Abstract
Background
The survival and function of transplanted pancreatic islets is limited, owing in part to disruption of islet-matrix attachments during the isolation procedure. Using polymer scaffolds as a platform for islet transplantation, we investigated the hypothesis that replacement of key extracellular matrix components known to surround islets in vivo would improve graft function at an extrahepatic implantation site.
Methods
Microporous polymer scaffolds fabricated from copolymers of lactide and glycolide were adsorbed with collagen IV, fibronectin, laminin-332 or serum proteins before seeding with 125 mouse islets. Islet-seeded scaffolds were then implanted onto the epididymal fat pad of syngeneic mice with streptozotocin-induced diabetes. Nonfasting glucose levels, weight gain, response to glucose challenges, and histology were used to assess graft function for 10 months after transplantation.
Results
Mice transplanted with islets seeded onto scaffolds adsorbed with collagen IV achieved euglycemia fastest and their response to glucose challenge was similar to normal mice. Fibronectin and laminin similarly promoted euglycemia, yet required more time than collagen IV and less time than serum. Histopathological assessment of retrieved grafts demonstrated that coating scaffolds with specific extracellular matrix proteins increased total islet area in the sections and vessel density within the transplanted islets, relative to controls.
Conclusions
Extracellular matrix proteins adsorbed to microporous scaffolds can enhance the function of transplanted islets, with collagen IV maximizing graft function relative to the other proteins tested. These scaffolds enable the creation of well-defined microenvironments that promote graft efficacy at extrahepatic sites.
Keywords: Islet transplantation, Biomaterial, Diabetes, PLG, Scaffold, Microenvironment
Type 1 diabetes mellitus (T1DM) affects an estimated 1.5 million Americans (1) and is characterized by autoimmune-mediated destruction of pancreatic β-cells, which results in absolute insulin deficiency (2–5). Although careful glucose monitoring combined with exogenous insulin administration can effectively control acute glycemia, secondary microvascular and macrovascular complications eventually afflict most type 1 diabetic subjects (6–8). β-cell replacement via transplantation of allogeneic islets has been explored as a potential curative treatment but clinical islet transplantation has thus far yielded disappointing results, with less than 10% of those transplanted remaining insulin independent after 5 years (9). Moreover, the stringent inclusion criteria for and shortage of donors, coupled with the requirement for two to four donor pancreata per recipient, limit the potential of this approach (10–12).
Reasons for the limited success of islet transplantation are multifactorial and likely related to the loss of vascular connections (13, 14) and disruption of cell-matrix contacts that occur during the isolation procedure (10). Basement membrane proteins present between intraislet endothelial and endocrine islet cells are primarily collagen IV, laminin, and fibronectin. These proteins engage integrins on the surface of islet cells to mediate adhesion, provide structural support, and activate intracellular chemical signaling pathways (15–17). During enzymatic digestion of the exocrine pancreas, these extracellular matrix (ECM) proteins are degraded, which interrupts cell-matrix interactions (18–20). Early islet cell death after transplantation may be related to a lack of integrin signaling resulting in apoptosis (20). Islets cultured on matrices containing ECM components, on the other hand, exhibited improved survival in vitro (21). Therefore, the provision of a matrix to support islet attachment may be an important requirement for maintaining the function and viability of transplanted islets.
As previously reported, microporous, biocompatible, biodegradable scaffolds fabricated from poly(lactide-co-glycolide) (PLG) were successfully used as platforms for islet transplantation in mice (22). This type of scaffold offers distinct advantages, including (i) a high surface area/volume ratio to enable nutrient and waste transport, (ii) an interconnected internal pore structure to allow for cell and blood vessel infiltration, (iii) sufficient mechanical rigidity to provide a platform for cell attachment and ease of implantation, and (iv) the ability to degrade over time, allowing for complete integration into the surrounding tissue. In addition to providing structural support, the scaffold surface can be modified with nondiffusible molecules, such as ECM components, to mediate cellular interactions that are necessary for cell attachment, growth, and proliferation (23). This surface modification enables manipulation of the local microenvironment so that the impact of factors in isolation or combination on graft efficacy can be determined.
In the present study, we investigated the ability and specificity of ECM proteins to promote the long-term function of islets transplanted onto microporous scaffolds coated with collagen IV, laminin or fibronectin, and implanted into a mouse model of diabetes. Consistent with a previous study, the epididymal fat pad was selected as the site of implantation due to its surgical accessibility, vascularization, and structural similarity to the greater omentum in humans (a potential extrahepatic site for clinical islet transplantation) (22, 24). Nonfasting and dynamic blood glucose data, weight measurements and immunohistochemistry results suggest that the composition of the local microenvironment surrounding transplanted islets is a key factor in promoting their long-term survival and function.
