Abstract
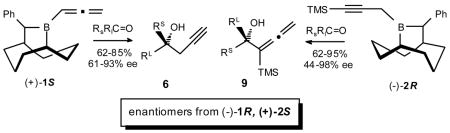
Simple and efficient Grignard procedures are reported for the syntheses of B-allenyl-10-(phenyl)-9-borabicyclo[3.3.2]decane (1) and its B-(Y-trimethylsilylpropargyl)- counterpart (2) in both enantiomeric forms. Both add selectively to ketones providing propargyl- and α-silylallenyl 3°-carbinols, respectively (i.e., 6 (61– 93% ee) and 9 (64–98% ee)). The air-stable boron by-product is efficiently recovered and recycled back to either 1 or 2. The ozonolysis and bromination of 9 provide non-racemic α-hydroxy acids and Y-bromopropynyl carbinols, respectively.
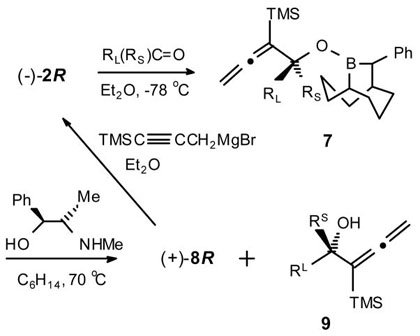
Recently, we reported the asymmetric allenyl- and propargylboration of aldehydes with the 10-trimethylsilyl-9-borabicyclo[3.3.2]decanes (10-TMS-9-BBDs).1 These new reagents provide efficient syntheses of non-racemic propargylic and α-allenylic carbinols, respectively. Moreover, the robust, rigid and recyclable nature of the BBD ring system makes these systems highly attractive alternatives to other methods.2 Neither process is known for prochiral ketones.3 The success of these and related SE2′ processes requires the formation of isomerically pure allenyl- or propargylborane reagents and Grignard procedures are now available for both. In related studies, we discovered that for asymmetric allylboration, the 10-TMS-9-BBD reagents are effective for aldehydes whereas the corresponding 10-Ph-9-BBD reagents are effective for ketones.4 We now wish to report the preparation of both enantiomerically pure forms of B-allenyl-10-Ph-9-BBD (1) and γ-trimethylsilyl-propargyl-10-Ph-9-BBD (2) and their additions to prochiral ketones for the highly selective asymmetric syntheses of propargyl- (5) and α-allenyl- (9) 3°-carbinols, respectively.
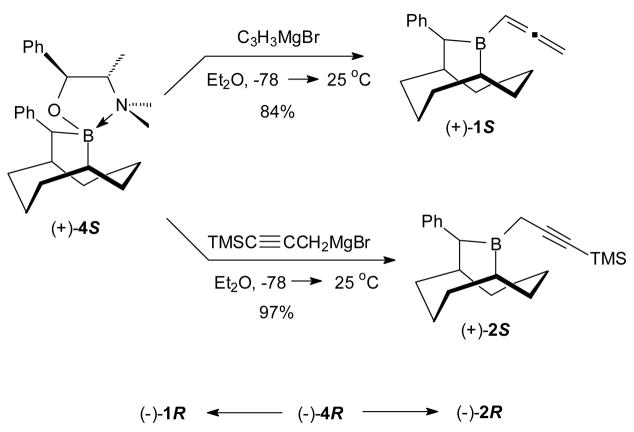
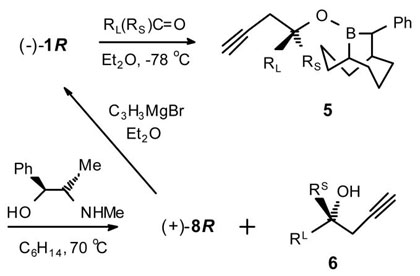
The thermally stable (±)-B-MeO-10-Ph-9-BBD (3), readily prepared from B-MeO-9-BBN, serves as a very convenient precursor to both (+)-4S and (−)-4R as pure crystalline compounds with a combined total yield of 67%.4 These complexes are air-stable and can be stored indefinitely. The reagents 1 were readily prepared in optically pure forms through the addition of allenylmagnesium bromide to the N-methylpseudoephedrine (NMPE) borinic ester complexes 4 (84%) (Scheme 1).5 The Grignard reagent derived from 3-bromo-1-TMS-1-propyne was also found to cleanly add to 4 to provide either (−)-2R or (+)-2S (97%) (Scheme 1).
Scheme 1.
