Abstract
The integrin Mac-1 plays a critical role in Fc receptor (FcR)-mediated antibody-dependent cellular cytotoxicity (ADCC). However, the mechanism by which Mac-1 facilitates the functions of FcγRIIA, a major FcR expressed on human leukocytes, is not fully understood. We report here that Mac-1 sustains cell adhesion, enhances cell spreading, and accelerates cell migration on pre-formed immune complexes (ICs) by directly interacting with FcγRIIA but not with the IC substrate. Coupling Mac-1 to FcγRIIA allows FcγRIIA to reside in the leading front of actin polymerization at the filopodial extension, and thus could potentially enhance the FcγRIIA-mediated cell spreading and migration. Direct interaction between Mac-1 and FcγRIIA is demonstrated by co-immunoprecipitation, by cell surface co-localization, and by solid-phase binding assays using recombinant αMI-domain and soluble FcγRIIA. Further mutational analysis identifies the E253-R261 sequence within the αMI-domain as part of the FcγRIIA binding interface within Mac-1. Altogether, these results demonstrate that FcγRIIA recognizes Mac-1 via the αMI-domain but not the lectin domain, a distinct feature from other FcRs, and that Mac-1 binding confers FcγRIIA with the ability to prolong cell adhesion as well as to spread and migrate on the ICs, leading to effective cell killing by ADCC.
Receptors recognizing the Fc fragment (FcR)1 of the immunoglobulin (Ig) molecules are critical to host defense by mediating leukocyte migration toward ICs deposited within the site of infections and by eliciting potent cytolysis of the IC-sensitized targets, such as malignant cells and invading pathogens (1–8). A number of clinical studies have demonstrated that FcγRIIA (CD32), a major FcR that recognizes the IgG subclass, is essential to the efficacy of several therapeutic antibody-based drugs, including Herceptin for HER-2/neu-positive breast cancer cells, Rituxan for non-hodgkins lymphoma, and the antibodies for melanoma (1,2,9). Yet, inappropriate engagement of FcγRIIA also causes autoimmune diseases in patients undergoing treatment using these therapeutic antibodies (10), and in transgenic mice overexpressing FcγRIIA (11).
The FcγRIIA-mediated antibody-dependent cytotoxicity (ADCC) is dependent on both FcγRIIA and the integrin Mac-1 (αMβ2, CR3, CD11b/CD18) (1,8,12,13). Despite the importance of Mac-1 in the FcγRIIA-mediated leukocyte functions, and the extensive studies conducted on Mac-1 interaction with various FcRs, including FcγRIIIB (CD16), FcαRI (CD89) and FcεRII (CD23), in addition to FcγRIIA (2–8,14–16), the molecular basis underlying Mac-1 recognition of these FcRs is still less understood. Based primarily on inhibition studies using lectin-domain-specific inhibitors such as N-acetyl-D-glucosamine (NADG) (13,15,17), the binding sites within Mac-1 for most of the FcRs are mapped to its lectin-domain in the α subunit. However, as Mac-1’s ability to promote FcγRIIA-mediated phagocytosis is insensitive to NADG inhibition (18), the nature of the FcγRIIA binding site within Mac-1 remains elusive.
Given the importance of Mac-1 in both FcγRIIA-mediated immunotherapy and autoimmune diseases (1,2,9,10), we sought to elucidate the molecular basis underlying the critical role of Mac-1 in the FcγRIIA-mediated processes, including sustained adhesion, cell spreading and cell migration on ICs. Here, we report the first biochemical evidence for a direct interaction between FcγRIIA and Mac-1, including 1) co-localization of these two proteins on the cell surface, 2) co-immunoprecipitation (co-IP) as a complex; 3) direct interaction between the recombinant αMI-domain of Mac-1 and soluble FcγRIIA, and 4) identification of the E253-R261 sequence within Mac-1 that is critical for FcγRIIA recognition. Moreover, our data shows that Mac-1 binding to FcγRIIA changes the spatial organization of FcγRIIA relative to actin filaments, and thereby potentially facilitates sustained cell adhesion, cell spreading, and cell migration toward IC-sensitized tumor cells, which in combination results in effective cytolysis of the malignant cells via FcγRIIA-mediated ADCC.
EXPERIMENTAL PROCEDURES
Materials
Human kidney 293 cells and the expression vector pCIS2XN were gifts from Dr. F. J. Castellino (Notre Dame, IN). NIF was kindly provided by Dr. E. F. Plow (Cleveland, OH). Dithiobis-succinimidylpropionate (DSP) was from Pierce (Rockford, IL). Fugene 6 was from Roche (Indianapolis, IN), and the β2-specific activating mAb MEM48 was from Biodesign (Kennebunk, ME). mAbs IV3, 44a, OKM1, M1/70 and IB4, as well as HL60 cells were from ATCC (Rockville, MD). All other reagents were the highest grade available from Sigma Chemical Co. (St Louis, MO) unless otherwise noted.
Establishment of stable cell lines
Differentiation of HL60 was induced with 1%DMSO for 6 days in RPMI-1640 plus 10% fetal bovine serum at a starting cell density of 1×105 cells/ml. Maturation of HL60 to neutrophils was confirmed by FACS analysis using mAb OKM1 (for Mac-1) and mAb IV3 (for FcγRIIA), and by morphological examination of Hema3 (Fisher Scientific)-stained Cytospin (Shandon) smears. To establish human 293 cell lines that express both FcγRIIA and Mac-1, the Mac-1-expressing 293 cells, which were established previously in the laboratory (19), were transfected with the expression vector pCIS2XN-FcγRIIA, where the cDNA of FcγRIIA was inserted into pCIS2XN at Xba I and Not I sites, and pcDNA3.1(+)-hygro, a hygromycin selection marker, using Fugene 6. To remove the 75-residue cytoplasmic tail of FcγRIIA, the extracellular and transmembrane domains of FcγRIIA were amplified by PCR using primers 5′AATCTAGACCATGGCTATGGAGACCCAAATGTCTCAG 3′ and 5′ACGTAGCGGCCGCTTACCTGCAGTAGATCAACGCCACTACAGCAGCAACAAT 3′, and the amplified fragments were inserted into pCIS2XN as described above. The correctness of the inserted FcγRIIA sequence was verified by DNA sequencing. After hygromycin selection, the cell lines with similar surface expressions were established by dual-color cell sorting using an αM-specific mAb (M1/70) and an FcγRIIA-specific mAb (IV3). To exclude possible clonal effects, multiple independent clones were used for each cell line in the study.
Cell adhesion and spreading assays
Cell adhesion to the ICs was conducted in 24-well non-tissue culture plates. Briefly, a 24-well polystyrene plate (BD Biosciences) was coated in the center with 70 μl bovine serum albumin (BSA, 1mg/ml) and followed by 70 μl anti-BSA IgG (150 μg/ml in DPBS) (16). The plate was then blocked with 300μl of 0.5% polyvinylpyrrolidone (Sigma) in DPBS. A total of 5 × 105 293 cells or 2×106 phorbol 12-myristate-13-acetate (PMA)-stimulated HL60 cells in 200 μl of Hanks’ balanced salt solution (HBSS) containing 1mM CaCl2, 1mM MgCl2 and 10 mM HEPES in the presence or absence of 10 μg/ml β2-specific activating mAb (MEM48), 25nM NIF, 150mM NADG, 150mM α-methylmannoside (Mannoside), or 150mM glucose were added to each well and incubated at 37°C. At different time points, 200 μl of 4% paraformaldehyde (PFA) was added to the plates to terminate cell adhesion/detachment, and the non-adherent cells were removed by washing with DPBS. The remaining adherent cells were quantified by 0.5% Crystal Violet staining as described previously (19). For cell spreading, 1×105 cells were added into each well and incubated at 37°C for 30 to 60 minutes, and then fixed with 4% PFA. Cell spreading was quantified under phase contrast microscope (20X objectives) based on four randomly picked fields in each well, and calculated as the ratio of spread vs total cells. Cell spreading was further verified by three-dimensional confocal fluorescence imaging as described below.
