Abstract
During postnatal development, the dendrites of spinal motor neurons are refined in an activity-dependent manner that can be influenced by blocking activation of N-Methyl-d-Aspartate (NMDA) receptors. In late postnatal life, dendritic refinement ceases, and dendrite architecture is unaffected by NMDA antagonists; however the molecular substrate for limiting dendritic plasticity is not understood. During late postnatal development, expression of the NR3B NMDA receptor subunit, a putative dominant-negative subunit that reduces glutamate-induced ionic currents, is upregulated within motor neurons. To investigate whether increasing NR3B expression may contribute to the loss in late development of activity-dependent dendritic reorganization in the spinal cord, we over-expressed NR3B in cultured spinal motor neurons, and compared its effects on dendrite morphology with the effects of pharmacological blockade of NMDA receptors. We found that over-expression of the NR3B receptor subunit increased the length and complexity of dendritic arbor, and increased numbers of dendritic filopodia, suggesting that NR3B promotes the addition of branch segments in developing motor neurons. In contrast, blockade of NMDA receptor activity by the NMDA antagonist AP5 had little effect on the overall length or complexity of dendritic arbor. Instead, treatment with AP5 resulted in significant reorganization of dendritic arbor in a manner that favored addition of dendritic segments of high branch orders, at the expense of those closer to the cell body. These results suggest that expression of the NR3B subunit may participate in activity-dependent reorganization of dendritic architecture, but via a mechanism that may be inconsistent with loss of NMDA receptor activity.
Keywords: motoneuron, dendrite branching, filopodia, activity-dependent development, AP5, dominant-negative
During a restricted period of postnatal development, the dendrites of spinal cord motor neurons undergo dramatic morphological refinement in an activity-dependent manner (Kalb, 1994; Inglis et al., 2000); however the molecular mechanisms that govern these processes remain poorly understood. In common with several other neuronal systems (Cramer and Sur, 1995; Katz and Shatz, 1996; Cline, 2001; Wong and Ghosh, 2002), activity at the N methyl-d-Aspartate (NMDA) type of glutamate receptor is a key determinant of the mature morphology of motor neurons, via a process which likely favors active, synchronously firing synaptic elements at the expense of those that fire asynchronously or are inactive. Consistent with this hypothesis, blockade of NMDA receptor activity in the spinal cord during the postnatal developmental period results in a loss of volume and complexity of dendritic arbor (Kalb, 1994; Inglis et al., 1998). However, in late postnatal life, refinement of dendritic architecture ceases to be sensitive to NMDA receptor blockade (Kalb, 1994); the mechanism that restricts activity-dependent refinement at later time-points has yet to be elucidated.
Expression levels of NMDA receptor subunits in the spinal cord are substantially regulated during development (Watanabe et al., 1994; Stegenga and Kalb, 2001; Brown et al., 2002; Oshima et al., 2002), and regulation of subunit expression has direct implications for the properties of NMDA receptors. Functional NMDA receptors are composed of an NR1 subunit and at least one NR2 (NR2A-D) subunit, or a combination of NR1, NR2 and NR3 (NR3A or B) subunits (Hollmann and Heinemann, 1994; Ciabarra et al., 1995; Sucher et al., 1995; Dingledine et al., 1999; Nishi et al., 2001). In the spinal cord, mRNAs for all NR2 subunits and for NR3A are downregulated to varying degrees over the embryonic and early postnatal periods of development (Watanabe et al., 1994; Stegenga and Kalb, 2001; Fukaya et al., 2005), consistent with lower NMDA receptor-mediated synaptic currents (Feldmeyer and Cull-Candy, 1996; Cull-Candy and Leszkiewicz, 2004), and coincident with the emergence of mature electrophysiological characteristics (Walton and Navarrette, 1991).
In contrast, mRNA for the NR3B subunit, localized predominantly within motor neurons (Nishi et al., 2001; Chatterton et al., 2002; Matsuda et al., 2003), is first detected during the second postnatal week (Fukaya et al., 2005), and is upregulated thereafter, attaining maximal levels at postnatal day 21. This expression profile is the inverse of NR2 subunit expression, and high levels of NR3B mRNA coincide with cessation of activity-dependent refinement of motor neuron dendrites (Kalb, 1994). Consistent with its putative role as a dominant-negative subunit (Nishi et al., 2001), co-expression of NR3B with NR1 and NR2 as been shown to reduce NMDA receptor currents (Chatterton et al., 2002), calcium permeability (Matsuda et al., 2002), and whole-cell currents (Nishi et al., 2001). This developmental switch in the NMDA receptor phenotype, therefore, may contribute to the mechanism through which NMDA receptor activity is diminished, and activity-dependent reorganization of motor neuron dendritic arbor is restricted.
To investigate whether NR3B expression could be responsible for limiting aspects of dendrite outgrowth and refinement, we performed a quantitative morphological study of motor neuron dendrites following expression of NR3B cDNA in mammalian motor neurons growing in mixed embryonic spinal cord cultures. The use of these cultures enabled us to image discrete dendritic elements such as filopodia, which may be predictive of dendritic branching, but which are not easily distinguished in motor neurons via other means of imaging. To determine whether the effects of NR3B on dendrite morphology are likely to be due to a reduction in NMDA receptor activity, we compared the results of NR3B expression with pharmacological blockade of NMDA receptors with the competitive NMDA receptor antagonist, DL-2-amino-5-phosphonovalerate (AP5).
EXPERIMENTAL PROCEDURES
Animals
Timed pregnant and adult Sprague Dawley rats were obtained from Charles River laboratories and maintained with continuous access to food and water in a 12/12 hour light/dark cycle. All animal protocols followed were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Tulane University Institutional Animal Care and Use Committee.
Protein extraction and Western Blotting
To determine whether NR3B is developmentally regulated in the rat spinal cord, tissue was isolated from animals at various ages and NR3B detected using Western Blot. Whole spinal cord tissue was collected at embryonic day 16 (E16), postnatal days 1, 7, 14, 21, 28 (P1, P7, P14, P21, P28) and from adults, and homogenized in lysis buffer (0.5 M HEPES, 3 M NaCl, 1 M MgCl2, 0.5 M EDTA, 0.1 M DTT, 10% SDS, 10% deoxycholate, 0.3% TritonX-100 and 2% protease inhibitor cocktail). Cell lysates were then centrifuged for 30 minutes at 13000 rpm at 4°C. Supernatents were maintained at −80°C till use. Protein concentration was estimated using the Lowry test (DC protein Assay kit, BioRad, Hercules, CA). In each lane of a SDS-polyacrylamide gel (4.5% stacking, 10% separating), 20µg of protein was loaded and resolved by SDS-PAGE. Proteins were then electrophoretically transferred onto a 0.2µm polyvinyl difluoride membrane. The membrane was blocked using tris-buffered saline with 0.1% Tween-20 and 5% non-fat dry milk and incubated overnight at 4°C with a primary antibody against NR3B (rabbit polyclonal, Upstate Biotechnology, Lake Placid, NY; 1:1000 in 1% Normal goat serum (NGS), Invitrogen, Carlsbad, CA). The membrane was then incubated for 1 hour at room temperature with an alkaline phosphatase-conjugated secondary antibody (goat anti-rabbit, 1:2000 in tris-buffered saline; Vector Laboratories, Burlingame, CA). Signals were visualized using the Immun-Star AP Chemiluminescence substrate (BioRad, Hercules, CA), apposed to Amersham Hyperfilm ECL (GE Healthcare, Piscataway, NJ). The intensity of NR3B expression bands were quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA). For these measurements, a pixel intensity threshold was set to exclude unlabeled parts of the gel. Pixel intensity and area of each band was obtained using the “show region statistics” function of MetaMorph, and the total intensity (pixel · cm2) was determined by the product of pixel intensity and area of each band (Schridde et al., 2006). Relative band intensities were calculated as percent maximal expression, with the band displaying the highest total pixel intensity set as 100% expression.
