Abstract
During early fasting, increases in skeletal muscle proteolysis liberate free amino acids for hepatic gluconeogenesis in response to pancreatic glucagon. Hepatic glucose output diminishes during the late protein-sparing phase of fasting, when ketone body production by the liver supplies compensatory fuel for glucose-dependent tissues 1–4. Glucagon stimulates the gluconeogenic program by triggering the dephosphorylation and nuclear translocation of the CREB regulated transcription coactivator 2 (CRTC2; also known as TORC2), while parallel decreases in insulin signaling augment gluconeogenic gene expression through the de-phosphorylation and nuclear shuttling of Forkhead Box O1 (FOXO1) 5–7. Here we show that a fasting-inducible switch, consisting of the histone acetyl-transferase (HAT) P300 and the nutrient-sensing deacetylase Sirtuin 1 (SIRT1), maintains energy balance through the sequential induction of CRTC2 and FOXO1. Following glucagon induction, CRTC2 stimulated gluconeogenic gene expression through an association with P300, which we show here is also activated by de-phosphorylation at Ser89 during fasting. In turn, P300 increased hepatic CRTC2 activity by acetylating it at Lys628, a site that also targets CRTC2 for degradation following its ubiquitination by the E3 ligase Constitutive Photomorphogenic Protein (COP1) 8. Glucagon effects were attenuated during late fasting, when CRTC2 was down-regulated due to SIRT1-mediated deacetylation and when FOXO1 supported expression of the gluconeogenic program. Disrupting SIRT1 activity, by liver-specific knockout of the SIRT1 gene or by administration of SIRT1 antagonist, increased CRTC2 activity and glucose output, while exposure to SIRT1 agonists reduced them. In view of the reciprocal activation of FOXO1 and its coactivator peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC-1α) by SIRT1 activators 9–12, our results illustrate how the exchange of two gluconeogenic regulators during fasting maintains energy balance.
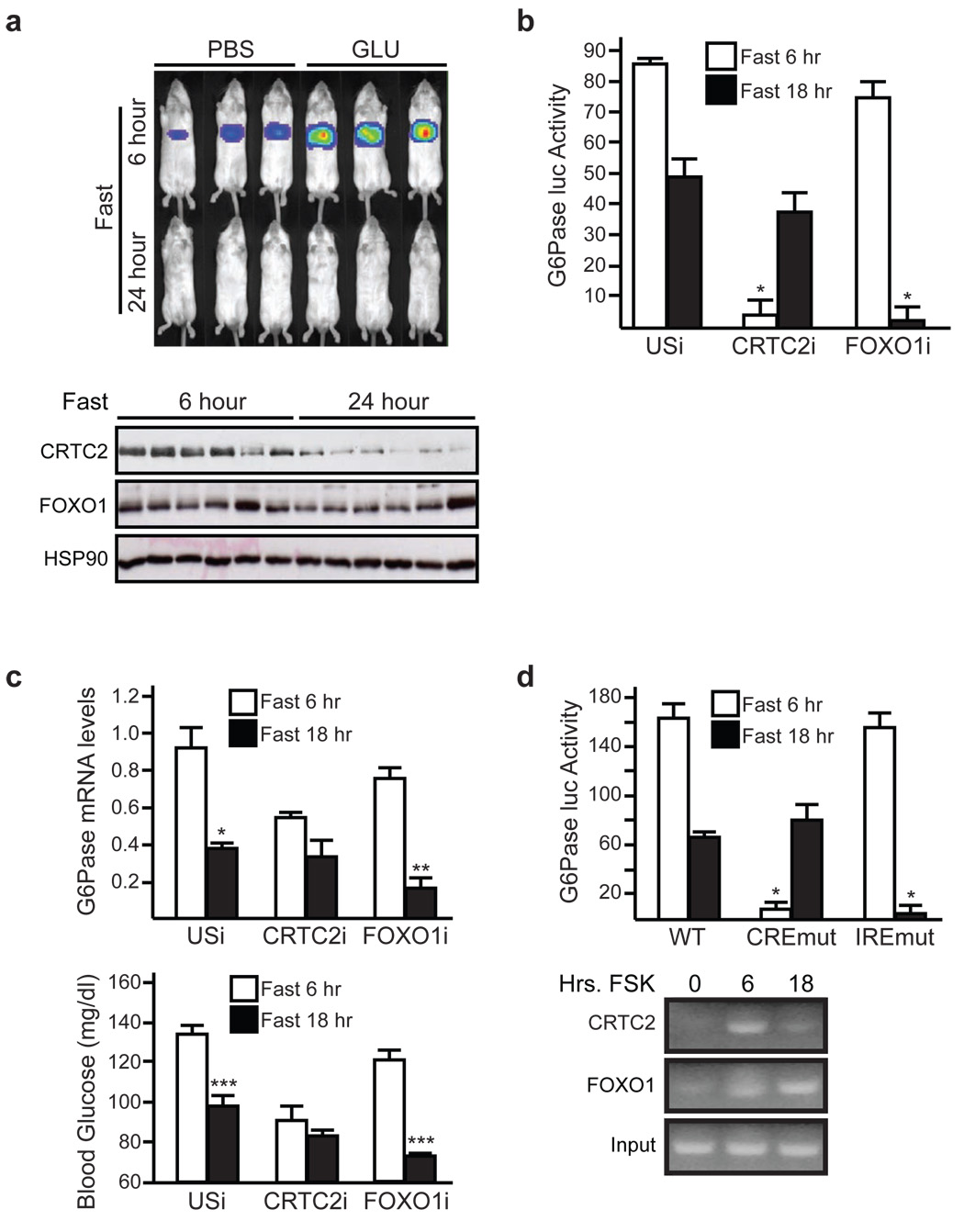
We compared the effects of short and long-term fasting on hepatic CRTC2 activity using an Adenoviral CRE-luciferase (Ad-CRE-luc) reporter. Fasting induced Ad-CRE-luc activity after 6 hours; these effects were augmented by intraperitoneal (IP) glucagon injection (fig. 1a, sup. fig. 1). Hepatic Ad-CRE-luc activity returned to near basal levels after 18–24 hours fasting, when circulating ketone bodies were highest and when hepatic gluconeogenesis was reduced (fig. 1a, top; sup. fig. 2) 13. In keeping with the decrease in gluconeogenic gene expression, hepatic CRTC2 protein amounts were also down-regulated in response to prolonged fasting (fig. 1a, bottom; sup. figs. 1 and 3).