Immunoprecipitation
Single cell suspension, prepared from 293 cells expressing FcγRIIA or FcγRIIA/Mac-1, were incubated with NIF, NADG, or glucose in HBSS containing 10mM HEPES, 1mM CaCl2 and 1mM MgCl2 at room temperature for 15 min, and then their surface receptors were crosslinked with a 12Å-long thiol-cleavable and amine-reactive crosslinker DSP (2mM) for 30 min. After washing, the residual active crosslinker was inactivated with 100mM ethanolamine, pH8 in the above HBSS buffer and then the cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 2 mM Na3VO4, 10 μg/ml leupetin, 10 μg/ml aprotinin, and 1 mM PMSF). The cell lysates, prepared by centrifugation, was then incubated with an FcγRIIA-specific mAb (IV3) at 4°C for 60 min, and then with Protein A-Sepharose (Amersham, Piscataway, NJ). After washing, the immunoprecipitates were eluted in SDS-PAGE loading buffer, separated by SDS-PAGE in the presence of β-mercaptoethanol, and transferred to PVDF membranes. The presence of Mac-1 in the immunoprecipitates was visualized by immunoblot using a rabbit anti-αM cytoplasmic tail antibody (ARC23) and an HRP-conjugate of goat anti-rabbit IgG.
Live cell imaging
Cells expressing FcγRIIA with and without Mac-1 were allowed to adhere to the ICs on a 24-well non-tissue culture plate at 37°C for 25 min in F-12/DMEM containing media alone, NIF (100nM), NADG (150mM) or glucose (150mM), as described above for the cell adhesion assays. After washing to remove the non-adherent cells, cell migration was monitored using the Neue LiveCell System (Camp Hill, PA) at 37°C and 5%CO2 for 10 hours. Time lapse images were taken in 2-minute intervals and image analysis was performed using MetaMorph (Universal Imaging).
Confocal laser-scanning immunofluorescence microscopy
Cells expressing FcγRIIA with and without Mac-1 were allowed to adhere to the IC-coated coverslips, as described for the cell adhesion assays. The adherent cells were fixed with 4% PFA, blocked with 10% calf serum plus 2% BSA, and then incubated with goat anti-human CD32 (R&D) and mouse anti-Mac-1 (OKM1). After washing, the cells were then stained with an Alexa 488 conjugate of donkey anti-goat IgG and an Alexa 568 conjugate of goat anti-mouse IgG or Alexa 488-phalloidin for actin filament (Molecular probes) after permeabilization with 0.4% Triton X-100. Coverslips were mounted in Fluosave (Calbiochem) and confocal fluorescence images were obtained using Bio-Rad Radiance 2000 laser scanning confocal system equipped with a Nikon Eclipse E800 microscope (BioRad; Hercules CA). Image analysis and three-dimensional image reconstruction were performed using Volocity (Improvision).
Fluorescence lifetime imaging microscopy (FLIM)
Cells were stained with goat anti-human CD32, with or without mouse anti-Mac-1 (OKM1) or a control mouse IgG, and followed by incubation with an Alexa 488 conjugate of anti-goat IgG and an Alexa 568 conjugate of anti-mouse IgG as described above. FLIM images were acquired using a BioRad Radiance 2000 multiphoton microscope, equipped with a high-speed Hamamatsu MCP detector. Excitation at 800nm was empirically determined to excite Alexa 488, but not Alexa 568. Donor (Alexa488) fluorophore lifetimes were fit to two exponential decay curves to calculate the fraction of fluorophores within each pixel that interact with an acceptor. As a negative control, Alexa 488 lifetime was measured in the absence of acceptor (Alexa 568), which showed lifetimes equivalent to FITC-IgG alone or in solution (20).
FACS analysis
A total of 106 cells expressing expressing FcγRIIA, Mac-1, or FcγRIIA plus Mac-1 in HBSS were incubated with 1μg mAb IV3 (for FcγRIIA) or OKM1 (for Mac-1) for 30 min. A subtype-matched mouse IgG served as a control. After washing with PBS, cells were mixed with RPE-conjugated Donkey anti-mouse IgG (Jackson ImmunoResearch) for another 30 min. Cells were then washed and resuspended in 500μl of DPBS. FACS analysis was performed using FACScan (BD Biosciences), counting 10,000 events. Mean fluorescence intensities were quantified using the FACScan program.
Preparation of soluble FcγRIIA
The FcγRIIA extracellular domain was amplified by PCR using the following primers 5′TATAGATCTGATGGCTATGGAGACCCAAATGTCTCAG3′ and 5′ACGTAGCGGCCGCTTAAGGTGAAGAGCTGCCCATGCT3′, and then cloned into the baculovirus transfer vector pACGP67A within Bgl II and Not I sites. After confirmation of its correct sequence by DNA sequencing, pACGP67A-sFcγRIIA was transfected, together with the Baculogold linearized Baculovirus DNA (BD Biosciences), into the sf9 cells. Single colonies were picked from the plaque assay plates and expanded, and the presence of sFcγRIIA in the condition media was determined by immunoblot using goat anti-human CD32 antibody. For large scale production, sFcγRIIA was purified using an IV3 affinity column. The purity of the recombinant sFcγRIIA was verified by both SDS-PAGE and by immunoblot as above.
Solid phase binding assays
To test direct interactions between soluble FcγRIIA and the αMI-domain, 96-well microtiter plates (Immulon 4B, Dynex Technologies Inc, Chantilly, VA) were coated with the ICs as described above and followed by incubation with soluble FcγRIIA. After washing and blocking with 2% BSA, GST or GST-αMI-domain (10 μg/ml; produced in E. coli (21)) in 1% BSA-TBST (20 mM Tris, 150 mM NaCl, 1mM Ca2+, 1 mM Mg2+, 0.05% Tween 20, pH 7.4), was added in the presence or absence of different inhibitors and incubated for 2–3 hours at RT. After washing with TBST, bound GST-αMI-domain was detected using anti-GST conjugated to horseradish peroxidase (HRP), and the HRP substrate 3,3,5,5,-tetramethylbenzidine (KPL, Gaithersburg, MD). Reciprocally, the plate was coated with either GST or GST-αMI-domain and blocked with BSA. Soluble FcγRIIA was then added and incubated at RT for 2 hours. After washing, bound FcγRIIA was detected using goat anti-human CD32, and followed by HRP-donkey anti-goat IgG and its substrate as described above.
