Abstract
While monosynaptic bulbospinal projections to phrenic motoneurons have been extensively described, little is known about the organization of phrenic premotor neurons in the adult rat spinal cord. As interneurons may play an important role in normal breathing and recovery following spinal cord injury, the present study has used anterograde and transneuronal retrograde tracing to study their distribution and synaptic relations. Exclusive unilateral, first-order labeling of the phrenic motoneuron pool with pseudorabies virus demonstrated a substantial number of second-order, bilaterally-distributed cervical interneurons predominantly in the dorsal horn and around the central canal. Combined transneuronal and anterograde tracing revealed ventral respiratory column projections to pre-phrenic interneurons suggesting some propriospinal relays exist between medullary neurons and the phrenic nucleus. Dual-labeling studies with pseudorabies virus recombinants also showed pre-phrenic interneurons integrated with either contralateral phrenic or intercostal motoneuron pools. The stability of interneuronal pseudorabies virus labeling patterns following lateral cervical hemisection was then addressed. Except for fewer infected contralateral interneurons at the level of the central canal, the number and distribution of phrenic-associated interneurons was not significantly altered two weeks post-hemisection (i.e. when the earliest post-injury recovery of phrenic activity has been reported). These results demonstrate a heterogeneous population of phrenic-related interneurons. Their connectivity and relative stability after cervical hemisection raises speculation for potentially diverse roles in modulating phrenic function normally and post-injury.
Keywords: Pseudorabies Virus, Crossed-phrenic phenomenon, Interneurons, Phrenic, Plasticity, Respiration, Spinal cord injury
INTRODUCTION
Recent evidence has led to an increasing appreciation for the injured spinal cord’s neuroplasticity potential (Ballermann and Fouad, 2006; Bareyre et al., 2004; Edgerton et al., 2004; Morris et al., 2003; Pearson, 2001; Weidner et al., 2001). For example, it is now recognized that interneurons can play important roles in mediating behavioral improvements by establishing novel intraspinal relay pathways (Bareyre et al., 2004; Courtine et al., 2008; Harkema, 2008). However, the neural substrate underlying spontaneous or therapeutically amplified functional recovery after SCI remains poorly defined. Hence, greater definition of synaptic circuitries associated with specific recovery processes is required, not only to interpret the outcomes of promising experimental interventions (e.g., Fouad et al., 2005; Houle et al., 2006), but also reveal how connectivity within various intraspinal functional domains may modulate neuroplasticity after SCI (Fouad and Pearson, 2004; Pearson, 2001).
Compromised breathing is a major consequence of cervical SCI and is the greatest cause of morbidity and mortality (Jackson and Groomes, 1994; Mitchell and Johnson, 2003; Winslow and Rozovsky, 2003). However, some natural recovery can occur (Bluechardt et al., 1992; Ledsome and Sharp, 1981), and the most widely studied experimental illustration of respiratory neuroplasticity is the “crossed phrenic phenomenon” (CPP). Following a cervical (C2) spinal hemisection (HMx), the ipsilateral hemidiaphragm is paralyzed due to interruption of axons from neurons in the ventral respiratory column (VRC, a.k.a. rostral ventral respiratory group - rVRG (Dobbins and Feldman, 1994)) in the medulla that project monosynaptically onto ipsilateral phrenic motoneurons (PhMNs) at C3-C6 (Ellenberger and Feldman, 1988; Ellenberger et al., 1990). Partial recovery of ipsilateral hemidiaphragm function can be evoked by contralateral phrenicotomy in a variety of species (Goshgarian, 2003). A spontaneous CPP-like recovery of PhMN output can also occur weeks (Fuller et al., 2006; Fuller et al., 2003; Golder and Mitchell, 2005; Vinit et al., 2007) to months (Golder et al., 2001; Nantwi et al., 1999) after a C2HMx alone. However, neurophysiological recordings have demonstrated only modest improvements in ipsilateral phrenic nerve activity even as long as three months post-injury (Fuller et al., 2008; Fuller et al., 2006).
One explanation for limited PhMN recovery is that a population of non-respiratory interneurons may chronically inhibit PhMN output in the injured spinal cord (Zimmer and Goshgarian, 2007). Other findings suggest respiratory neuroplasticity can be enhanced by pharmacological modulation of mono- or polysynaptic circuits (Golder et al., 2008; Zimmer and Goshgarian, 2006; 2007). To date, CPP-mediated recovery has been solely attributed to the activation of decussating axonal collaterals from intact, contralateral VRC fibers which terminate monosynaptically on ipsilateral PhMNs (Goshgarian et al., 1991; Moreno et al., 1992). Spinal interneurons with projections to PhMNs have been characterized anatomically and electrophysiologically in a number species (Aoki et al., 1980; Bellingham, 1999; Duffin and Iscoe, 1996; Palisses et al., 1989; Yates et al., 1999). However, the distribution, synaptic relationships, and potential functional roles of pre-phrenic interneurons in the normal or lesioned spinal cord of the rat are less defined.
The present study offers neuroanatomical evidence for the presence of a diverse population of cervical interneurons with direct connections to PhMNs in normal adult rats. Some pre-phrenic interneurons appear to receive projections from bulbospinal VRC fibers. This is contrary to previous reports stating that medullary projections to the phrenic nucleus are exclusively monosynaptic. In addition, a contingent of these cells appears to be anatomically integrated with the left and right PhMN pools and between phrenic and intercostal circuits. Quantitative analyses suggest that the majority of interneuronal-PhMN connections are not overtly altered two weeks following C2HMx when early spontaneous recovery via the CPP has been reported (Fuller et al., 2008; Fuller et al., 2006; Golder and Mitchell, 2005). These collective neuroanatomical findings are consistent with previous neurophysiological findings and raise the possibility that interneurons may contribute to the modulation of PhMN activity in the normal and injured spinal cord more than previously recognized.
MATERIALS AND METHODS
Adult female Sprague-Dawley rats (250-300 grams) were obtained from Harlan Scientific and housed at the McKnight Brain Institute Animal Care Facility at the University of Florida. All experimental procedures were conducted with IACUC approval and following NIH guidelines. A total of 51 animals were used in this study.
Surgical Methods
All animals used for this study were anesthetized by injection of xylazine (10mg/kg s.q.) and ketamine (120mg/kg i.p.). Once the chosen procedure was completed, anesthesia was reversed via injection of yohimbine (1.2mg/kg s.q.). Upon recovery animals received buprinorphine (0.03mg/kg s.q., analgesic) and sterile lactated Ringers solution (5ml s.q., to prevent dehydration). For studies involving spinal hemisection (n=4), an incision was made extending from the base of the skull to the third cervical segment (C3), and a subsequent laminectomy was made at the second cervical segment (C2). A dural incision was then made and a lateral hemisection performed on the left side of the spinal cord with a microscalpel and gentle aspiration. Using this approach completeness of the lesion was readily visible and reproducible. The dura was closed with interrupted 10-0 sutures and durafilm placed over the dura. Muscle was sutured in layers and the skin closed with wound clips.