Mixed Spinal Cord Cultures
Mixed spinal cord cultures were prepared from embryonic day 16 (E16) rat embryos as described previously (Prithviraj et al., 2008). Briefly, spinal cords were dissected, and dissociated by treatment with trypsin (0.25%) for 15 minutes at 37°C, followed by trituration through three Pasteur pipettes of decreasing bore size. Cells were then resuspended in culture media consisting of MEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (10,000U/ml), 2mM glutamine and 20mM glucose and plated on glass bottom culture dishes (12mm diameter glass bottom; MatTek Corp., Ashland, MA) coated with poly-d-lysine (100µg/ml) and laminin (10µg/ml). Poly-d-lysine was obtained from Sigma-Aldrich (St. Louis, MO) while all other tissue culture reagents were obtained from Invitrogen (Carlsbad, CA).
Transfection of Cultured Neurons
Cultured cells were transfected with CMV-driven expression plasmids containing cDNA sequences of NR3B or green fluorescent protein (GFP) using a lipid-based transfection protocol (Lipofectamine 2000, Invitrogen, Carlsbad, CA) described previously (Prithviraj et al., 2008). Briefly, on the sixth day in vitro (DIV6) cells were transfected with 100ng of NR3B cDNA and co-transfected with 50ng of GFP cDNA for visualization purposes. Control cultures were transfected with 50ng of GFP alone.
In a second series of experiments, neuronal cultures were treated with the competitive NMDA receptor antagonist AP5 (Sigma, St. Louis, MO). In these experiments, DIV6 cultures were transfected with 50ng of GFP for visualization purposes, and then treated with a 100µM solution of AP5 immediately following the end of transfection. AP5 remained in the culture dish until the neurons were imaged. Control cultures were transfected with 50ng of GFP, but received no drug treatment.
Imaging of Cultured Neurons
GFP-expressing motor neurons were imaged twenty-four hours post transfection using an inverted microscope (Zeiss Axiovert, 200M) with fluorescence optics. Prior to imaging, culture media in the dishes was replaced with warmed, colorless, buffered external media (Robert et al., 2000), and allowed to equilibrate to 37°C. Dishes were maintained at 37°C using plate and objective heaters during the imaging procedures. Motor neurons in culture were readily distinguished from other neurons by the size of their cell body and the presence of a long axon and extensive dendritic trees (Carriedo et al., 1996; Vandenberghe et al., 2000; Zona et al., 2006; Prithviraj et al., 2008). However, motor neuron identity was confirmed post-imaging in a series of neurons with immunocytochemistry for SMI-32, a marker for non-phosphorylated neurofilaments, present in abundance in the axons and dendrites of motor neurons (Carriedo et al., 1996; Prithviraj et al., 2008). Within this group, all motor neurons selected on the basis of morphology were subsequently found to be positive for SMI-32 (Figure 1). For quantitative measurements, five to ten GFP-expressing motor neurons were chosen from each dish, at random. Time-lapse imaging was carried out using the Zeiss Axiovision image capture software with the Zeiss Axiocam. For each neuron, images were taken once every 5 seconds for a period of 2 minutes (25 frames total). Neurons showed no signs of injury such as blebbing or necrosis during the imaging procedure.
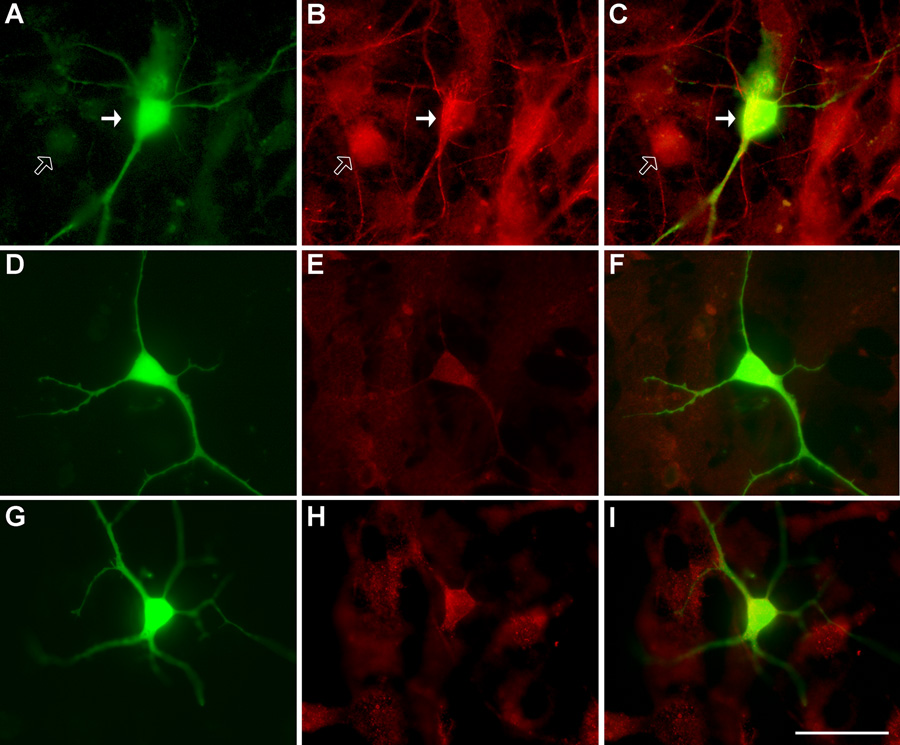
Figure 1. SMI-32 and NR3B imunoreactivity in transfected spinal motor neurons.
(A) representative image of a motor neuron transfected with GFP, (B), SMI-32 immunoreactivity, and (C), overlaid images. Filled arrows point to a transfected, SMI-32 positive neuron while the unfilled arrow points to a neighbouring, non-tranfected neuron, negative for SMI-32 immunoreactivity. (D), GFP-transfected control neuron, (E), NR3B immunoreactivity of control neuron in (D), and (F), overlaid image. (G), representative image of a neuron transfected with GFP and co-transfected with NR3B, (H), NR3B immunoreactivity of neuron in G, and (I), overlaid image. Note that control motor neurons display NR3B immunoreactivity, indicative of endogenous NR3B. Neurons transfected with NR3B show increased NR3B immunoreactivity, approximately double that of non-transfected surrounding cells. Scale bar represents 50µm.