RESULTS
Cell adhesion to ICs is dependent on FcγRIIA but not on Mac-1
It has been reported that FcγRIIIB (CD16) and FcαRI (CD89), which do not contain an ITAM (22) sequence within their cytoplasmic domains, require Mac-1 for sustained cell adhesion and the formation of close cell contacts with the IC-coated targets (3–5,23). However, FcγRIIA, which possesses an ITAM motif within its cytoplasmic domain and can signal in the absence of other ITAM-containing subunits, still depends on Mac-1 for its ADCC activity toward the IC-tagged targets (1,6,8). To understand the role of Mac-1 in FcγRIIA-mediated functions, we first evaluated whether Mac-1 is required for cell adhesion to ICs, using human 293 cells (which do not express endogenous Mac-1 or FcγRIIA) that express either Mac-1 alone or Mac-1 plus FcγRIIA. Cell adhesion was conducted on IC-coated 24-well non-tissue culture plates, and the adherent cells were quantified by staining with crystal violet as we described previously (19). In addition, specific contributions of FcγRIIA and Mac-1 to cell adhesion were further verified using their corresponding specific inhibitors (mAb IV3 and NIF (21)), respectively. We found that the 293 cells expressing either FcγRIIA alone or both FcγRIIA and Mac-1 adhered to the ICs (Fig. 1A). In contrast, no specific adhesion to ICs was observed for Mac-1-expressing cells, even upon integrin activation by addition of a β2-activating antibody (MEM48) (24) (Fig. 1A). Confirming the specificity of the assay, no adhesion was seen for mock-transfected cells. Moreover, adhesion by the FcγRIIA/Mac-1-expressing cells was blocked by the FcγRIIA-specific mAb IV3 but not by a control IgG. Addition of the Mac-1-specific inhibitor NIF had no effect (Fig. 1A), although it completely blocked Mac-1-mediated cell adhesion to the Mac-1 ligand fibrinogen (data not shown). Together, these results demonstrated that adhesion of the FcγRIIA/Mac-1-expressing cells to ICs is mediated by FcγRIIA, and that Mac-1, with or without activation, does not directly support cell adhesion to this substrate.
Figure 1. FcγRIIA but not Mac-1 is responsible for cell adhesion to ICs.
A) Cell adhesion is dependent on FcγRIIA. Human 293 cells expressing either FcγRIIA or Mac-1 alone or FcγRIIA plus Mac-1 were allowed to adhere to IC-coated 24-well non-tissue culture plates in the presence or absence of a β2-activating mAb (MEM48), an FcγRIIA-specific blocking mAb (IV3), a Mac-1-specific inhibitor (NIF), or a non-immune IgG for 20 min at 37°C. After washing, the adherent cells were quantified by the crystal violet method. Verifying specificity, no significant cell adhesion was observed for mock-transfected 293 cells. B) Sustained cell adhesion is dependent on Mac-1. Human 293 cells expressing either FcγRIIA alone or both FcγRIIA and Mac-1 were allowed to adhere to the IC-coated 24-well plates in the presence or absence of NIF or mAb IV3. At different time points, PFA was added to stop cell adhesion/detachment, the plates were washed and the adherent cells were quantified. The time of peak cell adhesion (25 min post-cell addition) was designated as time zero of cell detachment. *, p<0.01 by Student’s t-test. C) Sustained cell adhesion is independent of the Mac-1 lectin-domain. The above study was conducted on the FcγRIIA/Mac-1-expressing cells in the presence of NADG, Mannoside, or NIF. Glucose was used as a negative control. D) Sustained neutrophil adhesion requires Mac-1. Differentiated HL60 cells were activated by PMA, washed and then allowed to adhere to the IC-coated plates in the presence or absence of NIF at 37°C. At different time points, cell detachment was determined as above. *, p<0.01; **, p<0.001 by Student’s t-test. Data shown are the means ±SD of three independent experiments.
Mac-1 promotes sustained cell adhesion by FcγRIIA: involvement of the αMI-domain
To assess the possibility that Mac-1 facilitates the FcγRIIA-dependent ADCC activity by supporting sustained leukocyte adhesion to ICs, we evaluated cell detachment at different time points. In these experiments, cells expressing FcγRIIA with or without Mac-1 were added to IC-coated plates at 37ºC and at different time points, PFA was added to stop cell adhesion/detachment and to fix the cells. The non-adherent cells were then removed by washing and the adherent cells were quantified by Crystal Violet staining. We found that the FcγRIIA-expressing cells adhered rapidly to the ICs, such that more than 90% of the input cells became adherent in 25 minutes (peak adhesion; assigned as time 0 of cell detachment), and thereafter they gradually detached from the IC-coated plates. Expression of Mac-1 in FcγRIIA/293 cells had no effect on the initial phase of cell adhesion, evidenced by the similar number of adherent cells at peak adhesion (Fig. 1B, time 0). However, cells expressing FcγRIIA alone showed rapid detachment from the IC-coated plates. At 40 min after peak adhesion, very few cells remained adherent (Fig. 1B). In contrast, the FcγRIIA/Mac-1-expressing cells maintained adhesion and did not detach for an extended period of time (more than 60 minutes), indicating that Mac-1 is required for sustained cell adhesion (Fig. 1B). Confirming the specific requirement of Mac-1 in this process, treatment of the FcγRIIA/Mac-1-expressing cells with NIF, an αMI-domain-specific inhibitor of Mac-1 (21), abolished the sustained adhesion, but did not affect the initial cell adhesion (Fig 1B). Furthermore, addition of the FcγRIIA-specific mAb IV3 completely blocked cell adhesion, even in the presence of Mac-1 (Fig 1B). Since Mac-1 is known to interact with the FcRs via its lectin-like domain (17), we evaluated the role of this domain in supporting sustained adhesion by FcγRIIA using the lectin-domain specific inhibitor NADG (17). Addition of NADG or α-methylmannoside (Mannoside) at 150mM, a concentration that is effective in blocking co-capping between Mac-1 and FcγRIII (17), had no effect on sustained cell adhesion. Similarly, addition of its control polysaccharide glucose did not have any effect either (Fig. 1C). These results suggest that the ability of Mac-1 to sustain the FcγRIIA-mediated cell adhesion is dependent on the αMI-domain but not the lectin-like domain.
To determine if sustained leukocyte adhesion to ICs is similarly dependent on Mac-1, we used DMSO-differentiated HL60 cells as representatives of human neutrophils, which express both Mac-1 and FcγRIIA (25). The differentiated HL60 cells were activated with PMA and washed. The activated cells were then allowed to adhere to immobilized ICs in the presence or absence of NIF at 37°C, and at different time points, PFA was added into the plates to stop cell adhesion/detachment. The non-adherent cells were then removed by washing and the remaining adherent cells were quantified. Results from these experiments showed that similar to the 293 cells expressing recombinant FcγRIIA and Mac-1, the differentiated HL60 cells maintained adhesion to the ICs for an extended period of time and addition of NIF significantly accelerated cell detachment (p=0.01 at 35 min and p<0.001 at 55 min and 75 min) (Fig 1D). Together, these experiments demonstrated that sustained neutrophil adhesion to the ICs, a process that is important for ADCC (3,4,6) and for neutrophil activation (26), is dependent on Mac-1.
