Abstract
Objectives
To determine how a mother’s brain responds to her own baby’s facial expressions, comparing happy, neutral and sad face affect.
Methods
In an event-related functional MRI study, 28 first-time mothers were shown novel face images of their own 5–10 month-old baby and a matched unknown baby. Sixty unique stimuli from 6 categories (own-happy, own-neutral, own-sad, unknown-happy, unknown-neutral and unknown-sad) were presented randomly for 2 seconds each, with a variable 2–6 second inter-stimulus interval.
Results
Key dopamine-associated reward processing regions of the brain were activated when mothers viewed their own baby’s face, compared to an unknown baby face. These included the ventral tegmental area / substantia nigra regions, the striatum, and frontal lobe regions involved in 1) emotion processing (medial prefrontal, anterior cingulate and insula cortex), 2) cognition (dorsolateral prefrontal cortex) and 3) motor/behavioral outputs (primary motor area) (P<0.001, false discovery rate corrected [FDR] q<0.05). Happy, but not neutral or sad own-infant faces, activated nigrostriatal brain regions interconnected by dopaminergic neurons (P<0.0005, FDR q<0.05), including the substantia nigra and dorsal putamen. A region-of-interest analysis revealed that activation in these regions was related to positive infant affect (happy>neutral>sad) for each own-unknown baby face contrast.
Conclusions
When first-time mothers see their own baby’s face, an extensive brain network appears to be activated, wherein affective and cognitive information may be integrated and directed toward motor/behavioral outputs. Dopaminergic reward-related brain regions are activated specifically in response to happy, but not sad, baby faces. Understanding how a mother responds uniquely to her own baby, when smiling or crying, may be the first step in understanding the neural basis of mother-infant attachment.
Keywords: attachment, dopamine, maternal responsiveness, mother-child relations, neuroimaging
INTRODUCTION
Starting from the early post-partum period, mothers demonstrate a unique ability to recognize different sensory cues from their own babies, including visual (1;2), auditory (3) or olfactory (4) cues. These stimuli, such as a hunger cry or smiling face, are powerful motivators for a mother to respond, either through caregiving, physical touch, speech or play. Animal research suggests that infant-responsive maternal behavior is causally related to the offspring’s long-term developmental outcome in a number of domains, including cognitive development (5;6) , stress reactivity (7–9) and maternal behavior in adulthood (7;10). Factors that restrict a mother’s ability to respond to her baby’s cues, such as depression (11), substance abuse (12) or even prolonged mother-infant separation (13) may result in adverse developmental outcomes for children (11;12;14;15). In addition, the ability to link these sensory cues with the underlying needs of an infant, and differentially respond to such needs, is thought to be the basis for establishing secure mother-infant attachment (15–17). Thus, a mother’s behavioral and brain response to her infant’s cues may be an important predictor of infant development.
Over recent years, several research groups have sought to better understand how a mother’s brain responds to her child’s auditory or visual cues, using functional MRI (fMRI) (18–23). One common theme emerging from these studies has been the possible role of the mesocorticolimbic dopamine system in processing reward-based signals and motivating maternal care, as seen in animal models (see review (24)). Several studies have shown that the striatum, a key projection of midbrain dopamine neurons which includes the putamen and caudate head, is activated in response to face images of a mother’s own child compared to unknown (or familiar but unrelated) children (22;23), as well as to infant cry stimuli (18). Similar activation patterns have been seen in response to pictures of romantic partners (22), beautiful faces (25) and sexual stimuli (26), suggesting a link between brain reward circuits and attachment.
However, some maternal response studies have failed to show striatal activation (20;21), among other important differences. The amygdala, for example, is strongly activated in some studies (20;23), but deactivated in another (22). Since the amygdala plays an important role in processing face affect (27), and its response may be modulated by dopamine (28;29), differences in baby face affect may have been a confounding factor. Although most baby face studies sought to standardize face affect, none of them specifically controlled for variation in affect, or examined response differences related to facial affect. In addition, most prior studies have had a small sample size (10 or fewer subjects) or used a suboptimal fixed effects analysis (24) which prevents generalization of the results to the population from which the sample was drawn (30).
This study includes a relatively large sample of first-time mothers and their babies, specifically comparing maternal brain responses to baby face stimuli grouped into happy, neutral and sad affect. We predicted that “own baby” faces compared to “unknown” faces, would activate dopamine-associated reward-processing brain regions, including the ventral striatum and prefrontal cortex, and that the contrast in these regions would be greater for smiling baby faces than neutral or sad faces. Based on pilot results (31) and results from infant cry studies (18), we also predicted that sad faces from a mother’s own baby compared to an unknown baby would activate the anterior cingulate cortex, which is involved in conflict monitoring (32), and both the insula and amygdala, regions often associated with negative emotion processing (27). Together these response patterns would help us to better define the neural basis of human mother-infant attachment.
METHODS
Subjects
This cohort is part of larger longitudinal study of mother-infant attachment, including 43 women who were enrolled during the third trimester of pregnancy. Subjects were recruited from prenatal clinics, local church groups, and poster, magazine and internet advertisements. Each woman was screened for recruitment by phone or by completing an online questionnaire. Inclusion criteria included: first-time singleton pregnancy, right handedness, non-smoking during pregnancy, not currently on psychotropic medications, and no contraindications for MRI scanning (such as metal implants or severe claustrophobia). At the time of the fMRI scanning visit, approximately one year after enrollment, 5 women were lost to follow-up or declined further participation and 10 were unable to be scanned (9 due to a second pregnancy and one because of a past history of seizures), leaving 28 scanned women. During the second scanning run, data was only available for 26 women, because of unacceptable head motion in one case, and scanner failure in another.
The protocol was approved by the Institutional Review Board at Baylor College of Medicine, Houston, Texas, and all subjects provided written informed consent.
Experimental Design
Prenatal Session
During the third trimester of pregnancy, enrolled women provided sociodemographic information from which was calculated the Hollingshead SES score. They also participated in a variety of psychometric tests, including the Adult Attachment Interview, the Personality Disorder Questionnaire 4+ (PDQ4+), the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD) and the Beck Depression Inventory (BDI)(33).
Videotaping Session
At around 7 months post-delivery, each baby was videotaped in a standard setting at the Human Neuroimaging Laboratory, Baylor College of Medicine. Smiling faces were elicited by the experimenters interacting with the babies using a variety of age-appropriate toys, and crying faces were obtained by leaving the baby alone in the room (observed from behind a one-way mirror) with the video camera recording facial expressions. The mothers did not observe the videotaping, to ensure that each baby face image was novel when presented during the subsequent scanning session. At this visit, the mothers also updated their demographic information and completed another BDI.