Figure 1. Sequential activation of CRTC2 and FOXO1 during fasting.
a, Ad-CRE-luc activity (top) and CRTC2 protein amounts (bottom) in mice fasted for 6 or 24 hours. Intra-peritoneal glucagon injection indicated. b, and c, Effect of 6 or 18 hour fasting on Ad-G6Pase-luc activity (b), G6Pase mRNA amounts (c), and blood glucose concentrations (c) in mice infected with Ad-CRTC2i, Ad-FOXO1i, or (USi) control virus (n=4, (*) P< .05; (**) P< .02; (***) P< .01). d, Top, activities of wild-type or mutant Ad-G6Pase-luc reporters defective in CREB (CREmut) or FOXO1 (IREmut) binding. Mice were fasted for 6 or 18 hours as indicated (n=4, *;P<.05). Bottom, chromatin immunoprecipitation (ChIP) assay showing binding of myc-tagged FOXO1 or Flag-epitope tagged CRTC2 to the G6Pase promoter in HepG2 hepatocytes exposed to FSK for 6 or 18 hours. For panels b, c, d, data are means ± s.e.m.
The E3 ligase COP1 has been shown to silence the gluconeogenic program during refeeding through the ubiquitin-dependent degradation of CRTC2 8. Although undetectable in 6 hour fasted mice, ubiquitinated CRTC2 protein amounts increased after 18 hours fasting (sup. fig. 3). Indeed, prolonged exposure to glucagon triggered CRTC2 degradation in primary hepatocytes; these effects were blocked by treatment with proteasome inhibitor MG132 and by RNAi-mediated depletion of COP1 (sup. figs. 4, 5).
By contrast with CRTC2, hepatic FOXO1 protein levels remained constant during fasting, suggesting that these transcriptional regulators are differentially regulated (fig. 1a, bottom; sup. fig. 3). To test this idea, we used an Adenoviral G6Pase-luciferase (Ad-G6Pase-luc) reporter, which contains FOXO1 and CREB binding sites that mediate induction of the G6Pase gene during fasting 14–18. Relative to feeding, hepatic G6Pase-luc activity increased markedly after 6 hours fasting (sup. fig. 6). By contrast with the complete suppression of Ad-CRE activity thereafter, however, Ad-G6Pase-luc activity decreased by only 50% after 18 hours (fig. 1b).
We performed knockdown studies to determine the regulatory contributions of CRTC2 and FOXO1 during fasting. RNAi-mediated depletion of hepatic CRTC2 reduced the gluconeogenic profile, which includes Ad-G6Pase-luc activity, gluconeogenic gene expression, and circulating blood glucose concentrations in short-term fasted mice, while depletion of FOXO1 had only modest effects at this time (fig. 1b,c; sup. fig. 7). By contrast, FOXO1 knockdown substantially reduced the gluconeogenic profile after 18 hours of fasting, when CRTC2 was degraded.
We tested the relative importance of CREB and FOXO1 promoter binding sites for G6Pase gene expression during short and long-term fasting. Mutation of the cAMP response element (CRE) blocked Ad-G6Pase-luc induction during short term fasting but had no effect during long-term fasting (fig. 1d). Conversely, mutation of FOXO1 binding sites (insulin response elements; IREs) disrupted G6Pase reporter activity during long-term but not short-term fasting. We observed similar effects of CREB and FOXO1 binding sites on G6Pase promoter activity in cultured HepG2 cells exposed for 6 or 18 hours to forskolin (FSK) (sup. fig. 8). Consistent with this activation profile, CRTC2 occupancy over the G6Pase promoter was maximal after short-term exposure to FSK and returned to baseline levels after 18 hours (fig. 1d, bottom; sup. fig. 8). By contrast, FOXO1 occupancy was low at 6 hours and increased after 18 hours, indicating that CRTC2 and FOXO1 likely regulate gluconeogenic gene expression sequentially in response to fasting.
