Abstract
Objectives
The aim of this study was to assess the pharmacokinetics and tolerability of Biolimus A9 eluted from Nobori coronary stents.
Background
The release kinetics and pharmacokinetics of drugs delivered via coronary stents have been shown to play an essential role in the efficacy and safety of drug eluting stents.
Methods
20 patients with coronary artery disease were treated with single 14 mm (10 patients) or 28 mm long stent (10 patients). Blood samples were drawn at 16 time points to determine the pharmacokinetics of Biolimus A9. At seven time points, complete laboratory and toxicology panels were assessed to screen for potential Biolimus A9 toxicity. The primary endpoint of the study was the systemic blood concentrations of Biolimus A9 after 28 days and 6 months as measured using highly specific and sensitive liquid chromatography- tandem mass spectrometry assay.
Results
At 28 days, 6 patients (30%) had quantifiable Biolimus A9 concentrations in blood. The highest Biolimus A9 blood concentration measured in any sample was 32.2 pg/mL. The median time to maximum concentration was 2 hours, ranging from 0.05 hours to 3 months. Six months after stent implantation, only 1 of 20 patients had measurable Biolimus A9 concentrations at the lowest level of quantification, while at 9 months no sample had quantifiable Biolimus A9 concentrations. Laboratory and toxicology assessments did not indicate any impact of Biolimus A9 on the evaluated parameters.
Conclusion
Results of this study suggest that systemic exposure to Biolimus A9 was very low and that Biolimus A9 was well tolerated.
Keywords: coronary restenosis, angioplasty, drug-eluting stent, Biolimus A9, pharmacokinetics
Introduction
Stent-based drug delivery is a revolutionary approach to mitigate hyperplastic growth of smooth muscle cells after injury induced by percutaneous procedure and stent implantation1–4. The success of drug eluting stents (DES) is associated with effective delivery of potent therapeutics to the target site in a biologically active form at an effective concentration over a sufficiently long time period. Therefore, rational design and optimization of drug eluting stents is a complex process that requires careful consideration of factors that govern local pharmacokinetics within the arterial wall, such as drug distribution, drug physicochemical properties, local biological tissue properties and stent design.
Unlike currently approved drug eluting stents utilizing drugs originally developed for other indications, Biolimus A9 has specifically been developed for local delivery to coronary arteries5. Biolimus A9 is a novel rapamycin derivative that, like sirolimus, inhibits smooth muscle cell proliferation via binding to the FK-binding protein and subsequent inhibition of the mammalian target of rapamycin (mTOR)6–8. The chemical structure of Biolimus A9 (C55H87NO14, molecular weight 986.28 Da) consists of a 31-membered triene macrolide lactone that preserves the core sirolimus ring structure with a 2-ethoxyethyl group addition to the hydroxy group at position C(40) of the sirolimus molecule (Figure 1). One rationale for the ethoxyethyl group was to increase lipophilicity and, thus, to improve uptake by the coronary vessel wall and reduce risk of systemic immunosuppression and toxicity.
Figure 1.
The newly developed Nobori Biolimus A9 eluting stent (Terumo Europe, Leuven, Belgium) has several unique features. The most important are biodegradable polymer carrier (poly lactic acid), and coating only on the abluminal stent surface. The later feature allows direct release of Biolimus A9 into the vessel wall and, enhanced by its high lipophilicity, fast uptake by the surrounding tissue. A minimal amount of drug is expected to be released into peripheral circulation.
Recently conducted clinical trials with Biolimus A9 eluting stents have demonstrated its high efficacy in reducing late lumen loss post coronary intervention6,9–13.
NOBORI PK was the first human study to evaluate pharmacokinetics of Biolimus A9 eluted from Nobori stents. The second objective of this study was to determine tolerability and safety of this new pharmacological compound as assessed by a series of hematology and biochemistry laboratory tests and thorough a frequent adverse events monitoring.
METHODS
Study Design and Patients
The study was a prospective, non-randomized, open-label multicenter trial conducted in three centers (see Annex 1). Twenty patients were included in the study.
The study was conducted in full compliance with Good Clinical Practice (1996), the Declaration of Helsinki, ISO 14155 and all other applicable regulatory requirements. The study protocol was approved by the ethics committees of all participating centers, by the Agency for Drugs and Medical Devices, and Ministry of Health of Serbia. Study risk and benefits were explained to all patients and they were enrolled only after having signed informed consent. The patients were free to withdraw from the study for any reason at any time.