Baby face still images encompassing various affect levels – happy, neutral and sad – were then captured from the videotape. Using a facial affect coding scheme, based on Cole et al (34), these images were classified by a trained research assistant into one of five affect groups: very happy, happy, neutral, sad or very sad. Excellent inter-observer reliability was demonstrated, based on 466 double coded images (Pearson correlation coefficient 0.925, 2-tailed, P<0.001). Control baby face images, unknown to each mother, were collected from the babies of other enrolled mothers or mothers involved in the pilot study. Each subject baby was matched to a single control baby, with an equal number of face images from each affect group. Wherever possible, the two babies were also matched on age and race. In cases of mixed race, the matching was based on a combination of race, complexion and hair color. Gender was matched if there were any obvious distinguishing features, such as earrings or longer hair. Each baby had been videotaped in a gender-neutral white jumpsuit. All images were standardized for size, orientation and background using Adobe Photoshop.
Scanning Session
A minimum of 3 months after the videotaping session, each mother attended a scanning session at the Human Neuroimaging Laboratory. Immediately prior to scanning, the mother participated in a one-hour long semi-structured interview, the Parent Development Interview (35), which prompted the mother to reflect on her relationship with her child. This provided a common setting for each mother prior to viewing the baby face images in the scanner.
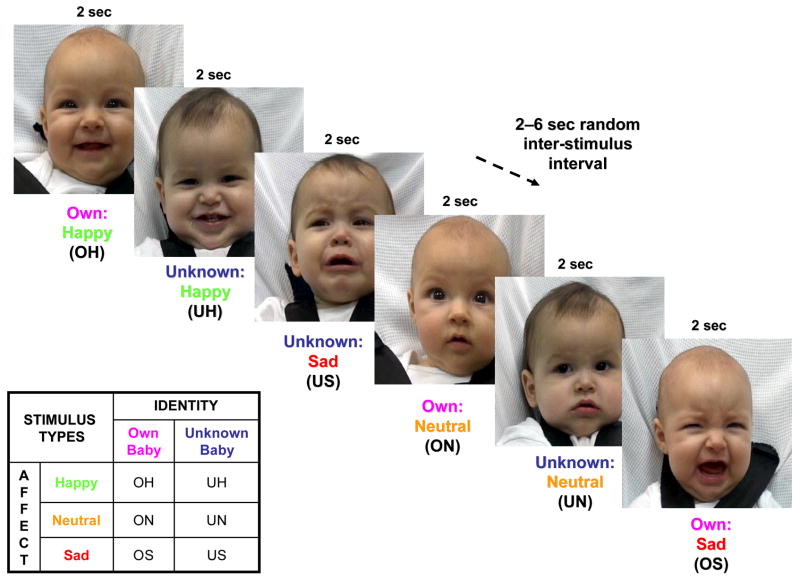
The mother then participated in two functional MRI runs, each time passively viewing a series of 60 unique baby face images, 30 of her own baby and 30 of an unknown baby face. Each mother was informed that her “brain activity will be monitored using functional MRI while she is shown pictures of her own baby and babies unknown to her” (recruitment brochure). Using an event-related fMRI design, randomly presented images were viewed for 2 seconds, with a random inter-stimulus interval of 2, 4 or 6 seconds (Figure 1). The 60 images were equally divided into 3 affect groups – happy, neutral or sad, with the intensity of happy and sad affect balanced between the “own” and “unknown” faces. The order of the images from each of the 6 groups (OH, ON, OS, UH, UN, US) was pseudo-randomized within and between each run, but not between subjects. There were no significant differences in the timing of “own” and “unknown” baby face images (natural log of mean presentation times, paired samples t-test, t=−0.73, df=29, P=0.47), or in the OH>UH, ON>UN or OS>US comparisons (df=9; Happy: t=1.52, P=0.16; Neutral: t=0.72, P=0.49; Sad: t=−1.69, P=0.13).
Figure 1.
Baby face presentation paradigm in functional MRI experiment. Ethnically-matched still baby face images were presented for 2 seconds, followed by a variable 2–6 second period of a blank screen. The 6 stimulus types outlined were presented in random order.
All imaging was performed using a 3 Tesla Siemens Allegra head-only MRI scanner. Visual images were generated using a computer controlled LCD projector, and presented to the mother via an overhead mirror display. High-resolution T1-weighted structural images (192 slices, in plane resolution 256 x 256; field of view [FOV] 245mm; slice thickness 1mm) were acquired first. Regional brain activation was assessed by measuring changes in blood-oxygen-level-dependent functional MRI signal (BOLD-fMRI). Subjects underwent two whole-brain functional runs of around 185 scans each (gradient recalled echo planar imaging; 37 slices; repetition time [TR] 2000 msec; echo time [TE] 25 msec; flip angle, 90 degrees; 64 x 64 matrix [in plane resolution]; FOV 220mm; slice thickness 3mm; gap thickness 1mm). Slices were positioned 30 degrees to the anterior commissure / posterior commissure (ACPC) line in the axial plane, downward from posterior to anterior, which (along with a reduced TE and slice thickness) has been shown to optimize visualization of the orbitofrontal cortex (36).
After the scanning session, each mother was asked to rate each of the baby face images on how they thought the baby was feeling, as well as their own feelings of pleasure or arousal, using an adaptation of the Self-Assessment Manikin (37). Each mother also completed the Wechsler Test of Adult Reading, as a predictor of IQ (38), and repeated the BDI. At around 14 months of age, all but one of the children were assessed for general development, using The Screening Test of the Bayley Scales of Infant and Toddler Development, 3rd edition (39). They also participated in a child assessment of attachment, using the Strange Situation Procedure (40).
Data Processing and Analysis
Imaging data for each subject were preprocessed in BrainVoyager QX, version 1.7.9 (41) and analyzed in version 1.8.6 and 1.9.9, using the following steps:
Head motion correction was performed using trilinear/sinc interpolation by spatial alignment of all brain volumes to the first volume by rigid body transformations. One subject had more than 2 mm translation (2.3 mm) during Run 1, and analyses were repeated before and after excluding this subject. A single subject also had unacceptable head motion during Run 2 (3.2 mm translation and 3.5 mm rotation), and was excluded from further analyses.
Slice scan time correction was performed using sinc interpolation, based on the repetition time (TR) and order of slice scanning (ascending interleaved). Following linear trend removal, low frequency nonlinear drifts of 3 or less cycles were removed using a temporal high pass filter. Spatial smoothing was not performed.
The anatomical dataset underwent iso-voxel scaling to 1 x 1 x 1 mm resolution and was transformed into sagittal orientation. It was then transformed into ACPC and Talairach standard space using sinc interpolation (42). Functional runs for each subject were coregistered with the anatomical 3-dimensional dataset, iso-voxel transformed to 3 x 3 x 3 mm resolution, and then transformed into standard ACPC and Talairach coordinate space, resulting in normalized 4-dimensional volume-time course data. For presentation purposes, the final activation map was interpolated into a 1 x 1 x 1 mm resolution.