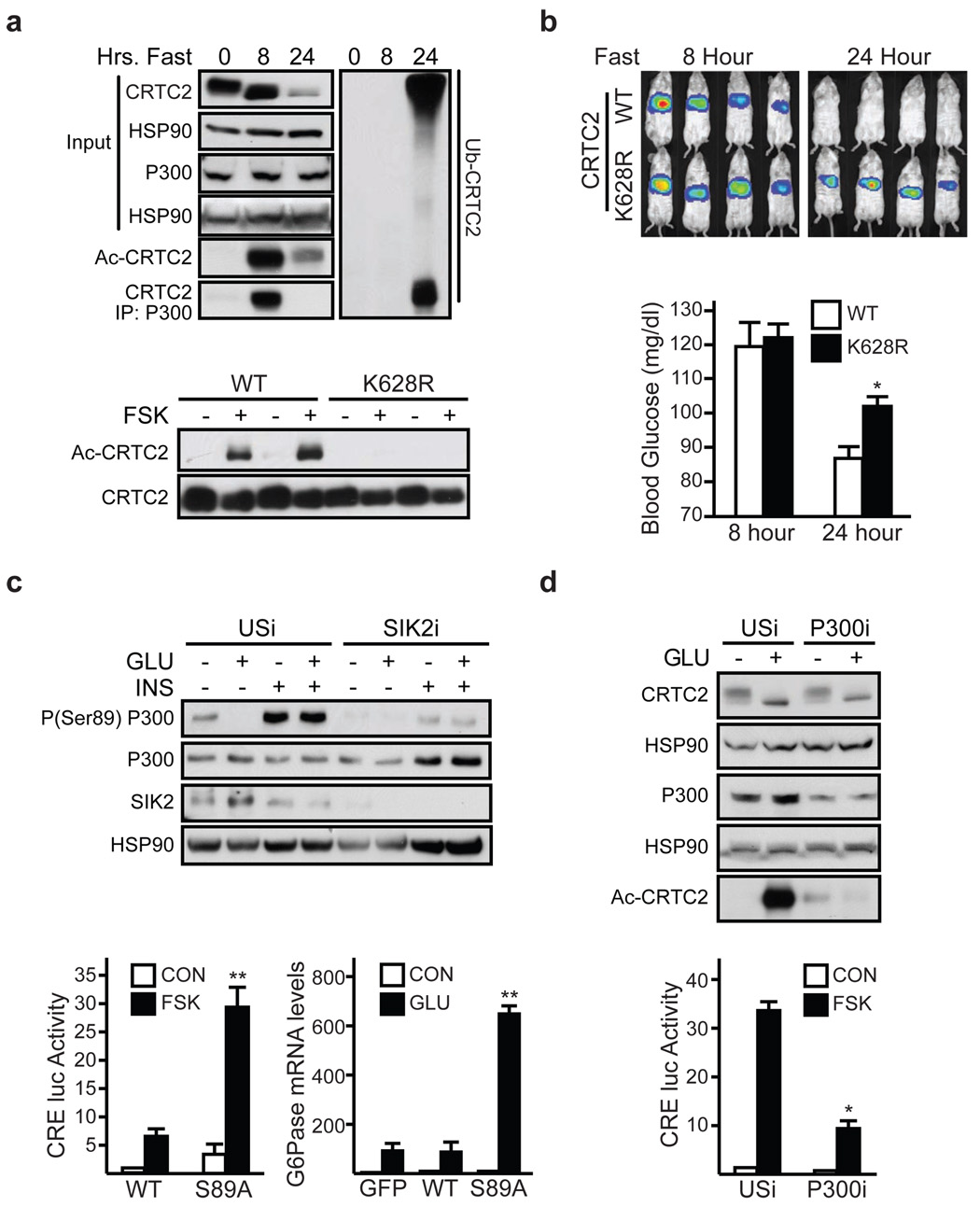
We reasoned that CRTC2 activity during fasting may be regulated through lysine acetylation, because this modification has been shown to protect certain activators against ubiquitin-mediated degradation 19. Supporting this idea, fasting led to CRTC2 acetylation after 8 hours and to CRTC2 ubiquitination after 24 hours (fig. 2a, top). Exposure of primary hepatocytes to glucagon also triggered CRTC2 acetylation; these effects were reversed by subsequent exposure to insulin (sup. fig. 9).
Figure 2. CBP/P300 promote CRTC2 acetylation during fasting.
a, Top, immunoblot of acetylated or ubiquitinated hepatic CRTC2 proteins in 8 and 24 hour fasted mice. Recovery of CRTC2 from P300 immunoprecipitates shown. Bottom, immunoblot of acetylated CRTC2 in HEK293T cells expressing wild-type or Lys628Arg mutant CRTC2. Exposure to FSK indicated. b, Hepatic Ad-CRE-luc activity (top) and circulating glucose levels (bottom) in 8 hr. and 24 hr. fasted mice expressing wild-type or Lys628Arg mutant CRTC2. For blood glucose, (n=3, P <.05). c, Top, immunoblot of phospho-Ser89 P300 protein amounts in primary hepatocytes exposed to glucagon (2 hrs.) followed by insulin (1hr). Effect of Ad-SIK2 RNAi relative to control (USi) shown. Bottom, Ad-CRE-luc reporter activity (left) and G6Pase mRNA amounts (right) in primary hepatocytes expressing wild-type or Ser89Ala mutant P300. Exposure to FSK or glucagon (6hrs.) indicated (n=3; P < .001). d, Top, effect of Ad-P300 RNAi on amounts of acetylated CRTC2 (top) in hepatocytes exposed to glucagon for 1 hour. Bottom, effect of Ad-P300 RNAi on Ad-CRE-luc reporter activity (bottom) in hepatocytes exposed to FSK for 6 hrs (n=3, P < .001). For panels b, c, d, data are means ± s.e.m.
Using mass spectrometry to characterize residues in CRTC2 that undergo acetylation, we found a single site at Lys628, also corresponding to the principal ubiquitination site in CRTC2 (sup. fig. 10) 8. We confirmed these findings using wild-type and Lys628Arg mutant CRTC2 constructs; exposure to FSK increased the acetylation of wild-type but not Lys628Arg mutant CRTC2 (fig. 2a, bottom). Consistent with an important role for Lys628 in modulating CRTC2 activity, Ad-CRE-luc activity, circulating glucose levels, and CRTC2 protein amounts were increased in mice expressing mutant Lys628Arg CRTC2 compared to wild-type CRTC2 during prolonged fasting (fig. 2b, sup. fig. 11).