Neuroanatomical Tracers
Retrograde tracers used in this study were two recombinants of the Bartha strain of pseudorabies virus (PRV) or Cholera toxin B-subunit (CT-B 0.1% in dist illed water. While PRV is transynaptically transported and will infect the entire phrenic circuit over time, CT-B is a monosynaptic tracer and will label only those cells projecting to the site of tracer application (Yates et al., 1999). Therefore only PhMNs should be CT-B labeled. The two PRV recombinants were used in this study were PRV152 (8.0-9.9×108 pfu/ml) and PRV614 (2.0×108 pfu/ml), which expressed either an enhanced green fluorescent protein or monomeric red fluorescent protein, respectively. In other experiments, mini-ruby (biotin dextran amine conjugated with tetramethylrhodamine - Molecular Probes, 10,000 kDa) was used to anterogradely label projections from the medullary VRC region to the cervical spinal cord.
Propagation of Pseudorabies Virus
PRV152 was generously provided by Dr. Lynn W. Enquist (Princeton University). Additional stocks of PRV152 and PRV614 were obtained from Dr Bruce Banfield (University of Colorado). Biosafety Level II practices (U.S. Department of Health and Human Services, 1988) were employed in all phases of PRV usage. Details pertaining to the preparation of these viruses has been previously described (Banfield et al., 2003; Card and Enquist, 1999). For virus prepared locally (University of Florida), seed stocks of each were grown on porcine kidney (PK15) cells obtained from the American Type Culture Collection. The PK15 cells were propagated at 37°C, 5% CO2 with humidity in MEM Medium with Earl’s salts (Invitrogen) supplemented with nonessential amino acids, 10% fetal bovine serum and antibiotics (250 U of penicillin/ml, 250 μg of streptomycin/ml). Viral stocks were then produced by infecting near-confluent PK15 monolayers at a multiplicity of infection of 0.01. After exhibiting 100% cytopathic effect, the cells and medium were harvested and centrifuged at 16,000 x g for 40 min, resuspended in 1/100th of the original volume of medium, and then subjected to two rounds of freeze-thaw. The stocks were subsequently aliquoted into volumes necessary for each experiment and stored at -80°C. The aliquots were titrated for infectious virus on monolayers of PK-15 by standard plaque assay under agarose.
Retrograde Neuroanatomical Tracing
PhMN projections to the diaphragm were retrogradely labeled using either PRV152 alone (40-50μl; n=41) or in combination with CT-B (1:25, ∼1μl of each; n=6). An incision was made along linea alba and the skin and muscles were retracted to expose the diaphragm. Tracer was administered to the left half of the diaphragm by topical application (i.e., “painted” onto the muscle surface). Animals were left to survive for 48 to 72 hours following tracer application. This time-course was based in pilot data showing primary (PhMN) labeling at 48 hours, and extrapolated to subsequent transneuronal labeling.
In some experiments, the left and right halves of the diaphragm were labeled with PRV152 and PRV614, respectively and animals were left for 64 hours following PRV application (n=4). In another aspect of investigation, the diaphragm was infected with PRV152 and PRV614 was delivered to the three caudal-most intercostal muscle segments, ipsilateral to the labeled diaphragm, via either injection (n=1) or topical application (n=3). Animals were left to recover for 64 hours after which tissues were collected for histology. Application of separate PRV recombinants in this manner can be used to examine the relationship between different circuits (left vs. right phrenic and phrenic vs. intercostal circuits).
Tracing Controls
Phrenicotomy
A subset of animals (n=2) were anesthetized as above and an incision was made in the skin overlying the trachea. The sternocleidomastoid muscle and connective tissue was blunt dissected and laterally retracted to reveal the left brachial plexus and phrenic nerve. The phrenic nerve was then severed with iridectomy scissors and the cut ends deflected from each other. The muscle was sutured and the skin stapled. PRV was then administered to the ipsilateral hemidiaphragm as described above.
Cervical Sympathectomy
Superior cervical ganglionectomy was performed in another subset of animals (n=4) by using a ventral approach as described for the phrenicotomy above. An incision was made over the left carotid artery and the muscles deflected laterally. The carotid sheath was blunt dissected, the superior cervical ganglion isolated and excised. The ipsilateral hemidiaphragm was then labeled with PRV as described.
Vagotomy
Using the same surgical approach as described for the cervical sympathectomy, the left vagus nerve was isolated from the carotid artery, transected using iridectomy scissors and the severed ends deflected away from the site of transection (n=2). The muscles and skin were then sutured and stapled respectively. The ipsilateral hemidiaphragm was then labeled with PRV.
Anterograde Neuroanatomical Tracing
Anesthetized animals (n=10) were placed into a stereotaxic frame and an incision was made dorsally from caudal end of the skull to the first cervical segment. Muscles overlying the vertebral column were blunt dissected and retracted to reveal the cisterna magna. A small amount of the medial occipital bone was removed and an incision was made through the fissure and dura to expose the caudal medulla and obex. A Carbostar-3 microelectrode (Kation Scientific, Fig. 8), was used for both electrophysiological recording and iontophoretic delivery of tracer. The pipette was connected to both an audio monitor (Grass AM10) and CED Power 1401 Digitizer (connected to a PC) via an extracellular amplifier (ExAmp-20kb, Kation Scientific, M2100). One of the two glass barrels was filled with mini-ruby (10% in sterile saline (0.9%)) and a fine diameter, insulated tungsten electrode was inserted into the same barrel for iontophoresis. The pipette was stereotaxically lowered into the dorsal surface of the medulla in the region of the VRC (or rostral ventral respiratory group; rVRG (Dobbins and Feldman, 1994)), ∼500μm rostral and 2000μm lateral to the obex, until bursting was heard that correlated with inspiratory activity (rib-cage movement).
Figure 8.
Neurophysiologically-guided anterograde tracing of axons originating within the VRC (a.k.a., rVRG see text) is illustrated. Sections were labeled with Vectastain ABC to detect Miniruby (conjugated with biotin, brown) and counter-stained with cresyl violet. (A) Discrete stereotaxic localization of inspiratory medullary neurons was facilitated by using a Carbostar-3 electrode (B, image courtesy of Kation Scientific). Miniruby (10,000kDa BDA conjugated with rhodamine) was then iontophoretically delivered (see Methods). In a subset of animals (n=4) the rVRG recording was matched with diaphragm EMG recordings (C) to ensure correct placement of the electrode in the medulla. Note that the heartbeat was also detected in left-side diaphragm EMG traces. Anterogradely-labeled axons were observed in horizontal sections of the cervical spinal cord at the level of the phrenic motoneurons (D) and bilaterally around the central canal. Boutons were observed on the soma of motoneurons (asterisk in D) and between cells, presumably on dendrites (black arrowheads in D). Boutons were also found around the soma of neurons at the central canal (E). Labeled axons were seen crossing the spinal midline (white arrowhead in F). Rostro-caudal orientation in D-F is from top to bottom. Scale bars are 1mm (A), and 10 (B) 25 (D, E) and 50 microns (F). SEM = scanning electron microscope. cc = central canal.