For each functional run of the event-related data, a BrainVoyager protocol file was created, representing the timing of each stimulus event. The six baby face stimulus types in the design matrix included Own-Happy (OH), Own-Neutral (ON), Own-Sad (OS), Unknown-Happy (UH), Unknown-Neutral (UN) and Unknown-Sad (US) (Figure 1). Each predictor was then convolved with a double-gamma hemodynamic response function (43). Using the General Linear Model (GLM), group effects were evaluated using a random effects analysis, with a % time course transformation applied to each run of each subject separately. In the random effects analysis, statistical maps were created for each individual subject before being subjected to a second level of statistical analysis, allowing generalization to the sample population of first-time mothers. Main effects and possible interaction effects of infant “identity” and “affect” (Figure 1) were explored using 2-factor repeated measure ANOVAs (F-test, df=2,54). Group t-maps (2-tailed, df=27) were also generated after specifying a particular contrast in stimulus types (e.g. OH>UH), and were visualized on an averaged 3D anatomical image, which was created from all of the individual subject images.
The false discovery rate (FDR) approach (44) was used to correct for multiple comparisons at a threshold of q<0.05, which accepts 5% of the discovered (supra-threshold) voxels as false positives. A cluster threshold of 100 mm3 (or approximately 4 voxels) was used, except in the brainstem, where a threshold of 30 mm3 (or around 1 voxel) was used to reveal activation of smaller nuclei. Anatomical regions were confirmed using the automated “Talairach Daemon” (searching for “nearest gray matter”) (45), and manually, using a human brain atlas (46). Brodmann Areas (BA) were defined using the BrainVoyager Brain Tutor (47).
Hemodynamic responses to event types (% BOLD signal change) were averaged and standardized across subjects, and plotted against time to create an event-related averaging plot for anatomical regions-of-interest. A random effects GLM analysis was performed on each volume individually.
RESULTS
Description of Subjects
The 28 mothers who participated in this study had a mean age of 29 years, were racially diverse (representative of the Houston population (48)), and middle to upper class (based on the Hollingshead Four-Factor Index of Social Status (49)), with 75% having completed higher education. Only one mother scored outside the normal range on WTAR-predicted WAIS-III IQ scores (range 81–120, median and mode=112) (Table 1). One other mother was classified as having “mild” depression symptoms based on the Beck Depression Inventory during the videotaping session, but none of the mothers reported significant symptoms during subsequent visits. There were no self-reports of current or past alcohol or drug abuse problems, or involvement in substance abuse treatment programs. However, 61% of mothers screened positive for one or more personality disorders on the PDQ4+, including 8 mothers for obsessive-compulsive and 8 for avoidant personality disorder (but none for borderline personality on the MSI-BPD). Although 93% of mothers reported returning to work by the time of the scanning session, 54% were still breastfeeding, and 43% reported that they were not separated from their child for more than 20 hours per week. Except for one baby born at 36 weeks gestation, the babies were full-term. At 14 months of age, only one child scored in the “at risk” range in one of five subscales of the Bayley Scales for Infant and Toddler Development, and this score was at the upper limit of the range. For this child, 3 out of the other 4 developmental scores were in the “competent” range. All other children were in the “competent” or “emerging” range for each developmental subscale, including cognition, expressive and receptive communication, fine motor, and gross motor development (Table 1).
Table 1.
Demographic information for study cohort (at time of scanning unless noted).
| No. | Variables | Mean ± SD | Range | |
|---|---|---|---|---|
| 1 | Age of mother, y | 30.2 ± 5.0 | 20–42 | |
| 2 | Age of baby – videotaping session, mth | 6.7 ± 1.6 | 5–10 | |
| 3 | Age of baby – scanning session, mth | 10.7 ± 2.3 | 7–17 | |
| 4 | Hollingshead SES score (joint with partner)* | 49.1 ± 12.7 | 24–66 | |
| 5 | Maternal IQ (WTAR-predicted WAIS-III) | 108.7 ± 9.2 | 81–120 | |
| 6 | Maternal Race (n) | |||
| - White, non-Hispanic | 13 | |||
| - African American | 7 | |||
| - Hispanic | 4 | |||
| - Other | 4 | |||
| 7 | Maternal Education (n) | |||
| - Post-graduate degree | 13 | |||
| - College/University degree | 9 | |||
| - Incomplete college | 6 | |||
| 8 | Marital Status (n) * | |||
| - Married | 20 | |||
| - Single/never married | 4 | |||
| - Unmarried cohabitation | 3 | |||
| 9 | Child Development at 14 months (n) * | Competent | Emerging | At Risk |
| - Cognitive | 21 | 5 | 1 | |
| - Receptive Communication | 25 | 2 | 0 | |
| - Expressive Communication | 21 | 6 | 0 | |
| - Fine Motor | 25 | 2 | 0 | |
| - Gross Motor | 27 | 0 | 0 | |
Data missing for one subject.
Maternal Brain Responses
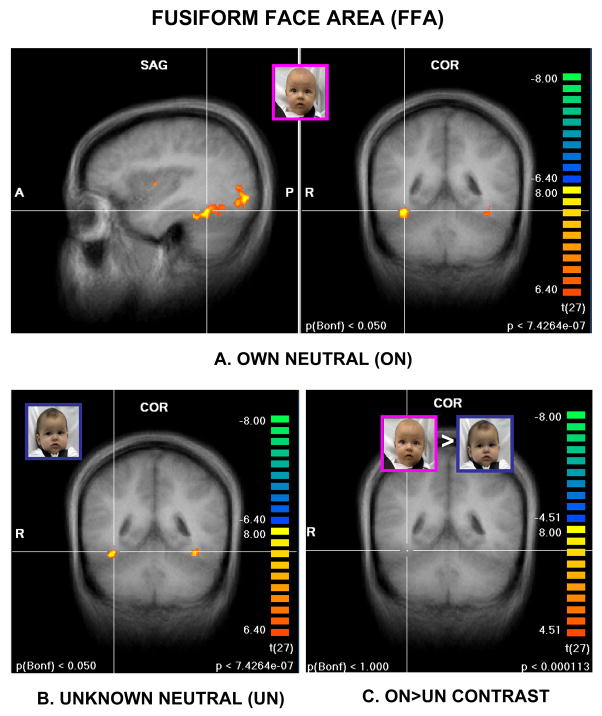
Before addressing the specific hypotheses of this study, we examined maternal brain responses to affect-neutral baby faces compared to the no-face baseline. As expected, face stimuli activated brain regions along the ventral visual pathway from the primary visual cortex to the temporal lobe, including the fusiform gyrus and the so-called “fusiform face area” (Figure 2A&B) (50). However, after contrasting “own” and “unknown” baby faces (ON>UN), no significant activation remained, even at lowered statistical thresholds (Figure 2C). Thus, there was no significant difference in posterior visual pathway response between the own and unknown baby face stimuli.