CRTC2 has been found to promote CREB target gene expression through an association with the HAT paralogs CREB Binding Protein (CBP) and P300 20. Indeed, short-term fasting increased the CRTC2:P300 interaction in liver, while long-term fasting disrupted it (fig. 2a). Exposure to glucagon or FSK also triggered this association in primary hepatocytes; these effects were blocked by subsequent exposure to insulin (sup. figs. 5, 12).
In the course of studies to determine how insulin and glucagon regulate the P300:CRTC2 interaction, we noticed that, similar to CRTC2, P300 and CBP also contain a consensus recognition motif for the Salk Inducible Kinase 2 (SIK2) at Ser89 in P300 (ΨXBS/TXSXXXΨ, where Ψ is a hydrophobic residue and B is a basic amino acid; P300: LLRSGSSPNL). Indeed, phosphorylation of P300 at Ser89 has been reported to inhibit its transcriptional activity, although the underlying mechanism is unclear 21,22. Under basal conditions, P300 was phosphorylated at Ser89 in primary hepatocytes (fig. 2c, top). Consistent with the upregulation of hepatic SIK2 activity during feeding and inhibition during fasting 8, amounts of Ser89-phosphorylated P300 increased when cells were exposed to insulin; and they decreased after treatment with glucagon. RNAi-mediated depletion of SIK2 reduced amounts of Ser89-phosphorylated P300 in cells exposed to insulin, indicating that P300 is likely a direct substrate for this kinase. Moreover, SIK2 immunoprecipitates were competent to phosphorylate wild-type but not S89A mutant P300 in vitro (sup. fig. 13).
To investigate the potential role of Ser89 phosphorylation in regulating P300 activity, we prepared a phosphorylation-defective (S89A) P300 expression virus. Relative to wild-type P300, mutant S89A P300 associated with CRTC2 more efficiently in cells exposed to glucagon and insulin (sup. fig. 14). Furthermore, S89A P300 was more active than wild-type P300 in potentiating Ad-CRE-luc reporter activity and gluconeogenic gene expression, confirming the importance of P300 de-phosphorylation for CRTC2 induction (fig. 2c, bottom).
Because they have intrinsic HAT activity, CBP/P300 might be expected to modulate CRTC2 activity in part through acetylation. Indeed, over-expression of CBP increased amounts of acetylated CRTC2 in cells exposed to FSK or to staurosporine, a SIK2 kinase inhibitor (sup. fig. 15). The effects of P300/CBP appear direct, because purified recombinant P300 protein was capable of acetylating a CRTC2 polypeptide containing the Lys628 acetylation site in vitro (sup. fig. 15). Conversely, RNAi-mediated depletion of P300 reduced CRTC2 acetylation and decreased Ad-CRE-luc activity in hepatocytes exposed to glucagon (fig. 2d, sup. fig. 16).
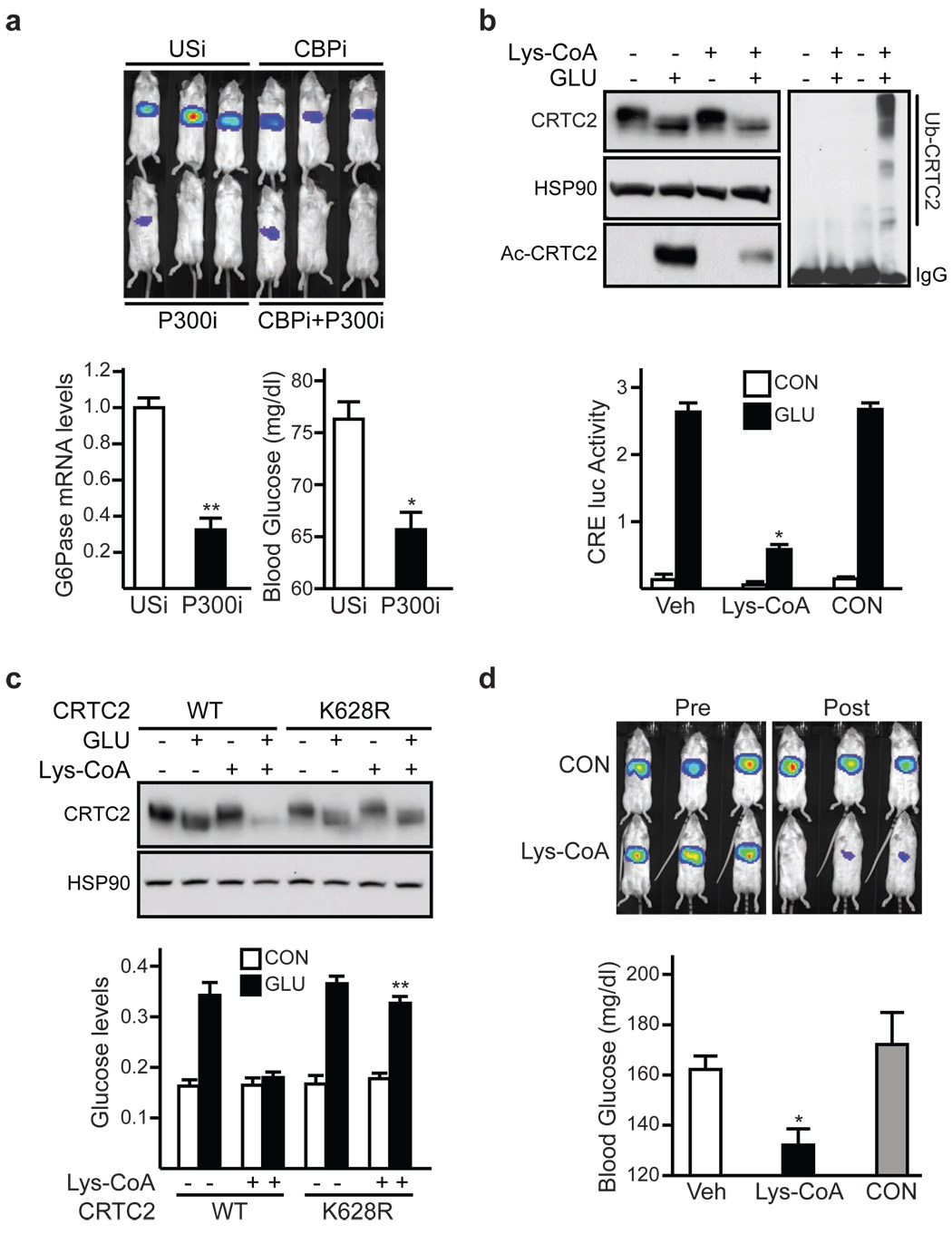
We evaluated the role of P300 and CBP in modulating gluconeogenesis via CRTC2. RNAi-mediated depletion of hepatic P300, and to a lesser extent CBP, reduced the gluconeogenic profile in 6 hour fasted mice (fig. 3a). Hepatic CRTC2 protein amounts were also decreased in P300-depleted mice, suggesting that P300 is required to prevent hepatic CRTC2 degradation during fasting (sup. fig. 16).