A subset of these animals (n=4) was used to verify the location of the VRC by correlating bursts in physiological activity with inspiratory muscle activity. The diaphragm was surgically exposed and fine insulated tungsten electrodes were implanted in the left hemidiaphragm. The muscles and skin were then stapled as described above. The Carbostar-3 microelectrode and the EMG electrodes were connected to the same digitizer via a differential AC amplifier (AM systems, 1700). Localization of the VRC was defined by bursting activity from cells in the left medulla that were in synchrony with ipsilateral hemidiaphragm EMG recordings and with inspiratory movements of the rib-cage. As implantation of EMG electrodes in the diaphragm resulted in minor muscle injury, this approach could not be used in experiments exploring potential VRC projections to PRV-labeled PhMNs and interneurons. Therefore, in subsequent animals the VRC was located by only correlating bursts from cells in the medulla with rib cage movements only.
Once the VRC was located, tracer (10% Miniruby diluted in 0.9% sterile saline) was administered by iontophoresis (∼5.5mA, alternating 7 seconds on and 7 seconds off) for approximately 30 minutes. The electrode/pipette was then slowly withdrawn, the muscle was sutured in layers and the skin closed with wound clips. Animals were left to recover for 11-12 days following injection and transcardially perfused for histological analysis (n=6). To simultaneously demonstrate VRC projections relative to PRV-infected PhMNs and interneurons, animals with prior VRC injections were re-anesthetized and PRV applied to the hemidiaphragm (ipsilateral to the VRC delivery site) for dual-labeling. Those animals were then euthanized 64 hours later.
Histology and Immunohistochemistry
At the end of each experiment, animals were given a lethal dose of Beuthanasia® (9:1, sodium pentobarbitone to phenytoin solution) and intracardially perfused with paraformaldehyde (4% w/v in 0.1M PBS, pH=7.4). Brainstem and spinal cord tissue was then dissected and post-fixed by immersion in 2% paraformaldehyde and stored at 4°C for sectioning. Transverse vibratome sections (40μm) were made through the brainstem and either longitudinal or transverse vibratome sections (40μm) were made through the cervical spinal cord. One additional animal was processed for plastic sectioning as described below. Tissue from dual-tracing experiments labeled with PRV152 and either PRV614 (both expressing fluorophores) or Miniruby (conjugated with a red fluorophore) were slide mounted and coverslipped. Tissue labeled with only PRV152 was processed for bright-field microscopy. The optimal dilution of all antibodies was determined by repeated immunohistochemical studies. Negative immunohistochemical controls consisted of sections where either the primary or secondary antibody was omitted, as has been described previously (Dziegielewska et al., 2000). These control sections always appeared blank. In addition, tissue sections from negative control animals (not exposed to any tracers) showed no labeling.
Longitudinal sections were used for quantitative analysis of PRV labeling. Prior to incubation with the primary antibody, sections were washed in PBS (0.1M (pH=7.4), 3 × 5 minutes), blocked against endogenous peroxidase activity (30% methanol, 0.6% hydrogen peroxide in 0.1M PBS, incubated for 1-2 hours), re-washed in PBS, and blocked against non-specific protein labeling (10% serum in 0.1M PBS with 0.03% Triton-X). Sections were then incubated at 4°C overnight using primary antibodies against PRV (rabbit anti-PRV (Rb133/Rb134, raised against whole, purified PRV particles that were acetone inactivated), 1:10,000 - generously provided by Dr. Lynn Enquist). The following day, tissue was washed in PBS (0.1M, 3 × 5 minutes), incubated for 2 hours at room temperature in a biotinylated secondary antibody (donkey anti-rabbit, Jackson Immunocytochemicals, 1:200), re-washed in PBS (3 × 5 minutes) and incubated for another 2 hours in an avidin-biotin complex (ABC, Elite Vectastain Kit, Vector Labs). Sections were then given a third series of washes in PBS and antigen was visualized with diaminobenzidine (DAB, Sigma). Immunoprecipitation and SDS-page has demonstrated that these PRV antibodies are specific for the major capsid, tegument and viral membrane proteins of PRV (Card et al., 1990; Card et al., 1991). Specificity of labeling in the present study was determined by immunohistochemistry on tissue from negative control animals (not exposed to PRV). Sections from these animals showed no labeling.
One animal (64 hours following PRV delivery to left hemidiaphragm) was used for analysis of PRV labeled cells in semi-thin (i.e., 2μm) sections. Transverse Vibratome sections (100μm) were immunolabeled with PRV antibodies as described above. Sections were then thoroughly washed in PBS to ensure complete removal of DAB and post-fixed in a mixture of paraformaldehyde and glutaraldehyde (4% and 3.5% respectively in 0.1M PBS, pH=7.4) and stored at 4°C for two nights. The tissue was then processed and embedded in epoxy resin (Electron Microscopy Sciences) as previously detailed (Lane et al., 2007). PRV-infected cells were identified in semi-thin section counterstained with Toluidine blue.
Tissue that was dual-labeled with PRV152 and CT-B was incubated overnight in primary antibodies against PRV (as described above) and CT-B (polyclonal goat antiserum to purified cholera toxin B-subunit (choleragenoid - isolated from Vibrio cholerae type Inaba 569B), List Biological Laboratories, Product No. 703, Lot No. 7032A5, 1:10,000). This antiserum labels a single immunoprecipitation band against 0.5mg/ml of purified cholera toxin B subunit at a dilution of 1:16 on Western blot (manufacturers technical information). The following day, tissue was washed in PBS, incubated in fluorescently conjugated secondary antibodies (donkey anti-rabbit FITC - 1:100 and donkey anti-goat Texas Red - 1:100, Jackson Immunocytochemicals), re-washed and slide mounted for fluorescence microscopy. Tissue sections from negative control animals (not used for tracing) showed no labeling with either antibody.
Microscopy and Quantitative Analyses
Sections were examined using either brightfield or fluorescence microscopy. Brightfield and standard fluorescence photographs were taken using a Zeiss AxioPhot microscope with an AxioCam HRc digital camera linked to a PC. In addition, an Olympus IX81 Confocal microscope was to examine dual BDA-PRV labeled sections. Confocal images were obtained using a Hanamatsu ORCA-ER digital camera. Image size and contrast were standardized using Adobe Photoshop 7.0 (Adobe Systems, Inc.). No color correction profiles were used at any stage. All images were collected in a RGB (red-green-blue) color format. Supplementary images in magenta-green (Supp. Figs. 2-5) were made in Adobe Photoshop by copying information from the red channel and pasting this into the blue channel. Using this method the red labeling (PRV614 or Miniruby) becomes magenta and overlapping areas of red and green become white.
Using bright field microscopy, PRV-positive PhMNs and second-order cells with visible nuclei were counted at 10x magnification in consecutive longitudinal cervical spinal cord sections from each animal. Only cells with a visible nucleus were counted and an Abercrombie correction was used (T/[T+h], where T = section thickness and h = diameter of the nuclei in the z-axis) (Guillery, 2002). The average diameter of nuclei for each cell population was measured using KS-400 analysis software (KS Imaging Systems, ver. 3.0, Carl Zeiss Vision GmbH).