MATERIALS AND METHODS
Protein Adsorption to Scaffolds
PLG scaffolds were prepared as previously described (25) and treated in the manner described below on the day before islet isolation and seeding. For protein adsorption, dry scaffolds were immersed in 0.5 N NaOH for 1 min (26) followed by immersion in an excess of water. Scaffolds were dried at room temperature before placing in 70% EtOH. Scaffolds were again dried before being placed into individual wells of a 24-well dish. Collagen IV (50 μL at 1 mg/mL; Sigma), fibronectin (50 μL at 1 mg/mL; Sigma), laminin-332 (formerly termed laminin-5 and hereafter referred to as “laminin”; 50 μL of conditioned cell culture media from 804G cells containing approximately 1 mg/mL of laminin-332 [27]), or serum-containing media (RPMI-1640 media [Gibco-BRL, Grand Island, NY] supplemented with 10% heat-inactivated fetal calf serum [Hyclone, Logan, UT], 100 U/mL penicillin-G, 100 mg/mL streptomycin sulfate, and 1 mmol/L L-glutamine; hereafter referred to as “SCM”) were added to the scaffold and incubated at room temperature for 2 hours, followed by addition of 50 μL of the same component to each scaffold. Scaffolds were then incubated with 95% humidity at 37°C overnight to allow for protein adsorption. Before islet seeding, 100 μL of fresh SCM was applied to the top of each scaffold.
Protein adsorption to the scaffold surface was assessed using the picrosirius stain (28). After overnight incubation, scaffolds were washed three times with phosphate-buffered saline (PBS) to wash away any unbound protein. After staining, scaffolds were visualized by light microscopy to identify adsorbed proteins.
Animals and Induction of Diabetes
Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) between 8 and 12 weeks of age were used as islet donors and transplant recipients. Four days before islet transplantation, graft recipient mice were injected intraperitoneally with 220 mg/kg of streptozotocin (Sigma, St. Louis, MO) to chemically induce irreversible diabetes (29). Nonfasting blood glucose levels were measured in whole blood samples obtained from the tail of the animals using a One Touch Basic glucose monitor (Lifescan, Milpitas, CA). Mice were used in these studies only if they had blood glucose measurements greater than 300 mg/dL on consecutive days before transplantation. The blood glucose levels of donor mice were also checked before islet isolation to verify that they were metabolically normal. All studies were approved by the Northwestern University Animal Care and Use Committee.
Islet Isolation, Scaffold Seeding, and Transplantation
Islet isolation and scaffold seeding were performed as previously described except that each recipient only received 125 islets (22). After isolation, islets were seeded onto each scaffold in a minimal volume of media by applying them to the scaffold and allowing them to filter into the microporous structure. Examination of the tissue culture media after removal of the scaffolds demonstrated that greater than 95% of the islets stayed on the scaffolds after seeding. Scaffolds were then incubated at 37°C in 5% CO2 and 95% air for 30 min. At that time, 20 μL of SCM was added to the top of each scaffold and returned to the incubator. After a 60-min incubation, 5 mL of SCM was added to the tissue culture well in which each scaffold was placed and returned to the 37°C incubator for 30 min before transplantation.
Recipient mice were anesthetized with an intraperitoneal injection of Avertin (250 mg/kg body wt) and the abdominal region was shaved and prepped in a sterile manner. After a short, midline lower abdominal incision, the right epididymal fat pad was identified and spread on the shaved, exterior abdominal surface. Scaffolds preseeded with islets were then placed on and wrapped by the fat pad and returned to the intraperitoneal cavity. Scaffolds not seeded with islets but incubated overnight in SCM were transplanted as negative controls. The wound was closed in two layers. Mice were allowed free access to food and water postoperatively and were routinely checked throughout the duration of the study for any signs of infection around the surgical site.
Assessment of Graft Function
After transplantation, nonfasting blood glucose and weight measurements were taken between 12:00 and 17:00 as described above using the following schedule: everyday during the first postoperative week, every other day during weeks 2 to 5, once per week during weeks 6 to 25, and once per month thereafter until the conclusion of the study. Grafts were considered to be functional if glucose levels were maintained at less than 200 mg/dL and mice did not reconvert to a hyperglycemic state for the duration of the study. After graft removal at the end of postoperative week 42, blood glucose levels were monitored for 72 hr, at which time the mice were sacrificed.
Intraperitoneal glucose tolerance tests (IPGTTs) were performed at 4 and 40 weeks after transplantation to assess the grafts’ ability to respond to glucose challenges. After a 6 hr fast, 2 g/kg of 50% dextrose (Abbott Labs, North Chicago, IL) was injected intraperitoneally. Blood glucose levels were measured at baseline (before injection), 15, 30, 60, and 120 min after glucose injection. Area under the curve (AUC) for each animal was calculated using the trapezoidal rule (30). The area corresponding to the baseline glucose measurement multiplied by 120 min was subtracted from the total AUC calculated to account for any baseline differences between the animals.
Histological Analysis
Histological analysis was performed to characterize the morphology of transplanted islets and to quantify islet area and vascular density. On postoperative day 7 or 297, fat pads containing islet grafts were explanted and fixed in 4% paraformaldehyde. Fixed specimens were embedded in paraffin or Tissue-Tek O.C.T. compound (Miles Scientific, Elkhart, IN), and 5 μm paraffin or 10 μm cryosections were prepared, respectively. Immunohistochemistry was performed to confirm the presence of β-cells using guinea pig anti-insulin antibody (1:100; Zymed, South San Francisco, CA) and a biotinylated goat anti-guinea pig immunoglobulin (1:1000; Vector, Burlingame, CA), followed by streptavidin-horseradish peroxidase, which was revealed by staining with 3,3′-diaminobenzidine (DAB). Sections were counter-stained with hematoxylin. Paraffin sections were also stained with hematoxylineosin according to standard protocols. Digital images were acquired using a Spot camera via the accompanying image analysis software (Diagnostic Instruments, Inc., Sterling Heights, MI) attached to a Nikon Eclipse 50i microscope (Nikon, Tokyo, Japan).