Figure 2.
Activation of ventral visual pathway, including the fusiform face area (Talairach coordinates 36, -46, -17) by own-neutral, as well as unknown-neutral baby faces A. Coronal and sagittal views of activation from own-neutral (ON) baby faces, compared to no-face baseline B. Coronal view of activation from unknown-neutral (UN) baby faces, compared to no-face baseline. C. Contrast between ON>UN, showing no remaining activation of visual pathway or fusiform face area. (A and B: P<0.000001, Bonferroni correction P<0.05, C: P<0.0001, uncorrected; cluster threshold 100 mm3).
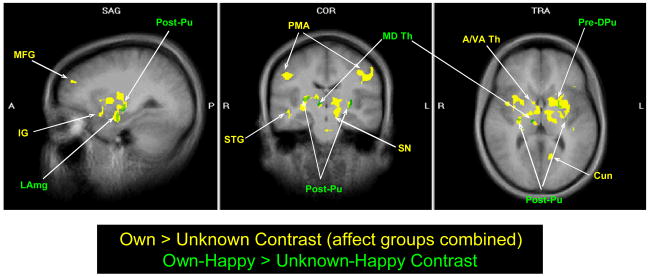
Next, we tested our first hypothesis, regarding the main effect of infant “identity” (O>U) on maternal brain response. From the first scanning run, this revealed activation of forebrain regions involved in 1) emotion processing (medial prefrontal, anterior cingulate and insula cortex), 2) cognition (dorsolateral prefrontal cortex) and 3) motor/behavioral outputs (primary motor area, BA 4) (F=13.6 to16, df=1,27, P<0.001, FDR corrected q<0.05). Also activated were striatal and midbrain regions including the ventral striatum, head of caudate, putamen, ventral tegmental area (VTA) and substantia nigra. Other significant areas included regions of the inferior, middle and superior temporal gyri (including the fusiform gyrus and temporal pole), the lateral amygdala, thalamic nuclei and the hypothalamus (Table 2, Figure 3). No brain region was significantly activated by infant “affect” as a main effect (F>15.66, df=2,54, using a random effects model, FDR corrected q<0.05), nor was an “identity x affect” interaction effect seen, with or without FDR correction. No significant activation was seen for any contrast during the second scanning run, where the baby face stimuli from Run 1 were repeated and data from 2 subjects were missing.
Table 2.
Areas of significant activation from own vs. unknown baby face contrast (all affect groups combined, i.e. OH+ON+OS > UH+UN+US) (t-test, df=27, P<0.001, FDR corrected q<0.05; all cluster thresholds = 100 mm3, except midbrain regions). All regions-of-interest P<0.0001. Talairach coordinates (x, y, z) represent center-of- gravity mean values for each region-of-interest. A large areas of activation involving the lentiform nuclei was divided manually according to anatomical regions.
| Region-of-Interest / Cluster (Brodmann Area, BA) | Right Hemisphere
|
Left Hemisphere
|
||
|---|---|---|---|---|
| x, y, z | Z-score O>U | x, y, z | Z-score O>U | |
| Frontal Lobe
| ||||
| Medial Prefrontal Cortex | ||||
| Superior frontal gyrus – medial (BA 6/9) | 1, −2, 60 | 4.55 | −7, 39, 25 | 4.41 |
| Superior frontal gyrus (BA 9/10) | 3, 59, 29 | 4.97 | - | - |
| Lateral Orbitofrontal Cortex | ||||
| Inferior frontal gyrus (BA 47) | - | - | −43, 23, 1 | 5.09 |
| Dorsolateral Prefrontal Cortex | ||||
| Inferior frontal gyrus (BA 44) | 48, 8, 28 | 4.74 | - | - |
| Middle frontal gyrus (BA 9) | - | - | −24, 48, 32 | 4.17 |
| Primary Motor Area / Somatosensory Cortex | ||||
| Pre- / Post-central gyrus (BA 4) | 45, −17, 37 | 4.86 | - | - |
|
| ||||
| Parietal / Occipital Lobe
| ||||
| Post-central gyrus (BA 3/40) | 20, −27, 51 | 4.54 | −46, −17, 37 | 4.69 |
| Lingual gyrus (BA 18/19) | - | - | −15, −56, −2 | 4.48 |
|
| ||||
| Temporal Lobe (Lateral)
| ||||
| Middle temporal gyrus (BA 21) | - | - | −56, −38, −5 | 4.57 |
| Middle temporal gyrus / temporal pole (BA 38) | 48, 3, −13 | 5.36 | −47, 3, −12 | 4.97 |
| Superior temporal gyrus (BA 22/21) | 39, −41, 12 | 4.84 | −39, −28, 4 | 5.14 |
| Inferior temporal / fusiform gyrus (BA 37) | 38, −53, −7 | 4.30 | −41, −44, −17 | 5.09 |
|
| ||||
| Limbic Lobe / Sub-Lobar Regions
| ||||
| Basal Ganglia | ||||
| Ventral striatum (pre-commissural) | - | - | −13, 6, 4 | 4.88 |
| Dorsal putamen (pre- commissural) | 22, 5, 4 | 4.03 | −23, 2, 4 | 4.74 |
| Putamen (post-commissural) | 24, −17, 9 | 4.83 | −29, −12, 0 | 5.10 |
| Putamen (post-commissural) – superior | - | - | −26, −10, 10 | 4.80 |
| Dorsal caudate (pre-commissural) | 9, 5, 11 | 4.15 | −14, 2, 16 | 4.98 |
| Thalamus / Hypothalamus | ||||
| Medial dorsal / centromedial thalamus | 7, −20, 2 | 5.39 | - | - |
| Ventral anterior / lateral thalamus | 4, −7, 4 | 5.52 | −9, −9, 4 | 5.20 |
| Ventral anterior / lateral thalamus | 14, −10, 8 | 4.87 | −11, −16, 4 | 4.75 |
| Hypothalamus | 3, −8, −6 | 4.80 | −5, −8, −7 | 4.59 |
| Medial Temporal Lobe | ||||
| Lateral superior amygdala | - | - | −27, −6, −13 | 5.62 |
| Parahippocampal gyrus (BA 36) | 42, −37, −9 | 5.77 | - | - |
| Insula Cortex | ||||
| Insula (inferior) | 32, −3, −7 | 5.69 | - | - |
| Insula | 40, 3, 4 | 4.77 | −31, −5, 2 | 5.23 |
| Insula (posterior) / planum polare | 42, −19, −6 | 5.10 | - | - |
| Cingulate Cortex | ||||
| Anterior cingulate cortex – pregenual (BA 24/32) | - | - | −2, 37, 13 | 4.97 |
| Anterior cingulate cortex – pregenual (BA 24) | - | - | −3, 13, 32 | 4.62 |
| Middle cingulate cortex (BA 24) | 1, −2, 42 | 4.92 | - | - |
| Posterior cingulate cortex – retrospenial (BA 31) | - | - | −5, −53, 17 | 4.48 |
| Posterior cingulate cortex – retrospenial / cuneus (BA 17) | - | - | −8, −64, 10 | 4.75 |
|
| ||||
| Midbrain (Cluster threshold = 30 mm3)
| ||||
| Ventral tegmental area vicinity (midline) | 1, −16, −15 | 5.56 | - | - |
| Substantia nigra vicinity | - | - | −8, −23, −9 | 4.93 |
| Red nucleus vicinity | 3, −21, −7 | 5.23 | −3, −21, −8 | 5.46 |
|
| ||||
| Cerebellum
| ||||
| Cerebellum | 38, −48, −25 | 4.78 | −34, −38, −28 | 4.97 |
| Anterior cerebellum | - | - | −2, −45, −39 | 4.69 |
Figure 3.