The average number of cells in each anatomical region (e.g. the dorsal horn) was represented for each time point following PRV delivery (± standard error of the mean; SEM). The number of positive labeled interneurons (secondary labeling) was then expressed as a function of the number of labeled phrenic motoneurons to control for any variation in primary labeling between animals. Statistical significance was determined by analysis using SigmaStat software (ver. 3.5). Comparison between treatment groups was performed using either t-test or ANOVA, and the Tukey test was used for multiple pair-wise comparisons. Ninety-five percent confidence intervals were used in all tests.
RESULTS
PRV infection of phrenic motoneurons
Ipsilateral PhMNs were the only infected neurons observed at spinal levels forty-eight hours following administration of PRV to the left hemidiaphragm. There was no evidence of bilateral PhMN pool labeling at this or any later survival interval. These PhMNs exhibited cytological and topographic features, including dendritic arborization patterns, as previously described by others (Dobbins and Feldman, 1994; Goshgarian and Rafols, 1981). Sections of cervical spinal cord (Figs. 1A, B, 2D, 3C) showed PRV-positive PhMNs extending from C3-C6. The PhMN pool appeared as a nearly continuous column of cells, usually comprised of distinct clusters of four or more motoneurons seen at intervals of approximately 100 microns throughout the phrenic nucleus. Between 56-64 hours following PRV application to the left hemidiaphragm, the number of infected PhMNs appeared to increase relative to that seen at 48 hours post-PRV application (see below). No glial cell infection was seen at this time (determined on a morphological basis in vibratome and semi-thin sections). However, by 64 hours, there was a noticeable infiltration of mononuclear cells around infected PhMNs observed in plastic semi-thin sections (Fig. 2D). At 72 hours post-PRV delivery, PhMN labeling appeared equally robust, although there seemed to be less variability in PhMN infection between animals than at 64 hours (see below). Early lysis of infected cells was observed 72 hours post-PRV delivery. Therefore all quantitative findings reported below are limited to the 64hr survival interval.
Figure 1.
A transverse spinal cord section is shown that was obtained from approximately the C3 spinal level ∼58 hours following PRV labeling of the left hemidiaphragm. In addition to infected ipsilateral phrenic motoneurons (* in A and B), some PRV-positive cervical interneurons (**) were also observed (A and C). No labeling was detected in the brainstem at this time point. This suggests that the cervical interneurons are second-order labeled. CC = central canal. Scale bar is 500 (A), and 100 (B & C) micrometers.
Figure 2.
Transverse semi-thin (2μm) plastic sections labeled with antibodies to PRV (brown) and counterstained with Toluidine blue, 64 hours following delivery of PRV to the left hemidiaphragm. Labeled cells were visible in the dorsal horn (arrowheads in A, B) and at the level of the central canal (arrowheads in C). B is a higher magnification image from A (*). At this time post-PRV there is an accumulation of mononuclear cells (**) around PRV-labeled PhMNs (D). This most likely reflects imminent lysis of the earliest infected cells and thus provides an indirect, but useful, index of second-order vs. primary infection. Scale bar is 100 (A), 50 (C) and 25 (B, D) micrometers.
Figure 3.
Three representative longitudinal sections are shown that were obtained from a serial section series of cervical spinal cord stained with PRV-specific antibody. Schematic diagrams of transverse spinal cord sections indicate the approximate horizontal level that each image was obtained. At 64 hours post-diaphragm PRV delivery, immunopositive cells were observed bilaterally in the dorsal horn (A) and more extensively in around the central canal (B). Cells within the dorsal horn are more sparsely distributed throughout the cervical cord (arrowheads in A) than those cells at the level of the central canal (B). The majority of labeled cells were observed ipsilateral to the labeled phrenic motoneuron pool (C). Cells within the dorsal horn and at the level of the central canal are shown at higher power in Fig. 4. Definitive second-order labeling of cells medial and lateral to the phrenic motoneuron pool was not determined in this study (see text). Emphasis in this study is thus restricted to PRV-immunopositive cells dorsal the level of the phrenic motoneuron pool. Rostro-caudal orientation is from right to left. Scale bar is 1 millimeter. NB. A high resolution version of this image is available as a Supplementary Figure (Supp. Fig. 1).
PRV infection of cervical spinal interneurons
Previously, Dobbins and Feldman (1994) reported PRV transynaptic infection of cervical interneurons consistent with the time for replication and transfer across one synapse: (46-52 hours). At 56-64 hours post-PRV in the present study, similar interneuronal labeling was observed in lamina VII near the lamina VII-X border and dorsal to the central canal in lamina X (Figs. 1A, C, 2C, 3B, 4B, C) and in the dorsal horn (Figs. 2A, B, 3A, 4A). In contrast with the exclusive ipsilateral labeling of PhMNs, second-order cells were bilaterally distributed (Fig. 3B, 4B, C), although the majority were found ipsilateral to the infected phrenic nucleus (Figs. 3C).
Figure 4.
High magnification images of putative pre-phrenic interneurons seen in longitudinal sections through the dorsal horn (A) and at the level of the central canal (B and C). Labeled interneurons were distributed bilaterally in all cervical segments. The dendritic processes from the majority of labeled cells lateral to the central canal were orientated in a rostro-caudal direction. However, some cells located more medially (over the central canal) had bilaterally directed processes some of which crossed the spinal midline (asterisk in B and C). Rostro-caudal orientation is from right to left. Scale bar is 200 (A, B) and 100 micrometers (C).
Most cervical interneuron labeling was largely restricted to the level of the PhMN pool. While some interneurons were also seen as far rostrally as C1, and caudal to the labeled PhMN pool, these cells were fewer in number. Lastly, a contingent of PRV-infected neurons was observed immediately lateral and medial to the PhMN pool in the ventral horn. Cells peripheral to the tightly clustered PhMN pool were excluded from the quantitative analyses described below, as an unequivocal distinction between putative interneurons and PhMNs could not be made.
Relative to PhMN diameters (∼30-40μm), second-order neurons around the central canal and in the dorsal horn were smaller on average (∼20-30μm). Designation of these second-order infected cells as putative interneurons is consistent with their size range and their gray matter locations. The later infection of these cells was also indicated by the lack of mononuclear cell accumulations as described around PhMNs above. The Rexed laminar distribution of cells was confirmed from transverse vibratome and plastic-embedded tissue sections (Figs. 1A, C, 2A-C). The number of cells varied considerably between transverse sections leaving the impression of a modest population of pre-phrenic interneurons in any one section. Serial horizontal tissue sections gave a clearer perspective of the relative number and rostral-caudal distribution of these PRV-labeled cells which spanned the entire distance of the cervical spinal cord to which this study was limited (Figs. 3). Except for occasional clusters of second-order cells, most were sufficiently separated from each other consistent with what was seen in transverse sections.