Patients who were at least 18 years of age, with ischemic heart disease due to de novo lesions in native coronary arteries were considered for enrolment. Angiographic inclusion criteria were: a reference vessel diameter of 2.5 mm to 3.5 mm and a lesion length ≥ 5 mm and ≤ 25 mm. Major clinical exclusion criteria were: left ventricular ejection fraction <30%, myocardial infarction (MI) within the preceding 48 hours, intolerance to aspirin, heparin, clopidogrel bisulfate, ticlopidine, and drugs similar to Biolimus A9 (sirolimus, everolimus, zotarolimus), paclitaxel, contrast media, and stainless steel; platelet count <100,000 or >700,000 cells/mm3 or a white blood cell count <3,000 cells/mm3, serum creatinine concentrations >2.0mg/dL (or >150 μmol/L); current participation in other investigational trials; any coronary interventional procedure within 30 days before or planned within 60 days after the implantation of study stent; planned surgery within 6 months; stroke or transient ischemic attack within the previous 3 months; gastrointestinal bleeding. The main angiographic exclusion criteria were significant (>50%) stenosis proximal or distal to the treated lesion; previous stenting anywhere in the territory of treated vessel; any DES implanted one year preceding the intervention, total occlusion (TIMI flow 0 and I), left main or ostial target lesion, severe calcification; evidence of thrombus or severe tortuousity.
Patients who met all of the inclusion criteria and none of the exclusion criteria were considered for enrolment into the study.
The treatment of vessels other than the target vessel was allowed during the same procedure, but only bare metal stents could be implanted.
In the initial protocol, clinical follow up at six time points was scheduled (48 h, 7 and 28 days, 3, 6 and 9 months), however the protocol was amended to extend the follow-up period to 5 years with yearly assessments.
Study Device
The Nobori drug eluting stent system comprises four components: the stainless steel S-stent and its delivery catheter, a drug carrier, poly-lactic acid (PLA), and an anti-proliferative drug compound, Biolimus A9 (Biosensors International, Ltd. Singapore). PLA has been used in a variety of medical applications and the final products of its degradation are carbon dioxide and water. The Nobori drug-eluting stent is coated only abluminally with the matrix containing Biolimus A9 and PLA (15.μg each per 1 millimeter of stent length). The drug-polymer matrix is designed to release the drug as the polymer degrades over 6–8 months.
Study Endpoints
The primary study endpoint was the Biolimus A9 concentration at 28 days, and 6 months after Nobori stent implantation.
The following secondary endpoints were assessed: The values of major biochemistry and hematology parameters at 48 h, 7 days, 28 days, 3, 6 and 9 months after stent implantation as compared to baseline values; the development of any of the following known potential Biolimus A9 toxicities: nausea, diarrhea, fever, rash, epistaxis, bruising and itching. Other adverse events including major adverse clinical events, such as death, myocardial infarction, and surgical or percutaneous target vessel revascularization, were also recorded.
Intervention
All patients were pre-treated with aspirin and clopidogrel or ticlopidine. Non-target lesions, if any, were treated first with bare-metal stents, followed by treatment of the target lesions with Nobori Biolimus A9-eluting stents. All lesions were predilated. Clopidogrel or ticlopidine were continued for at least 12 weeks. Available Nobori stent sizes were 14 and 28 mm long and 2.5, 3.0 and 3.5 mm in diameter. The amounts of Biolimus A9 on the individual implanted stents are listed in Table 3.
Table 3.
Nobori Stent Sizes, Biolimus A9 Doses and Pharmacokinetics.