The asymmetric allenylboration of representative ketones was examined with 1. Rapid reaction is observed with methyl ketones (3–12 h, −78 °C). The resulting 3°-carbinols 6 are obtained efficiently (62–85%) in high ee (61–93%) (Table 1). However, with propiophenone, the addition is much slower (2 d, 25 °C), with 5b being produced in 76% ee. Notably, even very challenging substrates such as 2-butanone and methyl vinyl ketone give 6 with highly respectable levels of selectivities (i.e., 74 (c) and 61% (h) ee, respectively).
Table 1.
Allenylboration of RLRSCO with 1
 | |||||
|---|---|---|---|---|---|
| RL | RS | 1 | series | 6(%)a | % eeb (abs config) |
| Ph | Me | S | a | 74 | 91 (S) |
| Ph | Me | R | a | 85 | 93 (R) |
| Ph | Et | R | b | 65 | 76 (R) |
| Et | Me | R | c | 71 | 74 (S) |
| Bu | Me | S | d | 80 | 81 (R) |
| i-Pr | Me | R | e | 71 | 84 (R) |
| t-Bu | Me | S | f | 66 | 83 (S) |
| TMS | Me | S | g | 62 | 90 (R) |
| CH2=CH | Me | S | h | 64 | 61 (S) |
The a series was performed with both (−)-1R and (+)-1S. The aS, b, f and h series were conducted employing an oxidative work-up. For the remaining examples, the intermediate 5 was isolated and converted to 6 and either 4 or 8 was recovered (69–81%) via the NMPE work-up procedure.
Product ee determined by conversion to the Alexakis esters6 and analysis by 31P NMR.
Encouraged by these very positive results with the allenylboration of ketones with 1, we turned our attention to the corresponding propargylboration of representative ketones with 2. Based upon our earlier studies with the propargyl- vs allenylboration of aldehydes with the corresponding 10-TMS-9- BBD systems,1 we expected even higher enantioselectivities from 2 than we had observed with 1.
The asymmetric propargylboration of representative ketones and was examined with 2 in THF at −78 °C to obtain good to excellent yields (62–95%) of the corresponding α-allenyl 3°-carbinols 9 with methyl ketones generally exhibiting high selectivities (78–98% ee) (Table 2). Reaction times for these substrates varied considerably from 3–36 h for the methyl ketones. The aryl ketones containing electron-withdrawing groups (i.e., Table 2, series e, and f) were the slowest in this group. Even less reactive is the ethyl ketone, propiophenone, whose propargylation requires 52 h at −78 °C. However, even highly demanding substrates such as 2-hexanone, give 9 with high selectivity (i.e., 9b, 84% ee).
Table 2.
Propargylboration of RLRSCO with 2
 | |||||
|---|---|---|---|---|---|
| RL | RS | 1 | series | 9(%)a | % eeb (abs config)c |
| Ph | Me | S | a | 81 | 97 (R)d |
| Bu | Me | S | b | 62 | 84 (R) |
| c-C6H9 e | Me | S | c | 67 | 91 (R) |
| p-MeOC6H4 | Me | R | d | 95 | 92 (S) |
| p-BrC6H4 | Me | S | e | 80 | 98 (R) |
| p-O2NC6H4 | Me | S | f | 79 | 98 (R) |
| 2-C4H3Se | Me | S | g | 71 | 78 (S) |
| 2-C4H3Oe | Me | S | h | 82 | 80 (R) |
| Ph | Et | S | i | 63 | 64 (R) |
In each case, the crystalline by-product 8 was recovered (50–85%) using a pseudoephedrine (PE) work-up (i.e., (1R,2R)-(−)-PE for (+)-8S and (1S,2S)-(+)-PE for (−)-8R). This process is both more economical and convenient than with NMPE which is not currently available in both enantiomeric forms. Moreover, 8 is easily converted back to 2 (>95%) through the same general Grignard procedure used for 4.
Product ee was determined by reaction of 8 with phosphorus CDA and analysis by 31P NMR.
Configuration predicted by analogy to 9a.
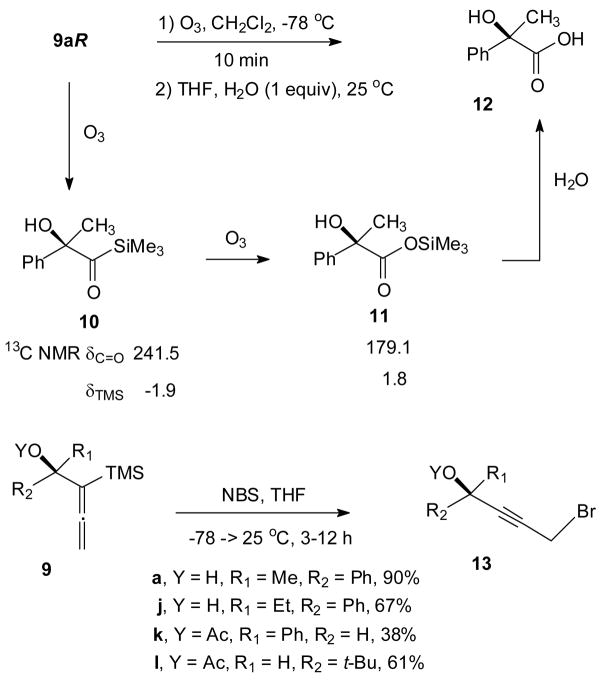
Allenic alcohol 9a was converted to the known 2-hydroxy-2-phenylpropionic acid and the sign of rotation was compared with the reported value.