Quantification of Islet Size and Vascular Density
Assessment of islet size and vascular density was performed in grafts removed after 297 days of implantation. For each condition, three randomly chosen paraffin-embedded grafts were serially sectioned as described above. Note that for the serum condition, the grafts used were from animals whose diabetes had been reversed. The first section containing insulin positive cells was identified and labeled “base section.” Starting at 50 μm after the base section, and then at approximately 60 μm intervals thereafter, slides were selected for insulin-IHC and hematoxylineosin (H&E) staining. Five slides per tissue sample per condition were collected in this manner, each set representing a depth within the scaffold of approximately 300 μm. Few islets were observed at greater depths within the graft. One section on each slide was stained for insulin whereas the other was stained using H&E. The section stained for insulin was used for verification of islet location, whereas the H&E section was used for identification of blood vessels. Pictures were taken using a 40X objective as described above and assembled into composite images in Adobe Photoshop CS3 Extended (Adobe Systems Inc., San Jose, CA). Using Photoshop, the area of each islet was measured and the corresponding number of intraislet vessels was counted after blinding the observer to the condition being evaluated.
Statistical Analysis
All values are reported as the mean±SEM. Differences in the number of days to reach euglycemia between experimental groups were compared using the Kaplan-Meier survival analysis and the log-rank test. Statistical analyses for comparison of weight and IPGTT data, and all bar graphs in Figure 6, were performed by using Student’s t test. A P-value of less than 0.05 was considered statistically significant.
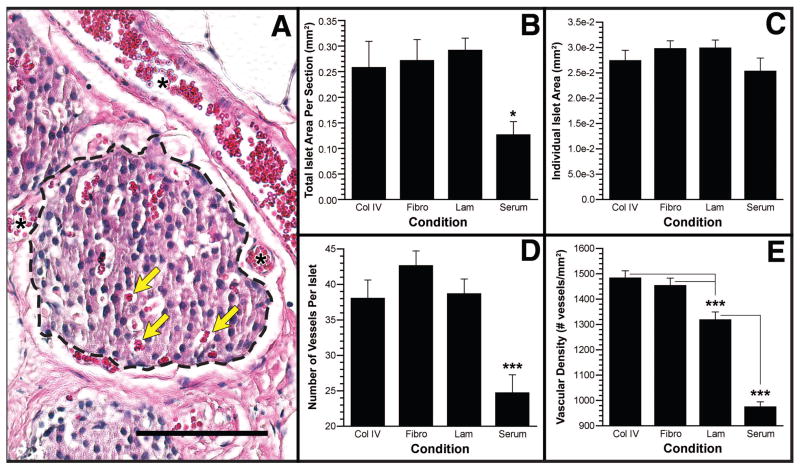
FIGURE 6.
Quantification of islet area and vascular density. (A) An example H&E stained section indicating how islet boundaries were established (dashed line) to calculate area and intraislet vessels (yellow arrows). Examples of large vessels seen adjacent to islets and periislet vessels are marked with an asterisk. Total islet area per tissue section (B), area per islet (C), vessels per islet (D), and vessel density (E) for the four conditions tested. Data are presented as mean±SEM. *P<0.05; ***P<0.001. Scale bar indicates 100 μm.
RESULTS
Protein Adsorption to Scaffolds
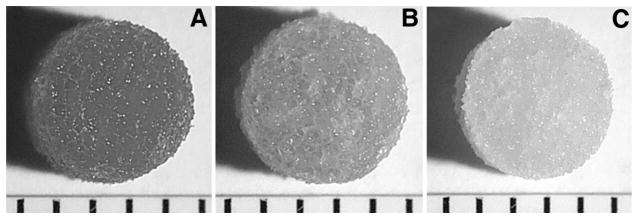
Protein adsorption was visualized to determine an appropriate protein concentration and duration of incubation that would provide a homogeneous distribution throughout the scaffold. Hydrolyzed-scaffolds incubated with collagen IV (Fig. 1A) demonstrated extensive protein adsorption throughout the scaffold, whereas nonhydrolyzed scaffolds (Fig. 1B) demonstrated a lower staining intensity as well as an inconsistent distribution of staining in the scaffold. Hydrolyzed-scaffolds incubated in PBS showed no staining (Fig. 1C). Increasing the concentration of collagen IV from 0.00 to 3.71 mg/mL increased the intensity of staining, as did increasing the time of incubation from 1 to 16 hr (data not shown). Examination of scaffold cross-sections after staining confirmed that protein adsorption was homogenous throughout the entire scaffold volume. These experiments were repeated using fibronectin and laminin with similar results (data not shown). On the basis of these results, overnight incubation of hydrolyzed scaffolds in 1 mg/mL of the selected ECM component was used in all subsequent studies.
FIGURE 1.