Figure 3. P300/CBP modulate hepatic CRTC2 activity.
a, Top, Ad-CRE-luc activity in 6 hour fasted mice expressing Ad-CBP RNAi, Ad-P300 RNAi, or Ad-USi. Bottom, G6Pase mRNA amounts (left) and blood glucose levels (right) in mice expressing Ad-P300 RNAi relative to control. (n=3; * P <.001; ** P <.01). b, Effect of P300/CBP HAT inhibitor Lys-CoA-TAT or control TAT peptide on CRTC2 acetylation (top) and Ad-CRE-luc activity (bottom) in primary hepatocytes exposed to glucagon. (n=3; P <.001). c, Effect of Lys-CoA-TAT on CRTC2 protein amounts (top) and on glucose output (bottom) from primary hepatocytes expressing wild-type or Lys628Arg mutant CRTC2 (n=3; P < .001). d, Top, Ad-CRE-luc activity in 6 hour fasted mice imaged prior to (Pre) or following (Post) IP injection of Lys-CoA-TAT or TAT peptide. Bottom, blood glucose concentrations in 8 hr. fasted mice injected with Lys-coA-TAT or TAT control. For blood glucose levels (n=6; P < .001). For panels a, b, c, d, data are means ± s.e.m.
We tested the importance of P300/CBP HAT activity for CRTC2-dependent gluconeogenesis. Addition of a cell permeable P300/CBP HAT inhibitor Lys-CoA-TAT 23,24 to cultured hepatocytes reduced amounts of acetylated CRTC2 and correspondingly enhanced CRTC2 ubiquitination and degradation (fig. 3b, top). Ad-CRE-luc reporter activity and glucose output were consequently down-regulated in hepatocytes exposed to Lys-CoA-TAT (3b, bottom); these effects were blocked in cells expressing acetylation/ubiquitination-defective Lys628Arg CRTC2 but not wild-type CRTC2 (fig. 3c; sup. fig. 17). Demonstrating the importance of CBP/P300 HAT activity for hepatic glucose production through CRTC2, Lys-CoA-TAT administration also reduced the gluconeogenic profile in fasted mice (fig. 3d; sup. fig. 17).
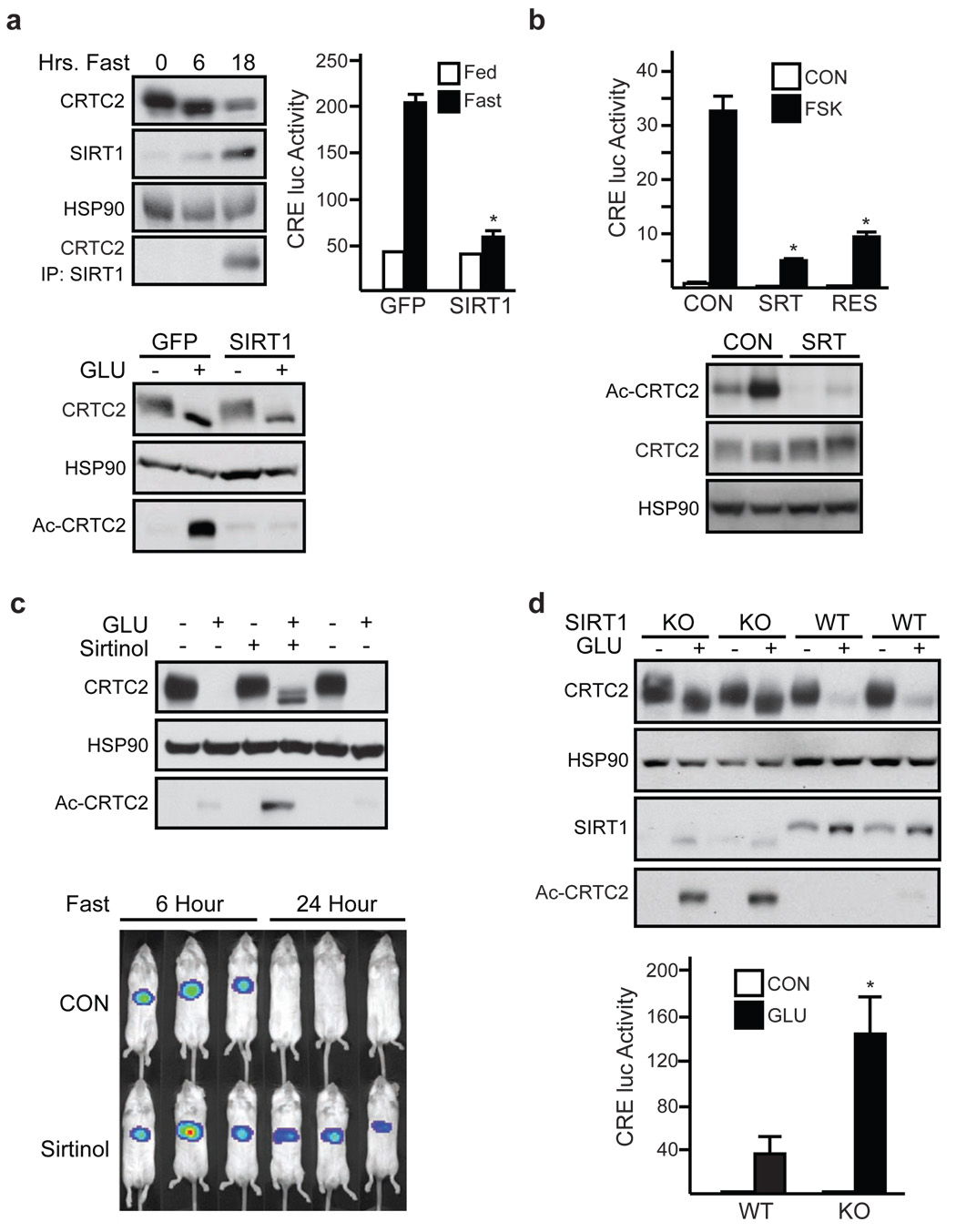
Having seen that hepatic CRTC2 is deacetylated during prolonged fasting, we considered the involvement of a CRTC2 deacetylase in this process. Recently, the histone deacetylase SIRT1 has been shown to promote energy balance by modulating cellular gene expression in response to nutrient deprivation 25. Indeed, SIRT1 activators have been found to improve glucose homeostasis in insulin resistant mice by reducing hepatic gluconeogenesis, although, paradoxically, they increase the activity of FOXO1 and its coactivator PGC-1α 26 9,11,27. Hepatic SIRT1 protein accumulated after 18 hours fasting, when CRTC2 acetylation and protein amounts were correspondingly reduced (fig. 4a, top left) 9. In line with these changes, we recovered CRTC2 from IPs of SIRT1 prepared from livers of long-term but not short-term fasted mice. Moreover, Ad-SIRT1 over-expression in primary hepatocytes reduced amounts of acetylated and total CRTC2 protein and decreased Ad-CRE-luc activity following exposure to glucagon (fig. 4a, bottom; sup. figs. 18, 19). We observed similar inhibitory effects of Ad-SIRT1 on Ad-CRE-luc reporter activity and circulating blood glucose levels in vivo (fig. 4a, right; sup. fig. 19).