Most putative interneurons were multi-polar and exhibited pyramidal-shaped cell bodies. Dendrites of PRV-positive interneurons lateral to the central canal could be visualized for appreciable distances in single sections coursing in a rostro-caudal direction parallel to the central canal. Other cells were oriented perpendicular to the central canal, and their dendrites extended for shorter distances in a medial and lateral direction. Labeled neurons dorsal to the central canal had bilaterally directed dendritic processes that could be visualized traversing the midline (e.g. asterisk in Fig. 4B, C). Based on observations from transverse sections, labelling at the level of the central canal in horizontal sections will be referred to as laminae VII and X.
Examination of brainstem sections from six animals indicated that PRV infection of cervical interneurons at 64 hours occurred prior to significant labeling of medullary respiratory neurons. PRV labeling of cells in the VRC was not observed until 72 hours post-PRV delivery (n=5), as described by Dobbins and Feldman (1994). However, some labeling was observed in the lateral reticular (LRN) and raphe nuclei at 56 (n=2) and 64 hours (n=1) post-PRV delivery. This latter observation was highly variable (seen in only 3 out of 8 animals) and a complement of cervical interneurons was still detected in animals that had no brainstem labeling.
Control Experiments Demonstrate PhMN-Dependent Second-Order Labeling of Cervical Interneurons
The secondary (non-PhMN) labeling seen at 64 hours suggested the presence of a population of pre-phrenic interneurons, based on the observed cytoarchitectural pattern of PRV infection. To support this interpretation, experiments were performed in which both PRV and CT-B were simultaneously applied to the left hemidiaphragm (Billig et al., 2001). Dual retrograde labeling of the PhMN pool was clearly evident at 64 hours, but none of the second-order PRV-positive cells were labeled by CT-B (Fig. 5).
Figure 5.
To determine the pattern of first- and second-order labeling, dual retrograde tracing studies with PRV (transynaptically transported) and cholera toxin B-subunit (monosynaptically transported) were used. Sixty-four hours following delivery of these tracers to the hemidiaphragm, both PhMNs (A, transverse section) and interneurons (B, longitudinal section) were PRV-labeled (green); however, only motoneurons were labeled with the cholera toxin (red). Dual-labeled cells thus appear yellow. Rostro-caudal orientation in B is from left to right. Scale bar is 50 (A) and 100 (B) micrometers. NB. A magenta-green version of this image is available as a Supplementary Figure (Supp. Fig. 2).
Additional control experiments were conducted to eliminate possible labeling of these putative interneurons from non-PhMN sources (see also, Dobbins and Feldman, 1994). No infected neurons were detected in the dorsal motor nucleus of the vagus in any experiment, indicating that virus did not escape from the diaphragm to infect the parasympathetic innervation of abdominal organs (Card et al., 1990). To further verify that cervical neurons were not infected by diffusion of virus to non-target structures, a phrenicotomy was conducted prior to PRV delivery in two animals. With this approach, no PRV-infected cells were detected at either cervical or thoracic levels. In three animals, the ipsilateral vagus nerve was transected prior to delivery of PRV to the diaphragm; this did not alter the distribution of infected cervical neurons.
Lastly, a superior cervical ganglionectomy ipsilateral to the PRV152-labeled left hemidiaphragm was performed in four animals to factor out any possible contribution to our original labeling results due to sympathetic innervation of blood vessels in the diaphragm. Following these lesions, no infected neurons were observed in the intermediolateral cell column, but labeling of cervical interneurons persisted as described above. Thus, it seems unlikely that cervical interneurons were infected secondary to sympathetic nervous system circuitry.
Although many tracers have the potential for being transported both anterogradely and retrogradely, PRV-Bartha has been shown to be only transported in the latter direction (Card et al., 1998; Card et al., 1993). In other experiments prior to this study (Schrimsher and Reier, unpublished), cervical interneuron labeling was maintained even when dorsal rhizotomies (C3-C7) were performed prior to PRV delivery. In light of that and earlier reported evidence (Dobbins and Feldman, 1994), this control was omitted from the present study.
Quantitative analysis of phrenic motoneuron-associated interneurons
As our qualitative observations suggested, the number of PRV-positive PhMNs significantly increased between 48 (38.5 ±16.3; n=4) and 64 hours (243.3 ±40.9; n=4) following PRV delivery (P=0.006). However, there was no statistical difference between the number of PhMNs labeled at 64 and 72 hours (223.8 ±9.9; n=4). The absolute number of second-order labeled cells also remained comparable between 64 and 72 hours (Table 1) though considerable variability was evident. Regionally, the number of labeled interneurons in laminae VII and X was greater on the ipsilateral compared to contralateral side at both 64 and 72 hours post-PRV (Table 1). Similarly, the number of interneurons observed in the ipsilateral dorsal horn at 64 (38.2 ±14.7) and 72 hours (36.7 ±12.8) post-PRV was greater than that seen on the contralateral side at each time (6.2 ±2.7 and 8.9 ±2.3 respectively).
Table 1.
Quantification of PRV-labeled cells in cervical spinal cord The mean numbers of PRV-positive cells counted within each gray matter region (±SEM) are presented at different times following delivery of PRV to the left diaphragm in uninjured animals and 2 weeks following lateral spinal cord hemisection (bold)
| Time post-PRV delivery | Phrenic Motoneurons | Dorsal Horn | Laminae VII and X | ||
|---|---|---|---|---|---|
| Ipsilateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| 48hr | 38.5 (±16.3) | 0.4 (±0.4) | 0.0 (±0.0) | 0.4 (±0.4) | 0.0 (±0.0) |
| 64hr | 243.3 (±40.9) | 38.2 (±14.7) | 6.2 (±2.7) | 110.5 (±23.9) | 48.1 (±14.2) |
| 72hr | 223.8 (±9.9) | 36.7 (±12.8) | 8.9 (±2.3) | 106.0 (±6.0) | 46.6 (±10.5) |
| 64hr (SCI) | 163.8 (±32.9) | 14.2 (±9.4) | 2.3 (±2.3) | 45.1 (±24.0) | 9.6 (±4.9) |
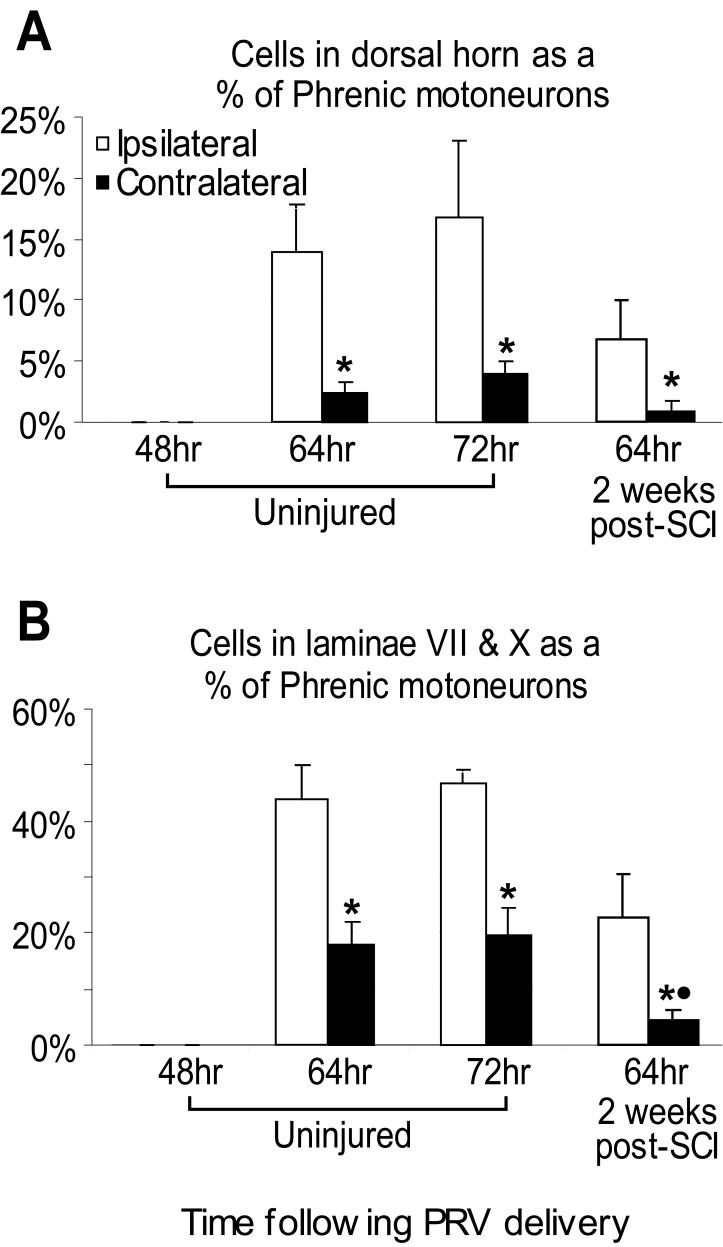