Maternal brain activation in response to own infant vs. unknown infant happy faces (green regions and labels: t-test, df=27, P<0.0001, FDR corrected q<0.05, cluster threshold 100 mm3) and all affect states combined (yellow regions and labels: F-test, df=1,27, P<0.001, FDR corrected q<0.05). Talairach coordinates -27, -16, 6. Abbreviation (left to right). Green labels: LAmg, lateral amygdala; Post-Pu, post-commissural putamen; MD Th, mediodorsal thalamus; Pre-DPu, pre-commissural dorsal putamen. Yellow labels: MFG, middle frontal gyrus; IG, insula gyrus; STG, superior temporal gyrus; PMA, primary motor area; SN, substantia nigra; A/VA Th, anterior / ventroanterior thalamus; Cun, cuneus.
As hypothesized, significant areas of activation were seen when the mothers were shown happy faces of their own baby compared to an unknown baby (OH>UH) (P<0.0005, FDR corrected q<0.05). Five specific regions of activation were seen in the limbic area (with a cluster threshold of 100 mm3), and one in the midbrain (cluster threshold 30 mm3), including bilateral putamen, left substantia nigra region, right thalamus, and the left lateral superior amygdala (Table 3). These regions essentially overlapped regions of significance in the main effects “identity” analysis for O>U (Figure 3).
Table 3.
Areas of significant activation from own-happy vs. unknown-happy baby face contrast (t-test, df=27, P<0.0001, FDR corrected q<0.05). These regions-of-interest were then analyzed with respect to neutral and sad baby face contrasts. Talairach coordinates represent center-of-gravity mean values for each region-of-interest.
| Region-of-Interest / Cluster
|
Z-score
|
|||
|---|---|---|---|---|
| Anatomical Region | Talairach coordinates (x, y, z) | OH>UH | ON>UN | OS>US |
|
Cerebrum (Cluster threshold = 100 mm3)
| ||||
| R putamen (post-commissural) | 24, −17, 9 | 5.60*** | 2.70* | 1.43 |
| R medial dorsal / ventrolateral thalamic nucleus | 9, −18, 4 | 5.60*** | 2.64* | 0.09 |
| L dorsal putamen (pre-commissural) | −21, 2, 4 | 5.27*** | 2.88** | 2.25 |
| L putamen (post-commissural) / claustrum | −27, −14, −1 | 5.35*** | 2.38 | 0.78 |
| L lateral amygdala (superior) | −30, −6, −12 | 5.56*** | 2.44 | 2.28 |
|
| ||||
|
Midbrain (Cluster threshold = 30 mm3)
| ||||
| L substantia nigra (vicinity) | −9, −22, −12 | 5.76*** | 2.65* | 0.94 |
P<0.01,
P<0.005,
P<0.0001.
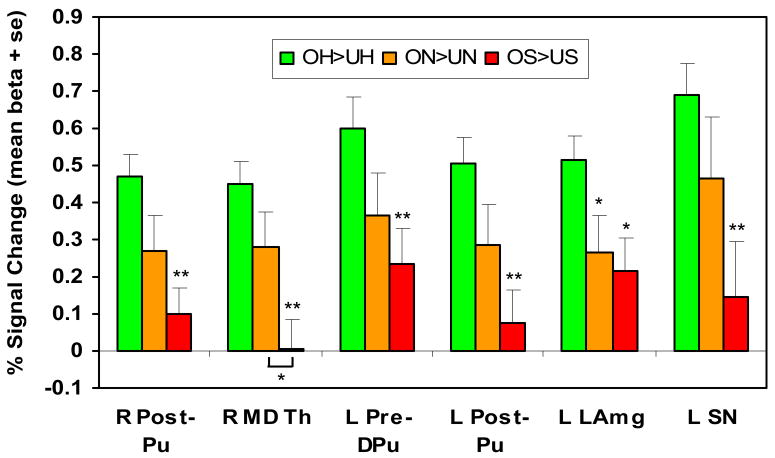
A region-of-interest random effects analysis was then performed in each of the six OH>UH regions separately (all P<0.0001; Table 3). To explore how these results varied with infant affect, and ensure that they were not due to baby face familiarity differences alone, the analyses were repeated for neutral and sad affect faces. Significant activation was seen in four of the six regions using the “Own-Neutral” vs. “Unknown-Neutral” (ON>UN) contrast, although, as predicted, at much lower levels of statistical significance (P<0.01). No region showed significant activation when contrasting own vs. unknown sad faces. In all six regions, there appeared to be a progressive decrease in the % signal change differences, across happy, neutral and sad affect (Figure 4). The response to sad affect was significantly less than for happy affect in each region (paired sample t-tests, 2-tailed, df=27, P<0.005, except amygdala: P<0.05). A significant difference was also seen between happy and neutral affect in one region, and between neutral and sad affect in another (both P<0.05).
Figure 4.
Progressive decrease in activation depending on infant affect (happy>neutral>sad) in specified regions-of-interest. Paired sample t-tests (2-tailed, df=27) comparing happy affect with neutral or sad, except as noted. Abbreviations (left to right). R Post-Pu, right post-commissural putamen; R MD Th, right medial dorsal thalamus; L Pre-DPu, left pre-commissural dorsal putamen; L Post-Pu, left post-commissural putamen; L LAmg, left lateral amygdala; L SN, left substantia nigra. * P<0.05, ** P<0.005.