| Subject | Stent | Total Exposure Biolimus A9 | t_max | Cmax | t_last | C_last | AUC_obs | C_28d | C_6M | C_9M |
|---|---|---|---|---|---|---|---|---|---|---|
| (μg) | [h] | [pg/mL] | [h] | [pg/mL] | [pg/mL·h] | [pg/mL] | [pg/mL] | [pg/mL] | ||
| 10_11 | 3.5×28mm | 440 | ND | <LLOQ | ND | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| 10_12 | 3.5×14mm | 230 | ND | <LLOQ | ND | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| 10_13 | 2.5×28mm | 451 | 3 | 11,4 | 3 | 11,4 | 8,2 | Missing | <LLOQ | <LLOQ |
| 10_15 | 3.0×28mm | 451 | 1 | 25,9 | 672 | 11,5 | 8289,9 | 11,5 | <LLOQ | <LLOQ |
| 10_16 | 2.5×14mm | 225 | 0,5 | 25,8 | 168 | 20,8 | 2855,3 | <LLOQ | <LLOQ | <LLOQ |
| 10_17 | 2.5×28mm | 451 | 1 | 32,2 | 48 | 10,7 | 766,9 | <LLOQ | <LLOQ | <LLOQ |
| 30_30 | 3.5×28mm | 440 | 0,05 | 24,3 | 3 | 10,6 | 38,0 | <LLOQ | <LLOQ | <LLOQ |
| 30_31 | 3.5×14mm | 230 | 1 | 15,9 | 672 | 13,0 | 4626,2 | 13,0 | <LLOQ | <LLOQ |
| 30_32 | 3.0×14mm | 225 | 2 | 25,6 | 672 | 11,1 | 7125,5 | 11,1 | <LLOQ | <LLOQ |
| 30_33 | 3.0×14mm | 225 | 0,05 | 22,1 | 168 | 15,9 | 2865,4 | <LLOQ | <LLOQ | <LLOQ |
| 30_34 | 3.0×28mm | 451 | 2 | 30,4 | 168 | 13,0 | 2663,4 | <LLOQ | <LLOQ | <LLOQ |
| 40_40 | 2.5×14mm | 225 | 3 | 12,5 | 48 | 10,9 | 520,9 | <LLOQ | <LLOQ | <LLOQ |
| 40_41 | 3.0×14mm | 225 | 1 | 11,7 | 2160 | 11,6 | 8637,7 | <LLOQ | <LLOQ | <LLOQ |
| 40_42 | 3.0×28mm | 451 | 3 | 10,3 | 2160 | 10,3 | 8025 | <LLOQ | <LLOQ | <LLOQ |
| 40_44 | 3.5×28mm | 440 | ND | <LLOQ | ND | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| 40_45 | 3.5×28mm | 440 | 2160 | 30,3 | 2160 | 30,3 | 33096,5 | 10,4 | <LLOQ | <LLOQ |
| 40_46 | 3.0×14mm | 225 | 3 | 17,7 | 168 | 14,6 | 1222,6 | <LLOQ | <LLOQ | <LLOQ |
| 40_47 | 3.5×14mm | 230 | 2 | 17,7 | 4320 | 10,3 | 22881,5 | 10,9 | 10,3 | <LLOQ |
| 40_49 | 3.0×28mm | 451 | 672 | 17,0 | 2160 | 15,7 | 29378,7 | 17,0 | <LLOQ | <LLOQ |
| mean | 336,55 | 178,4 | 17,4 | 984,4 | 11,7 | 7000,0 | 4,1 | 0,5 | <LLOQ | |
| SD | 112,99 | 554,3 | 10,2 | 1246,1 | 7,0 | 10156,4 | 6,1 | 2,4 | ||
| CV% | 310,7 | 58,8 | 126,6 | 60,3 | 145,1 | 149,1 | 435,9 | |||
| median | 2,0 | 17,7 | 420,0 | 11,4 | 2855,3 | <LLOQ | <LLOQ | |||
| minimum | 225 | 0,05 | <LLOQ | 3 | <LLOQ | <LLOQ | <LLOQ | <LLOQ | ||
| maximum | 451 | 2160 | 32.2 | 4320 | 30,3 | 33096,5 | 17.0 | 10,3 | ||
| 14 mm | ||||||||||
| mean | 225,6 | 1,6 | 16,6 | 1047,0 | 12,0 | 5637,2 | 3,9 | 1,1 | <LLOQ | |
| SD | 2,4 | 1,1 | 8,0 | 1490,4 | 5,6 | 7092,9 | 5,9 | 3,4 | ||
| CV% | 70,5 | 48,5 | 142,3 | 46,4 | 125,8 | 150,7 | 300,0 | |||
| median | 225 | 1,5 | 17,7 | 420,0 | 11,6 | 2865,4 | <LLOQ | <LLOQ | ||
| minimum | 225 | 0,05 | <LLOQ | 48 | <LLOQ | <LLOQ | <LLOQ | <LLOQ | ||
| maximum | 230 | 3 | 25,8 | 4320 | 20,8 | 22881,5 | 13.0 | 10,3 | ||
| 28 mm | ||||||||||
| mean | 446,6 | 355,3 | 18,2 | 921,8 | 11,4 | 8226,7 | 4,3 | <LLOQ | <LLOQ | |
| SD | 5,7 | 766,0 | 12,3 | 1047,6 | 8,4 | 12572,1 | 6,7 | |||
| CV% | 215,6 | 67,6 | 113,7 | 74,3 | 152,8 | 155,5 | ||||
| median | 451 | 2,5 | 20,7 | 420,0 | 11,1 | 1715,2 | <LLOQ | |||
| minimum | 440 | 0,05 | <LLOQ | 3 | <LLOQ | <LLOQ | <LLOQ | |||
| maximum | 451 | 2160 | 32,2 | 2160 | 30,3 | 33096,5 | 17.0 | |||
Only one Nobori stent was allowed to be implanted per patient. In case of a dissection, a bare metal stent implantation was mandatory.