Co-localization between Mac-1 and FcγRIIA on the cell surface
Cell adhesion and detachment are controlled by actin polymerization and the re-organization of the cytoskeleton. To see if Mac-1 prolongs FcγRIIA-mediated cell adhesion by changing the spatial organization between FcγRIIA and actin polymerization, we visualized the distributions of Mac-1, FcγRIIA, and actin filaments by confocal laser scanning fluorescence microscopy. For these experiments, the FcγRIIA/Mac-1-expressing cells were allowed to adhere to the ICs on coverslip and then fixed with paraformaldehyde. Surface FcγRIIA and Mac-1 was stained with a goat anti-human CD32 (for FcγRIIA) and a murine mAb OKM1 (for Mac-1) respectively, followed by their corresponding secondary antibodies conjugated to either Alexa 488 or Alexa 568. The results showed that FcγRIIA (in green) co-localized well with WT Mac-1 (in red) on the cell surface (top panels). Verifying the specificity of the immunostaining, no fluorescence was observed for non-immune control IgG-stained samples (bottom panels) under pthe same instrument settings. To see if Mac-1 also colocalizes with FcγRIIA on DMSO-differentiated HL60 cells, confocal fluorescence microscopy experiments were conducted and representative confocal images were presented in Fig. 2A (Panel b). Overall, approximately 80% Mac-1 were colocalized with FcγRIIA, and strong colocalization was primarily found at focal adhesion sites and in filopodial extensions.
Figure 2. Expression of Mac-1 alters spatial distribution of FcγRIIA.
A) Co-localization between Mac-1 and FcγRIIA. Human 293 cells expressing Mac-1/FcγRIIA (a and d) or Mac-1/FcγRIIA(CT-) (c), and DMSO-differentiated HL60 cells (b) were allowed to adhere to ICs, fixed with 2% PFA and then stained for FcγRIIA (goat anti-human CD32) and Mac-1 (mAb OKM1) (a-c) or control goat and mouse IgG (d), followed by staining with Alexa 488-donkey anti-goat IgG and Alexa 568 goat anti-mouse IgG. Confocal fluorescence images were acquired using Bio-Rad Radiance 2000 system and co-localization was shown in yellow. Bars shown represent 10μm. The images shown are representative of 40 images taken from three independent experiments. B) FLIM between Mac-1 and FcγRIIA on the cell surface. DMSO-differentiated HL60 cells were stained with the donor antibody (goat anti-FcγRIIA) in the absence (a, b) or the presence of the acceptor antibodies specific for Mac-1 (OKM1) (c) or a control mouse IgG (d), followed with Alexa 488-anti-goat IgG and Alexa 568-anti-mouse IgG. a) Surface fluorescence staining for FcγRIIA. b–d) FLIM images were taken at the z-section of the cell surface and presented in pseudocolors from red to blue, showing the fluorescence lifetimes (in picoseconds) of Alexa 488 in the absence (b) or presence (c and d) of the acceptor Alexa 568. C) Mac-1 expression alters the spatial distribution of FcγRIIA relative to actin filaments. The adherent and fixed human 293 cells expressing either FcγRIIA alone (a), Mac-1 and FcγRIIA (b) or Mac-1 and FcγRIIA(CT-) (c, d) were stained with goat anti-human CD32 (a, b, and c) or mouse anti-Mac-1 (OKM1) (d), washed, and then permeabilized with 0.4% Triton X-100. These cells were then stained with Alexa 568-conjugated donkey anti-goat (a, b, and c) or goat anti-mouse IgG (d), respectively, and Alexa 488-phalloidin (for actin filaments). A series of two dimensional confocal fluorescence images at different z-sections were collected as in A), and three-dimensional reconstruction was performed by deconvolution using the Volocity program. Bars shown represent 10μm. The images shown are representative of approximately 20 images taken for each cell type. These experiments were repeated twice with similar results.
To further corroborate the above finding, we measured molecular proximity between Mac-1 and FcγRIIA on the HL60 cell surface by fluorescence lifetime imaging microscopy (FLIM) (20). This high resolution imaging technique is based on fluorescence resonance energy transfer (FRET) that occurs if two fluorophores are in very close proximity (<100Å), resulting in the shortening of the donor fluorescence lifetime. Accordingly, we labeled DMSO-differentiated HL60 cells simultaneously with a goat anti-FcγRIIA IgG and a mouse anti-Mac-1 (OKM1) or a control IgG, followed by staining with an Alexa 488-anti-goat and an Alexa 568-anti-mouse antibody, and the fluorescence lifetimes were determined using a multiphoton confocal microscope. The results showed that the presence of Mac-1 (i.e. the acceptor Alexa 568) significantly shortened the average fluorescence lifetime of the anti-FcγRIIA fluorophore (i.e. the donor Alexa 488) from 2293 ± 285 to 1324 ± 504 ps (1899 ± 237 ps with a control IgG; OKM1 vs. IgG, p= 0.00187, n=11), indicating that Mac-1 and FcγRIIA resided <100Å from each other on the cell surface. Fig. 2B shows a representative pseudocolor-coded images of surface co-localization between Mac-1 and FcγRIIA (i.e. the z-section is set at the height of the cell surface), where uniformly bluish image (thus long fluorescence lifetime) was obtained in the absence of the acceptor (Panel b) or in the presence of a non-immune control IgG (Panel d), and a red to green-filled image representing short fluorescence lifetime (therefore close proximity) was obtained in the presence of the acceptor Mac-1 fluorophore (Panel c).
Expression of Mac-1 changes the relative spatial organization between actin and FcγRIIA
To determine if expression of Mac-1 changes the relative spatial distribution of FcγRIIA and the actin filaments, the above cells were first stained with goat anti-CD32 (for FcγRIIA), fixed and permeabilized with 0.4% Triton X-100. The cells were then stained with Alexa 488-labeled phalloidin for actin filaments and Alexa 568-anti goat IgG. A series of two dimensional (x-y) confocal fluorescence images at different z-positions were collected, and these images were then used to construct three-dimensional images by deconvolution using Volocity, which allows us to better visualize the spatial distributions of FcγRIIA and the actin filament. As shown in Fig. 2C, without Mac-1, the cells spread poorly with short cell extensions, where FcγRIIA (in red) stayed behind the actin filament staining (in green) (panel a). In contrast, co-expression of FcγRIIA and Mac-1 allowed the cells to spread on ICs, and exhibited a flat cell body and long filopodial extensions. Most importantly, when Mac-1 is present, FcγRIIA (in red) resided at the front of the actin filament (panel b). Taking together, these data suggest that Mac-1 binding to FcγRIIA alters its relative spatial distribution to actin polymerization, and thus potentially facilitates FcγRIIA-mediated cell adhesion and spreading on the ICs.