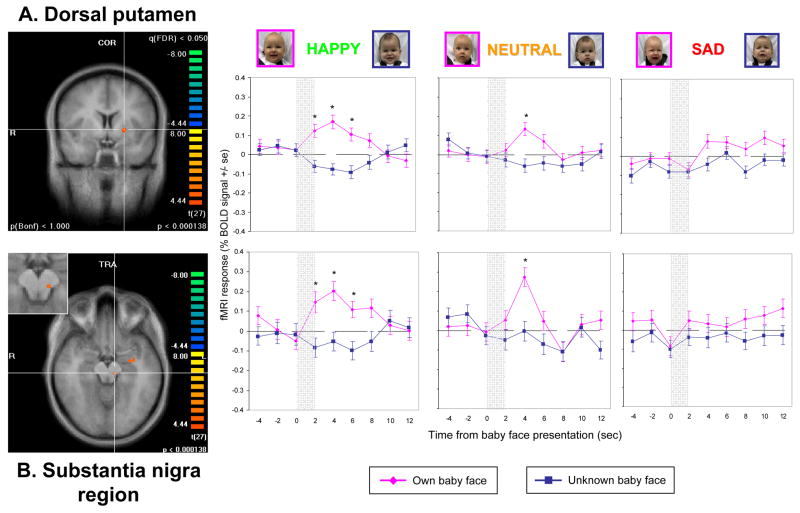
When the BOLD signal change was examined over time in each of these regions, the change from baseline fMRI response coincided precisely with the presentation onset of the baby face stimuli, and significant differences between the OH and UH stimuli responses were seen. As an example, in Figure 5 the left dorsal putamen and substantia nigra area, two key interconnecting dopaminergic brain regions, showed a significant fMRI-BOLD response to own-happy faces but much less to neutral faces, and no response difference was seen in the sad face contrast.
Figure 5.
Hemodynamic brain response of mothers viewing their own baby’s face, compared to an unknown baby face in (A) the left dorsal putamen, and (B) the left substantia nigra (enlarged view inset) (P<0.0001, FDR corrected q<0.05). Event related averaging graphs for each region, separated by affect group.
Thus, although no significant “affect x identity” interaction effect was seen, these findings suggest that infant affect has a moderating effect in each of these 6 dopamine-associated brain regions, and that familiarity did not fully explain the results of the OH>UH contrast analysis.
Finally, we examined differences in maternal brain response to sad-affect baby faces. Compared to the no-face baseline, both “own” and “unknown” sad faces produced widespread brain activation, including the specifically hypothesized regions, anterior cingulate, insula and amygdala (t-test, df=27, P<0.001, FDR corrected q<0.01). However, as with the ON>UN contrast, no significant regions of activation remained after contrasting OS with US (at P<0.001, cluster 30 mm2, uncorrected).
Behavioral Rating of Baby Faces
On viewing the baby face images outside the scanner, the mothers’ own feelings were highly correlated with how they imagined the baby to be feeling (r=0.82, p<0.001). Crying baby faces, regardless of identity, resulted in more negative affective responses from the mothers, but the mothers’ emotional responses were more tightly correlated with their own baby’s affect than for unknown baby faces (Own: r=0.87; Unknown: r=0.80). That is, the mothers were more sensitive to their own babies’ emotional states, than to unknown baby faces (slope own = 0.84, slope unknown = 0.49, P<0.05, two sample t-test, 2-tailed). The mothers also rated their feelings as being more “aroused” or intense for their own baby, compared to unknown baby faces (P<0.01, two sample t-test, 2-tailed).
DISCUSSION
As almost any mother will attest, seeing one’s own baby smile is a uniquely pleasurable and rewarding experience. But what’s in a smile, when we consider a mother’s brain response? And how is seeing one’s own baby linked to motivated behavior? This study shows that when first-time mothers observe their own baby’s face, all of the key dopamine-associated reward-processing regions of the brain are activated, including the midbrain VTA / substantia nigra regions, the striatum and the prefrontal cortex, as well as the primary motor area. Smiling, but not neutral or sad, faces specifically activate nigrostriatal brain regions interconnected by dopaminergic neurons (51), with a graded response dependent on infant affect (happy>neutral>sad).
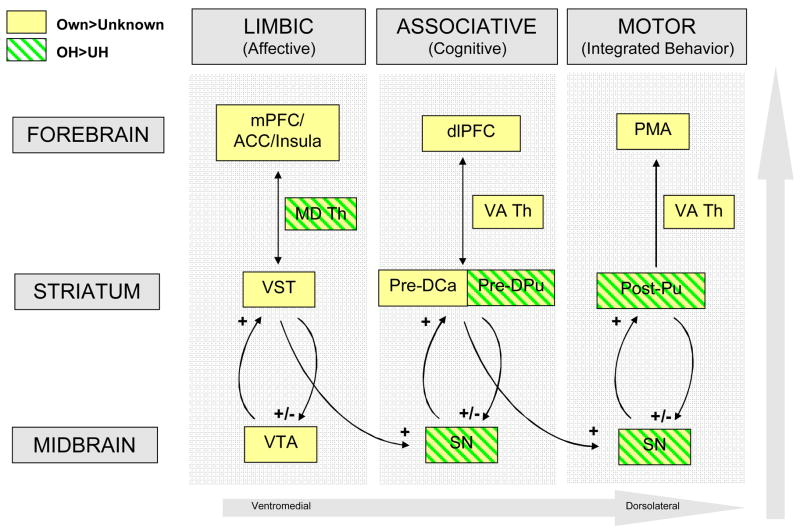
Two other studies have also shown maternal brain activation in the VTA / substantia nigra and the striatum in response to child-related stimuli (Bartels and Zeki (22) for face stimuli of older children and Lorberbaum et al (18) for infant cry stimuli). In primates, Haber et al (51) demonstrated important anatomical feed-forward loops between the striatum and the VTA / substantia nigra region, suggesting that these striatonigrostriatal circuits funnel information between ventromedial (limbic), central (associative) and dorsolateral (motor) striatal regions (Figure 6). Each striatal region is integrally connected to a corresponding region of the midbrain’s VTA and substantia nigra via ascending and descending dopaminergic neurons. Likewise, there are corresponding connections between the striatum and the forebrain, including those involved in emotion processing (medial prefrontal, anterior cingulate, insula), cognition (dorsolateral prefrontal) and motor/behavioral outputs (primary motor area) (51). Thus, the striatum is believed to be an important relay station between the limbic and motor systems, integrating affective information from limbic regions with cognitive information from the prefrontal cortex, in shaping motor/behavioral responses.
Figure 6.
Own vs. unknown baby faces activate prominent dopaminergic brain regions involved in cognitive, affective and motor information processing. Own-happy>Unknown-happy contrast (green shade boxes) and Own>Unknown contrast (all affect states combined; yellow boxes). Abbreviations (top to bottom). Green labels: MD Th, medial dorsal thalamus; Pre-DPu, pre-commissural dorsal putamen; Post-Pu, post-commissural putamen; SN, substantia nigra. Yellow labels: mPFC, medial prefrontal cortex; ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; PMA, primary motor area; VA Th, ventral anterior thalamus; VST, ventral striatum; Pre-DCa, pre-commissural dorsal caudate; VTA, ventral tegmental area. OH, Own-happy baby faces; UH, Unknown-happy baby faces.