Assessments
Blood samples (3 mL) for the measurements of Biolimus A9 concentrations in whole blood were collected into 3 mL EDTA plastic tubes (Venosafe, Terumo Europe, Leuven, Belgium) before the procedure and after 2–5, 15, 30, 45 min, 1, 2, 3, 8, 24, 48, and 72 hrs, 7 and 28 days, 3, 6 and 9 months. Blood samples were immediately snap frozen in a mixture of dry ice and isopropanol, and stored in good laboratory practice (GLP) compliant freezers at −80°C until shipped on dry ice to the central analytical laboratory at the University of Colorado Health Sciences Center by specialized courier service (World Courier). Sample stability during transport was established by shipment and analysis of blank blood samples that were enriched with known amounts of Biolimus A9. No significant deviations from the nominal concentrations after shipment were noted confirming stability of Biolimus A9 in blood during shipment.
Blood samples for blood chemistry, biochemistry and hematology were collected before the procedure and 48 h, 7 and 28 days, 3, 6 and 9 months after stent implantation.
Before hospital discharge and at all follow-up visits the angina status, concomitant medications and any adverse event that occurred at and after the stenting procedure were recorded. All adverse events were classified as mild, moderate or severe and possibly, probably or definitely drug/device-related.
Sample Processing and Analysis of Biolimus A9 Concentrations by Liquid Chromatography- Tandem Mass Spectrometry (LC-MS/MS)
Biolimus A9 was quantified in blood using a validated modification of an LC-MS/MS assay that was originally developed for sirolimus and several of its derivatives14,15 with a lower limit of quantification of 10 pg/mL.
Extraction procedure
The only manual step during the extraction of blood samples was protein precipitation. The protein precipitation solution (methanol/0.2 M ZnSO4, 7:3, v/v) contained the internal standard zotarolimus (Abbott, Abbott Park, IL) at a concentration of 4 ng/mL. Zotarolimus was chosen since the other alternatives sirolimus and everolimus were found to be Biolimus A9 metabolites as well as potential degradation products. Three hundred kL protein precipitation solution was added to 300 kL blood. After vortexing (5 min) and centrifugation (4°C, 8000 g, 5 min), the supernatant was transferred into a glass HPLC vial.
Three hundred microliters of the samples were injected onto a 10 · 2 mm extraction column (Keystone Scientific, Bellefonte, PA) filled with Hypersil ODS-1 of 10 μm particle size (Shandon, Chadwick, UK). Samples were washed with a mobile phase of 20% methanol and 80% 0.1% formic acid. The flow was 5 mL/min and the temperature for the extraction column was set to 65°C. After 1 min, the switching valve was activated and the analytes were eluted in the backflush mode from the extraction column onto a 50 · 4.6 mm C8, 3.5 7μm analytical column (Zorbax XDB C8, Agilent Technologies, Palo Alto, CA). The mobile phase consisted of methanol and 0.1% formic acid supplemented with 1 μmol/L sodium formate. The following gradient was run: time 0 min: 87% methanol, 2 min: 100% methanol, 3.5 min: 100% methanol. The MS/MS was run in the positive mode. For Biolimus A9, the following ion pair was detected: m/z= 1008.6 [M+Na]+ → 409.5. Zotarolimus, the internal standard, was detected using the transition m/z= 988.6 [M+Na]+→ 369.5.
After the analysis was completed, peaks were integrated and the results were printed. Biolimus A9 concentrations were corrected based on the internal standard and quantified using the calibration curves that were included in each batch. All calculations were carried out by the Applied Biosystems Analyst Software (version 1.4.1.).
Assay Performance and Stability
The lower limit of quantitation (LLOQ) was 10 pg/mL and the assay was linear from 0.01–100 ng/mL (r2 ≥ 0.99). At the tested concentration levels of 0.025 ng/mL, 0.1 ng/mL, and 1 ng/mL intra-day accuracies in human EDTA blood were between 100.6%, 96.8% and 86.4%, respectively, and intra-day precisions were 5.3%, 6.8% and 3.7%. Inter-day accuracies were between 96.8%, 102.3% and 98.1%, and inter-day precisions 12.7%, 7.1% and 7.7%. No matrix interferences, ion suppression, or carry-over was detected. Biolimus A9 in extracted samples was stable in the autosampler for at least 24 hours and blood samples could undergo three freeze-thaw cycles.
