0.96 (s, 3H), 0.71 (s, 3H). ESI-MS: calculated for C21H30O3, 330.2, found 353.3 [M+ Na]+. 1H NMR (500 MHz, CDCl3) for compound 5b: δ 5. 63 (dd, J = 8.0 Hz, 3.0 Hz, 1H), 5.56 (m, 1H), 3.66 (m, 1H), 2.50–2.58 (m, 2H), 2.31 (t, J = 15 Hz, 25 Hz, 1H), 2.18–2.25 (m, 2H), 2.05–2.14 (m, 2H), 1.90–1.97 (m, 3H), 1.73–1.82 (m, 3H), 1.52 (m, 1H), 1.28–1.41 (m, 3H), 0.98 (s, 3H), 0.83 (s, 3H). ESI-MS: calculated for C19H26O2, 286.2, found 309.3 [M + Na]+. 1H NMR (500 MHz, DMSO) for compound 5c: δ 5.58 (dd, J = 10 Hz, 4.0 Hz, 1H), 5.43 (m, 1H), 4.71 (m, 1H), 3.65 (m, 1H), 2.63 (t, J = 10 Hz, 1H), 2.34 (m, 1H), 2.15 (m, 1H), 2.10 (s, 3H), 1.20–2.08 (m, 14H), 0.95 (s, 3H), 0.58 (s, 3H). ESI-MS: calculated for C21H30O2, 314.2, found 337.3 [M + Na]+.
UVB irradiation and preparation of secosteroids, lumisterols and tachysterols (5c serves as an example)
A methylene chloride solution of 5c (3.65 mg, 1 mg mL−1) was subjected to UV irradiation for 5 min in a quartz cuvette, using a Biorad UV Transilluminator 2000 (Biorad, Hercules, CA). The spectral characteristics of the UVB (280–320 nm) source were published previously25 and its strength (4.8 ± 0.2 mW cm−1) was measured routinely using a digital UVB Meter Model 6.0 (Solartech Inc., Harrison Twp, MI). The reaction mixture was incubated, as indicated (RT or 37°C), for 14 h and selected products were purified by RP-HPLC chromatography, as described below. The major products, pre-D-, D-, T- and L-like, were identified on the basis of their retention time and UV absorption spectra followed by MS and NMR measurement. A modification to the method was made to allow irradiation in 400 μL glass HPLC vials for 20–30 min. The initial test showed that the results of irradiation were similar to a quartz cuvette (glass vials were found to transmit at least 50% of irradiation at 290 nm and above). Both methanol and ethanol were successfully used as solvents for irradiation. To rule out a thermal effect of irradiation a sham irradiation was performed by irradiating the same amount of sample in a vial covered with aluminum foil.
General procedure for Reverse Phase-HPLC (RP-HPLC) chromatography
HPLC analyses were performed using a Waters HPLC-system equipped with a diode-array detector (Waters Associates, Milford, MA). The reaction mixture (2–50 μL) of irradiated 5–7 dienes (50–200 μg) was injected by an autosampler onto an Atlantis C18 column (Waters, IL) running mobile phase of 30% methanol–water at a flow rate of 1.5 mL min−1. Fractions were collected every 15 s and were reanalyzed by RP-HPLC. Fractions containing above 95% of pure compound (for 240 nm and 280 nm spectra) were pooled and used for further characterization. Chromatographic conditions were optimized to achieve best separation for each product.
MS/NMR data collection
Mass spectra were recorded using a Bruker Esquire-LC/MS Spectrometer equipped with an electrospray ionization (ESI) source. The sample was run in 100% methanol at a sample flow rate of 5.0 μL min−1. All NMR measurements were performed on a Varian Unity Inova-500 MHz spectrometer (VarianNMR Inc., Palo Alto, CA) using a 4 mm Nanoprobe, 3 or 5 mm probe. CDCl3 was used for the initial study of androsta- and pregna-5,7-dienes, whereas deuterated methanol (D-methanol) was used to study vitamin D-like and T-like derivatives. Temperature was regulated at 20 °C (±0.1 °C). Chemical shifts were referenced to NMR solvent peaks.