Co-immunoprecipition between Mac-1 and FcγRIIA from total cell lysates
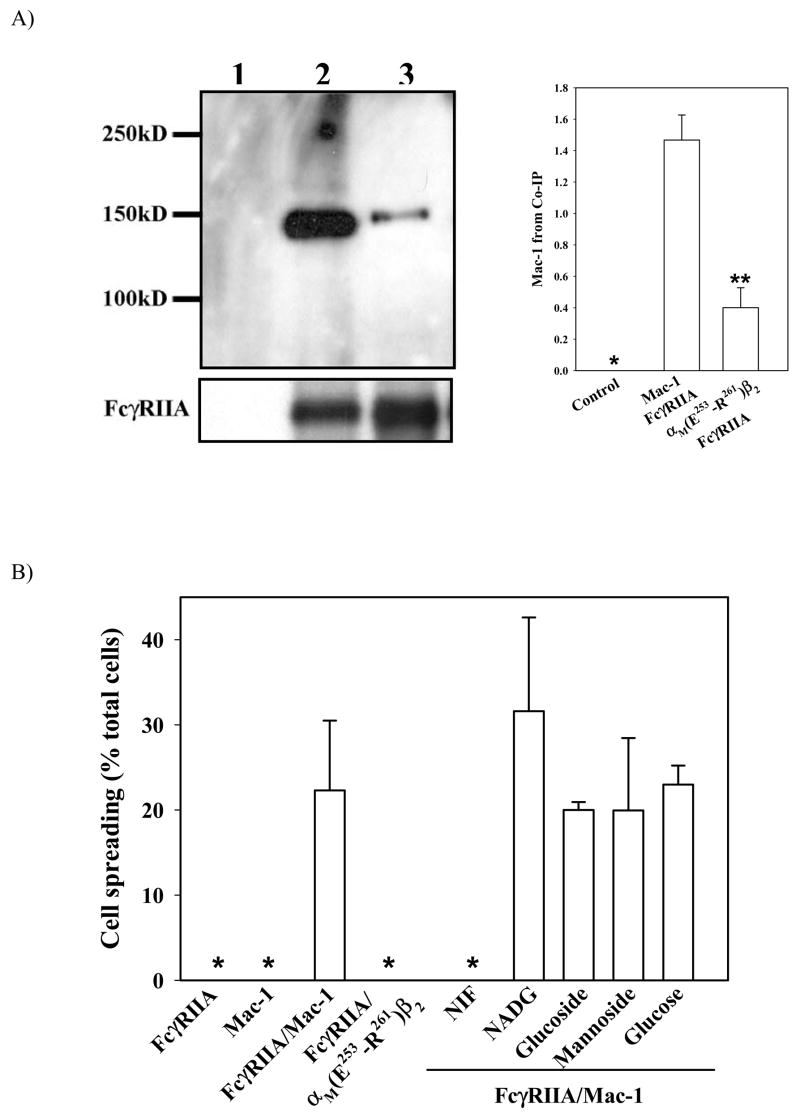
In agreement with the above co-localization experiments, Mac-1 has been shown to co-cap with a number of FcRs on the cell surface (7,14,27). To determine if Mac-1 and FcγRIIA co-localize by direct associations, we conducted co-IP experiments (Fig. 3A) using 293 cells that express only Mac-1 (lane 1), FcγRIIA (lane 2) or both receptors (lanes 3–5). Given the dynamic nature of the Mac-1/FcγRIIA interaction (28), the potentially transient protein complex on the cell surface was crosslinked by a short-length thiol-cleavable crosslinker (spacer length=12Å). The resulting cell lysates were then incubated with either an FcγRIIA-specific mAb (IV3) or a control IgG at 4°C and the Mac-1/FcγRIIA complex was then pulled down with Protein A-Sepharose. The presence of Mac-1 in the immpunoprecipitates was detected by immunoblot using a polyclonal antibody specific for the 165 kDa αM subunit (ARC23). The results showed that immunoprecipitation with the FcγRIIA-specific mAb IV3 (lane 4) but not a control IgG (lane 3) specifically precipitated Mac-1 from the total cell lysates (Fig. 3A), thus verifying the specificity of the co-IP. Further confirming the specificity, the FcγRIIA-specific mAb IV3 failed to precipitate Mac-1 from lysates prepared from the cells expressing only Mac-1 (lane 1). In addition, when co-IP was conducted in the presence of the Mac-1-specific antagonist NIF (lane 5), significantly less Mac-1 was obtained (Fig. 3C). Immunoblot with an anti-actin antibody demonstrated equal loading (data not shown), and stripping and re-probing of the above PVDF membrane with a goat anti-CD32 antibody confirmed the presence of FcγRIIA in the immunoprecipitation mixtures (Fig. 3B).
Figure 3. Mac-1 co-immunoprecipitates with FcγRIIA.
Human 293 cells expressing Mac-1, FcγRIIA, or both in the presence or absence of NIF were crosslinked with DSP and then lysed in cell lysis buffer. Co-IP was conducted using mAb IV3 against FcγRIIA, and the immunoprecipitated complex was separated by SDS-PAGE and immunoblotted with a polyclonal antibody (ARC23) against the cytoplasmic tail of the Mac-1 α subunit (A) and the membrane was stripped and re-probed with an anti-FcγRIIA antibody (B). Equal loading was confirmed by immunoblot with anti-actin antibody (not shown). Quantification of Mac-1 in Co-IP was performed using an Image Station 2000R (Kodak) and expressed in arbitrary units (C). Data shown are the means ±SD of two independent experiments. * indicates a value below 0.001; **, p<0.01 by Student’s t-test.
Mac-1 binding to FcγRIIA is mediated by its αMI-domain
The observations that the αMI-domain-specific inhibitor of Mac-1, NIF (29), inhibited the co-IP between Mac-1 and FcγRIIA (Fig. 3) and blocked Mac-1’s ability to sustain the FcγRIIA-mediated cell adhesion to the ICs (Fig. 1) suggested that the αMI-domain may be involved in Mac-1 binding to FcγRIIA. Such a requirement of the αMI-domain in Mac-1 binding to the FcRs is unexpected as all other FcRs interact with the lectin-like domain of Mac-1 (13,15,17). To determine if the αMI-domain interacts directly with FcγRIIA, we prepared a recombinant GST fusion of the αMI-domain in E. coli and a soluble FcγRIIA in sf9 cells. To mimic the conditions used in the cell adhesion experiments, recombinant soluble FcγRIIA was immobilized onto the ICs, which were pre-coated onto a 96-well plate. After blocking with BSA, GST or GST-αMI-domain was added in the presence of 1mM Ca2+ and 1mM Mg2+ to the IC-coated wells, and bound αMI-domain was detected using a polyclonal antibody specific for GST. We found that GST-αMI-domain, but not the control GST, bound soluble FcγRIIA, which could be blocked by addition of NIF or an αM-specific mAb 44a (Fig. 4). Verifying specificity, GST-αMI-domain did not bind to ICs in the absence of FcγRIIA, and a non-immune control IgG or a β2-specific mAb (IB4) had no effect on the αMI-domain binding to FcγRIIA. Furthermore, no inhibition on αMI-domain binding to FcγRIIA was observed for either NADG or its control glucose at 150mM.
Figure 4. Direct binding of the αMI-domain to soluble FcγRIIA.
Soluble FcγRIIA was immobilized onto IC-coated 96-well microtiter plates, followed by blocking with BSA. GST or GST-αMI-domain was then added in the presence or absence of NIF, mAb 44a (recognizing the αMI-domain), mAb IB4 (recognizing the β2 subunit), or non-immune control IgG (NIgG), as well as NADG or its control glucose for 2 hours at RT. The bound GST-αMI-domain was detected using anti-GST conjugated to horseradish peroxidase (HRP), and the HRP substrate 3,3,5,5,-tetramethylbenzidine. The specificity of the assay was verified by lack of GST binding to FcγRIIA. Data shown are the means ±SD of two independent experiments. *, P=0.04.