Statistical and Pharmacokinetic Analyses
Sample size
This was a non-randomized, single arm, clinical trial with the primary endpoints Biolimus A9 concentrations at 28 days and 6 months after stent implantation. Since it was a single arm, observational study, the statistical analysis was mainly descriptive. The number of observations was within the typical range for this type of pharmacokinetics study.
Pharmacokinetics
Data were analyzed after all queries had been successfully resolved and the database had been locked. Pharmacokinetic parameters were evaluated using non-compartmental analysis as implemented in the WinNonlin software (version 5.0 professional, Pharsight, Mountain View, CA). The following pharmacokinetic parameters were estimated: AUC0-τ: area-under-the-time-concentration curve over the observation period, Clast: the last quantifiable concentration, Cmax: maximum concentration, tlast: the time of the last quantifiable concentration, tmax: time-to-maximum concentration. In several samples of one patient clots were observed and the Biolimus A9 concentrations for this patient was not included in the pharmacokinetic analysis. The 3, 6, and 9-month samples of this patient could be analyzed and all Biolimus A9 concentrations were found below the LLOQ. Thus, 19 patients were included in the pharmacokinetic analysis, but all 20 in the clinical and safety analysis.
Statistical Analysis
Data were entered into and analyzed using the SAS software package (version 9.1., SAS Institute, Cary, NC). If not mentioned otherwise, means ± standard deviations are reported.
RESULTS
Twenty patients with de novo stenosis of native coronary arteries were enrolled in the study. The study had 100% follow-up compliance at all time points. The mean age of the patients was 53.7± 7.8 years. Cardiovascular risk factors in the study patient population included high prevalence of hypertension (70%), hypercholesterolemia (90%), history of cigarette smoking (95%) and prior myocardial infarction (55%). For further details, please see Table 1. The lesion characteristics are shown in Table 2. All stents were successfully implanted, but one patient suffered non-Q-Wave myocardial infarction due to a long spiral dissection which required implantation of two additional bare metal stents.
Table 1.
Baseline Demographics and Clinical Characteristics
| Number of Patients | 20 |
| Age | 53.7±7.8 |
| Male Gender, % | 75.5 |
| Left Ventricular Ejection Fraction, % | 53.7 ± 9.4 |
| Angina status, % (n/total) | |
| Asymptomatic | 5(1/20) |
| Stable Angina | 70 (14/20) |
| Unstable Angina | 25 (5/20) |
| Single Vessel Disease, %(n/total) | 45 (9/20) |
| Multiple Vessel Disease, %(n/total) | 55 (11/20) |
| History of MI, %(n/total) | 55 (11/20) |
| History prior PCI, %(n/total) | 10 (2/20) |
| History prior CABG, %(n/total) | 5 (1/20) |
| History Diabetes, %(n/total) | 20 (4/20) |
| History Hypertension, %(n/total) | 70 (14/20) |
| History Cigarette Smoking, %(n/total) | 95 (19/20) |
| Previous | 68 (13/19) |
| Current | 32 (6/19) |
| Hypercholesterolemia, %(n/total) | 90 (18/20) |
Table 2.
Procedural and Lesion Characteristics
| Coronary Artery Treated, | % (n/total) |
| Left Anterior Descending | 50 (10/20) |
| Right Coronary Artery | 35 (7/20) |
| Left Circumflex | 15 (3/20) |
| Type of Lesion¨* | |
| A | 25 (5/20) |
| B1 | 20 (4/20) |
| B2 | 45 (9/20) |
| C | 10 (2/20) |
| Procedure Success | 95 (19/20) |
| Device Success | 100 (20/20) |
AHA/ACC: American Heart Association/American College of Cardiology
Biolimus A9 Concentrations
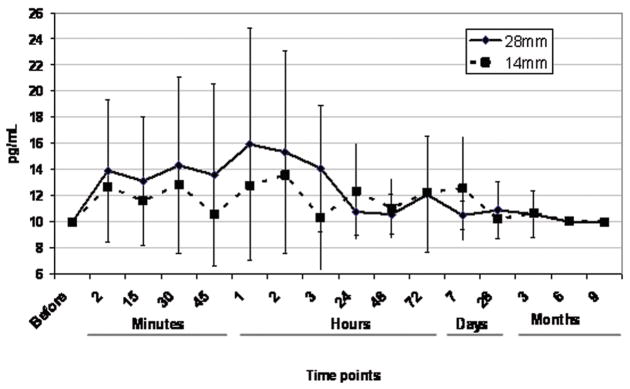
After 28 days, 6 of 20 subjects (30%) had measurable concentration of Biolimus A9. The median concentration was 17.7 pg/mL with concentrations ranging from below LLOQ to 32.2 pg/mL (Table 4). The median concentration was 16.6 pg/mL and 18.2 pg/mL for patients with 14 mm or 28 mm, respectively. Those values were not significantly different. Nine months after stent implantation, Biolimus A9 concentrations were below the LLOQ in all samples.
