Abstract
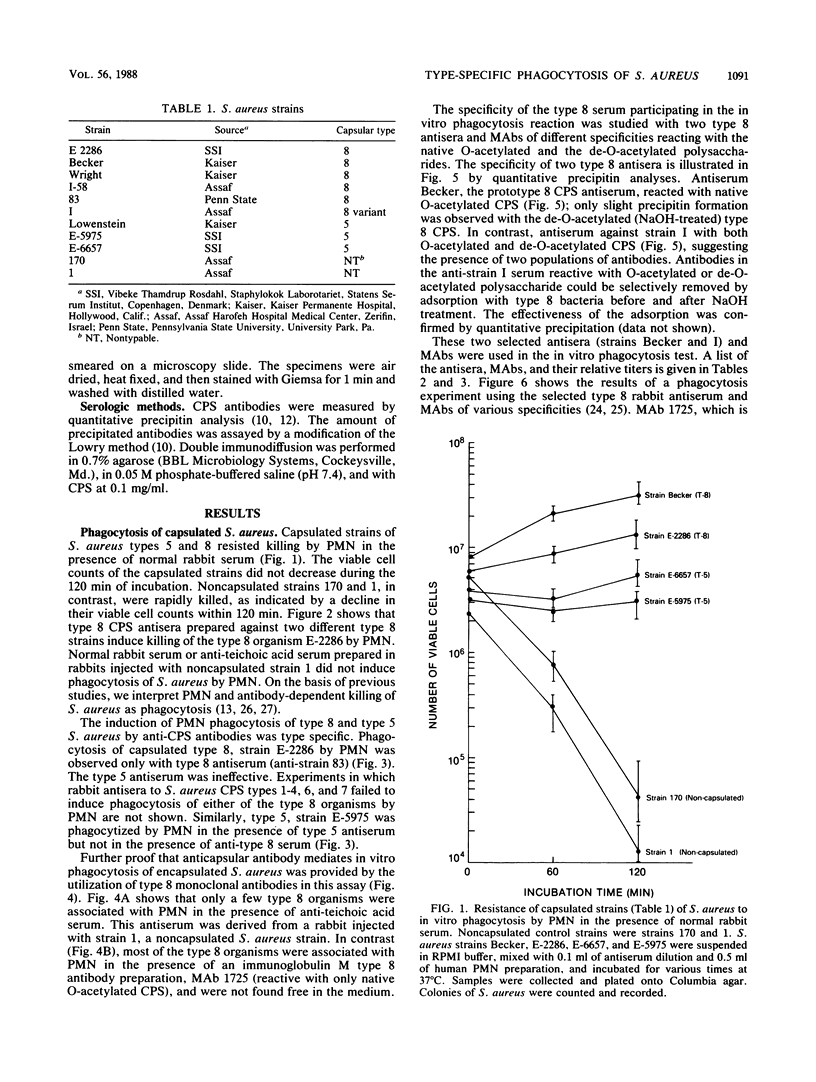
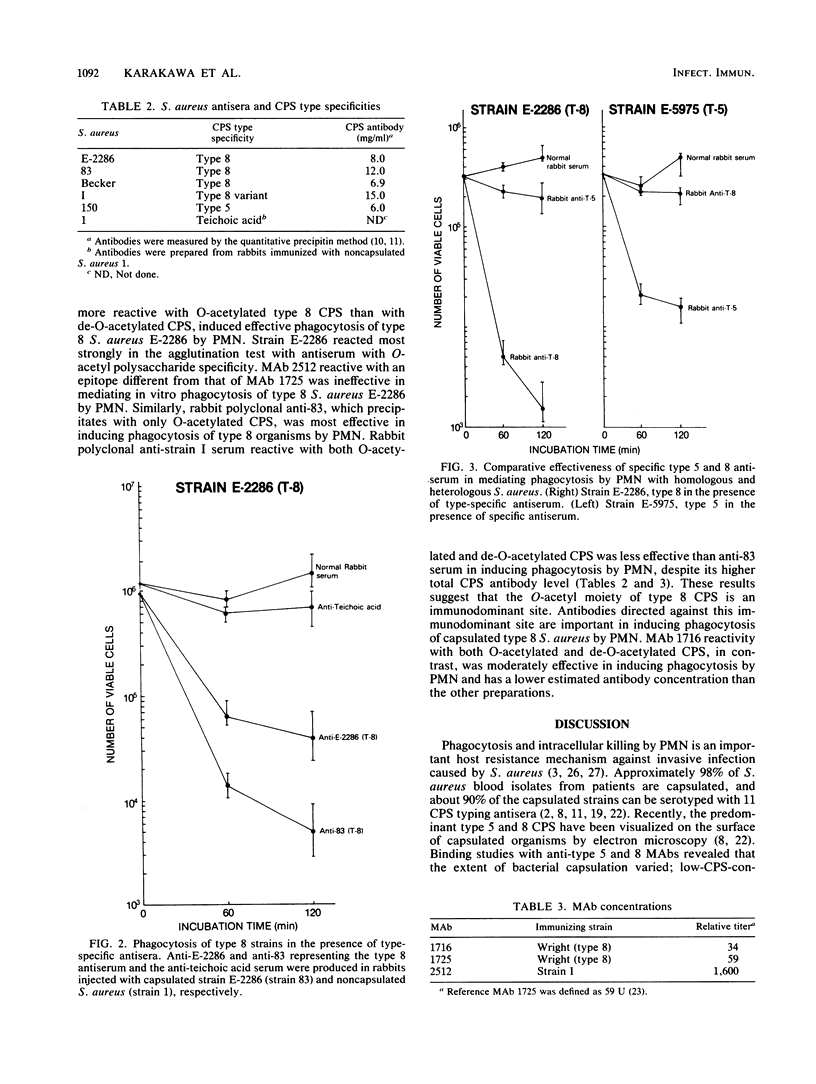
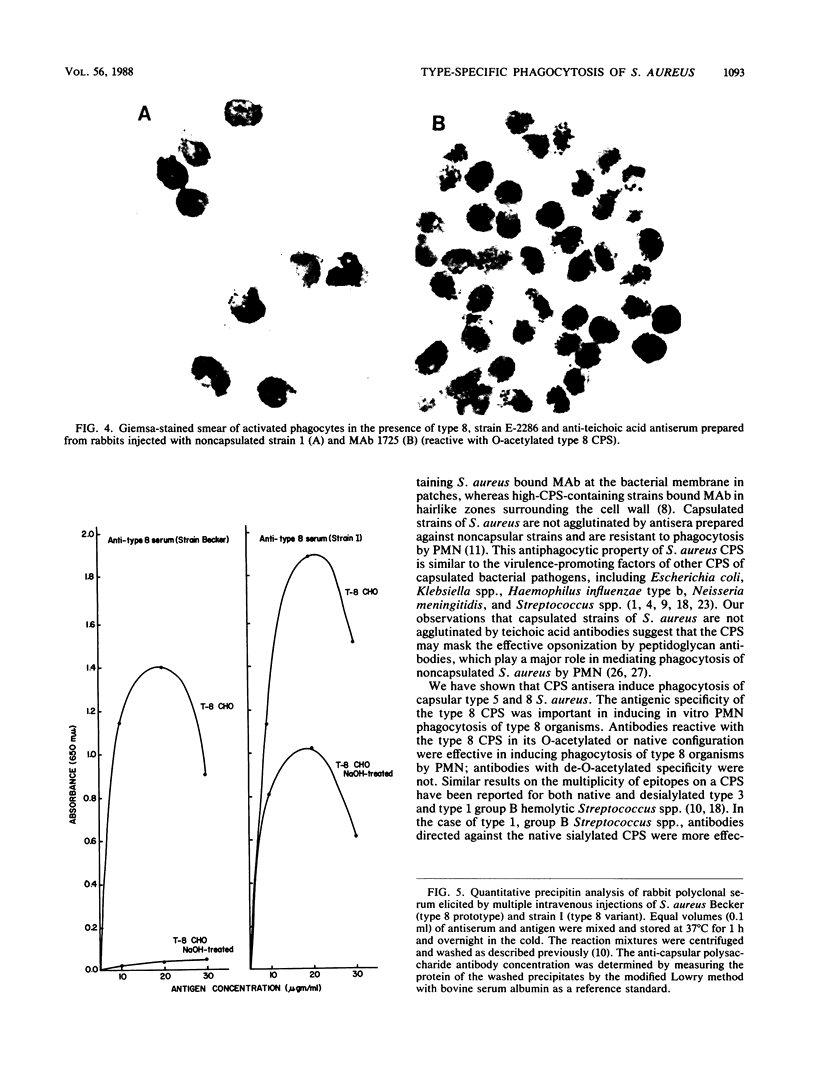
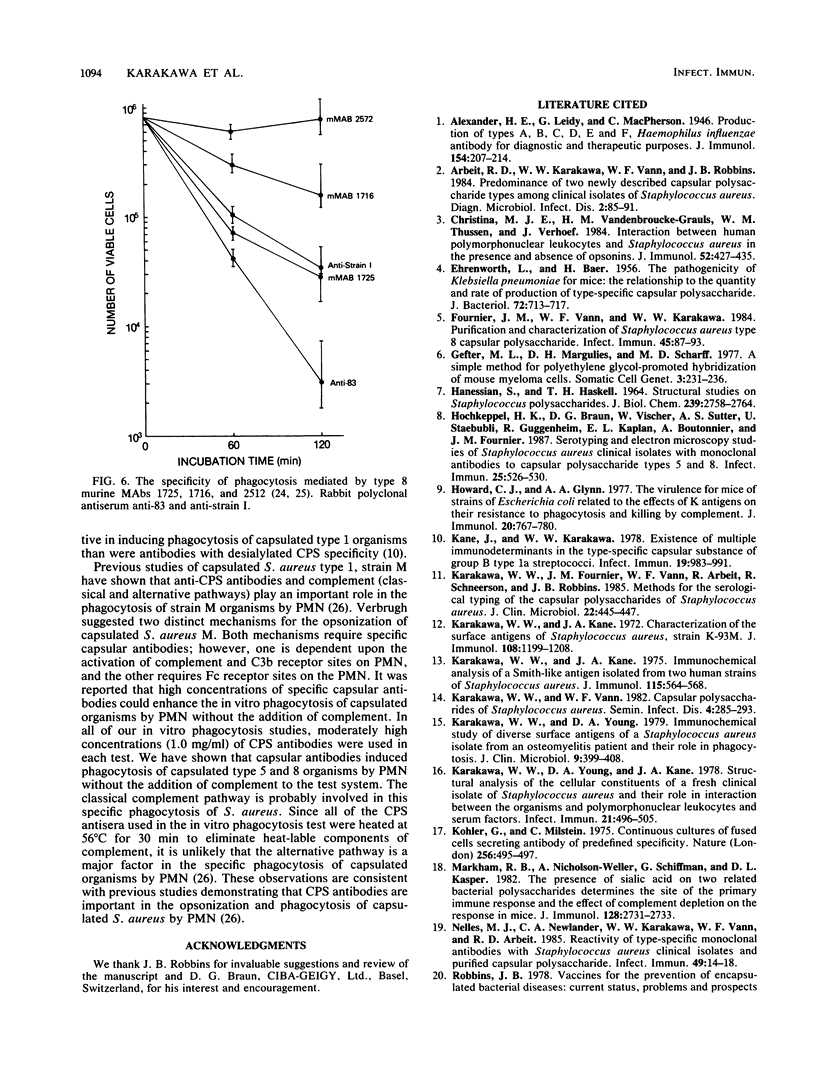
Capsular types 5 and 8, which account for about 70% of Staphylococcus aureus strains isolated from the blood of patients, resisted in vitro phagocytosis by human polymorphonuclear leukocytes (PMN). Antisera and monoclonal antibody to type 5 and 8 capsular polysaccharides (CPS) induced type-specific in vitro phagocytosis of capsulated organisms by PMN. Antibodies directed against the O-acetyl moiety of the type 8 CPS were more effective in inducing phagocytosis of type 8 organisms by PMN. Either type-specific antiserum or monoclonal antibody reactive with the native O-acetylated type 8 CPS was most effective in inducing in vitro phagocytosis of type 8 organisms by PMN. These results provide further evidence that CPS of S. aureus are associated with host immunity to this organism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- BAER H., EHRENWORTH L. The pathogenicity of Klebsiella pneumoniae for mice: the relationship to the quantity and rate of production of type-specific capsular polysaccharide. J Bacteriol. 