Given considerable variability in PhMN labeling between animals, the extent of regional interneuronal labeling was next expressed as a proportion of infected PhMNs (Fig. 6). There was no significant change in secondary labeling between 64 and 72 hours post-PRV delivery. However, the PhMN-interneuron ratio of labeled cells in the dorsal horn and in laminae VII and X of the ipsilateral spinal cord was significantly greater than on the contralateral side at both time points (Fig. 6). Within the dorsal horn 64 hours post-PRV delivery, the cervical interneurons represented 14% of infected PhMNs on the ipsilateral side (Fig. 6A), compared with 2% on the contralateral side. Similarly, a much higher proportion of interneurons was seen in ipsilateral laminae VII/X (∼45%) (Fig. 6B) compared to the contralateral side (∼20%). These results also indicate a much higher number of pre-phrenic labeled cells in the intermediate gray compared with the dorsal horns. Overall, the proportion of all second-order (presumed interneuronal) to PhMN labeling approximated 80% (representing both ipsi- and contralateral labeling).
Figure 6.
The number of PRV-positive cells counted in the dorsal horn (A) and in laminae VII and X (B) are presented as a proportion of the number of PRV-positive PhMNs (±SEM). Forty eight hours following delivery of PRV to the left diaphragm (n=4), no cells other than the PhMNs were labeled. Sixty-four (n=4) to seventy-two hours (n=4) post-PRV delivery, a significant number of cells were observed bilaterally in the dorsal horn and at the level of the central canal. The number of cells within each area relative to labeled PhMNs remained constant between 64 and 72 hours. However, the number of cells labeled in both the ipsilateral dorsal horn and in laminae VII and X was significantly (P<0.05) greater than seen contralaterally (*). Two weeks following injury (n=4), there is a reduction in the number of cells observed in all topographical regions. The cell number around the central canal post-injury, contralateral to the labeled hemidiaphragm (●), was statistically less than seen in uninjured animals 64 hours post-PRV delivery (P=0.03)
Laterality of phrenic motoneuron-associated interneuronal projections
Bilateral distribution of interneurons in laminae VII and X raised the possibility that some phrenic-associated interneurons may project to both the left and right PhMN pools. To test this, left and right hemidiaphragms were labeled with PRV152 and PRV614, respectively. Phrenic motoneurons were labeled on each side in a similar pattern with no indication of PhMN co-infection. Interneurons infected with each viral recombinant were again observed bilaterally in laminae VII and X. Though many of these neurons were labeled with one or the other recombinant, some double-labeled cells were seen (Fig. 7). This confirms that at least a subpopulation of these interneurons is integrated with both left and right phrenic circuitry.
Figure 7.
Shown are longitudinal sections of the cervical spinal cord 64 hours following co-administration of PRV152 (green) and PRV614 (red) to the left and right halves of the diaphragm, respectively. Bilaterally-labeled interneurons were observed (A) as shown previously. In addition, there was a sub-population of bilaterally distributed interneurons at the level of the central canal that were labeled with both PRV variants (arrowheads). Rostro-caudal orientation is from top to bottom. Scale bar is 25 (A) and 100 micrometers (B). NB. A magenta-green version of this image is available as a Supplementary Figure (Supp. Fig. 3).
VRC fibers closely approximate infected phrenic motoneuron-associated interneurons
In addition to their innervation of the PhMN pool, anterogradely-labeled VRC axons have been shown to extend collaterals to immediately surrounding gray matter areas at the level of the lamina VII/X interface (see Figs. 4 and 5 in Goshgarian et al., 1991). However, the significance of that finding was not pursued. Because these regions correspond to the location of most cervical interneurons described in the present study, another series of experiments was conducted to determine the potential innervation of these interneurons by VRC fibers. For this purpose, the left VRC was first located using a combination of stereotaxic coordinates and electrophysiological recordings of respiratory-related discharges (see Methods; Fig. 8C). Iontophoretic injections of Miniruby were subsequently made. These injections resulted in discrete neuronal labeling predominantly within the tegmental field immediately lateral to nucleus ambiguus (Fig. 8A). The majority of these cells were observed within 200μm lateral and ventrolateral of nucleus ambiguus, within the VRC. Some minor labeling was also seen within the ipsilateral spinal trigeminal nucleus, which given its close approximation to the injection site, may have been directly labeled by iontophoresis. Labeled fibers were observed within the medulla both ipsi- and contralateral to injection, but no bouton-like profiles were visible. Although Miniruby is predominantly transported in an anterograde direction (Reiner et al., 2000), some retrogradely labeled cells were also observed in the region of the VRC on the contralateral side and within the medullary raphe.
The distribution of labeled fibers within the cervical spinal cord could be divided into three regions: 1) bilaterally at the level of the phrenic motor nucleus, 2) bilaterally around the central canal (laminae VII and X) and 3) the ipsilateral dorsal horn. The first region was consistent with physiologically guided injection into the VRC. Initial experiments also confirmed the accuracy of VRC injections using immunohistochemistry for BDA and cresyl violet counterstaining. Labeled fibers were observed coursing longitudinally in the ventromedial white matter of the cervical spinal cord (as has been reported previously (Lipski et al., 1994)) and the ventral gray matter. These axons extended into the phrenic pool and boutons were observed in close approximation to cell soma and within the surrounding dendritic field (Fig. 8D). Boutons were also observed in close proximity to interneurons and associated proximal dendrites (Fig. 8E). This observation may indicate synaptic contacts. As shown by Goshgarian et al. (1991) some labeled VRC axons crossed at the midline (Fig. 8F) and were directed towards the opposite ventral horn. Although of the present study did not label the contralateral PhMN pool, the trajectory of those fibers appeared consistent with the location of the phrenic nucleus.