Figure 4. SIRT1 attenuates CRTC2 activity during fasting.
a, Top left, Immunoblot of hepatic CRTC2 protein recovered from SIRT1 IPs from ad libitum fed and 6 hour or 18 hour fasted mice. Right, effect of Ad-SIRT1 expression on Ad-CRE-luc activity in 8 hr. fasted mice (top) and on CRTC2 acetylation in hepatocytes exposed to glucagon (bottom). (n=3; P <.001). b, Top, Ad-CRE-luc activity in primary hepatocytes exposed to SIRT1 activators resveratrol or SRT1720 (n=3; P <.001). Bottom, immunoblot showing amounts of acetylated CRTC2 in livers of Zucker fa/fa obese rats maintained on chow supplemented with SRT1720 (n=4; P <.05). c. Top, immunoblot of acetylated and total CRTC2 protein in primary hepatocytes exposed to Sirtinol and/or glucagon for 4 hours. Bottom, Ad-CRE-luc activity in 6 or 24 hour fasted mice following sirtinol administration. d, Total and acetylated CRTC2 protein amounts (top) and Ad-CRE-luc activity (bottom) in primary hepatocytes from control or liver-specific Sirt1−/− mice. Exposure to glucagon (6 hours) indicated (n=4; P <.05). For panels a, b, d, data are means ± s.e.m.).
We reasoned that SIRT1 activators may also attenuate the gluconeogenic program during fasting through inhibition of CRTC2. Induction of SIRT1 activity with SRT1720 26 or resveratrol reduced amounts of acetylated CRTC2 and lowered Ad-CRE-luc activity in primary hepatocytes exposed to FSK or glucagon (fig. 4b, top; sup. fig. 20). Consistent with its ability to lower circulating blood glucose concentrations in part through inhibition of hepatic gluconeogenesis 26, SRT1720 also decreased amounts of acetylated hepatic CRTC2 in Zucker fa/fa rats (fig. 4b, bottom). By contrast, SRT1720 did not alter Ad-CRE-luc activity or glucose output from hepatocytes expressing acetylation/ubiquitination-defective Lys628Arg CRTC2 (sup. figs. 17, 21).
Based on these results, we tested, conversely, whether SIRT1 inhibitors increase CRTC2 activity. Exposure of primary hepatocytes to the SIRT1 antagonists sirtinol and nicotinamide enhanced CRTC2 acetylation and Ad-CRE-luc reporter activity in glucagon-stimulated cells (fig. 4c, top; sup. fig. 22). IP sirtinol administration also augmented hepatic Ad-CRE-luc reporter activity during prolonged fasting, when CRTC2 activity is normally down-regulated (fig 4c, bottom).