Protein adsorption to scaffolds. Photomicrographs of scaffolds stained with picrosirius red after 1 mg/mL collagen IV was adsorbed. The scaffolds were treated by base hydrolysis (A) or were untreated (B). Negative control for base-hydrolyzed scaffold by incubation with PBS (C). Indicator marks at bottom of images are 1 mm apart.
ECM Proteins Improve Islet Function After Transplantation
Subsequent experiments investigated the ability of collagen IV, fibronectin, and laminin—ECM proteins known to be present in pancreatic islets in vivo—to enhance islet function after transplantation. In addition to transplanting islets onto scaffolds coated with collagen IV, fibronectin and laminin, a fourth group of mice was transplanted with scaffolds that had been incubated in SCM before islet seeding. As a negative control, a fifth group of mice was implanted with scaffolds that had been incubated in SCM but not seeded with islets before implantation. In these studies, a syngeneic animal model was used, which allowed for investigation of the impact of various ECM components on graft success without complicating effects from immunosuppressive agents.
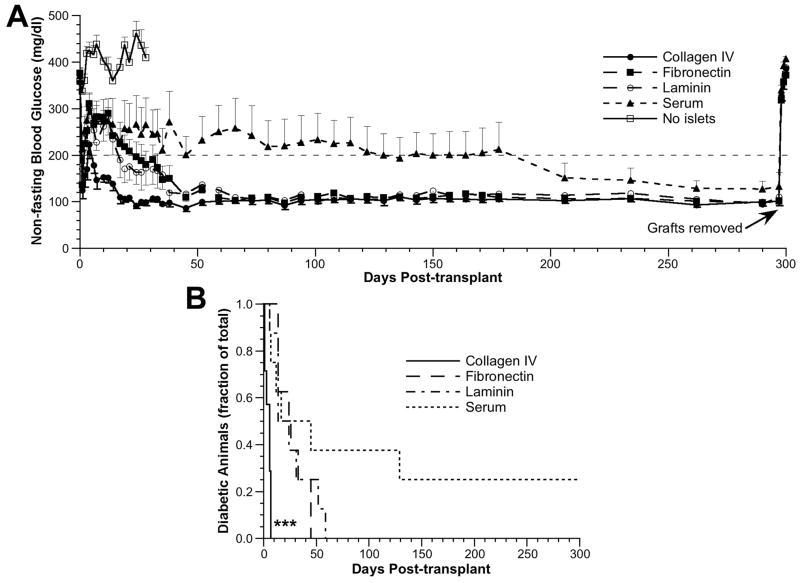
Mice transplanted with scaffolds preadsorbed with collagen IV achieved euglycemia most rapidly, with a mean time to euglycemia of 4.4±1.0 days (100% converted; n=7), compared with 26.9±4.6 days (100% converted; n=8) for the fibronectin group, 26.8±6.8 days for the laminin group (100% converted; n=8) and 36.0±18.1 days (75% converted; n=8) for the serum group (Fig. 2A). Mice implanted with scaffolds lacking islets (n=8) remained hyperglycemic with glucose levels between 282 and 547 mg/dL before being sacrificed on day 28. All other mice were maintained until day 297 posttransplantation, at which time the fat pad containing the graft was removed from each animal. In all cases, euglycemic animals reverted to a state of hyperglycemia within 24 hr after scaffold removal, confirming that the islets contained within the fat pad were responsible for sustaining euglycemia (Fig. 2A). The time to euglycemia for the collagen IV group was significantly less than that of the other groups as determined by the log-rank test applied to a Kaplan-Meier survival curve (P<0.001 for collagen IV vs. fibronectin, laminin and serum) (Fig. 2B). None of the other pair-wise comparisons had significance at the P=0.05 level.
FIGURE 2.
Glucose regulation after islet transplantation. (A) Nonfasting blood glucose levels from day 0 (day of transplant) through day 300 posttransplant. ●, collagen IV group (n=7); ■, fibronectin group (n=8); ○, laminin group (n=8); ▲, serum group (n=8); □, no islet group (n=8). Data are presented as mean glucose level±SEM (one-sided error bars used for clarity). (B) The fraction of diabetic animals that converted to euglycemia over time for scaffolds coated with collagen IV (solid line), fibronectin (dashed line), laminin (dash-dot line), and serum proteins (dot-dot line). ***P<0.001, collagen IV vs. all other conditions.
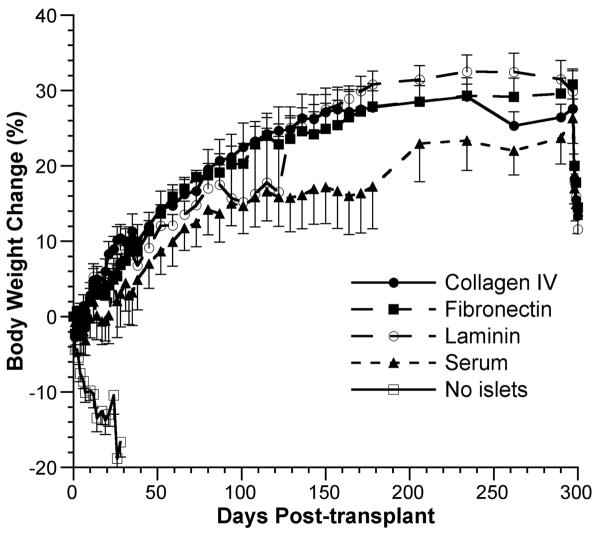
Consistent with the blood glucose levels, mice transplanted with islets exhibited similar increases in body weight from day 0 (day of transplant) to day 297 [27.6±1.3% for the collagen IV group, 30.8±2.1% for the fibronectin group, 29.9±2.7% for the laminin group, and 26.3±3.3% for the serum group] (Fig. 3). Although the serum group consistently exhibited a lower percent change in weight compared with the three experimental groups, these differences were not statistically significant at any time point as determined by Student’s t test (P>0.05). Mice in the negative control group lost an average 16.6%±2.0% of their body weight before being sacrificed on day 28.