Results
The total synthesis of vitamin D3 analogs with a modified side chain is usually complicated due to a lack of efficient methodology and suitable precursors. However, several groups successfully used a multistep protocol, based on a Wittig–Horner reaction. In this method the A ring and C, D rings moiety are prepared separately, and then attached together. In order to avoid complexity of this approach we used the classic and naturally occurring pathway of synthesis, where vitamin D3 is a product of UVB photolysis of 7DHC (Scheme 1). The big advantage of this method is that a variety of 3-acetylated 5-diene precursors is commercially available. Additionally, usage of UVB irradiation allows the generation and characterization of not only vitamin D-like compounds, but also of other products with tachysterol-like and lumisterol-like structures (see Schemes 1 and 2 for details).
Synthesis of 5,7 dienes
The synthesis of 5c from pregnenolone acetate (2c) was initially carried out by a bromination/dehydrobromination method, followed by hydrolysis of the acetyl group at C-3.26 However, this standard procedure resulted in a mixture of 95% 3β-hydroxypregna-4,6-dien-20-one (6c) and only 5% 3β-hydroxypregna-5,7-dien-20-one (5c). This mixture of isomers was separated by silica gel-AgNO3 chromatography27 and products were identified by their distinctly different UV (λmax 233, 238, 248 nm for 4,6-diene and λmax 262, 272, 283, 294 for 5,7-diene) and NMR spectra. To improve the yield of the desired 5,7-diene, the alternative method for the synthesis of 3β-hydroxypregna-5,7-dien-20-one (5c) and the other 5,7-dienes (5a and 5b) was adopted24,27 (see Scheme 2).
Compounds 4a and b were synthesized from the same precursor 2 as 5a, b and c except a deprotection reaction was carried on simultaneously with reduction of the carbonyl group. Interestingly, only synthesis of androsta-5,7-diene-3β,17β-diol (4a) resulted in a mixture of 4,6- and 5,7-dienes, where 5,7-diene constituted 95% of the mixture after initial purification. The 4,6-diene was subsequently removed by silica gel-AgNO3 chromatography.27
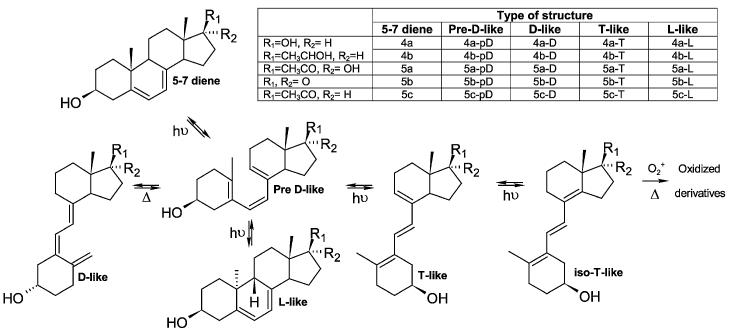
Physicochemical properties (UV and MS data) of synthesized androsta- and pregna-5,7-dienes are summarized in Table 1. The detailed NMR data are presented in the electronic supplementary information, ESI, Table S1.‡ NMR chemical shifts for 5b and 5c are in agreement with those previously published.27
Table 1.