The E253-R261 sequence within the αMI-domain is critical to Mac-1 recognition of FcγRIIA
If the αMI-domain is the major binding interface between Mac-1 and FcγRIIA, we anticipated that mutations within the αMI-domain should abolish Mac-1 recognition of FcγRIIA. In addition, since NIF competed effectively with FcγRIIA for binding to the αMI-domain (Fig. 4), we expected that the FcγRIIA binding site may partially overlap the NIF binding site, which was localized previously by us to E253-R261 and its surrounding sequences (29). Thus, we assessed if αM(E253-R261)β2, a mutant Mac-1 in which the E253-R261 sequence (EDVIPEADR) has been replaced with its homologous sequence from the αLI-domain, could form a complex with FcγRIIA in the co-IP experiments. Our previous work confirmed that mutating E253-R261 did not alter the gross conformation of the αM(E253-R261)β2 mutant receptor, based on studies using a panel of conformation-dependent mAbs (29). Fig. 5A shows that unlike WT Mac-1 (lane 2), significant less amount of αM(E253-R261)β2 was co-immunoprecipitated by FcγRIIA (lane 3). No protein was precipitated using an irrelevant control IgG, thus confirming the specificity of the assay (lane 1). Together, these data confirm a critical role of the αMI-domain, especially the E253-R261 sequence, in FcγRIIA binding to Mac-1.
Figure 5. A critical role of segment E253-R261 within the αMI-domain in Mac-1/FcγRIIA recognition and in FcγRIIA-mediated cell spreading.
A) Co-IP between FcγRIIA and either WT or a mutant Mac-1, αM(E253-R261)β2, was conducted and quantified as above. The amount of FcγRIIA present in the co-IP was verified by stripping the membrane and re-probing with an anti-FcγRIIA antibody (bottom panel). Quantification of Mac-1 in Co-IP was performed using an Image Station 2000R (Kodak) and expressed in arbitrary units (right panel). Data shown are the means ±SD of two independent experiments. * indicates a value below 0.001; **, p<0.01 by Student’s t-test. B) Cell spreading. Cells expressing FcγRIIA, Mac-1, FcγRIIA/Mac-1, or FcγRIIA/αM(E253-R261)β2 were allowed to adhere to IC-coated plates as described in Fig. 1, in the presence or absence of NIF, NADG, β-methyl D-glucoside (gulcoside), α-methylmannoside (mannoside), or glucose at 37ºC for 50 min. Cell spreading was quantified manually under the phase-contrast microscope, and confirmed by three-dimensional confocal fluorescence microscopy. * indicates less than 0.5%. Data shown are the means ±SD of duplicate experiments and are representative of three independent experiments.
Mac-1 is critical to FcγRIIA-mediated cell spreading on Ics
To test the role of the αMI-domain in Mac-1’s ability to promote FcγRIIA-mediated cell spreading on ICs, we conducted cell spreading experiments in the presence or absence of different inhibitors, including NIF (for the αMI-domain), NADG and α-methylmannoside (for the lectin-domain), and glucose. Cell spreading was initially assessed by phase contrast microscopy and then confirmed based on three-dimensional confocal fluorescence images as described in Fig. 2B. No cell spreading was observed for cells expressing FcγRIIA or Mac-1 alone after incubation at 37 °C for 50 minutes (Fig. 5B). In contrast, a significant number (~23%) of the cells that expressed both FcγRIIA and Mac-1 spread, which could be inhibited by addition of NIF, but not NADG, α-methylmannoside or the control glucose, indicating that the αMI-domain but not the lectin-domain plays a major role in promoting cell spreading. In further support of this conclusion, the αMI-domain mutant αM(E253-R261)β2, that did not interact well with FcγRIIA (Fig. 5A), failed to support FcγRIIA-mediated cell spreading (Fig. 5B). Altogether, these data demonstrated that the αMI-domain but not the lectin-domain of Mac-1 functions critically in the FcγRIIA-mediated cell spreading on ICs.
Mac-1 functions to promote FcγRIIA-dependent cell migration on ICs: an essential role of the αMI-domain
Recent studies show that leukocyte migration to the site of inflammation is also mediated by the FcRs. In addition, these investigators found that such an FcR-dependent leukocyte migration requires Mac-1 (4). Given our above observations that Mac-1, via its αMI-domain, binds FcγRIIA and changes its relative spatial organization to the actin filaments, we anticipated that the αMI-domain may also be critical to the FcγRIIA-dependent cell migration on ICs. To examine this possibility, we performed cell migration experiments using human 293 cells that express FcγRIIA or Mac-1 alone, FcγRIIA plus Mac-1, and different combinations of FcγRIIA and Mac-1 mutants. The migration assay was performed on IC-coated 24-well plates at 37°C and 5%CO2 for 10 hours with time-lapse images captured at 2-minute intervals, and migration of these different cells were quantified by randomly picking four view fields and calculating the net linear distances migrated by the cells within these fields using MetaMorph. The results showed that the FcγRIIA-expressing cells did not spread or form cell extensions on ICs. These cells oscillated randomly at their original positions and no significant movement was observed. The cells expressing both FcγRIIA and Mac-1 spread and formed filopodial extensions, and exhibited enhanced cell migration (Fig. 6). To help study the role of Mac-1 in FcγRIIA-dependent migration, and to exclude potential contributions of the FcγRIIA-ITAM-mediated intracellular signaling to cell migration, we established additional cell lines that express the cytoplasmic domain-truncated FcγRIIA, FcγRIIA(CT-), with or without Mac-1. Removal of the cytoplasmic tail in the absence of Mac-1 slightly enhanced cell migration (Fig. 6). In further support of Mac-1 binding to FcγRIIA via its extracellular αMI-domain, cytoplasmic domain deletion of FcγRIIA had no effect on its colocalization with Mac-1 (Fig. 2A, panel c). Most importantly, FcγRIIA(CT-) was still capable of relocating to the focal adhesion sites/filopodial extensions (Fig. 2C, panel c) in a manner similar to that of Mac-1 (Fig. 2C, panel d). These results suggest that FcγRIIA linkage to the cytoskeleton is likely mediated by Mac-1 and not via its own cytoplasmic domain. Interestingly, cells expressing FcγRIIA(CT-) plus Mac-1 spread very well and migrated actively on ICs (Fig. 6; p<0.01, 269 cells counted). Most importantly, we found that migration of the cells expressing FcγRIIA(CT-) and Mac-1 was inhibited by addition of NIF (Fig. 6; p<0.01, 102 cells counted), and by mutating the E253-R261 sequence within the αMI-domain (Fig. 6; p<0.04, 124 cells counted), indicating that the αMI-domain plays a critical in Mac-1’s ability to promote cell migration on ICs.
Figure 6. Mac-1 promotes FcγRIIA-dependent cell migration.
Cells expressing different combinations of FcγRIIA or its mutant FcγRIIA (CT-) with Mac-1 or its mutant αM(E253-R261)β2 were allowed to adhere to the ICs on a 24- well non-tissue culture plate at 37°C with or without NIF. After washing to remove the non-adherent cells, cell migration was monitored using the Neue LiveCell System at 37°C and 5%CO2. Time lapse images were taken with a 10X objective every 2 minutes. To quantify cell migration, three to four random fields with a minimum of 70 cells were selected for each experiment and the net linear distance migrated was calculated for each individual cell using Metaview (Universal Imaging) and then averaged. Data shown are the means ±SD of three independent experiments.
DISCUSSION
The major findings of this study are 1) Mac-1 binds directly to FcγRIIA via its αMI-domain, a mechanism that is unique among the many FcRs; 2) the FcγRIIA binding interface is located within a region surrounding residues E253-R261; and 3) Mac-1 binding to FcγRIIA changes the spatial distribution of FcγRIIA relative to the actin filaments, and thereby potentially contributes to the FcγRIIA-mediated sustained cell adhesion, cell spreading, and cell migration on IC-deposited targets. Thus, our study represents the first molecular characterization of Mac-1 binding to FcγRIIA and provides direct evidence for a physical interaction between Mac-1 and FcγRIIA, which may be useful to our understanding of the critical role of Mac-1 in FcγRIIA-mediated tumor cell killing by ADCC (1).