In responding to infant social cues, whether positive or negative, mothers need to integrate both affective and cognitive information about their baby, and evaluate competing demands, before choosing the most appropriate behavioral response (52;53). For example, a distressed baby usually evokes an empathic emotional response from a mother, as well as cognitive processes to determine, based on past experience and knowledge, possible causes and remedies for her baby’s distress. Likewise, a smiling baby face usually leads to positive affective arousal in a mother, associations with other rewarding experiences and contingent behavioral responses, such as smiling, caressing or playing.
The difference in striatal and midbrain responses seen in this study between happy, neutral and sad affect (Table 3; Figures 4 and 5) is consistent with other studies which show preferential activation for more appetitive or rewarding stimuli (54), including faces rated as more beautiful (25) or monetary reward (55). Non-human primate studies have shown that the firing rate of dopaminergic neurons is increased in response to “positive prediction errors”, meaning unexpected natural or conditioned rewards (56). Perhaps a mother’s own baby’s unexpected smile, for example, may activate dopamine circuits via a similar mechanism. In rat dams, extracellular dopamine release in the ventral striatum is associated with an increase in maternal behaviors, with the dopamine signal preceding the onset of the behavior (57). Although functional MRI only measures blood-oxygen level dependent changes in brain activity, together these studies suggest that positive sensory cues from infants, such as a smiling facial expression, may stimulate dopamine release in the striatum, and promote responsive maternal care.
In this population of mothers, own-happy baby faces tended to activate associative and motor regions of the striatum, rather than the more affect-related regions of the ventral striatum and the VTA (51) (Figure 6). However, these regions were activated when all affect groups were combined in the O>U contrast (Table 2). Given that the OH>UH contrast used only one-third the number of images used in the O>U contrast (20 vs. 60), this may simply reflect insufficient statistical power. In fact, when statistical thresholds were lowered in the OH>UH contrast, a similar activation pattern was seen (data not shown). However, further research will explore whether this pattern varies with maternal characteristics, such as adult attachment classification, where affective and cognitive brain responses have been hypothesized to be key distinguishing features (52).
The fact that the mothers did not have a stronger response to their own baby’s crying face, compared to an unknown baby’s, was also surprising. It appears that, at least in this sample of mothers, the brain responds equally to own and unknown baby faces in distress. This was evident from the contrast between sad faces and baseline, which revealed widespread activation in response to both own and unknown sad baby faces, though with a similar pattern for each. Thus, in the contrast between OS and US, no significant activation remained. However, it is possible that differences in timing of the two conditions could have biased the results, with earlier images expected to produce a stronger hemodynamic response. Although the timing difference between own-sad and unknown-sad images was not statistically significant (t= -1.69, P=0.13), own-sad images were seen somewhat earlier than unknown-sad images. This would, however, have biased the results in favor of own-sad images, rather than unknown-sad. Another possible explanation is that individual mothers respond differently to their own baby’s sad face, some feeling distress themselves, others inhibiting their own negative affect. Future work looking at adult attachment strategies may reveal important individual differences in maternal brain response to sad infant affect.
One limitation of this study is that the mothers were scanned at varying times post-partum (between 7 and 17 months), viewing baby faces ranging from 5 to 10 months in age (Table 1). Although there is no published fMRI data on the question, mothers may respond differently to their infant at differing ages, which may have influenced our results. Also, some key maternal brain regions identified in animal studies, such as the medial preoptic area (10) and ventral bed nucleus of the stria terminalis, were not activated in this study. However, other fMRI studies have only demonstrated activation of these areas in mothers of younger babies, during the first few months of life (18, 24), suggesting that these regions may be more important during the early postpartum period.
While individual variation seen within this population (such as breastfeeding duration, mother-infant separation, and psychopathology risk) is another limitation in interpreting study findings, it also presents an opportunity for further research into the significance of these individual differences. In addition to understanding how prior experience may influence maternal brain responses, the present paradigm might also enable investigators to explore how these response patterns relate to current maternal behavior. For example, the difference in response between own and unknown happy faces in these dopamine-associated regions may be an index of the reward value or salience of the infant’s face to the mother, which may in turn relate to maternal sensitivity or child neglect. This may further our understanding of brain processes that mediate the effect of prior experience on current maternal behavior.
Individual differences in affective and cognitive brain responses are fascinating topics for ongoing and future research. In some mothers, for example, a crying baby may trigger an angry response, or even physical abuse (58), rather than empathic caregiving. Likewise, in cases of maternal depression (11) or substance abuse (12), a smiling face may repeatedly fail to illicit positive caregiving. Depressed individuals show a decreased emotional response to happy faces, decreased accuracy in recognizing facial expressions, and increased memory for negative faces (59). Cocaine, a common drug of abuse among child-bearing-age women, and which activates both mesocorticolimbic and nigrostriatal dopamine systems (60–62), appears to compete with natural infant-related reward signals (63). This may relate to relatively high rates of child neglect in cocaine exposed mothers (64).
Important questions which are currently being examined include: What are the effects of maternal depression or substance abuse on brain responses to infant cues? How do brain responses predict differences in maternal sensitivity or attachment? What effect may these response differences have on a child’s subsequent development or attachment security?
How a mother responds to her infant’s behavioral cues may have an important role in shaping future child development. This study takes us one step closer to understanding the underlying brain processes and pathways involved in this important dyadic relationship.
Acknowledgments
This research was supported by NIH Grant K23 HD43097 (L.S.), GCRC MO1RR00188 (L.S.), Baylor CHRC: Pediatrics Mentored Research Program K12 HD41648 (L.S.), Kane Family Foundation (P.R.M.), NINDS grant NS-045790 (P.R.M.) and NIDA grant DA-11723 (P.R.M.). We would like to thank Laura Lomax-Bream for advice on study design and Jonathan Wrathall for assistance with conducting the experiments and analyzing data.
Sources of Support: NIH Grant K23 HD43097 (L.S.), GCRC MO1RR00188 (L.S.), Baylor CHRC: Pediatrics Mentored Research Program K12 HD41648 (L.S.), Kane Family Foundation (P.R.M.), NINDS grant NS-045790 (P.R.M.) and NIDA grant DA-11723 (P.R.M.).