UV and MS spectral data for steroidal 5,7 dienes and derivatives
| Structure type | No | Parental compound | UV max/nm | Predicted MW | MS+ | Ref. |
|---|---|---|---|---|---|---|
| 4,6-diene | 6c | 2c | 232, 240, 249 | 314.46 | 337.3 [M + Na]+ | 30 |
| 5,7-diene | 4a | 1 | 262, 272, 281, 292 | 288.42 | 311.3 [M + Na]+ | 31,32b |
| 4b | 2b | 263, 272, 282, 293 | 316.48 | 339.25 [M + Na]+ | 32b | |
| 5a | 2a | 263, 272, 281, 293 | 330.46 | 353.25 [M + Na]+ | 24,32b | |
| 5b | 2b | 263, 271, 282, 292 | 286.41 | 309.3 [M + Na]+ | 17,18,31,32b | |
| 5c | 2c | 262, 272, 283, 294 | 314.46 | 337.3 [M + Na]+ | 22,31,32b | |
| Pre-D-like | 4a-pD | 4a | 260 | 288.42 | NDa | New |
| 4b-pD | 4b | 260 | 316.48 | NDa | New | |
| 5a-pD | 5a | 260 | 330.46 | NDa | New | |
| 5b-pD | 5b | 260 | 286.41 | NDa | New | |
| 5c-pD | 5c | 260 | 314.46 | NDa | 22 | |
| D-like | 4a-D | 4a | 264 | 288.42 | 311.3 [M + Na]+ | 32b |
| 4b-D | 4b | 265 | 316.48 | 339.25 [M + Na]+ | 22,26,32b | |
| 5a-D | 5a | 265 | 330.46 | 353.25 [M + Na]+ | 32,33b | |
| 5b-D | 5b | 264 | 286.41 | 309 [M + Na]+ | 32,34 | |
| 5c-D | 5c | 265 | 314.46 | 337.3 [M + Na]+ | 26,32,35b | |
| L-like | 4a-L | 4a | 262, 271, 282 | 288.42 | 311.3 [M + Na]+ | New |
| 4b-L | 4b | 262, 272, 281 | 316.48 | 339.25 [M + Na]+ | New | |
| 5a-L | 5a | 264, 273, 281 | 330.46 | 353.25 [M + Na]+ | New | |
| 5b-L | 5b | 261, 272, 280 | 286.41 | NDa | New | |
| 5c-L | 5c | NDa | NDa | NDa | 36b | |
| T-like | 4a-T | 4a | 272, 281, 291 | 288.42 | NDa | 34 |
| 4b-T | 4b | 272, 280, 291 | 316.48 | NDa | New | |
| 5a-T | 5a | 271, 280, 290 | 330.46 | 353.25 [M + Na]+ | New | |
| 5b-T | 5b | 271, 280, 289 | 286.41 | 309 [M + Na]+ | New | |
| 5c-T | 5c | 274, 281, 290 | 314.46 | 337.3 [M + Na]+ | 22 | |
| isoT-like | 5c-iT | 2c | 233, 238, 248 | 314.46 | 337.3 [M + Na]+ | New |
| isoT-like (oxide) | 4a-iT | 4a | 234, 251, 260 | 320.42 | 343 [M + Na]+ | New |
| 5a-iT | 5aiT | 238, 249, 260 | 362.46 | 385.15 [M + Na]+ | New |
ND - not determined.
Patents.