Quantitative Analyses of Dendrite Morphology
To provide a comprehensive measure of dendrite complexity and length, we employed the following parameters of dendrite morphology: number of primary dendrites; total amount of dendritic arbor; number of branch points and branch tips per neuron. These parameters have previously been used to provide a comprehensive overview of dendrite architecture (Inglis et al., 2002; Prithviraj et al., 2008). To determine whether our treatment had any effects on the distribution of arbor within specific portions of the dendritic tree, for example in newer as opposed to older dendrite branches, we also analyzed imaged neurons according to their branch order (Prithviraj et al., 2008). For these analyses, any dendrite emerging directly out of the cell body was considered to be a first-order branch; these bifurcate to give rise to second-order branches and so on. Three dimensional image stacks collected during time-lapse imaging procedures were analyzed using either digitized Camera Lucida software (Neurolucida, MBF Bioscience, Williston, VT), or Scion Image (Scion, Frederick, MD).
Immunocytochemistry
After imaging, motor neuron identity was confirmed immunocytochemically as described previously (Prithviraj et al., 2008). Briefly, following transfection and imaging, dishes were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 minutes and then washed in PBS for an additional 30 minutes. Cells were subsequently incubated overnight at room temperature with a primary antibody against SMI-32 (mouse monoclonal, Lot # 14813301, Sternberger Monoclonals, Lutherville, MD; 1:1000 dilution in 1% NGS with 0.1% TritonX-100). Cells were rinsed for 30 minutes with PBS and incubated at room temperature for one hour in a rhodamine-TRITC conjugated secondary antibody (goat anti-mouse, Lot # 0612048666, Jackson, Woodgrove, PA; 1:200 in 1% NGS).
Immunocytochemical methods were also used to estimate the relative levels of NR1, NR2A and NR3B following transfection. For these experiments, culture dishes were fixed and washed as above, and incubated overnight, at 4°C, with primary antibody against NR1 (mouse monoclonal, Lot # 386049, Abcam, Cambridge, MA; 1:300), NR2A (rabbit polyclonal, Lot # 0512018469, Chemicon, Temecula, CA; 1:200) or NR3B (rabbit polyclonal, Lot # 22395, Upstate Biotechnology, Lake Placid, NY; 1:500) in 1% NGS with 0.1% TritonX-100. Cells were then rinsed for 30 minutes with PBS, followed by addition of a rhodamine-TRITC conjugated secondary antibody (goat anti-mouse, Lot # 25040642, Chemicon, Temecula, CA; 1:200) or goat anti-rabbit, Lot # 36590A, Invitrogen, Carlsbad, CA; 1:200) in 1% NGS for one hour at room temperature. Following incubation with the secondary antibody, all cells were rinsed in PBS and coverslipped, and immunoreactivity was detected by fluorescence microscopy.
The percentage increase in NR3B expression in transfected cells as compared to non-transfected cells imaged from the same coverslip was estimated using Metamorph (Molecular Devices, Sunnyvale, CA). For these measurements, fluorescence intensities (gray levels) were measured in the cell bodies of five transfected cells and compared to those of non-transfected cells in the same coverslip.
Statistical Analyses
All statistical analyses were performed using Statview (version 5.0; SAS Inc). For analyses of dendrite parameters, ANOVA was used for statistical comparisons of experimental groups, with Scheffé post-hoc test for pairwise comparisons between groups. For analyses of dendrites by branch order, treatment groups were compared using Repeated Measures ANOVA, with Scheffé post-hoc test. Probabilities were estimated using the exact F statistic and degrees of freedom. Fluorescence intensity analyses of NR3B in control and NR3B-transfected cells were compared statistically using an unpaired, two-tailed Student’s t test. For all statistical tests, significance was set at p<0.05.
RESULTS
Effects of transgene expression on NMDA immunoreactivity
To estimate the expression levels of NR3B expression following transfection, gray level pixel intensities (0–255) of immunofluorescence were measured in transfected and non-transfected cells were measured using MetaMorph (Molecular Devices, Sunnyvale, CA). Cells transfected with NR3B exhibited an average pixel intensity of 157 ± 13 while non-transfected cells had an average intensity of 77 ± 10. This increase was significant (Figure 1; t = 4.943; p = 0.0011), and is of a similar magnitude to our previous study in which transfected glutamate receptor subunits are expressed at about twice that of non-transfected, neighboring neurons (Prithviraj et al., 2008). Over-expression of NR3B produced a small, but insignificant increase in immunofluorescence of NR1 and NR2A. For NR1, the average pixel intensity was 101 ± 4.6 before transfection, and 110± 4.9 after transfection of NR3B. Similarly, for NR2A, average pixel intensites measured before and after transfection were 101 ± 4.6 and 110± 4.9 respectively (Supplementary figure 1). These results suggest that, in agreement with a previous study (Nishi et al., 2001), NR3B over-expression has little effect on the expression rates of other NMDA receptor subunits in cultured neurons.
Developmental regulation of NR3B protein in the rat spinal cord
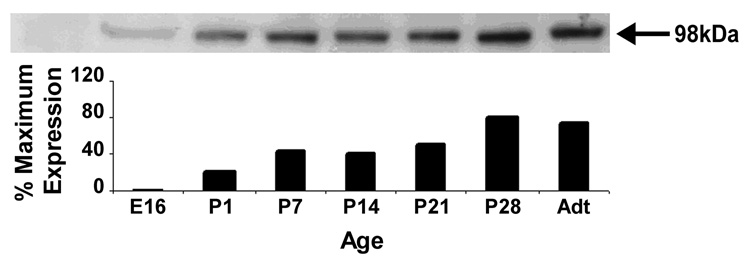
To determine the developmental expression of NR3B protein, Western blot analysis was performed on tissue from rat spinal cords isolated at various developmental stages from embryonic day E16 to adult (Figure 2). Expression of NR3B at E16 was low, but detectable. Expression rose markedly by postnatal day 1 (P1), and thereafter increased gradually, reaching maximal levels at P28. Our results are in agreement with a previous study which reported substantial upregulation of NR3B mRNA in developing motor neurons during the postnatal period (Fukaya et al., 2005).
Figure 2.
Top: Western blot analysis of developmental expression of NR3B in the spinal cord, collected from rats at E16, P1, P7, P14, P21, P28, and adults (Adt). Arrow points to the 98kDa band representing NR3B protein. Note that NR3B was present at all ages analyzed, but was increased over the course of postnatal development. Bottom: bar graph illustrating relative intensities of each band of the blot depicted above. Data are expressed as a percentage of the maximal band intensity (pixel · cm2), which was observed to occur P28.