Mac-1 and the FcRs are the two major receptors involved in innate immunity against foreign pathogens, including phagocytosis, production of reactive oxygen intermediates and various cytolytic enzymes, as well as ADCC. Although each of the receptors recognizes its own ligands (C3bi and the ICs, respectively) and elicits distinct biological responses, numerous studies have demonstrated that Mac-1 and FcRs function cooperatively to promote cell killing (2–8,14–16). Interactions between FcRs and Mac-1 are mostly studied indirectly by their ability to co-cap on the cell surface (15) and by the ability of Mac-1 to promote FcR-mediated leukocyte functions, including phagocytosis (6,18), activation (5), and ADCC (6,8). However, no biochemical studies demonstrating a direct interaction between Mac-1 and FcRs have been reported. Based primarily on the inhibition studies with the lectin-domain-specific inhibitors, such as NADG, it is generally believed that FcRs interact with Mac-1 via its lectin domain (15). However, since NADG does not block Mac-1’s ability to promote FcγRIIA-mediated phagocytosis (18), the molecular basis underlying FcγRIIA recognition of Mac-1 is unknown. In this work, we found that FcγRIIA interacts primarily with the αMI-domain, but not the lectin-domain of Mac-1, which agrees well with this earlier study (18). In addition to our study, binding of soluble FcγRIIIA (CD16) to surface Mac-1 and CD11C/CD18, based exclusively on FACS analysis, has been reported (14). However, CD16 binding to Mac-1 can be inhibited by antagonists that are specific for either the αMI-domain (mAb 904) or the lectin-domain (NADG) (14). Furthermore, no direct interaction between Mac-1 and murine CD16 was found in a recent study (30). Thus, more studies are needed to clarify this confusion and to definitively localize the CD16 binding site within Mac-1.
The “switchblade” model of integrin activation predicts that the active integrin exists in an extended conformation (31), where the αMI-domain likely resides approximately ~ 250 Å above the cell membrane (32). Given the relatively small size (a total of ~170 residues in D1 and D2) of FcγRIIA (33), it is not clear how these two receptors interact with each other above the cell membrane. One possibility is that FcγRIIA on one cell interacts with Mac-1 on another cell, which we consider unlikely, based on the following observations: 1) the FcγRIIA- and Mac-1-expressing cells did not undergo heterotypical aggregations (unpublished observation); 2) cell spreading and migration requires co-expression of Mac-1 with FcγRIIA on the same cell (Fig. 5B and Fig. 6); and 3) Mac-1 colocalizes with FcγRIIA at the cell extensions of individual cells (Fig. 2A). Another possibility is that, analogous to the interaction between uPAR and integrins (34), FcγRIIA may interact with the integrins that exist in the bent conformation, where the αMI-domain would reside very close to the membrane (32). Should this be the case, FcγRIIA, like uPAR, might also be capable of modulating the activity of Mac-1. Further experiments will be required to test these different hypotheses.
Our data shows that Mac-1 binding to FcγRIIA alters the spatial distribution of FcγRIIA relative to actin filaments (Fig. 2B), and thereby promotes its sustained cell adhesion, spreading and migration on ICs (Figs. 1B, 5B, and 6). Consistent with our observations, Kusunoki et al. reported that sustained neutrophil adhesion to ICs is mediated by FcγRIIA but not by FcγRIII, and requires the presence of Mac-1 (35). An alternative mechanism is that FcγRIIA functions indirectly to promote cell spreading and migration by activating Mac-1, which in turn binds to ICs. However, our observations that 1) Mac-1 did not support cell adhesion to ICs with or without activation (Fig. 1A); 2) the FcγRIIA-specific blocking antibody IV3 inhibited Mac-1-mediated cell adhesion by differentiated HL60 in the presence of PMA (data not shown); and 3) the recombinant αMI-domain did not interact directly with the ICs in solid-phase binding assays (Fig. 4) suggest that the ICs are not a ligand of Mac-1. Thirdly, it is possible that FcγRIIA may initiate intracellular signaling, such as activation of Ca2+ influx, PI-3K, and MAPK (14), via its ITAM sequence or via engagement of Mac-1 (“outside-in” signaling), and thereby contribute to the sustained cell adhesion, spreading and migration. In this regard, our observation that deletion of the entire cytoplasmic tail of FcγRIIA, including the ITAM sequence, did not abrogate, but rather promoted cell migration (Fig. 6) is intriguing. It is likely that the ITAM sequence, which has been reported to recruit both SHP-1 and SHIP, may actually inhibit sustained cell adhesion, spreading and migration. Further experiments to investigate these possible mechanisms are underway.
In summary, we demonstrated that Mac-1 promotes the FcγRIIA-mediated functions, including cell adhesion, spreading and migration by binding directly to FcγRIIA and modulating its surface distribution. We identified a novel binding site within Mac-1 for FcγRIIA that is located within the αMI-domain but not the lectin domain. We have for the first time demonstrated a direct interaction between Mac-1 and FcγRIIA, based on both co-immunoprecipitation experiments using total cell lysates and solid-phase binding assays using purified proteins. The information provided in this study may help us better understand how Mac-1 contributes to the FcγRIIA-mediated functions, and thus may be useful in the design of therapeutic agents that can either enhance leukocyte killing of invading bacteria and fungi as well as malignant cells, or block the deleterious FcR-induced autoimmune diseases.
Acknowledgments
We are grateful to Sharron Brown for her help with protein expression in sf9 cells and Drs. Castellino and Plow for valuable reagents.
This work was supported in part by grants from the National Institute of Health (NHLBI R01 HL61589-01) and from American Heart Association (0240208N). Dr. Li Zhang is an established investigator of the American Heart Association.
Footnotes
FcR, receptor for the Fc fragment of immunoglobulin; IC, immune complex; ITAM, immunoreceptor tyrosine-based activation motifs; NIF, neutrophil inhibitory factor; PMA, phorbol 12-myristate-13-acetate.