Effect of UVB irradiation on androsta- and pregna-5,7-dienes transformation to D-like, T-like and L-like products
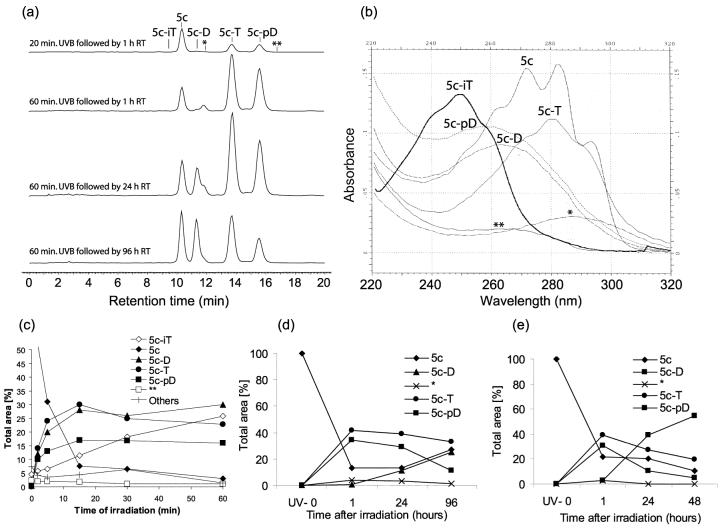
The UV conversion of androsta- and pregna-5,7-dienes were performed using a UVB light source (4.8 ± 0.2 mW cm−2) with maximum emission spectrum in the range of 280–320 nm.25 The photolysis reaction and subsequent time-dependent conversion of products were analyzed by a HPLC equipped with a diode array detector which enabled very rapid monitoring of products by characteristic UV spectra.8 Products of irradiation were characterized based on their retention time related to the substrate and UV spectra. This enabled us to define compounds with longer retention times (when compared to the precursor) such as L-like, D-like, T-like and pre-D-like substances (see for details: Table 1; Fig. 1 and Fig. S2–S5‡). Compounds with shorter retention times were mainly isotachysterols, oxidized isotachysterols or others with maximum absorption at 250 nm (±5 nm). A low dose of UVB irradiation (20 min of irradiation; Fig. 1a) of 5c resulted in the formation of previtamin D-like product 5c-pD (λmax at 260 nm; (3β,6Z)-9,10-secopregna-5(10),6,8-trien-3-ol) and only small amounts of 5Z,7E-3β-hydroxy-9,10-secopregna-5,7,10(19)trien-20-one (5c-D) with maximum UV absorption at 265 nm (isomerization of pre-D to D requires time, see next paragraph for details). Additional products were 5c-T - λmax at 274, 281, 290 nm and two unknown products with λmax at 265 and 290 nm (Fig. 1b). The presence of 5c-L - 3β-hydroxy-9β,10α-pregna-5,7-dien-20-one - was not detected after irradiation of 5c (Fig. 1b), but analogous L-like products were detected after irradiation of other 5,7-dienes (see Fig. S2b, S3b and S4b‡). Experiments with 5c showed maximum production of 5c-pD after 15 min. The UVB irradiation for 30 and 60 min resulted in an increased formation of a product with a λmax at 290 (about 20%) and a slight decrease in the concentration of other products (Fig. 1c). Additionally, the formation of several products with shorter retention times and with characteristic λmax below 250 nm, and gradual disappearance of D-like, T-like and L-like compounds were observed after 30 and 60 min of irradiation (Fig. S1‡). The high UVB dose caused further transformation of vitamin D-like compounds, similar to those observed for vitamin D3 in human skin.9 However, in contrast to 7DHC photolysis, the products of long irradiation of androsta- and pregna-5,7-dienes probably had structures similar to isotachysterol and its oxidized derivatives.10 For example, potential isotachysterols 4a-iT and 5a-iT with spectra characteristic (λmax 238, 249, 260 nm ±5 nm) had the molecular weight of parental compound plus 32 (O2) and 23 (Na+), as shown by mass spectrometry (Table 1). Unfortunately, we were not able to further characterize isoT-like compounds because of their relatively short life time.
Fig. 1.
Dynamics of UVB-driven photolysis of 3β-hydroxypregna-5,7-diene-20-one (5c). (a) Compound 5c was irradiation for 20 min (top chromatogram) or 60 min (others). Samples were incubated in the dark, at room temperature (20 °C) and analyzed by RP-HPLC 1, 24 and 96 h after irradiation. Chromatograms were recorded at 280 nm. (b) Representative UV spectra of 5c and products of its irradiation. Spectra were normalised to fit in a scale. (c) UVB dose (time of irradiation) dependent of conversion of 5c monitored by relative quantification of substrate to products. Equal amounts of 5c were irradiated for 0, 2, 5, 15, 30 and 60 min, incubated for 24 h at room temperature and analyzed by RP-HPLC. (d and e) Temperature dependent isomerization of 5c irradiation products. The relative changes in amount of substrate and products after irradiation for 15 min followed by incubation for various time (as shown) at 20 °C (d) or 37 °C (e). The results on panels c, d and e were expressed as a percentage of total area under the selected peak (at 280 nm) to the total area of all peaks at 280 nm.