Effects of over-expression of NR3B on motor neuron dendrite morphology
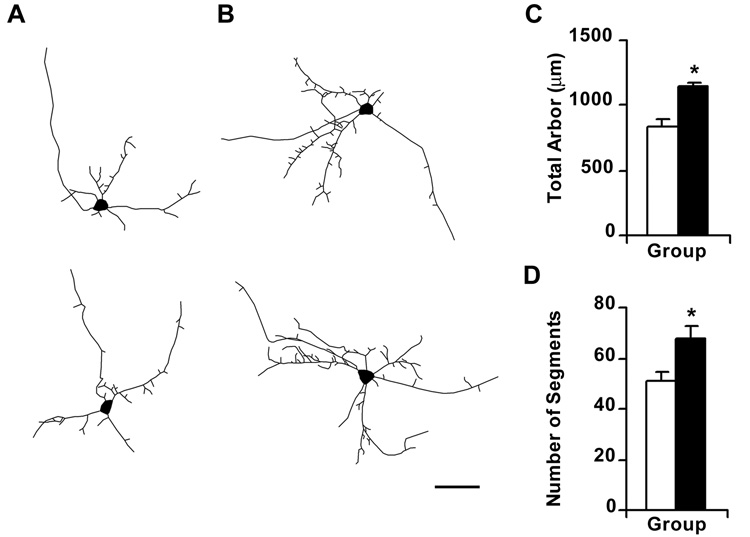
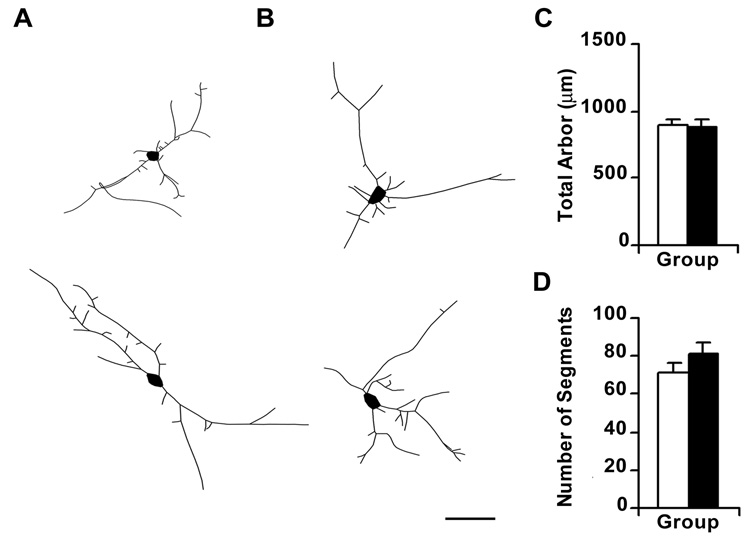
Approximately 24 hours after transfection, neurons in which NR3B was overexpressed had a significantly greater amount of dendritic arbor (Table 1; Figure 3), compared to controls expressing GFP alone (F1,59 = 18.216; p < 0.0001), but the number of primary dendrites was similar between treatment groups (F1, 59 = 0.1; p = 0.7526; Scheffé post-hoc test). These results suggest that NR3B expression does not promote the emergence of new dendritic trees but limits its effects to dendritic trees that are already established. NR3B expression also increased the complexity of dendrites, as indicated by a significant increase in the number of dendritic segments (F1,59 = 8.541; p = 0.0049) (Figure 3) and branch tips per neuron (F1,59 = 8.513; p = 0.0050). In contrast, there was no significant difference in the average length of dendritic segments following NR3B expression (F1,59 = 0.887; p = 0.3502) suggesting that additional branch segments were likely to attain a normal length.
Table 1.
Effects of NR3B expression on key architectural parameters of motor neuron dendrites
| Neuron Parameter | GFP | NR3B | F(1,59) ; p |
|---|---|---|---|
| Total dendrite arbor per cell (µm) | 834 ± 38 | 1142 ± 61* | F = 18.22; p < 0.0001 |
| Average length of segment (µm) | 17.8 ± 0.1 | 16.5 ± 0.8 | F = 0.887; p = 0.3502 |
| Primary dendrites | 5.8 ± 0.3 | 6.0 ± 0. 3 | F = 0.100; p = 0.7526 |
| Branch points | 22.6 ± 1.7 | 30.9 ± 2.3* | F = 8.478; p = 0.0051 |
| Branch tips | 28.4 ± 1.8 | 36.9 ± 2.3* | F = 8.513; p = 0.0050 |
| Number of Filopodia | 28.7 ± 2.0 | 36.9 ± 2.6* | F = 6.301; p = 0.0148 |
Numbers represent mean ± SEM of GFP (n = 30) or NR3B (n=31) expressing neurons. F and p values were created with ANOVA.
Significant difference from GFP (Scheffé post hoc test).
Figure 3.
Over-expression of NR3B causes an increase in total amount and complexity of dendritic arbor in transfected neurons. Digitized camera lucida tracings of representative control neurons expressing GFP (panel A) and of neurons over-expressing NR3B and co-transfected with GFP (panel B). Scale bar represents 50µm. (C, D), Bar graphs representing changes in total dendritic arbor, and number of dendritic segments, respectively, following NR3B over-expression. Filled bars represent neurons over-expressing NR3B whereas unfilled bars represents GFP-transfected controls. Cells over-expressing NR3B showed significant increases in the total amount of dendritic arbor (F1,59 = 18.216; p < 0.0001) and the number of dendritic segments (F1,59 = 8.541; p = 0.0049), as compared to controls. * Represents a significant difference from GFP-expressing control neurons.
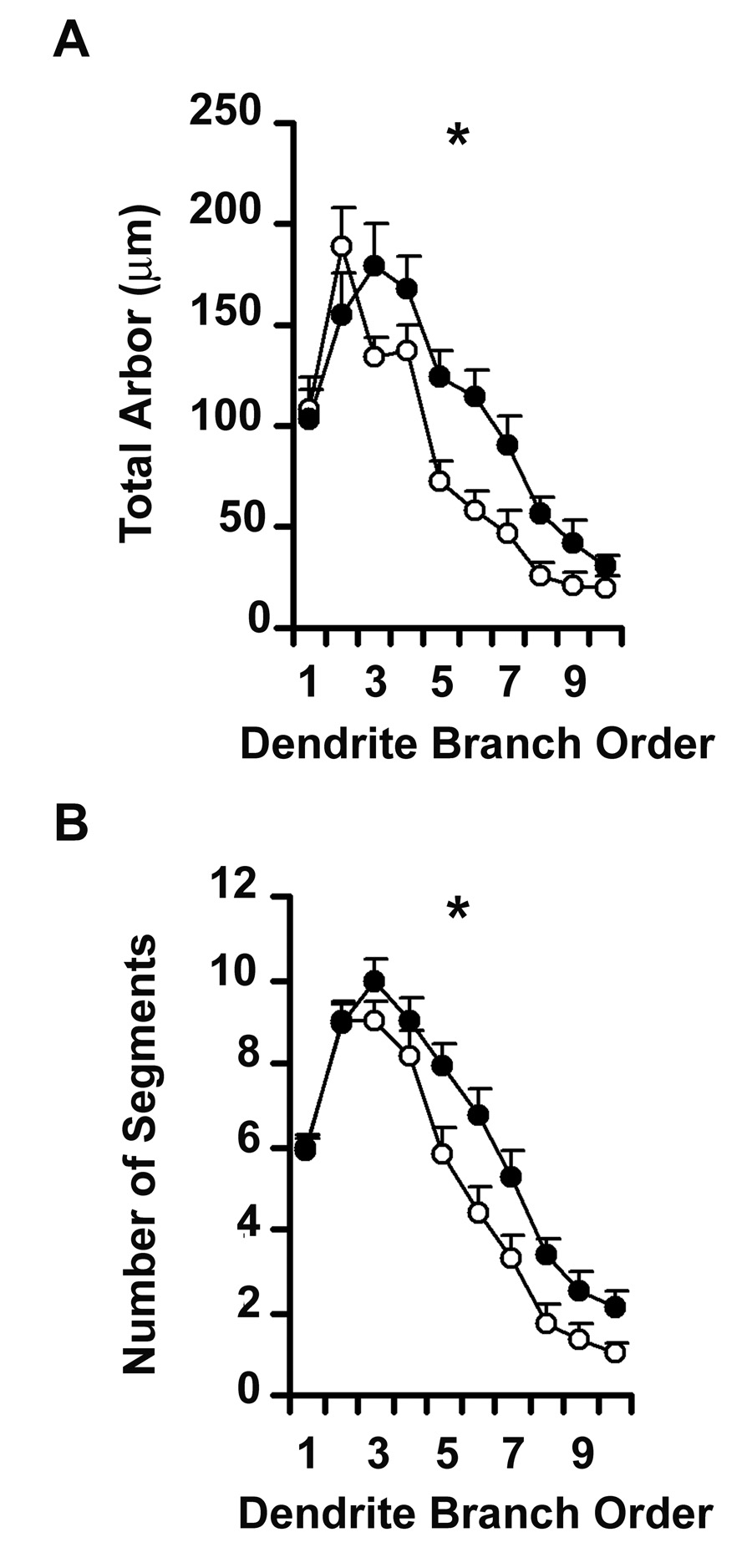
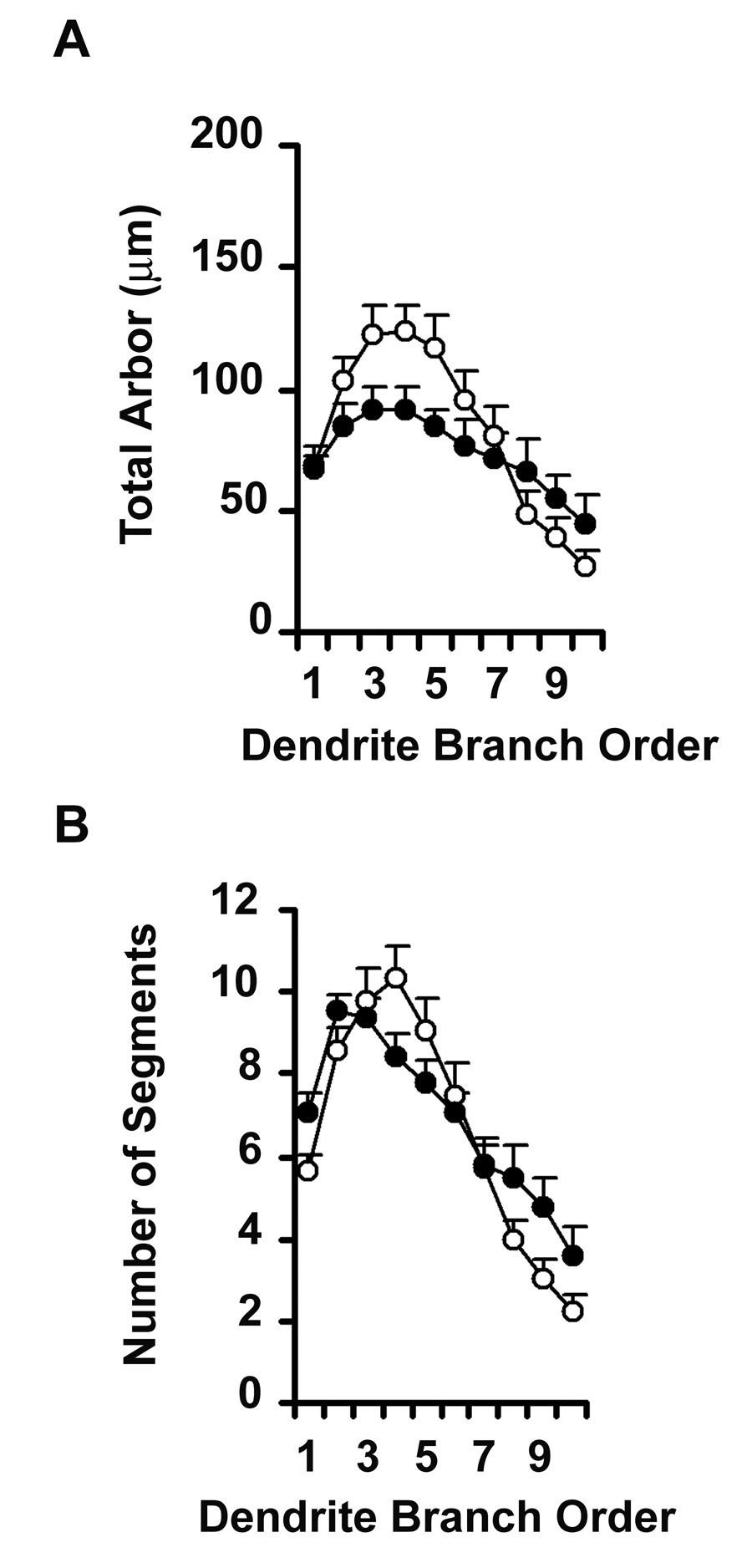