1-cyclohexenyl, 2-thienyl and 2-furyl.
The absolute stereochemistry for 9a was determined by its conversion to the known atrolactic acid (12) through simple ozonolysis (Scheme 2). This oxidation is greatly facilitated by the α-TMS substitution in 9 which permits the ozonolysis to proceed directly to 11 through 10.1a To discover other potential applications for this α-silylallenyl functionality, we examined the NBS-mediated bromodesilylation of 9a,j together with two examples of O-Ac 2°-carbinols 9k,l. The reactions are highly regioselective producing the corresponding propargyl bromides 13 cleanly in non-racemic form. This simple synthesis of these interesting polyfunctional compounds represents another useful feature of the TMS substitution3b,c in 2 which leads to 9.
Scheme 2.
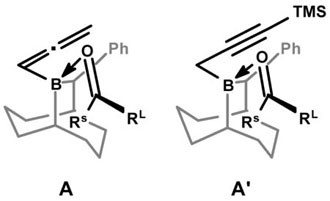
As previously described,4 the 10-substituted-9-BBD ring clearly defines a “chiral pocket” as illustrated in the energetically favored pre-transition state complexes A and A′ (see below) for the allenyl- and propargylboration processes with the reagents 1R and 2R, respectively. Since BBD systems are stable, easily prepared in either enantiomerically pure form, highly reactive, environmentally friendly, recyclable and exhibit high selectivities over a wide range of unsymmetrical ketones, their applications to the asymmetric allenyl- and propargylboration to ketones represent highly useful new processes.
Supplementary Material
Acknowledgments
The support of the NSF (CHE-0517194), and NIH (S06GM8102) is gratefully acknowledged.
Footnotes
Supporting Information Available: Full experimental procedures, analytical data and selected spectra for 1, 2, 6, 9–13 and derivatives (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hernandez E, Soderquist JA. Org Lett. 2005;70:5397. doi: 10.1021/ol051886k.Lai C, Soderquist JA. Org Lett. 2005;7:799. doi: 10.1021/ol0476164.See also: Burgos CH, Canales E, Matos K, Soderquist JA. J Am Chem Soc. 2005;127:8044. doi: 10.1021/ja043612i.
- 2.(a) Ikeda N, Arai I, Yamamoto H. J Am Chem Soc. 1986;108:483–486. doi: 10.1021/ja00263a020. [DOI] [PubMed] [Google Scholar]; (b) Haruta R, Ishiguro M, Ikeda N, Yamamoto H. J Am Chem Soc. 1982;104:7667–7669. [Google Scholar]; (c) Corey EJ, Yu C-M, Lee D-H. J Am Chem Soc. 1990;112:878. [Google Scholar]; (d) Brown HC, Khire UR, Narla G. J Org Chem. 1995;60:8130. [Google Scholar]; (e) Kulkarni SV, Brown HC. Tetrahedron Lett. 1996;37:4125. [Google Scholar]
- 3.For racemic processes, see: Zweifel G, Backlund SJ, Leung T. J Am Chem Soc. 1978;100:5561.Wang KK, Nikam SS, Ho CD. J Org Chem. 1983;48:5376.Wang KK, Liu C. J Org Chem. 1985;50:2578.Brown HC, Khire UR, Narla G. J Org Chem. 1995;60:8130. With chiral substrates, see: Alcaide B, Almendros P, Aragoncillo C, Rodriguez-Acebes R. J Org Chem. 2001;66:5208. doi: 10.1021/jo015704l.
- 4.Canales E, Prasad G, Soderquist JA. J Am Chem Soc. 2005;127:11572. doi: 10.1021/ja053865r. [DOI] [PubMed] [Google Scholar]
- 5.Short RP, Masamune S. J Am Chem Soc. 1989;111:1892. [Google Scholar]
- 6.Alexakis A, Frutos JC, Mutti S, Mangeney P. J Org Chem. 1994;59:3326. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.