References
- 1.Stockmeyer B, Beyer T, Neuhuber W, Repp R, Kalden JR, Valerius T, Herrmann M. Polymorphonuclear granulocytes induce antibody-dependent apoptosis in human breast cancer cells. J Immunol. 2003;171:5124–5129. doi: 10.4049/jimmunol.171.10.5124. [DOI] [PubMed] [Google Scholar]
- 2.van Spriel AB, van Ojik HH, Bakker A, Jansen MJ, van de Winkel JG. Mac-1 (CD11b/CD18) is crucial for effective Fc receptor-mediated immunity to melanoma. Blood. 2003;101:253–258. doi: 10.1182/blood.V101.1.253. [DOI] [PubMed] [Google Scholar]
- 3.van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, van de Winkel JG. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97:2478–2486. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- 4.Coxon A, Cullere X, Knight S, Sethi S, Wakelin MW, Stavrakis G, Luscinskas FW, Mayadas TN. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou MJ, Brown EJ. CR3 (Mac-1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc gamma RIII and tyrosine phosphorylation. J Cell Biol. 1994;125:1407–1416. doi: 10.1083/jcb.125.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majima T, Ohashi Y, Nagatomi R, Iizuka A, Konno T. Defective mononuclear cell antibody-dependent cellular cytotoxicity (ADCC) in patients with leukocyte adhesion deficiency emphasizing on different CD11/CD18 requirement of Fc gamma RI versus Fc gamma RII in ADCC. Cell Immunol. 1993;148:385–396. doi: 10.1006/cimm.1993.1120. [DOI] [PubMed] [Google Scholar]
- 7.Zhou MJ, Poo H, Todd RF, Petty HR. Surface-bound immune complexes trigger transmembrane proximity between complement receptor type 3 and the neutrophil’s cortical microfilaments. J Immunol. 1992;148:3550–3553. [PubMed] [Google Scholar]
- 8.Kushner BH, Cheung NK. Absolute requirement of CD11/CD18 adhesion molecules, FcRII and the phosphatidylinositol-linked FcRIII for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood. 1992;79:1484–1490. [PubMed] [Google Scholar]
- 9.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Tax WJ, Tamboer WP, Jacobs CW, Frenken LA, Koene RA. Role of polymorphic Fc receptor Fc gammaRIIa in cytokine release and adverse effects of murine IgG1 anti-CD3/T cell receptor antibody (WT31) Transplantation. 1997;63:106–112. doi: 10.1097/00007890-199701150-00020. [DOI] [PubMed] [Google Scholar]
- 11.Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol. 2002;14:798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- 12.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Stern A, Rosales C. Cross-talk between Fc receptors and integrins. Immunol Lett. 2003;90:137–143. doi: 10.1016/j.imlet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Gauchat JF, Mazieres N, Spagnoli R, Storkus W, Lotze M, Bonnefoy JY, Fridman WH, Sautes C. Soluble Fcgamma receptor type III (FcgammaRIII, CD16) triggers cell activation through interaction with complement receptors. J Immunol. 1996;157:1184–1192. [PubMed] [Google Scholar]
- 15.Zhou M, Todd RF, van de Winkel JG, Petty HR. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc gamma receptor III on human neutrophils. Possible role of lectin-like interactions. J Immunol. 1993;150:3030–3041. [PubMed] [Google Scholar]
- 16.Graham IL, Lefkowith JB, Anderson DC, Brown EJ. Immune complex-stimulated neutrophil LTB4 production is dependent on beta 2 integrins. J Cell Biol. 1993;120:1509–1517. doi: 10.1083/jcb.120.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehgal G, Zhang K, Todd RF, Boxer LA, Petty HR. Lectin-like inhibition of immune complex receptor-mediated stimulation of neutrophils. Effects on cytosolic calcium release and superoxide production. J Immunol. 1993;150:4571–4580. [PubMed] [Google Scholar]
- 18.Worth RG, Mayo-Bond L, van de Winkel JG, Todd RF, Petty HR. CR3 (alphaM beta2; CD11b/CD18) restores IgG-dependent phagocytosis in transfectants expressing a phagocytosis-defective Fc gammaRIIA (CD32) tail-minus mutant. J Immunol. 1996;157:5660–5665. [PubMed] [Google Scholar]
- 19.Zhang L, Plow EF. Overlapping, but not identical, sites are involved in the recognition of C3bi, neutrophil inhibitory factor, and adhesive ligands by the alphaMbeta2 integrin. J Biol Chem. 1996;271:18211–18216. doi: 10.1074/jbc.271.30.18211. [DOI] [PubMed] [Google Scholar]
- 20.von Arnim CA, Tangredi MM, Peltan ID, Lee BM, Irizarry MC, Kinoshita A, Hyman BT. Demonstration of BACE (beta-secretase) phosphorylation and its interaction with GGA1 in cells by fluorescence-lifetime imaging microscopy. J Cell Sci. 2004;117:5437–5445. doi: 10.1242/jcs.01422. [DOI] [PubMed] [Google Scholar]
- 21.Muchowski PJ, Zhang L, Chang ER, Soule HR, Plow EF, Moyle M. Functional interaction between the integrin antagonist neutrophil inhibitory factor and the I domain of CD11b/CD18. J Biol Chem. 1994;269:26419–26423. [PubMed] [Google Scholar]
- 22.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 23.Tang T, Rosenkranz A, Assmann KJM, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, Cotran RS, Mayadas TN. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor- dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Ferzly M, Takagi J, Springer TA. Epitope Mapping of Antibodies to the C-Terminal Region of the Integrin beta(2) Subunit Reveals Regions that Become Exposed Upon Receptor Activation. J Immunol. 2001;166:5629–5637. doi: 10.4049/jimmunol.166.9.5629. [DOI] [PubMed] [Google Scholar]
- 25.Brackman D, Lund-Johansen F, Aarskog D. Expression of cell surface antigens during the differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3, retinoic acid and DMSO. Leuk Res. 1995;19:57–64. doi: 10.1016/0145-2126(94)00061-e. [DOI] [PubMed] [Google Scholar]
- 26.Jones SL, Knaus UG, Bokoch GM, Brown EJ. Two signaling mechanisms for activation of alphaM beta2 avidity in polymorphonuclear neutrophils. J Biol Chem. 1998;273:10556–10566. doi: 10.1074/jbc.273.17.10556. [DOI] [PubMed] [Google Scholar]
- 27.Poo H, Krauss JC, Mayo-Bond L, Todd RF, Petty HR. Interaction of Fc gamma receptor type IIIB with complement receptor type 3 in fibroblast transfectants: evidence from lateral diffusion and resonance energy transfer studies. J Mol Biol. 1995;247:597–603. doi: 10.1006/jmbi.1995.0166. [DOI] [PubMed] [Google Scholar]
- 28.Petty HR, Worth RG, Todd RF., III Interactions of integrins with their partner proteins in leukocyte membranes. Immunol Res. 2002;25:75–95. doi: 10.1385/IR:25:1:75. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Plow EF. Identification and reconstruction of the binding site within alphaMbeta2 for a specific and high affinity ligand, NIF. J Biol Chem. 1997;272:17558–17564. doi: 10.1074/jbc.272.28.17558. [DOI] [PubMed] [Google Scholar]
- 30.Jongstra-Bilen J, Harrison R, Grinstein S. Fcgamma-receptors induce Mac-1 (CD11b/CD18) mobilization and accumulation in the phagocytic cup for optimal phagocytosis. J Biol Chem. 2003;278:45720–45729. doi: 10.1074/jbc.M303704200. [DOI] [PubMed] [Google Scholar]
- 31.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 32.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal Structure of the Extracellular Segment of Integrin {alpha}V{beta}3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell KF, Powell MS, Hulett MD, Barton PA, McKenzie IF, Garrett TP, Hogarth PM. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat Struct Biol. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Czekay RP, Robillard L, Kugler MC, Zhang F, Kim KK, Xiong JP, Humphries MJ, Chapman HA. Regulation of alpha5beta1 integrin conformation and function by urokinase receptor binding. J Cell Biol. 2005;168:501–511. doi: 10.1083/jcb.200404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusunoki T, Tsuruta S, Higashi H, Hosoi S, Hata D, Sugie K, Mayumi M, Mikawa H. Involvement of CD11b/CD18 in enhanced neutrophil adhesion by Fc gamma receptor stimulation. J Leukoc Biol. 1994;55:735–742. doi: 10.1002/jlb.55.6.735. [DOI] [PubMed] [Google Scholar]