Since branch points appeared to be added to established dendritic trees, rather than through the emergence of new primary dendrites from the cell body, we examined the distribution of branch segments across the dendritic tree by analyzing segments according to their branch order (Figure 4). We confirmed that NR3B significantly increased the total amount of arbor across the cell (Repeated Measures ANOVA F1,59 = 16.108; p = 0.0002), but the effect was restricted to third order branches and higher (Group × Order, F9, 531 = 2. 336; p = 0.0138), indicating that the ability of NR3B to increase dendritic arbor is most likely limited to newer regions of dendrite. Repeated Measures ANOVA also confirmed the significant increase in the number of dendritic segments across the dendritic tree (F1, 59 = 7.499; p = 0.0081). There was a trend towards a significant group by order interaction (F9, 531 = 1.792; p = 0.0670), in favor of addition of higher order branch segments, consistent with the suggestion that the effect of NR3B tends to occur in newer regions of dendrite. The significant increase in total amount and complexity of dendrite arbor of neurons over-expressing the NR3B receptor subunit suggests that NMDA receptors containing NR3B may play an active role in establishment or maintenance of mature motor neuron dendrite architecture.
Figure 4.
Line graph representing NR3B mediated changes in dendrite morphology along the length of the dendritic tree, as a function of branch order. Unfilled circles represent GFP-transfected control neurons while filled circles represent neurons co-transfected with NR3B. Repeated Measures ANOVA revealed significant increases in (A) the total amount of dendritic arbor F1,59 = 16.108; p = 0.0002) and in (B) the number of dendritic segments (F1,59 = 7.499; p = 0.0081) of neurons transfected with NR3B relative to controls. * represent significant difference from GFP-transfected controls.
Effects of AP5 administration on motor neuron dendrite outgrowth and branching
To determine whether the effects of NR3B on dendrite morphology are likely to be due to a reduction in NMDA receptor activity, we examined the effects of pharmacological blockade of NMDA receptors on dendrite morphology with the competitive NMDA receptor antagonist, AP5. Compared to control cells, neurons treated with AP5 (100µM) displayed a small but significant increase in the number of primary dendrites per cell (Table 2; F1,49 = 5.208; p = 0.0269). However, there were no significant changes in the total amount of dendritic arbor (Figure 5; F1,49 = 0.034; p = 0.8545), number of dendritic segments (Figure 5; F1,49 = 1.620; p = 0.2091), or branch tips per neuron (F1,49 = 0.686; p = 0.4115). These effects are markedly different from those observed after NR3B expression, suggesting that NR3B does not mediate altered dendrite morphology simply via the reduction of NMDA receptor currents.