We tested the role of SIRT1 further using mice with a liver-specific knockout of the Sirt1 gene. Under basal conditions, CRTC2 protein amounts were comparable in primary cultures of Sirt1−/− and wild-type hepatocytes (fig. 4d, top). By contrast with the degradation of CRTC2 following prolonged exposure of wild-type cells to glucagon, however, CRTC2 protein amounts remained elevated in Sirt1−/− cells. We observed similar differences in hepatic CRTC2 protein amounts between wild-type and Sirt1−/− mice during fasting (sup. fig. 23). As a result, hepatic Ad-CRE-luc activity was elevated and unresponsive to sirtinol administration in fasted Sirt1−/− mice (sup. fig 23). Ad-CRE luc activity and gluconeogenic gene expression were also increased in Sirt1−/− hepatocytes following glucagon exposure, demonstrating the importance of this deacetylase in modulating CRTC2 activity (fig. 4d, bottom; sup. fig. 24).
Taken together, these results indicate that fasting signals increase the gluconeogenic program transiently through the acetylation of CRTC2 by P300/CBP (sup. fig. 25). During prolonged fasting, SIRT1 deacetylates CRTC2 and promotes its ubiquitin-dependent degradation via COP1. The reciprocal upregulation of FOXO1 activity by SIRT1 during this period appears critical in maintaining energy balance through its effects on glucose metabolism 6. Studies into the mechanism by which nutrient signals modulate P300 and SIRT1 activities should provide further insight into this process.
Methods Summary
In vivo imaging studies with adenoviral CRE-luc and G6Pase-luc reporters were performed with an IVIS 100 Imaging System 8. For nutritional studies, mice were evaluated under ad libitum, short term fasted (6–8 hrs), or long-term fasted (18–24 hrs) conditions. Adenoviral expression vectors and RNAi constructs for SIRT1, P300, and CBP were generated as described 7. Fasting gluconeogenesis was evaluated using 14C-labeled alanine as reported 28. Wild-type and SIRT1−/− primary hepatocytes were cultured, and glucose output was measured enzymatically following collection in medium containing lactate and pyruvate 8. P300/CBP HAT activity was inhibited by administration of Lys-coA-TAT in vivo and in cultured hepatocytes 24.
Methods
Adenoviruses and animals
Wild-type CRTC2, mutant Lys628Arg CRTC2, CRE-luc, RSVβ-gal and SIRT1 adenoviruses have been described 8,9. Ad-G6Pase luc reporter was constructed by insertion of G6Pase-luc into pShuttle vector and by transferring this cassette to AdEasy by non-homologous recombination. Ad-P300 RNAi was constructed using the sequence: ACTTACCAGATGAATTAA. For live imaging experiments, 109 plaque forming units (pfu) CRE-luc and 5×107 pfu RSV β-gal adenovirus were delivered to 8–10 week old male mice (Jackson Labs; Bar Harbor, ME) by tail vein injection. Mice were imaged on day 3–5 after adenovirus delivery. For other in vivo studies, 1×108 pfu of over-expressing or RNAi adenovirus was employed. SIRT1 liver specific knockout mice were generated by crossing a SIRT1 allele containing a floxed exon 4 29 with Cre-expressing mice driven by the liver-specific albumin promoter.
Measurement of Gluconeogenesis Rates in vivo
Fed, 6h and 24h fasted mice were injected intraperitoneally with labeled precursor (5mCi of [U-14C]alanine) and blood samples were analyzed after 30 minutes, when 14C incorporation into glucose is still linear. Gluconeogenesis rates in vivo were determined as described previously 28. Results are expressed in micromole of glucose synthesized per hour per gram body weight. The separation of labeled glucose from labeled alanine and other charged compounds was performed by ion exchange chromatography. A neutralized aliquot of blood barium hydroxide-zinc sulfate was passed on to a layered bed resin column, consisting of Dowex-1-X8 (200–400 mesh) in the formate form and Dowex-50W-X8 (200–400 mesh) in the H+ form. The sample was eluted with water, and labeled glucose contained in the eluate was assayed for radioactivity in a scintillation spectrometer. To determine whether glucose was indeed collected in the eluate, glucose was transformed into glucose 6-phosphate (G6P) by hexokinase in the presence of ATP. G6P was then absorbed into a column of Dowex-1-X8. By using this method more than 95% of the counts found in this eluate were absorbed on the column, showing that 95% of labeled substrate on the glucose eluate was glucose, converted to G6P after incubation with hexokinase and ATP. The method thus gives a reliable estimate of the 14C glucose formed from 14C alanine.
In vivo Imaging and Analysis
For imaging, mice were injected IP with 50mg/kg Nembutal (Abbott Laboratories, Chicago IL) and 100mg/kg sterile firefly D-luciferin (Xenogen, Alameda CA). Mice were imaged on the IVIS 100 Imaging System, and analyzed with Living Image software (Xenogen, Alameda CA). Luciferase activity from CRE-luc and G6Pase reporters were normalized to β-glactosidase activity from co-injected RSV-βgal plasmid as described previously 8. For studies comparing mutant and wild-type G6Pase reporters (fig. 1e), activities were also normalized to reporter DNA from hepatic lysates by Q-PCR analysis. For Lys-CoA-TAT studies, mice were injected IP with Lys-CoA-TAT (10nmole/gram body weight) or control TAT at the beginning of a 6 hour fast followed by IP injection with 100µg/kg glucagon (Sigma, St. Louis MO) one hour prior imaging. Lys-coA-TAT and control TAT peptides were synthesized as described previously 24. Absence of liver toxicity was confirmed by measuring plasma alanine aminotransferase activity (ALT) using a kit from Teco diagnostics (Anaheim, CA). Mouse tissues were prepared as previously described 8. Blood glucose values were determined using a LifeScan automatic glucometer.