FIGURE 3.
Change in body weight after islet transplantation. Percent change in body weight from day 0 through day 300 posttransplant. ●, collagen IV group (n=7); ■, fibronectin group (n=8); ○, laminin group (n=8); ▲, serum group (n=8); □, no islet group (n=8). Data are presented as mean percent change in weight±SEM (one-sided error bars used for clarity).
Specific ECM Proteins Improve Islet Response to Glucose Challenges
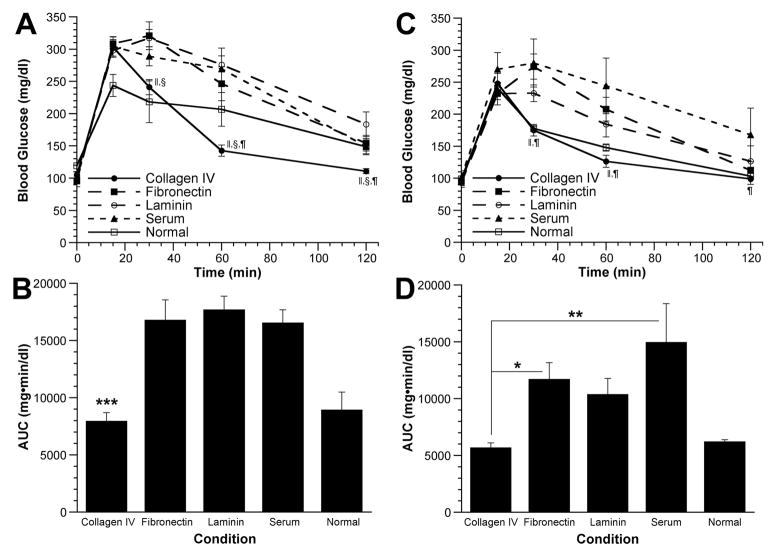
To further investigate the connection between ECM proteins and islet function, intraperitoneal glucose tolerance tests (IPGTT) were performed at 4 and 40 weeks posttrans-plant on mice in which euglycemia had been restored. For comparison, an IPGTT was also performed on nondiabetic, age-matched C57BL/6 mice (n=3) at both time points. At 4 weeks posttransplant, baseline fasting glucose levels were similar between the five groups of mice (Fig. 4A). At 30 min, however, glucose levels in the collagen IV and normal groups were significantly lower than in the fibronectin and laminin groups. Similarly, at 60 min, glucose levels in the collagen IV group were significantly lower than the laminin, fibronectin, and serum groups. At 120 min, glucose levels had returned to near baseline for all groups except for the laminin group, which was significantly higher than the collagen IV group. Glucose levels in the collagen IV and normal groups were similar at all time points. The area under the curve (AUC) for the collagen IV group was similar to that of the normal control mice but significantly less (P<0.001) than that of the other three treatment groups (Fig. 4B).
FIGURE 4.
Intraperitoneal glucose tolerance tests. An IPGTT was performed at 4 (A, B) and 40 weeks (C, D) after islet transplantation on animals that were euglycemic at that time. (A, C) Blood glucose levels as a function of time after glucose injection. ●, collagen IV group (n=7 at 4 weeks, n=7 at 40 weeks); ■, fibronectin group (n=5 at 4 weeks, n=8 at 40 weeks); ○, laminin group (n=6 at 4 weeks, n=8 at 40 weeks); ▲, serum group (n=4 at 4 weeks, n=6 at 40 weeks); □, normal control group (n=3 at 4 weeks, n=3 at 40 weeks). Data are presented as mean glucose level±SEM. ||, P<0.05, collagen IV vs. fibronectin; §, P<0.05, collagen IV vs. laminin; ¶, P<0.05, collagen IV vs. serum. (B, D) Areas under the glucose challenge curves (AUC) were calculated. Data are presented as mean AUC±SEM. *, P<0.05, collagen IV vs. fibronectin (at 40 weeks); **, P<0.01, collagen IV vs. serum (at 40 weeks); ***, P<0.001, collagen IV vs. fibronectin, laminin and serum groups (at 4 weeks). For clarity, statistically significant differences between the normal group and all other groups are not explicitly displayed.
Significant differences between groups were also found at 40 weeks posttransplant (Fig. 4C). Glucose levels in the collagen IV and normal groups were significantly lower than the fibronectin and serum groups 30 and 60 min after glucose injection. At 120 min after glucose injection, the collagen IV group still had glucose levels significantly lower than the serum group. Forty weeks posttransplant, the AUC for the collagen IV group (Fig. 4D) was similar to the normal group but significantly less than the fibronectin (P<0.05) and serum groups (P<0.01).