These findings were extended by Miniruby administration into the left medulla and topical application of PRV152 to the ipsilateral hemidiaphragm. Densely arborized Miniruby-positive fibers projected throughout the PRV-labeled phrenic pool (Fig. 9A, B). In some cases, these axons coursed parallel to longitudinally oriented PhMN dendrites. Bouton profiles were seen in high density in regions where PhMN cell bodies were clustered. Anterograde tracing of VRC axons also revealed projections into the dorsal horn and central grey matter (lamina VII and X). Some axons extended across the midline into the contralateral grey matter of the same region. Dual-labeling with left VRC Miniruby injection and ipsilateral hemidiaphragm PRV application showed arborizations of Miniruby-positive around the dendrites (Fig. 9C) and soma (Fig. 9D) of some PRV- labeled interneurons both ipsi- and contralateral to the injected VRC.
Figure 9.
Longitudinal sections through the cervical spinal cord. Miniruby (red) was iontophoretically delivered into the left rVRG and PRV152 (green) was administered to the ipsilateral diaphragm. Descending Miniruby-positive fibers from the medulla were observed forming boutons on and around PRV-labeled phrenic motoneurons (A & B). Many of these contacts were seen on the dendrites running rostro-caudally between motoneurons (A) and around the soma of these cells (B). Similar contacts were observed on the dendrites (arrowhead in C) and soma (arrowhead in D) of PRV-positive cervical interneurons distributed bilaterally around the central canal. Rostro-caudal orientation is from top to bottom. Scale bar is 50 (A) and 25 micrometers (B-D). NB. A magenta-green version of this image is available as a Supplementary Figure (Supp. Fig. 4).
Some Cervical Interneurons Exhibit Dual Projections to Phrenic and Intercostal Motoneuron Pools
Previous studies have described neurophysiological integration between phrenic and intercostal respiratory circuits (Bellingham, 1999; Decima et al., 1967). However, relatively little is known about anatomical connectivity between these regions in the rat. This was explored using dual application of PRV152 and PRV614 to the left hemidiaphragm and left intercostal muscles respectively. Sixty-four hours following PRV delivery, the left PhMN pool was positively labeled with PRV152 only. PRV152- and PRV614-positive interneurons were observed bilaterally throughout laminae VII and X of the cervical spinal cord. These neurons were predominantly labeled with only one of these tracers. Though few in number, dual-labeled cells were also detected (Fig. 10). These cells were bilaterally distributed from C3-C5 and C7, indicating that a subpopulation of these interneurons is integrated with both motoneuron pools.
Figure 10.

Longitudinal sections are illustrated of the cervical spinal cord 64 hours following dual-PRV labeling. PRV152 (green) was applied to the left diaphragm and PRV614 (red) to the left intercostal muscles. Interneurons labeled for both tracers were seen bilaterally throughout the cervical spinal cord, around the central canal. While the majority of cells were individually labeled for either PRV152 or PRV614, there was a sub-population of interneurons that were double-labeled (yellow, arrowheads). Rostro-caudal orientation is from top to bottom. Scale bar is 25 micrometers. cc = central canal. NB. A magenta-green version of this image is available as a Supplementary Figure (Supp. Fig. 5).
Phrenic Nucleus-Associated Interneurons Maintain Connections after High Cervical Hemisection
We next examined whether the normal pattern of PhMN-IN connectivity was preserved following C2HMx. PRV labeling was performed two weeks after injury since this is the earliest time at which the spontaneous CPP has been reported by us and others (Fuller et al., 2008; Fuller et al., 2006; Golder and Mitchell, 2005). At 64 hours post-PRV delivery (n =4), the distribution and general morphology of infected phrenic motoneurons appeared overtly unaltered (Fig. 11A). While the number of infected PhMNs was quantitatively less than that seen in control animals (163.8 ±32.9 compared to 243.3 ±40.9, see Table 1), this difference was not significant (P=0.208).
Figure 11.

Longitudinal sections are shown from animals 2 weeks following a lateral C2 hemisection and 64 hours following PRV delivery to the ipsilateral hemidiaphragm. Although there is an apparent decrease in the number of labeled cells compared with uninjured animals (Table 1), this difference was not statistically significant. The distribution of labeled phrenic motoneurons (A) and cervical interneurons (B) was comparable with uninjured animals. Labeled interneurons were also within close proximity to the lesion cavity (C). Rostro-caudal orientation is from right to left. Scale bar is 200 (A, B and inset in C) and 400 micrometers (C). cc = central canal
The distribution and morphology of interneurons after injury were comparable to that observed in uninjured animals (Fig. 11B, C), except that no cells were labeled in the first cervical segment (above the level of injury). However, there was a decrease in secondary labeling observed in the ipsi- and contralateral dorsal horn, laminae VII and X, and in the lateral and medial aspects of the gray matter at the level of the phrenic pool post-SCI (Table 1 and Fig. 6). The greatest change in cell number was seen in contralateral laminae VII/X (Table 1). Despite this apparent reduction, no significant differences were observed in the raw cell numbers between injured and uninjured animals (P=0.06).
To determine whether the decrease in secondary labeling reflected the reduced primary labeling (PhMNs) observed, the proportion of secondary to primary labeling was determined. While number of PRV-positive interneurons corresponded with a decline in PhMN labeling post-injury, there was a significant difference in ratio of secondary to primary labeling in contralateral laminae VII/X (P=0.03) compared with data from normal animals. However, no statistical difference was observed between injured and uninjured rats in any other topographical region. P values ranged from 0.09 (ipsilateral laminae VII/X) to 0.34 (contralateral dorsal horn).
DISCUSSION
While references to pre-phrenic interneurons in the rat have been made (Hilaire and Monteau, 1997), limited neuroanatomical evidence for such cells has been reported. Based on data from other species (Cleland and Getting, 1993; Iscoe and Duffin, 1996), pre-phrenic interneurons in the rat have been viewed as being primarily involved in mediating postural- or other nonrespiratory-related behaviors. Some findings suggest, however, that rat cervical interneurons may be integrated between descending pathways and PhMNs in the rat (Ling et al., 1995) and can influence PhMN bursting patterns, such as short-term potentiation (Hayashi and Fukuda, 1995). The phrenic circuitry features identified by this study are consistent with these and other neurophysiological observations. These findings demonstrate a substantial population of cervical pre-phrenic interneurons which may modulate PhMN activity and neuroplasticity, and integrate the phrenic and other motoneuron pools.