However, it cannot be ruled out that some of the products could represent suprasterols9 with the λmax 210 nm28, which was not detected because of the presence of methanol in HPLC running phase (methanol has strong absorbtion below 220 nm). Finally, it has to be noted that potential thermic effects of UV irradiation on the above process was excluded by negative results of “sham irradiation” control experiments (the samples were covered with aluminium foil and processed as experimental samples, see Fig. 1f in ESI‡ for details).
Time and temperature dependent conversion of pre-D-like compounds to D-like compound
After UVB-irradiation the pre-pregnacalciferol (5c-pD) was efficiently converted to 5c-D in a time-dependent manner. Usually 4–7 days at room temperature was sufficient for this conversion (Fig. 1c). Incubation at 37 °C effectively accelerated this process (Fig. 1e). Interestingly, higher temperature not only stimulated the conversion, but also moved equilibrium towards 5c-D formation, with decreases in other products.
Irradiation of other 5,7 dienes (compounds: 4a, 4b, 5a and 5b) resulted in similar pattern of products and UVB dose and time-dependent conversions (Fig. S2–S5‡).
Identification of L-like, D-like, and T-like compounds by combination of RP-HPLC, UV spectra, mass spectrometry and NMR
The initial identification of irradiation products was based on the retention time in relation to 3β-hydroxypregna-5,7-diene-20-one (5c), and unique UV spectra (Fig. 1, Table 1, Fig. S2–S5‡). Further characterization was performed after purification by RP-HPLC (see Experimental section for details) and the corresponding fractions of the selected peaks were analyzed by mass spectrometry. As predicted all D-like, L-like and T-like products had identical molecular weight corresponding to androsta- or pregna-5,7-diene precursor (Table 1).
The D-, L- or T-like irradiation products of androsta- and pregna-5,7-dienes of defined UV and mass spectra were subjected to NMR. The assignments of structures is based on 1H -NMR data and selected 2D experiments (COSY, TOCSY and HSQC). The detailed list of chemical shifts are shown in Tables S2–S4, see ESI‡. Identification was assigned based on expected chemical shifts and presence or absence of vinylic protons 6-CH and 7-CH; and methyl groups at C18, C19 and C21.
Structures of L-like derivatives (4aL, 4bL and 5aL) were confirmed based on different chemical shifts for the methyl group 19-CH3, which was shifted downfield about 0.20 ppm (±0.05 ppm) when compare with their precursors.
Although we were able to detect and characterize T-like and isoT-like compounds derived from androsta- and pregna-5,7-dienes, we found those compounds very reactive and unstable in deuterated chloroform, most probably due to trace acidic impurities of this solvent. Thus, only one structure (5a-T) was confirmed by NMR, despite the fact that compounds 4a-T, 4b-T, 5b-T and 5c-T were clearly identified by characteristic UV absorbance with λmax at 272, 280 and 290 nm (±2 nm). Since the presence of trace amount of HCl in 99.99% deuterated chloroform induced very fast structural degradation,29 we analyzed the structure of T-like compounds using deuterated methanol as solvent.
Fig. S7 in ESI‡ shows 1H NMR spectra for 3β,17β-dihydroxypregna-5,7-dien-20-one (5a) and its major irradiation products (5a-L, 5a-D, and 5a-T) as an example.