Cell Culture
HEK293T cells were transfected as previously described 20. For reporter studies, Ad-CRE-luc and RSVβ-gal infected hepatocytes (1 pfu/virus/cell) were exposed to glucagon (100 nM) for 6~12 hours. For drug studies, hepatocytes were pre-treated with Lys-CoA-TAT (20 µM) for 18 hours, Sirtinol (100 µM, Calbiochem) or SRT1720 (1 µM, Sirtris) for 1 hour and incubated with glucagon (100 nM) for 1~2 hours. Glucose output from primary hepatocytes was determined enzymatically following 1 hr collection in glucose-free M199 media supplemented with 10mM lactate and 1mM pyruvate. The corresponding hepatocytes were lysed in 100 µl buffer and protein concentration was determined by Bio-Rad protein assay reagent. Glucose production was expressed as nmol of glucose produced/hour/total protein.
mRNA Analysis
Total cellular RNAs from whole liver or from primary cultured hepatocytes were extracted using the RNeasy kit (Qiagen). mRNA levels were measured as described 8.
Immunoblot, immunoprecipitation and in vitro acetylation assay
Western blot and IP assays were performed as described 8. CRTC2 antisera were described 7. For in vitro acetylation assays, GST-CRTC2 (aa 601-692) fusion protein (1 µg) was incubated in reaction buffer (50 µl final volume) containing 50 mM Tris-Cl pH 8.0, 50 mM NaCl, 0.1 mM EDTA, 1 mM DTT, and 2 µM acetyl-CoA, in the presence or absence of baculovirus-expressed and purified P300 protein (90 ng). The reactions were carried at 30 °C for 30 min. GST-CRTC2 (aa 601–692) was pulled-down using Glutathione Sepharose 4B beads (Amersham). The acetylated proteins were detected by immunoblotting with the anti-acetyllysine antibody (Cell Signaling, Danvers, MA). Phospho (Ser89) P300 antiserum has been described 30.
Statistical analyses
Results are reported as mean ± SEM. The comparison of different groups was carried out using two-tailed unpaired Student‵s t test. Differences were considered statistically significant at P<0.05. All experiments were performed on at least two independent occasions.
Supplementary Material
Acknowledgements
We thank Michael Kahn for gift of phospho-specific P300 antiserum and Lilly Vera for excellent technical assistance. This work was supported by grants from the NIH, the Clayton Foundation for Medical Research, the Hillblom Foundation (Y.L.), and the Kieckhefer Foundation.
References
- 1.Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970;49:2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman MN, et al. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. Am J Physiol. 1980;239:E277–E286. doi: 10.1152/ajpendo.1980.239.4.E277. [DOI] [PubMed] [Google Scholar]
- 3.Goodman MN, McElaney MA, Ruderman NB. Adaptation to prolonged starvation in the rat: curtailment of skeletal muscle proteolysis. Am J Physiol. 1981;241:E321–E327. doi: 10.1152/ajpendo.1981.241.4.E321. [DOI] [PubMed] [Google Scholar]
- 4.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 5.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 8.Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 9.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 10.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 12.Gan L, et al. FoxO-dependent and -independent mechanisms mediate SirT1 effects on IGFBP-1 gene expression. Biochem Biophys Res Commun. 2005;337:1092–1096. doi: 10.1016/j.bbrc.2005.09.169. [DOI] [PubMed] [Google Scholar]
- 13.Badman MK, et al. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barthel A, et al. Differential regulation of endogenous glucose-6-phosphatase and phosphoenolpyruvate carboxykinase gene expression by the forkhead transcription factor FKHR in H4IIE-hepatoma cells. Biochem Biophys Res Commun. 2001;285:897–902. doi: 10.1006/bbrc.2001.5261. [DOI] [PubMed] [Google Scholar]
- 16.Hornbuckle LA, et al. Selective stimulation of G-6-Pase catalytic subunit but not G-6-P transporter gene expression by glucagon in vivo and cAMP in situ. Am J Physiol Endocrinol Metab. 2004;286:E795–E808. doi: 10.1152/ajpendo.00455.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ayala JE, et al. Conservation of an insulin response unit between mouse and human glucose-6-phosphatase catalytic subunit gene promoters: transcription factor FKHR binds the insulin response sequence. Diabetes. 1999;48:1885–1889. doi: 10.2337/diabetes.48.9.1885. [DOI] [PubMed] [Google Scholar]
- 18.Schmoll D, et al. Identification of a cAMP response element within the glucose-6- phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem J. 1999;338:457–463. [PMC free article] [PubMed] [Google Scholar]
- 19.Freiman RN, Tjian R. Regulating the regulators: lysine modifications make their mark. Cell. 2003;112:11–17. doi: 10.1016/s0092-8674(02)01278-3. [DOI] [PubMed] [Google Scholar]
- 20.Ravnskjaer K, et al. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. Embo J. 2007;26:2880–2889. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan LW, Gambee JE. Phosphorylation of p300 at serine 89 by protein kinase C. J Biol Chem. 2000;275:40946–40951. doi: 10.1074/jbc.M007832200. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, et al. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276:38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- 23.Guidez F, et al. Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein. Mol Cell Biol. 2005;25:5552–5566. doi: 10.1128/MCB.25.13.5552-5566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, et al. Synthesis and evaluation of a potent and selective cell-permeable p300 histone acetyltransferase inhibitor. J Am Chem Soc. 2005;127:17182–17183. doi: 10.1021/ja0558544. [DOI] [PubMed] [Google Scholar]
- 25.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 26.Milne JC, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motta MC, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 28.Ferre P, Pegorier JP, Marliss EB, Girard JR. Influence of exogenous fat and gluconeogenic substrates on glucose homeostasis in the newborn rat. Am J Physiol. 1978;234:E129–E136. doi: 10.1152/ajpendo.1978.234.2.E129. [DOI] [PubMed] [Google Scholar]
- 29.Cheng HL, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyabayashi T, et al. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.