The key pharmacokinetic parameters are shown in Table 3 and Biolimus A9 time-concentration curves are shown in Figure 2. Three patients did not have quantifiable Biolimus A9 concentrations in any of the blood samples collected. The time-to-peak concentration (tmax) showed large variability with a median of 2.0 hours and a range of 0.05 hours to 3 months (only patients with quantifiable Biolimus A9 concentrations were included in this analysis). The highest measured blood concentration at any time point was 32.2 pg/mL. The median maximum concentration (Cmax) was 17.7 pg/mL and ranged from <LLOQ to 32.2 pg/mL. The median systemic exposure as measured by the area-under-the-time-concentration curve (AUC) over the observation period was 2.9 ng/mL·h and ranged from <LLOQ to 33.1 ng/mL·h. There was also no difference in systemic exposure (AUC) between patients having 14 mm or 28 mm stents implanted. No evidence for early or late bursts of Biolimus A9 release was detected in the blood samples.
Figure 2.
Clinical Follow-up and Safety
Clinical and laboratory follow-ups showed good safety and tolerability of Biolimus A9. No significant changes compared with baseline were observed in any of the following parameters: red blood cells, hemoglobin, hematocrit, leukocytes, platelets, sodium, potassium, GOT, chloride, calcium, creatinine, and triglycerides. There was significant increase of creatine kinase and its MB fraction in one patient immediately after the procedure. This patient suffered non-Q-wave myocardial infarction due to a long spiral dissection. In several patients there was a decline in cholesterol levels, most likely because these patients were administered lipid lowering therapy after stent implantation. No patients reported any of the following: nausea, diarrhea, fever, rush, epistaxis, bruising or itching between hospital discharge and the 9-month follow-up visit. As of today, all patients in this study have been followed up for 2 years without reporting any potentially Biolimus A9-related adverse events nor major adverse cardiac events.
DISCUSSION
In this study, Biolimus A9 was found to be safe and well tolerated. The highest Biolimus A9 blood concentration measured in all samples was 32.2 pg/mL. The maximum concentrations of Biolimus A9 in patients after Nobori stent implantation is two orders of magnitude lower than that resulting from the lowest intravenous dose (0.0025 mg/kg) tested in a recently completed Biolimus A9 single ascending dose study in human (publication in preparation). No drug related adverse events were observed at this dose and the average maximum blood concentration in this study was 5.2 ± 4.4 ng/mL and thus 161-fold higher than the highest Biolimus A9 blood concentration measured in the present study after Nobori stent implantation.
The maximum concentrations recorded in our study were significantly lower than described by Vetrovec at al. for sirolimus eluting stents16. The maximum sirolimus concentration after implantation of a single sirolimus eluting stent in this study was 570 ± 120 pg/mL and after implantation of two stents 1005 ± 390 pg/mL. After 7 days, 17 out of 19 subjects still had sirolimus blood concentrations higher than 200 pg/mL. In comparison, the Cmax of Biolimus A9 in our study was 17.4 ± 10.2 pg/mL and thus 33–58- fold lower. The sirolimus AUC0-τ after implantation of a single Cypher stent was 55.1 ± 15.5 ng/mL·h (mean± standard deviation), while the Biolimus A9 AUC0-τ after implantation of a single Nobori stent was only 7.0± 10.2 ng/mL·h. The total drug content after implantation of two 18 mm long Cypher stents is similar to the content of one 28 mm long Nobori stent.