Table 2.
Effects of AP5 administration on key architectural parameters of motor neuron dendrites
| Neuron Parameter | GFP | AP5 | F(1,49) ; p |
|---|---|---|---|
| Total dendrite arbor per cell (µm) | 891 ± 51 | 876 ± 60 | F = 0.034; p = 0.8545 |
| Average length of segment (µm) | 13.5 ± 0.1 | 11.6 ± 0.6 | F = 2.964; p = 0.0914 |
| Primary dendrites | 5.6 ± 0.4 | 7.1 ± 0.4* | F = 5.208; p = 0.0269 |
| Branch points | 38.0 ± 2.5 | 41.1 ± 3.0 | F = 0.612; p = 0.4377 |
| Branch tips | 46.2 ± 3.0 | 49.9 ± 3.3 | F = 0.686; p = 0.4115 |
| Number of Filopodia | 7.3 ± 0.7 | 7.9 ± 1.4 | F = 0.162; p = 0.6895 |
Numbers represent mean ± SEM of GFP (n = 25) or GFP+AP5 (n=26) neurons. F and p values were created with ANOVA.
Significant difference from GFP (Scheffé post hoc test).
Figure 5. Administration of AP5 did not alter the amount or complexity of dendritic arbor of treated motor neurons.
(A, B), Digitized camera lucida tracings of representative control neurons expressing GFP (panel A) and of neurons expressing GFP and treated with a 100µM solution of AP5 (panel B). Scale bar represents 50µm. (C, D), Bar graph representing changes in total dendritic arbor (C) and number of dendritic segments (D) following AP5 administration. Filled bars represent neurons transfected with GFP and treated with AP5, whereas unfilled bars represent GFP-transfected controls neurons. The total amount of dendritic arbor (F1,49 = 0.034; p = 0.8545) and number of dendritic segments (F1,49 = 1.620; p = 0.2091) were unaltered by AP5 treatment, as compared to controls.
To investigate whether blockade of NMDA receptors may result in reorganization of arbor within a restricted region of the dendritic tree, in a manner that might be obscured by measuring total numbers of segments, we analyzed arbor according to the branch order. These analyses revealed that while AP5 did not change the total amount of arbor per cell (Repeated Measures ANOVA; F1,49 = 1.886; p = 0.1760), there was a significant change in the distribution of dendritic arbor length (Group × Order, F9,441 = 2.224; p = 0.0197) and in the number of dendritic segments (Group × Order, F9,441 = 3.650; p = 0.0002) across the dendritic tree (Figure 6). Thus, AP5 resulted in a reorganization of arbor across the dendritic tree, such that segments and arbor at lower dendritic orders were lost. These results suggest that blockade of NMDA receptor activity within the culture dish leads to elimination of some dendritic segments, specifically those older portions of the dendritic tree, while newer branch-points are spared.
Figure 6.
Line graph representing AP5 mediated changes in dendrite morphology along the length of the dendritic tree, as a function of branch order. Unfilled circles represent GFP-transfected control neurons while filled circles represent neurons treated with 100µM AP5. Repeated Measures ANOVA showed no significant differences in (A) the total amount of dendritic arbor (F1,49 = 1.886; p = 0.1760) or in (B) the number of dendritic segments (F1,49 = 0.251; p = 0.6186) of AP5 treated neurons, but revealed a significant change in the distribution of dendritic arbor (Group × Order, F9,441 = 2.224; p = 0.0197) and in the number of dendritic segments (Group × Order, F9,441 = 3.650; p = 0.0002) across the dendritic tree.
Effect of NR3B over-expression and AP5 administration on filopodial outgrowth in transfected motor neurons
Filopodia are highly dynamic structures that in motor neurons are likely to represent precursors to new branches (Vaughn et al., 1974; Ulfhake and Cullheim, 1988). Synapses formed on filopodia have been suggested to stabilize growth cones (Niell et al., 2004), permitting elongation of a dendritic branch segment (Vaughn et al., 1974). To determine whether regulation of NMDA receptor composition or activity regulates dendrite outgrowth via outgrowth of filopodia, we estimated the number of filopodia in neurons over-expressing NR3B or treated with AP5. Neurons transfected with NR3B were observed to have a significantly higher number of filopodia when compared to control neurons transfected with GFP alone (Table 1; F1,59=6.301; p=0.0148). These results suggest that NR3B facilitates dendritic outgrowth in motor neurons via the addition of new filopodia, that may in turn lead to branch segment addition. In contrast, neurons treated with AP5 showed no significant changes in the number of filopodia (Table 2; F1,49=0.162; p=0.6895), as compared to control GFP-expressing neurons. Thus, the effects of NR3B on dendrite outgrowth are distinct from those of NMDA receptor blockade.
DISCUSSION
The NR3B NMDA receptor subunit is reported to be expressed predominantly in motor neurons, where it is believed to function as a dominant-negative receptor subunit, that is, expression and incorporation of NR3B protein into NMDA receptors results in a reduction in cellular NMDA receptor activity (Nishi et al., 2001). In the present study we show that expression of the NR3B receptor subunit plays a role in determining the morphology of dendrites of spinal motor neurons. We find that NR3B protein expression is developmentally upregulated in the rat spinal cord, with the greatest degree of increase occurring between E16 and P1. Our results are consistent with previous studies employing in situ hybridization to measure developmental upregulation of NR3B mRNA in the ventral horn of the spinal cord (Fukaya et al., 2005), but suggest that upregulation occurs earlier than previously reported. Our ability to detect earlier NR3B expression may be due to the ability of Western blot techniques to detect small amounts of protein for which the mRNA may be below the detection limit of in situ hybridization. However, both studies are in agreement that NR3B is upregulated, and reaches its maximum level in the late postnatal or early adult period.
Modulation of NMDA receptor activity has been shown in many studies to have significant effects on dendrite outgrowth and refinement (Cramer and Sur, 1995; Katz and Shatz, 1996; McAllister, 2000; Cline, 2001; Wong and Ghosh, 2002); however the precise role for these receptors is unclear. Morphological changes in dendritic shape have been shown to correlate with NMDA receptor activity (Yuste and Bonhoeffer, 2001), and therefore downregulation of NMDA receptors during development would be expected to limit refinement of dendritic structures. In spinal motor neurons, application of NMDA receptor antagonists has been demonstrated to reduce outgrowth and branching of neurites (Cuppini et al., 1999). In addition, NMDA receptor antagonists administered to rodents during the postnatal period result in reduced complexity and volume of motor neuron dendrites (Kalb, 1994; Inglis et al., 1998; Hebbeler et al., 2002). By adulthood, NMDA receptor antagonists have no further effect on dendrite outgrowth (Kalb, 1994), suggesting that developmental regulation of NMDA receptor function, or the function of their downstream effectors, may be an important feature in maturation of dendrite architecture.
In the present study, we demonstrate that over-expression of NR3B increases in the total amount and complexity of dendritic arbor of transfected neurons as compared to controls. These results are important, because they imply that the molecular composition of NMDA receptors is an important determinant in the effects of NMDA receptor activation on dendritic outgrowth and refinement. Accordingly, a recent study has shown that genetic ablation of NR3B leads to impairment of motor coordination (Niemann et al., 2007); although the effects on dendrite morphology of reduced NR3B expression are as yet unknown, the present study suggests that the proper maturation of motor behavior is dependent at least in part on NR3B expression.