1956 Nov;72(5):713–717. doi: 10.1128/jb.72.5.713-717.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. M., Vann W. F., Karakawa W. W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984 Jul;45(1):87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- HANESSIAN S., HASKELL T. H. STRUCTURAL STUDIES ON STAPHYLOCOCCAL POLYSACCHARIDE ANTIGEN. J Biol Chem. 1964 Sep;239:2758–2764. [PubMed] [Google Scholar]
- Hochkeppel H. K., Braun D. G., Vischer W., Imm A., Sutter S., Staeubli U., Guggenheim R., Kaplan E. L., Boutonnier A., Fournier J. M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987 Mar;25(3):526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Kane J. A., Karakawa W. W. Existence of multiple immunodeterminants in the type-specific capsular substance of group B type Ia streptococci. Infect Immun. 1978 Mar;19(3):983–991. doi: 10.1128/iai.19.3.983-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Fournier J. M., Vann W. F., Arbeit R., Schneerson R. S., Robbins J. B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985 Sep;22(3):445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Characterization of the surface antigens of Staphylococcus aureus, strain K-93M. J Immunol. 1972 May;108(5):1199–1208. [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Immunochemical analysis of a Smith-like antigen isolated from two human strains of Staphylococcus aureus. J Immunol. 1975 Aug;115(2):564–568. [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A. Immunochemical study of diverse surface antigens of a Staphylococcus aureus isolate from an osteomyelitis patient and their role in in vitro phagocytosis. J Clin Microbiol. 1979 Mar;9(3):399–408. doi: 10.1128/jcm.9.3.399-408.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A., Kane J. A. Structural analysis of the cellular constituents of a fresh clinical isolate of Staphylococcus aureus, and their role in the interaction between the organisms and polymorphonuclear leukocytes in the presence of serum factors. Infect Immun. 1978 Aug;21(2):496–505. doi: 10.1128/iai.21.2.496-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Markham R. B., Nicholson-Weller A., Schiffman G., Kasper D. L. The presence of sialic acid on two related bacterial polysaccharides determines the site of the primary immune response and the effect of complement depletion on the response in mice. J Immunol. 1982 Jun;128(6):2731–2733. [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A., Karakawa W. W., Vann W. F., Arbeit R. D. Reactivity of type-specific monoclonal antibodies with Staphylococcus aureus clinical isolates and purified capsular polysaccharide. Infect Immun. 1985 Jul;49(1):14–18. doi: 10.1128/iai.49.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Vann W. F., Karpas A. B., Stein K. E., Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985 Oct 10;82(2):215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke-Grauls C. M., Thijssen H. M., Verhoef J. Interaction between human polymorphonuclear leucocytes and Staphylococcus aureus in the presence and absence of opsonins. Immunology. 1984 Jul;52(3):427–435. [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