It has to be noted that the current pharmacokinetic analysis has to be interpreted with caution since most concentrations were close to the lower limit of quantitation of the analytical assay (LLOQ, 10 pg/mL). It is very likely that especially early after stent implantation, Biolimus A9 concentrations listed as <LLOQ were not 0 pg/mL as it had to be assumed for calculation of the distribution statistics, but were rather between >0 and <10 pg/mL. It should also be taken into account that the analytical LC-MS/MS assay used in this study used the most sensitive analytical equipment available and was an order of magnitude more sensitive than any other assay used in the literature for similar studies.16
The high lipophilicity of Biolimus A9 (~10-fold higher than sirolimus) and the coating of the stent on the surface contacting the coronary artery wall, but not on the lumen side, contributed to a high diffusion rate into the tissue and low systemic exposure and blood concentrations. It seems reasonable to assume that Biolimus A9’s high lipophilicity and tight binding to its intracellular binding protein, the FK-binding protein, create a drug reservoir in the target tissue. Since the Nobori stent is a targeted sustained-drug release device, in addition to drug distribution and elimination, Biolimus A9 pharmacokinetics is significantly affected by polymer degradation and drug elution from the stent matrix.
There was no correlation between stent length or the Biolimus A9 amount on the stent and systemic drug exposure as measured by AUC. It can be speculated that one of the reasons is that due to the coating pattern of the stents, most of the drug is released from the stent directly into the surrounding coronary tissue. Based on the physicochemical properties of the drug, the stent design with only abluminal coating and our results, it can be hypothesized that drug concentrations in blood are governed by the release rates from the tissue reservoirs rather than being driven by elution from the stent.
This study was not powered to provide information related to clinical outcomes after implantation of Nobori stents. However, absence of any treatment-related adverse events between stent implantation and two year follow-up visits are worth noting.
Currently available clinical data with Nobori stents showed its excellent efficacy and safety11–13. Particularly appealing findings were extremely low rates of target lesion revascularization with complete absence of stent thrombosis up to 2 years follow-up of patients enrolled in NOBORI trials. Another interesting finding is better preserved endothelial function in coronary arteries treated with Nobori stent, contrary to the arteries treated with first generation drug eluting stents13. It remains to be seen during further follow-up visits and in larger studies as to whether the favorable release kinetics resulting in very low systemic exposure to Biolimus A9 in combination with a bioresorbable polymer keeps translating into good long term safety and efficacy.
CONCLUSIONS
Overall, the results of our Biolimus A9 pharmacokinetics study after Nobori stent implantation support the hypothesis that the higher lipophilicity of Biolimus A9 in combination with coating only on the surface of the stent that is in direct contact with the coronary vessel wall leads to more specific distribution into the target tissue and low systemic exposure. It is reasonable to expect that the low exposure to Biolimus A9 is the reason for the good tolerability and safety profile in the current study, in which not a single case of Biolimus A9-related toxicity was observed.
Acknowledgments
This study was supported by a grant from Terumo Corporation and the United States National Institutes of Health (NIH), grants R01 DK065094 (U.C.), 5 P30 DK048520, Mass Spectrometry Core (U.C.).
We are grateful to all patients who agreed to participate in this study and to Jamie Bendrick-Peart for samples receipt and analysis, M. Markovic, M. Lukic, V. Perovic, S. Popov, V. Erdelji, R. Simonovic, D. Udovica, S. Draskovic, C. Gavrancic, M. Vuckovic, who took greatest care to ensure that samples are frozen, stored and shipped under the most appropriate conditions and to all technicians and support staff in participating hospitals. Special thanks to H. Nagai, K. Ishihara, V. Borovicanin, C. Kreutz, N. Saito, K. Senshu and C. Dragos for invaluable help throughout the study.
Abbreviations
- t_max
time to maximum concentration
- Cmax
maximum concentration
- t_last
time to last quantifiable biolimus A9 concentration (< 10 pg/mL)
- C_last
last quantifiable biolimus A9 concentration
- AUC_obs
observed area-under-the-time-concentration curve (0--> T15 or T16, if applicable)
- C_28d
biolimus A9 concentration after 28 days (primary study end point)
- C_6M
biolimus A9 concentration after 6 months (primary study end point)
- C_9M
biolimus A9 concentration after 9 months (last time point)
- SD
standard deviation
- CV%
coefficient of variance
- ND
not determined since WinNonlin was unable to estimate the elimination constant
Non-standard abbreviations
- AUC
area-under-the-time-concentration curve
- Cmax
maximum blood concentration
- DES
drug-eluting stent
- HPLC
high-performance liquid chromatography
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LLOQ
lower limit of quantitation
- MACE
major adverse cardiac adverse events
- tmax
time to maximum blood concentration
Annex 1
NOBORI PK Study sites and investigators
Clinical Centre Serbia, Belgrade
M. Ostojic, M. Nedeljkovic, S. Stojkovic, B. Beleslin
Institute for Cardiovascular Disease Dedinje, Belgrade
D. Sagic, B. Milosavljevic, LJ. Mangovski, Z. Antonic, M. Colic, D. Topic
Institute for Cardiovascular Disease, Sremska Kamenica
R. Jung, D. Benz, D. Debeljacki, V. Ivanovic, D. Bikicki
Footnotes
For the calculation of distribution statistics, all values <LLOQ were assumed to be "0 pg/mL". All values are not normally distributed. Thus, means and standard deviations have to be interpreted with caution. Median and range (highlighted) are more relevant.