Conclusions
Based on an efficient and reproducible synthesis of androsta- and pregna-5,7-dienes (5b, 5c), and their hydroxylated derivative (4a, 4b and 5a), we were able to study in-depth their photo conversion into D-like derivatives under the standardized conditions (Scheme 3). The dynamics of the UVB-induced process were characterized, novel products were identified including L-like (4a-L, 4b-L and 5a-L) and T-like compounds (5a-T), and their structures described. It is important to emphasize that androsta- and pregna-5,7-dienes (compounds 4a, 4b, 5a-c) were previously detected in living organisms under physiological.16,17 and pathological (SLOS syndrome) conditions.13,15,24,18 Thus, the present finding open an exciting area relative to testing the hypothesis that steroidal 5,7-dienes can be photoconverted to secosteroids when delivered to or produced in the skin.37 If successful, this may lead to a new paradigm in photobiology. Furthermore, it has been reported that elimination of a cholesterol-type side-chain produces analogs of vitamin D3 without calcemic activity38 Thus, the library of D-, T- and L-like compounds described here represent promising candidates for evaluation of therapeutic utility in the treatment of various diseases, including cancer.
Scheme 3.
Photolysis of androsta- and pregna-5,7-dienes.
Supplementary Material
Acknowledgements
Supported by the grant # AR052190 from NIAMS to AS. Authors would like to thank Dr. Igor Rakow for the synthesis of 4b, 5a and 5b.
Footnotes
This paper was published as part of the themed issue in honour of Nicholas Turro.
Electronic supplementary information (ESI) available: HPLC chromatogram, UV spectra and additional experimental data for irradiation products of androsta- and pregna-5,7-dienes. NMR spectra for 5a, 5aD, 5aL and 5aT and Table of shifts for androsta- and pregna-5,7-dienes. See DOI: 10.1039/b809005j
References
- 1.Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed. Proc. 1978;37:2567–2574. [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci. USA. 1995;92:3124–3126. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF. Calcium and vitamin D. Diagnostics, and therapeutics. Clin. Lab. Med. 2000;20:569–590. [PubMed] [Google Scholar]
- 5.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutrition. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 6.Pfoertner K. [Photochemistry of the vitamin D series. II. Wavelength, dependence of photoisomerization of precalciferol] Helv. Chim. Acta. 1972;55:937–947. doi: 10.1002/hlca.19720550320. [DOI] [PubMed] [Google Scholar]
- 7.Dauben WG, Phillips RB. Wavelength-controlled production of previtamin D3. J. Am. Chem. Soc. 1982;104:355–356. [Google Scholar]
- 8.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 9.Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Met. 1989;68:882–887. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- 10.Jin X, Yang X, Yang L, Liu Z-L, Zhang F. Autoxidation of isotachysterol. Tetrahedron. 2004;60:2881–2888. [Google Scholar]
- 11.Tian XQ, Holick MF. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J. Biol. Chem. 1999;274:4174–4179. doi: 10.1074/jbc.274.7.4174. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto JK, Borch RF. Photoconversion of 7-dehydrocholesterol to vitamin D3 in synthetic phospholipid bilayers. Biochemistry. 1985;24:3338–3344. doi: 10.1021/bi00334a039. [DOI] [PubMed] [Google Scholar]
- 13.Nowaczyk MJ, Waye JS. The Smith-Lemli-Opitz syndrome: a novel metabolic way of understanding developmental biology, embryogenesis, and dysmorphology. Clin. Genetics. 2001;59:375–386. doi: 10.1034/j.1399-0004.2001.590601.x. [DOI] [PubMed] [Google Scholar]
- 14.Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64:481–490. doi: 10.1016/s0039-128x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 15.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. New England J. Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 16.Tait AD, Hodge LC, Allen WR. Biosynthesis of 3 beta-hydroxy-5,7-pregnadien-20-one by the horse fetal gonad. FEBS Lett. 1983;153:161–164. doi: 10.1016/0014-5793(83)80139-2. [DOI] [PubMed] [Google Scholar]