ECM Proteins Support Islet Architecture and Enhance Total Islet Mass
Islets seeded onto scaffolds coated with collagen IV maintained normal cell-cell interactions and intact islet architecture, which may be necessary for islet function. Seven days after implantation on collagen IV-coated scaffolds, the periphery of islets was in direct contact with the protein-coated scaffold surface (Fig. 5A; red arrows). Additionally, all islets were found to be located within a distance of approximately 400 μm from the surface on which they were seeded (data not shown). Similar results were observed using fibronectin- and laminin-coated scaffolds. However, on serum-coated scaffolds, islet architecture was disrupted. A fraction of the cell-cell interactions were disrupted leading to individual insulin-positive cells within the scaffold (data not shown).
FIGURE 5.
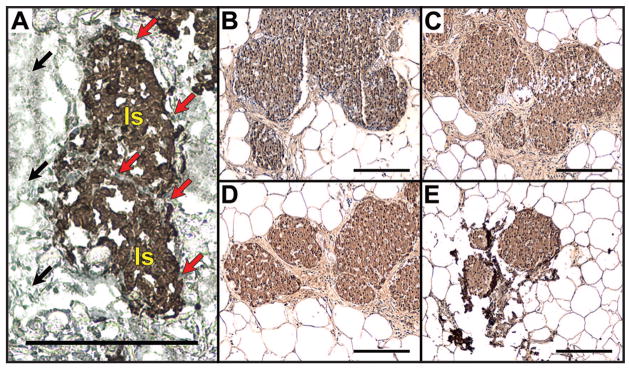
Histopathological assessment of explanted islet grafts. Immunohistochemical staining (brown) for insulin on a cryosection (cut perpendicular to the islet-seeded surface) taken from a collagen IV-coated scaffold explanted 7 days after implantation (A), or paraffin sections of collagen IV- (B), fibronectin- (C), laminin- (D) or serum-coated (E) scaffolds explanted 297 days after implantation. All scale bars indicate 200 μm. In panel A, two example islets are indicated by “Is,” black arrows indicate the edge of the scaffold, and red arrows indicate the scaffold surface in direct contact with the islets.
The architecture and size of transplanted islets were assessed for islet grafts explanted 297 days after transplantation. Immunohistochemical analyses revealed large numbers of insulin-positive cells that were well circumscribed by a basement membrane and organized into highly vascularized structures (Fig. 5, B–D). Although immunostaining for insulin was present in sections from the serum-coated scaffolds (Fig. 5E), total islet area was smaller relative to the other groups in all sections observed (Fig. 6B). For all conditions, scaffold material was not visible after 297 days of implantation, indicating that the polymer had degraded and that transplanted islets had become well integrated with the host tissue. Additionally, an abundance of larger vessels and periislet vessels (Fig. 6A, see asterisks) were observed next to and around the islets in all experimental conditions whereas few were observed in the serum condition.
ECM Proteins Enhance Islet Mass and Vascular Density
We subsequently quantified islet area and the number of intraislet blood vessels (Fig. 6, B–E). The three experimental ECM conditions had similar total islet area per section, which were significantly greater than the area measured for the serum condition (Fig. 6B; P<0.05). Additionally, the mean area per islet was not significantly different between the three experimental conditions and the serum control (Fig. 6C).
Assessment of vessel density revealed that the three ECM groups had significantly more intraislet microvessels than the serum control group (Fig. 6D; P<0.001). Measurement of vascular density (number of vessels/mm2) indicated that the collagen IV and fibronectin groups were similar, with both significantly greater than the laminin and serum groups (Fig. 6E; P<0.001). The vascular density of the laminin group was also significantly greater than the serum group (P<0.001). Interestingly, the vascular densities of islets transplanted on scaffolds coated with collagen IV (1484±27 vessels/mm2) and fibronectin (1455±28 vessels/mm2) are similar to those previously reported for native C57BL/6 islets. These values for vascular density are significantly greater than that reported for islets transplanted beneath the kidney capsule (31).
DISCUSSION
In the present study, we demonstrate for the first time that ECM components significantly improved the efficacy of islet grafts in an animal model of T1DM. The observed effect of ECM components on the restoration of euglycemia could be mediated by interactions between the adsorbed proteins and islets, between proteins and the host tissue, or a combination of the two. Previous reports have shown that ECM components interact with a variety of cell-surface integrins to affect intracellular processes such as β-cell survival (32), differentiation (33), proliferation (34), and insulin secretion (35). These in vitro findings establish the importance of integrin-mediated signaling on islet function and our in vivo findings, reported herein, extend these observations to demonstrate that ECM components significantly enhance the function of transplanted islets in an animal model of T1DM. Interestingly, whereas we find that collagen IV has a markedly positive impact on the function of transplanted islets, Kaido et al. (36) recently reported that islets cultured on collagen IV-coated tissue culture wells showed marked suppression of insulin gene transcription and significant glucose-independent insulin secretion. The major difference between these two approaches is that scaffolds provide islets with a 3-D matrix that supports and maintains the architecture and cellular organization found in native islets (22), whereas in vitro cultured islets gradually transition from spheroidal aggregates to monolayers (36). This beneficial effect of ECM proteins might be mediated by increased adhesive properties of ECM-adsorbed scaffolds, which could act to maintain the native architecture of islets and prevent them from escaping during or after transplantation, though previous work has shown that islets seeded onto control scaffolds remained associated with the scaffold after transplantation (22). This disruption of islet architecture may interfere with integrin-mediated signaling and paracrine interactions between islet cells (37). Additionally, Kaido et al. used adult human islets harvested from older donors (45–56 years old)—a factor known to negatively correlate with isolated islet function (38). Finally, the expansion of their primary islet cultures for 3 to 4 days before seeding on collagen IV-coated wells complicates a direct comparison, as significant islet cell apoptosis ensues 24 to 48 hr after isolation with in vitro cultured islets (20).