References
- 1.Anis RR, Karsch KR, Oberhoff M. An update on clinical and pharmacological aspects of drug-eluting stents. Cardiovasc Hematol Disord Drug Targets. 2006;6:245–255. doi: 10.2174/187152906779010755. [DOI] [PubMed] [Google Scholar]
- 2.Slavin L, Chhabra A, Tobis JM. Drug-eluting stents: preventing restenosis. Cardiol Rev. 2007;15:1–12. doi: 10.1097/01.crd.0000200844.16899.fc. [DOI] [PubMed] [Google Scholar]
- 3.Morice MC, Serruys PW, Sousa JE for the RAVEL study group. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 4.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 5.Grube E, Buellesfeld L. BioMatrix Biolimus A9-eluting coronary stent:: a next-generation drug-eluting stent for coronary artery disease. Expert Rev Med Devices. 2006;3:731–741. doi: 10.1586/17434440.3.6.731. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 7.Kahan BD. Sirolimus; a comprehensive review. Expert Opin Pharmacother. 2001;2:1903–1917. doi: 10.1517/14656566.2.11.1903. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 9.Grube E, Hauptman KE, Buellesfeld L, Lim V, Abizaid A. Six-month results of a randomized study to evaluate safety and efficacy of Biolimus A9 eluting stent with a bioerodable polymer coating. EuroInterv. 2005;1:53–57. [PubMed] [Google Scholar]
- 10.Costa RA, Lansky AJ, Abizaid A, Mueller R, Tsuchiya Y, Mori K, Cristea E, Leon MB, Sousa JE, Schmidt T, Hauptmann KE, Grube E. Angiographic results of the first human experience with the Biolimus A9 drug-eluting stent for de novo coronary lesions. Am J Cardiol. 2006;98:443–446. doi: 10.1016/j.amjcard.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier B, Serruys WP, Silber S, et al. Randomised comparison of Nobori, Biolimus A9-eluting coronary stent with a Taxus®, paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the Nobori 1 trial. EuroInterv. 2007;2:426–434. [PubMed] [Google Scholar]
- 12.Ostojic M, Sagic D, Belesli B, et al. for the NOBORI CORE Clinical Investigators. First clinical comparison of Nobori – Biolimus A9 eluting stent with Cypher – sirolimus eluting stent; NOBORI CORE 9 months angiographic and one year clinical outcomes. EuroInterv. 2008;3:574–579. doi: 10.4244/eijv3i5a103. [DOI] [PubMed] [Google Scholar]
- 13.Hamilos M, Ostojic M, Beleslin B, Sagic D, Magovski Lj, Stojkovic S, Nedeljkovic M, Orlic D, Milosavljevic B, Topic D, Karanovic N, Wijns W. Differential Effects of Drug Eluting Stents on Local Endothelium Dependent Coronary Vasomotion. J Am Coll Cardiol. 2008;51:2123–9. doi: 10.1016/j.jacc.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 14.Christians U, Jacobsen W, Serkova N, Benet LZ, Vidal C, Sewing KF, Manns MP, Kirchner GI. Automated, fast and sensitive quantification of drugs in blood by liquid chromatography-mass spectrometry with on-line extraction: immunosuppressants. J Chromatogr B. 2000;748:41–53. doi: 10.1016/s0378-4347(00)00380-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YL, Bendrick-Peart J, Strom T, Haschke M, Christians U. Development and validation of a high-throughput assay for quantification of the proliferation inhibitor ABT-578 using LC/LC-MS/MS in blood and tissue samples. Ther Drug Monit. 2005;27:770–778. doi: 10.1097/01.ftd.0000185766.52126.bd. [DOI] [PubMed] [Google Scholar]
- 16.Vetrovec GW, Rizik D, Williard C, Snead D, Piotrovski V, Kopia G. Sirolimus PK trial: a pharmacokinetic study of the sirolimus-eluting Bx Velocity stent in patients with de novo coronary lesions. Catheter Cardiovasc Interv. 2006;67:32–37. doi: 10.1002/ccd.20565. [DOI] [PubMed] [Google Scholar]