- 17.Tait AD, Hodge LC, Allen WR. The biosynthesis of 3 beta-hydroxy-5,7-androstadien-17-one by the horse fetal gonad. FEBS Lett. 1985;182:107–110. doi: 10.1016/0014-5793(85)81164-9. [DOI] [PubMed] [Google Scholar]
- 18.Tait AD, Santikarn S, Allen WR. Identification of 3 beta-hydroxy-5,7-pregnadien-20-one and 3 beta-hydroxy-5,7-androstadien-17-one as endogenous steroids in the fetal horse gonad. J. Endocrinol. 1983;99:87–92. doi: 10.1677/joe.0.0990087. [DOI] [PubMed] [Google Scholar]
- 19.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutrition. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 20.Bikle DD, Oda Y, Xie Z. Vitamin D and skin cancer: a problem in gene regulation. J. Steroid Biochem. Mol. Biol. 2005;97:83–91. doi: 10.1016/j.jsbmb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Plum LA, Prahl JM, Ma X, Sicinski RR, Gowlugari S, Clagett-Dame M, DeLuca HF. Biologically active noncalcemic analogs of 1alpha,25-dihydroxyvitamin D with an abbreviated side chain containing no hydroxyl. Proc. Natl. Acad. Sci. USA. 2004;101:6900–6904. doi: 10.1073/pnas.0401656101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murari MP, Londowski JM, Bollman S, Kumar R. Synthesis and biological activity of 3 beta-hydroxy-9,10-secopregna-5,7,10[19]-triene-20-one: a side chain analogue of vitamin D3. J. Steroid Biochem. 1982;17:615–619. doi: 10.1016/0022-4731(82)90562-3. [DOI] [PubMed] [Google Scholar]
- 23.Marwah P, Marwah A, Lardy HA. Microwave induced selective enolization of steroidal ketones efficient acetylation of sterols in semisolid state. Tetrahedron. 2003;59:2273–2287. [Google Scholar]
- 24.Guo LW, Wilson WK, Pang J, Shackleton CH. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids. 2003;68:31–42. doi: 10.1016/s0039-128x(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 25.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–1566. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 26.184112 EU Pat. 1986
- 27.Siddiqui AU, Wilson WK, Swaminathan S, Schroepfer GJ., Jr. Efficient preparation of steroidal 5,7-dienes of high purity. Chem. Phys. Lipids. 1992;63:115–129. doi: 10.1016/0009-3084(92)90028-n. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Yoshimoto S, Yasumura M. An improved procedure for the isolation of suprasterol2 I and II from a photochemical reaction mixture of ergocalciferol (vitamin D2) J. Nutr. Sci. Vitaminol. 1977;23:291–298. doi: 10.3177/jnsv.23.291. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal VK. A new procedure for the isomerization of vitamin D and its metabolites. J. Steroid Biochem. 1990;35:149–150. doi: 10.1016/0022-4731(90)90160-t. [DOI] [PubMed] [Google Scholar]
- 30.Djerassi C, Romo J, Rosenkranz G. Steroidal sapogenins. VIII. Steroids. 18. Synthesis of D7,9(11)-allopregnadien-3b-ol-20-one from diosgenin and from D5-pregnen-3b-ol-20-one. J. Org. Chem. 1951;16:754–760. [Google Scholar]
- 31.Antonucci R, Bernstein S, Giancola D, Sax KJ. Delta 5,7-Steroids. VI. The, preparation of Delta 5,7-steroidal hormones. J. Org. Chem. 1951;16:1126–1133. [Google Scholar]
- 32.7253293 US Pat. 2005
- 33.6372926 US Pat. 1997
- 34.Velluz L, Amiard G, Goffinet B. Etio analogs of precalciferol. Bull. Soc. Chim. France. 1957:882–886. [Google Scholar]
- 35.4891364 US Pat. 1990
- 36.989881 Br. Pat. 1965
- 37.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar CC, Tuckey R. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur. J. Biochemistry/FEBS. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holick MF, Garabedian M, Schnoes HK, DeLuca HF. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J. Biol. Chem. 1975;250:226–230. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.