Alternatively, adsorbed proteins may promote the infiltration of host cells, such as endothelial cells, into the scaffold (39), which interact with the grafted tissue. Endothelial cell infiltration promotes engraftment and revascularization of transplanted islets, which is essential to promoting their survival and function (40) and provides an explanation for the significantly increased total islet area in the ECM conditions relative to controls. Reduced vascularization of the serum-coated scaffolds may have limited nutrient availability, which could lead to cellular apoptosis. Enhanced graft revascularization may have also contributed to a better response during the IPGTT, although since the IPGTT results for the collagen IV condition were better than the other ECM conditions, despite having a similar vascular density as the fibronectin condition, other mechanisms in addition to revascularization may have contributed to enhanced islet engraftment and function, as discussed below. Thus, adsorbed proteins may exert their effects directly on endothelial cells to promote their infiltration into the scaffold (41).
The beneficial effects of ECM proteins could have also been mediated through interactions with integrins, which could promote islet cell survival and proliferation resulting in increased numbers of functioning β-cells. This interaction could also lead to an increase in the local concentration of vascular endothelial growth factor (VEGF-A) (13), which would stimulate both infiltration of host endothelial cells and expansion of donor intraislet endothelial cells. Therefore, the combination of direct and indirect effects of matrix components on transplanted islets could explain the observed improvement in outcome when islets were seeded on scaffolds adsorbed with ECM components.
In conclusion, we report that the presence of ECM proteins on microporous scaffolds leads to a pronounced decrease in the time required to reverse diabetes in C57BL/6 mice relative to noncoated scaffolds. Our approach is based on modification of the microenvironment surrounding islets to promote graft survival and function as well as to enhance integration with the recipient. Of the ECM components investigated, the provision of collagen IV was most effective at rapidly reversing streptozotocin-induced hyperglycemia in this animal model. This finding suggests that the composition of the islet microenvironment plays an important role in mediating the survival and function of transplanted islets. The scaffold provides a means to manipulate this environment and can be designed to support islet engraftment, and represents a significant departure from previous approaches in which biomaterials have been used for immunoisolation. Moreover, the ability to achieve euglycemia in so short a time with a single transplant of 125 islets (the average islet yield per pancreas is approximately 200) represents the successful application of a single-donor/single-recipient model of islet transplantation—a necessary benchmark that must be routinely achieved in human trials before clinical islet transplantation becomes widely practiced.
Acknowledgments
The authors thank Dr. Jonathan Jones for kindly providing the 804G conditioned media, Ms. Elizabeth Hughes for her assistance in collecting glucose measurements, and Dr. Alfred Rademaker for helpful discussions regarding the statistical analysis of the data.
This work was supported by National Institutes of Health grants F31 EB007118 (D.M.S.), R21 DK067833, RO1 DK52919, RO1 DK062641, RO1 DK063439, and RO1 EB003806; PPG # 4-2004-781 from the Juvenile Diabetes Research Foundation (D.B.K. and W.L.L.); and grants from Northwestern Memorial Foundation (D.B.K. and W.L.L.) and the Butz Foundation (W.L.L.).