In contrast to the results of NR3B expression, we found that treatment with the NMDA receptor antagonist AP5 resulted in rearrangement of dendritic arbor, with arbor being lost at older, more established parts of the dendritic tree. The effects we observe with AP5 differ from earlier studies, in which blockade of NMDA receptors results in loss of dendritic arbor (Cline and Constantine-Paton, 1990; Vogel and Prittie, 1995; Rajan and Cline, 1998; Rajan et al., 1999). One explanation for this may be differences in the time-courses of some experiments: our observations were made at approximately 24 hours after drug administration, and it is possible that more prolonged treatment with AP5 would result in a greater loss of dendritic arbor across the entire extent of the dendritic tree, similar to other studies. In addition, the effects of NMDA receptor blockers in vivo may differ substantially to the effects in vitro, in which NMDA receptor network activity is essentially silenced by the addition of AP5. However, our results are noteworthy because they suggest that altering NMDA receptor activity has bi-directional effects on dendrite growth, such that older regions of dendrites are more prone to elimination in the presence of NMDA receptor blockers, at the expense of newer, actively growing regions.
In accordance with its proposed role as a dominant negative subunit, NR3B expression has been shown to reduce NMDA receptor currents (Chatterton et al., 2002), calcium permeability (Matsuda et al., 2002), and whole-cell currents (Nishi et al., 2001). The carboxy-terminal region of NR3B has been shown to contain endoplasmic reticulum (ER) retention signal motifs, suggesting that one possible mechanism through which NR3B may act to reduce whole cell currents is via retention of assembled NMDA receptors within the lumen of the ER. However, a detailed study of NMDA receptor trafficking found that membrane expression of NR1 was increased in the presence of NR3B; these authors suggested that NR3B acts to mask ER retention signals present in NR1, enhancing surface expression of assembled NMDA receptors (Matsuda et al., 2003). Further, single channel conductance properties are reduced in neurons expressing NR3B, suggesting that NR3B exerts its effect on the electrophysiological properties of NMDA receptors trafficked to the membrane, rather than via retention of assembled receptors within the ER. Thus, the effects of NR3B in our studies are unlikely to be due simply to reductions in surface expression of NMDA receptors.
Since pharmacological blockade of NMDA receptor activity has previously been shown to reduce dendrite complexity in motor neurons (Kalb, 1994; Inglis et al., 1998), it might be expected that reduced NMDA receptor currents as a result of NR3B expression would similarly affect dendritic arbor. In this light, our finding that NR3B expression increased dendrite volume and complexity is somewhat surprising. However, studies in Xenopus oocytes have shown that combinations of NR1 and NR3B can function as excitatory glycine receptors that are unresponsive to glutamate or NMDA (Chatterton et al., 2002). It has been shown that co-expression of NR1, NR3A and NR3B receptor subunits in mammalian HEK293 cells can also give rise to glycine-activated currents (Smothers and Woodward, 2007). Activation of glycine receptors results in an increase in the total dendritic arbor of mouse spinal neurons (Tapia et al., 2000), suggesting that the increase in total amount and complexity of dendritic arbor we noticed following NR3B over-expression may be the result of glycinergic, rather than glutamatergic, activity, and may explain why NR3B expression has different effects to blockade of NMDA receptors by AP5. In the absence of glycine-activated currents following NR3B expression in hippocampal neurons (Matsuda et al., 2003), it is not yet clear whether NR3B expression is associated with glycinergic activity within mammalian neurons.
Alternatively, modest reduction in NMDA receptor activity may facilitate increased outgrowth by destabilizing localized regions of the dendritic tree, since activation of NMDA receptors has been show to stabilize some structural elements of dendrites, such as spines (Matus, 2005). In this regard, an interesting finding of our study was that over-expression of NR3B caused a significant increase in the number of filopodia of transfected cells. Filopodia are highly motile, transient structures that have been suggested to be precursors to dendritic segments (Vaughn et al., 1974; Ulfhake and Cullheim, 1988; Niell et al., 2004). It is possible that the reductions in NMDA receptor currents that have been observed to occur following NR3B expression (Nishi et al., 2001) permit local destabilization of the dendritic tree, facilitating new outgrowth of filopodia and subsequent branch extension.
In contrast to the effects of NR3B, we did not observe altered filopodial numbers in response to AP5 treatment. Our results differ from other studies in which NMDA receptor antagonists were reported to reduce filopodial growth in various brain regions (Rashid and Cambray-Deakin, 1992; Maletic-Savatic et al., 1999; Portera-Cailliau et al., 2003). However, it is possible that our failure to observe altered numbers of filopodia following AP5 is simply due to statistical difficulties in observing the typically very small numbers of filopodia that exist in motor neurons. Nevertheless, our results are important, because they suggest that control of filopodial formation by NMDA receptor activity is complex, and bi-directional. Indeed, it is possible that the differences between the effects of NR3B expression and pharmacological blockade of NMDA receptors may be due to differences in the magnitude of reduction of NMDA receptor currents. At least one study has shown that manipulating the permeability of AMPA glutamate receptors leads to bi-directional changes in dendrite outgrowth (Jeong et al., 2006). Thus, levels of NMDA receptor activity in developing motor neurons may be similarly linked intracellularly to activation of different pathways which promote branch extension or elimination.
CONCLUSION
Since NMDA receptor activity has been shown to promote activity-dependent reorganization of dendritic arbor in spinal motor neurons (Kalb, 1994; Inglis et al., 1998), altering the subunit composition of NMDA receptors during postnatal life is likely to influence the mature dendritic pattern of these neurons. Our results demonstrate that expression of the NR3B NMDA receptor subunit in developing motor neurons influences the complexity and amount of dendritic arbor, in a manner that is distinct from pharmacological blockade of the NMDA receptor. Our data suggest that the developmental upregulation of NR3B observed in spinal motor neurons has direct implications for mature dendritic architecture, and hence, the network properties of these neurons. Determining the molecular cues that regulate dendritic plasticity in later life will significantly enhance our understanding of those mechanisms through which mature neuronal architecture and connectivity are attained.
Supplementary Material
NR1 and NR2A immunoreactivity in cells overexpressing NR3B. (A) and (D) representative image of a motor neuron transfected with GFP and NR3B; (B), NR1 immunoreactivity, and (C), overlaid images. (E), NR2A immunoreactivity of neuron portrayed in (D); and (F), overlaid image. Scale bar represents 50µm.
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Yasunori Hayashi, Thomas Hughes and James Boulter for providing us with the plasmids used in this study. We also thank Crescent L. Combe and Sara M. Clark for their technical help and expertise. This work was supported by Awards from NSF (Award number 0446168); NIH/NIGMS CoBRE (1 P20 RR 15637) and the Louisiana Board of Regents (LEQSF (2003–2006)-RD-A-24).