References
- 1.Kaitz M, Good A, Rokem AM, Eidelman AI. Mothers' and fathers' recognition of their newborns' photographs during the postpartum period. J Dev Behav Pediatr. 1988 Aug;9(4):223–6. [PubMed] [Google Scholar]
- 2.Kaitz M, Rokem AM, Eidelman AI. Infants' face-recognition by primiparous and multiparous women. Percept Mot Skills. 1988 Oct;67(2):495–502. doi: 10.2466/pms.1988.67.2.495. [DOI] [PubMed] [Google Scholar]
- 3.Green JA, Gustafson GE. Individual recognition of human infants on the basis of cries alone. Dev Psychobiol. 1983 Nov;16(6):485–93. doi: 10.1002/dev.420160604. [DOI] [PubMed] [Google Scholar]
- 4.Kaitz M, Good A, Rokem AM, Eidelman AI. Mothers' recognition of their newborns by olfactory cues. Dev Psychobiol. 1987 Nov;20(6):587–91. doi: 10.1002/dev.420200604. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000 Aug;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 6.Weaver IC, Grant RJ, Meaney MJ. Maternal behavior regulates long-term hippocampal expression of BAX and apoptosis in the offspring. J Neurochem. 2002 Aug;82(4):998–1002. doi: 10.1046/j.1471-4159.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- 7.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999 Nov 5;286(5442):1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 8.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004 Aug;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 10.Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal Care Associated with Methylation of the Estrogen Receptor-alpha 1b Promoter and Estrogen Receptor-alpha Expression in the Medial Preoptic Area of Female Offspring. Endocrinology. 2006 Jun 1;147(6):2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 11.NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Dev Psychol. 1999 Sep;35(5):1297–310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- 12.Mayes LC, Feldman R, Granger RH, Haynes OM, Bornstein MH, Schottenfeld R. The effects of polydrug use with and without cocaine on mother-infant interaction at 3 and 6 months. Infant Behavior and Development. 1997;20(4):489–502. [Google Scholar]
- 13.NICHD Early Child Care Research Network. Child care and mother-child interaction in the first 3 years of life. Dev Psychol. 1999 Nov;35(6):1399–413. [PubMed] [Google Scholar]
- 14.Early Child Care Research Network NICHD. Does amount of time spent in child care predict socioemotional adjustment during the transition to kindergarten? Child Dev. 2003 Jul;74(4):976–1005. doi: 10.1111/1467-8624.00582. [DOI] [PubMed] [Google Scholar]
- 15.Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992 Mar;33(3):543–61. doi: 10.1111/j.1469-7610.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 16.Grienenberger JF, Kelly K, Slade A. Maternal reflective functioning, mother-infant affective communication, and infant attachment: exploring the link between mental states and observed caregiving behavior in the intergenerational transmission of attachment. Attach Hum Dev. 2005 Sep;7(3):299–311. doi: 10.1080/14616730500245963. [DOI] [PubMed] [Google Scholar]
- 17.Bowlby J. Attachment. 2. Vol. 1. London: Hogarth Press; 1969. Attachment and loss. [Google Scholar]
- 18.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002 Mar 15;51(6):431–45. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 19.Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003 Dec 15;54(12):1367–75. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 20.Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004 Aug 6;15(11):1825–9. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- 21.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004 Feb;21(2):583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004 Mar;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004 Aug 15;56(4):225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol & Psychiat. 2007;48(34):262–87. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001 Nov 8;32(3):537–51. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 26.Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002 May;125(Pt 5):1014–23. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006 May 1;30(4):1441–8. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, et al. Dopamine Modulates the Response of the Human Amygdala: A Study in Parkinson's Disease. J Neurosci. 2002 Oct 15;22(20):9099–103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Floresco SB, Tse MT. Dopaminergic Regulation of Inhibitory and Excitatory Transmission in the Basolateral Amygdala-Prefrontal Cortical Pathway. J Neurosci. 2007 Feb 21;27(8):2045–57. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- 31.Strathearn L, Li J, Montague PR. An fMRI study of maternal mentalization: Having the baby's mind in mind. NeuroImage. 2005;26(Supp 1):S25. [Google Scholar]
- 32.van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001 Dec;14(6):1302–8. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 34.Cole PM, Barrett KC, Zahn-Waxler C. Emotion displays in two-year-olds during mishaps. Child Dev. 1992 Apr;63(2):314–24. [PubMed] [Google Scholar]
- 35.Slade A. Parental reflective functioning: an introduction. Attach Hum Dev. 2005 Sep;7(3):269–81. doi: 10.1080/14616730500245906. [DOI] [PubMed] [Google Scholar]
- 36.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science. 2004 Oct 15;306(5695):503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 37.Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994 Mar;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 39.Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 40.Ainsworth MD, Bell SM. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970 Mar;41(1):49–67. [PubMed] [Google Scholar]
- 41.Goebel R. BrainVoyager [computer program]. Versions 1.7.9 to 1.9. Maastricht, The Netherlands: Brain Innovation; 2006. [Google Scholar]
- 42.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006 May;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998 Jan;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 44.Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. NeuroImage. 2002 Apr;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 45.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000 Jul;10(3):120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. 2. San Diego, CA: Elsevier; 2004. [Google Scholar]
- 47.Goebel R. BrainVoyager Brain Tutor [computer program]. Version 2.0. Maastricht, The Netherlands: Brain Innovation; 2007. [Google Scholar]
- 48.U.S. Census Bureau [homepage on the internet] State and County QuickFacts; Washington DC: [c2000 [cited 2007 April]]. Available from: http://quickfacts.census.gov/qfd/states/48/4835000.html. [Google Scholar]
- 49.Hollingshead AB. Four Factor Index of Social Status: Working Paper. 1985. Unpublished Work. [Google Scholar]
- 50.Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat Neurosci. 2006 Sep;9(9):1177–85. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- 51.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. J Neurosci. 2000 Mar 15;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crittenden PM, Claussen AH. The Organization of Attachment Relationships : Maturation, Culture, and Context. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 53.Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006 May 15;31(1):468–75. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 54.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996 Feb 1;379(6564):449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 55.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the Hemodynamic Responses to Reward and Punishment in the Striatum. J Neurophysiol. 2000 Dec 1;84(6):3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 56.Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997 Mar 14;275(5306):1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 57.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004 Apr 28;24(17):4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milner JS, Halsey LB, Fultz J. Empathic responsiveness and affective reactivity to infant stimuli in high- and low-risk for physical child abuse mothers. Child Abuse & Neglect. 1995 Jun;19(6):767–80. doi: 10.1016/0145-2134(95)00035-7. [DOI] [PubMed] [Google Scholar]
- 59.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006 Jan;19(1):34–9. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 60.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997 Sep;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 61.Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup Suckling Is More Rewarding Than Cocaine: Evidence from Functional Magnetic Resonance Imaging and Three-Dimensional Computational Analysis. J Neurosci. 2005 Jan 5;25(1):149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005 Dec;28(4):904–14. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 63.Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135(2):315–28. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasserman DR, Leventhal JM. Maltreatment of children born to cocaine-dependent mothers. Am J Dis Child. 1993 Dec;147(12):1324–8. doi: 10.1001/archpedi.1993.02160360066021. [DOI] [PubMed] [Google Scholar]