References
- 1.Eiselein L, Schwartz HJ, Rutledge JC. The challenge of type 1 diabetes mellitus. ILAR J. 2004;45:231. doi: 10.1093/ilar.45.3.231. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 3.Hamalainen AM, Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diab Rep. 2002;2:347. doi: 10.1007/s11892-002-0025-2. [DOI] [PubMed] [Google Scholar]
- 4.Yoon JW, Jun HS. Autoimmune destruction of pancreatic Beta cells. Am J Ther. 2005;12:580. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DB. Immunology: Insulin auto-antigenicity in type 1 diabetes. Nature. 2005;438:E5. doi: 10.1038/nature04423. [DOI] [PubMed] [Google Scholar]
- 6.Mohsin F, Craig ME, Cusumano J, et al. Discordant trends in microvascular complications in adolescents with type 1 diabetes from 1990 to 2002. Diabetes Care. 2005;28:1974. doi: 10.2337/diacare.28.8.1974. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM. Management of insulin-dependent diabetes mellitus. Drugs. 1992;44(suppl 3):39. doi: 10.2165/00003495-199200443-00006. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 9.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 10.Balamurugan AN, Bottino R, Giannoukakis N, et al. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas. 2006;32:231. doi: 10.1097/01.mpa.0000203961.16630.2f. [DOI] [PubMed] [Google Scholar]
- 11.Hering BJ. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 2005;79:1296. doi: 10.1097/01.tp.0000157321.55375.86. [DOI] [PubMed] [Google Scholar]
- 12.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 13.Lai Y, Schneider D, Kidszun A, et al. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation. 2005;79:1530. doi: 10.1097/01.tp.0000163506.40189.65. [DOI] [PubMed] [Google Scholar]
- 14.Pileggi A, Molano RD, Ricordi C, et al. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 15.Hamamoto Y, Fujimoto S, Inada A, et al. Beneficial effect of pretreatment of islets with fibronectin on glucose tolerance after islet transplantation. Horm Metab Res. 2003;35:460. doi: 10.1055/s-2003-41802. [DOI] [PubMed] [Google Scholar]
- 16.Jiang FX, Naselli G, Harrison LC. Distinct distribution of laminin and its integrin receptors in the pancreas. J Histochem Cytochem. 2002;50:1625. doi: 10.1177/002215540205001206. [DOI] [PubMed] [Google Scholar]
- 17.Kaido T, Yebra M, Cirulli V, et al. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 2004;279:53762. doi: 10.1074/jbc.M411202200. [DOI] [PubMed] [Google Scholar]
- 18.Paraskevas S, Maysinger D, Wang R, et al. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20:270. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Thomas F, Wu J, Contreras JL, et al. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130:333. doi: 10.1067/msy.2001.116413. [DOI] [PubMed] [Google Scholar]
- 20.Thomas FT, Contreras JL, Bilbao G, et al. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126:299. [PubMed] [Google Scholar]
- 21.Lucas-Clerc C, Massart C, Campion JP, et al. Long-term culture of human pancreatic islets in an extracellular matrix: Morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- 22.Blomeier H, Zhang X, Rives C, et al. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82:452. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Zhang X, Larson C, et al. The epididymal fat pad as a transplant site for minimal islet mass. Transplantation. 2007;84:122. doi: 10.1097/01.tp.0000266909.58117.e3. [DOI] [PubMed] [Google Scholar]
- 25.Jang JH, Bengali Z, Houchin TL, et al. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77:50. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park GE, Pattison MA, Park K, et al. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005;26:3075. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Baker SE, DiPasquale AP, Stock EL, et al. Morphogenetic effects of soluble laminin-5 on cultured epithelial cells and tissue explants. Exp Cell Res. 1996;228:262. doi: 10.1006/excr.1996.0325. [DOI] [PubMed] [Google Scholar]
- 28.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 29.Dufrane D, van Steenberghe M, Guiot Y, et al. Streptozotocin-induced diabetes in large animals (pigs/primates): Role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81:36. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 30.Cheung BW, Cartier LL, Russlie HQ, et al. The application of sample pooling methods for determining AUC, AUMC and mean residence times in pharmacokinetic studies. Fundam Clin Pharmacol. 2005;19:347. doi: 10.1111/j.1472-8206.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 31.Mattsson G, Jansson L, Nordin A, et al. Impaired revascularization of transplanted mouse pancreatic islets is chronic and glucose-independent. Transplantation. 2003;75:736. doi: 10.1097/01.TP.0000052592.92966.FE. [DOI] [PubMed] [Google Scholar]
- 32.Hammar E, Parnaud G, Bosco D, et al. Extracellular matrix protects pancreatic beta-cells against apoptosis: Role of short- and long-term signaling pathways. Diabetes. 2004;53:2034. doi: 10.2337/diabetes.53.8.2034. [DOI] [PubMed] [Google Scholar]
- 33.Jiang FX, Harrison LC. Extracellular signals and pancreatic beta-cell development: A brief review. Mol Med. 2002;8:763. [PMC free article] [PubMed] [Google Scholar]
- 34.Hayek A, Lopez AD, Beattie GM. Enhancement of pancreatic islet cell monolayer growth by endothelial cell matrix and insulin. In Vitro Cell Dev Biol. 1989;25:146. doi: 10.1007/BF02626171. [DOI] [PubMed] [Google Scholar]
- 35.Bosco D, Meda P, Halban PA, et al. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: Role of alpha6beta1 integrin. Diabetes. 2000;49:233. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- 36.Kaido T, Yebra M, Cirulli V, et al. Impact of defined matrix interactions on insulin production by cultured human beta-cells: Effect on insulin content, secretion, and gene transcription. Diabetes. 2006;55:2723. doi: 10.2337/db06-0120. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ihm SH, Matsumoto I, Sawada T, et al. Effect of donor age on function of isolated human islets. Diabetes. 2006;55:1361. doi: 10.2337/db05-1333. [DOI] [PubMed] [Google Scholar]
- 39.Rucker M, Laschke MW, Junker D, et al. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006;27:5027. doi: 10.1016/j.biomaterials.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Olsson R, Maxhuni A, Carlsson PO. Revascularization of transplanted pancreatic islets following culture with stimulators of angiogenesis. Transplantation. 2006;82:340. doi: 10.1097/01.tp.0000229418.60236.87. [DOI] [PubMed] [Google Scholar]
- 41.Tian B, Li Y, Ji XN, et al. Basement membrane proteins play an active role in the invasive process of human hepatocellular carcinoma cells with high metastasis potential. J Cancer Res Clin Oncol. 2005;131:80. doi: 10.1007/s00432-004-0614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]