Abbreviations Used
- ANOVA
Analysis of variance
- AP5
DL-2-amino-5-phosphonovalerate
- DIV
Days in vitro
- DTT
Dithiothreitol
- E
Embryonic day
- EDTA
Ethylenediaminetetraacetic acid
- ER
Endoplasmic reticulum
- GFP
Green fluorescent protein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MEM
Minimal Essential Medium (Eagle’s Medium)
- NMDA
N-methyl-d-aspartate
- NGS
Normal Goat Serum
- NR
N-methyl-d-aspartate receptor subunit
- P
Postnatal day
- PBS
Phosphate buffered saline
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TRITC
Tetramethyl Rhodamine Iso-Thiocyanate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brown KM, Wrathall JR, Yasuda RP, Wolfe BB. Quantitative measurement of glutamate receptor subunit protein expression in the postnatal rat spinal cord. Brain Res Dev Brain Res. 2002;137:127–133. doi: 10.1016/s0165-3806(02)00435-2. [DOI] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Cline HT, Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KS, Sur M. Activity-dependent remodeling of connections in the mammalian visual system. Curr Opin Neurobiol. 1995;5:106–111. doi: 10.1016/0959-4388(95)80094-8. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Cuppini R, Sartini S, Ambrogini P, Falcieri E, Maltarello MC, Gallo G. Control of neuron outgrowth by NMDA receptors. J Submicrosc Cytol Pathol. 1999;31:31–40. [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Feldmeyer D, Cull-Candy S. Functional consequences of changes in NMDA receptor subunit expression during development. J Neurocytol. 1996;25:857–867. doi: 10.1007/BF02284847. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Hayashi Y, Watanabe M. NR2 to NR3B subunit switchover of NMDA receptors in early postnatal motoneurons. Eur J Neurosci. 2005;21:1432–1436. doi: 10.1111/j.1460-9568.2005.03957.x. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Zuckerman KE, Kalb RG. Experience-dependent development of spinal motor neurons. Neuron. 2000;26:299–305. doi: 10.1016/s0896-6273(00)81164-2. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Furia F, Zuckerman KE, Strittmatter SM, Kalb RG. The role of nitric oxide and NMDA receptors in the development of motor neuron dendrites. J Neurosci. 1998;18:10493–10501. doi: 10.1523/JNEUROSCI.18-24-10493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Crockett R, Korada S, Abraham WC, Hollmann M, Kalb RG. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22:8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong GB, Werner M, Gazula VR, Itoh T, Roberts M, David S, Pfister B, Cohen A, Neve RL, Hollmann M, Kalb R. Bi-directional control of motor neuron dendrite remodeling by the calcium permeability of AMPA receptors. Mol Cell Neurosci. 2006;32:299–314. doi: 10.1016/j.mcn.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Kamiya Y, Matsuda S, Yuzaki M. Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res. 2002;100:43–52. doi: 10.1016/s0169-328x(02)00173-0. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Fletcher M, Kamiya Y, Yuzaki M. Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J Neurosci. 2003;23:10064–10073. doi: 10.1523/JNEUROSCI.23-31-10064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Growth of dendritic spines: a continuing story. Curr Opin Neurobiol. 2005;15:67–72. doi: 10.1016/j.conb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Niemann S, Kanki H, Fukui Y, Takao K, Fukaya M, Hynynen MN, Churchill MJ, Shefner JM, Bronson RT, Brown RH, Jr, Watanabe M, Miyakawa T, Itohara S, Hayashi Y. Genetic ablation of NMDA receptor subunit NR3B in mouse reveals motoneuronal and nonmotoneuronal phenotypes. Eur J Neurosci. 2007;26:1407–1420. doi: 10.1111/j.1460-9568.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima S, Fukaya M, Masabumi N, Shirakawa T, Oguchi H, Watanabe M. Early onset of NMDA receptor GluR epsilon 1 (NR2A) expression and its abundant postsynaptic localization in developing motoneurons of the mouse hypoglossal nucleus. Neurosci Res. 2002;43:239–250. doi: 10.1016/s0168-0102(02)00035-4. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prithviraj R, Kelly KM, Espinoza-Lewis R, Hexom T, Clark AB, Inglis FM. Differential regulation of dendrite complexity by AMPA receptor subunits GluR1 and GluR2 in motor neurons. Dev Neurobiol. 2008;68:247–264. doi: 10.1002/dneu.20590. [DOI] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38:357–368. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rashid NA, Cambray-Deakin MA. N-methyl-D-aspartate effects on the growth, morphology and cytoskeleton of individual neurons in vitro. Brain Res Dev Brain Res. 1992;67:301–308. doi: 10.1016/0165-3806(92)90231-k. [DOI] [PubMed] [Google Scholar]
- Robert A, Howe JR, Waxman SG. Development of glutamatergic synaptic activity in cultured spinal neurons. J Neurophysiol. 2000;83:659–670. doi: 10.1152/jn.2000.83.2.659. [DOI] [PubMed] [Google Scholar]
- Schridde U, Strauss U, Bräuer AU, van Luijtelaar G. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci. 2006;23:3346–3358. doi: 10.1111/j.1460-9568.2006.04865.x. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-D-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther. 2007;322:739–748. doi: 10.1124/jpet.107.123836. [DOI] [PubMed] [Google Scholar]
- Stegenga SL, Kalb RG. Developmental regulation of N-methyl-D-aspartate- and kainate-type glutamate receptor expression in the rat spinal cord. Neuroscience. 2001;105:499–507. doi: 10.1016/s0306-4522(01)00143-9. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JC, Cardenas AM, Nualart F, Mentis GZ, Navarrete R, Aguayo LG. Neurite outgrowth in developing mouse spinal cord neurons is modulated by glycine receptors. Neuroreport. 2000;11:3007–3010. doi: 10.1097/00001756-200009110-00036. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Cullheim S. Postnatal development of cat hind limb motoneurons. II: In vivo morphology of dendritic growth cones and the maturation of dendrite morphology. J Comp Neurol. 1988;278:88–102. doi: 10.1002/cne.902780106. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Ihle EC, Patneau DK, Robberecht W, Brorson JR. AMPA receptor current density, not desensitization, predicts selective motoneuron vulnerability. J Neurosci. 2000;20:7158–7166. doi: 10.1523/JNEUROSCI.20-19-07158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JE, Henrikson CK, Grieshaber JA. A quantitative study of synapses on motor neuron dendritic growth cones in developing mouse spinal cord. J Cell Biol. 1974;60:664–672. doi: 10.1083/jcb.60.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel MW, Prittie J. Purkinje cell dendritic arbors in chick embryos following chronic treatment with an N-methyl-D-aspartate receptor antagonist. J Neurobiol. 1995;26:537–552. doi: 10.1002/neu.480260407. [DOI] [PubMed] [Google Scholar]
- Walton KD, Navarrete R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord. J Physiol. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal distributions of the N-methyl-D-aspartate receptor channel subunit mRNAs in the mouse cervical cord. J Comp Neurol. 1994;345:314–319. doi: 10.1002/cne.903450212. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zona C, Pieri M, Carunchio I. Voltage-dependent sodium channels in spinal cord motor neurons display rapid recovery from fast inactivation in a mouse model of amyotrophic lateral sclerosis. J Neurophysiol. 2006;96:3314–3322. doi: 10.1152/jn.00566.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NR1 and NR2A immunoreactivity in cells overexpressing NR3B. (A) and (D) representative image of a motor neuron transfected with GFP and NR3B; (B), NR1 immunoreactivity, and (C), overlaid images. (E), NR2A immunoreactivity of neuron portrayed in (D); and (F), overlaid image. Scale bar represents 50µm.