Phrenic-Associated Cervical Interneurons and Temporal Patterns of PRV Infection
The second-order PRV infection at cervical spinal levels observed in the present study, significantly extend findings of an earlier investigation which demonstrated intraspinal labeling of cells in Rexed laminae VII and X of the adult rat (Dobbins and Feldman, 1994). However, the emphasis of that study was on medullary projections to PhMNs. The interneurons observed were not considered to reflect direct associations with primary respiratory pathways because labeling occurred concurrently with brainstem premotor neuron infection. Instead, it was suggested that the interneurons were integrated with a secondary pathway via ascending medullary projections.
In the present study, concomitant interneuron and brainstem labeling was inconsistent. Furthermore, possible second-order infection of spinal neurons via ascending ipsilateral projections was ruled out by comparable interneuron labeling patterns being seen in uninjured controls and animals with chronic C2HMx lesions. The majority of second-order PRV-positive cervical neurons observed in this study most likely represent bona fide interneurons with direct innervation of PhMNs. This interpretation challenges previous WGA-HRP (horseradish peroxidase-conjugated with wheat germ agglutinin) transynaptic tracing experiments. Studies using WGA-HRP failed to show any transneuronal labeling of interneurons either normally or after C2HMx in the adult rat (Moreno et al., 1992). For the present study, a separate series of WGA-HRP experiments was performed (not shown) which confirmed those findings. The disparity between transneuronal labeling results may reflect WGA-HRP, unlike PRV, being transported via physiologically active connections between cells (Harrison et al., 1984). Other variables (e.g., tracer concentration) may also be involved (Harrison et al., 1984).
Quantitative Assessment of the Pre-Phrenic Interneuron Population
Raw counts of second-order cervical neurons revealed a substantial number of cells in the dorsal horn, and laminae VII/X both ipsi- and contralateral to the labeled hemidiaphragm. These counts, however, may under-represent the magnitude of the interneuron population. This could be due to incomplete primary labeling of the PhMN pool as the total number of PhMNs labeled in this study was less than previously reported (Goshgarian and Rafols, 1981). To circumvent some variability, counts of second-order labeling were normalized as a function of infected PhMNs in each specimen. These results also demonstrated a substantial number of interneurons ipsi- and contralateral to the labeled PhMN pool throughout the entire cervical spinal cord. In laminae VII/X on the ipsilateral spinal cord alone, normalized data indicated approximately one interneuron for every two phrenic motoneurons. One caveat is that a proportion of labeled interneurons may represent part of a local polysynaptic circuit. The post-PRV survival periods used cannot discriminate between true second-order and subsequent local interneuronal labeling that may occur in a compressed post-infection interval.
Heterogeneity of Phrenic Interneurons
Given the bilateral gray matter distribution of these cells, it is likely they represent functional heterogeneity within each cytoarchitectural region (see below). To explore this possibility, dual PRV tracing was used to determine how pre-phrenic interneurons may be integrated between left and right phrenic circuitries and between phrenic and intercostal motoneuron pools. Although the use of two similar PRV variants for dual-tracing has been previously described, differences between recombinants can affect the rate of transport of each virus and neuronal infection, as well as co-infection (Banfield et al., 2003; Billig et al., 2000; Cano et al., 2004; Kim et al., 1999). At comparable titers, robust PRV co-infection has been described (Banfield et al., 2003). However, the titers of each PRV variant used in the present study differed slightly (PRV152: 9.9×108 pfu/ml; PRV614: 2.0×108 pfu/ml). Therefore, the present dual-labeling results may not accurately reflect the number of cells that project to both sides of the diaphragm. For this reason, only qualitative observations were made for this part of the study.
The distribution of PRV-labeled cells and the patterns of connectivity that we report suggest a substantial diversity in the rat phrenic nucleus. For example, the presence of interneurons around the central canal, some of which project to both PhMN pools, indicates some interneurons may serve as commissural cells that integrate PhMNs on each side of the spinal cord. In addition, the extensive distribution of pre-phrenic interneurons throughout the cervical spinal cord indicates that some pre-phrenic interneurons may integrate phrenic with other respiratory motor circuits. Evidence from other species suggest that cervical interneurons are involved with phrenic and other spinal functions such as: intercostal activity (Bellingham, 1999; de Troyer, 1998; Decima et al., 1969), abdominal contraction (Billig et al., 2000), postural control (Butler, 2007) and locomotion (Eldridge et al., 1987; Palisses et al., 1988; Viala, 1986; Viala et al., 1979). It is therefore possible that the cervical interneurons described here mediate a similar function. This is supported by evidence of a subpopulation of interneurons around the central canal with projections to both the phrenic and intercostal motoneuron pools. The absence of dual labeling in the motoneuron pools of interest (left vs. right phrenic motoneurons or phrenic vs. intercostal motoneurons) indicates that dual interneuron infection in these experiments was not a consequence of peripheral diffusion (e.g., between the right and left hemidiaphragm).
Supraspinal input to cervical interneurons
Electrophysiological observations suggest that projections from the VRC innervate pre-phrenic interneurons in some species (Davies et al., 1985; Fedorko et al., 1983). However, neuroanatomical evidence in the rat has been largely unsupportive (Dobbins and Feldman, 1994; Goshgarian et al., 1991). However, using isolated brainstem-spinal cord preparations, Juvin and Morin (2005) have recently reported that an equal proportion of direct and polysynaptic relay projections to PhMNs exist at birth with the former becoming increasingly predominant thereafter. Our results from experiments using combined PRV and anterograde tracing of VRC axons suggest that a contingent of respiratory-relay cervical interneurons may persist in the adult spinal cord consistent with other electrophysiological data (Hayashi and Fukuda, 1995; Ling et al., 1995).
It has been generally viewed that interneurons in the upper cervical spinal cord of the rat and other species could represent polysynaptic relays between medullary VRC neurons and PhMNs. For example, some C1-C2 respiratory interneurons in the rat extend collaterals into the region of the phrenic nucleus (Lipski et al., 1993; Tian and Duffin, 1996). Those projections, however, are extremely limited (Lipski et al., 1993) as our present findings would predict. PRV infection is a function of innervation density (Card et al., 1999) and few C1-C2 interneurons were observed relative to more numerous pre-phrenic interneurons at other cervical levels.
Consistent with other reports using a similar experimental approach (Ellenberger et al., 1990; Goshgarian et al., 1991), we observed prominent VRC innervation of ipsilateral PhMNs even with limited BDA labeling of the VRC. Crossed VRC projections towards the contralateral PhMN pool were also observed. Previous anterograde tracing studies of bulbospinal projections in rat not only revealed what appeared to be decussating direct projections to the phrenic nuclei, but also dense arborizations of VRC collaterals to laminae VII/X (Figure 4 in Goshgarian et al., 1991). In light of putative second-order interneurons now demonstrated in the same region, it seemed likely that some of these PRV-positive cells could receive projections from VRC axons. Although ultrastructural confirmation is required, routine fluorescence and confocal microscopy also revealed anterogradely-labeled axons terminating in close proximity to soma and dendrites of some PRV-infected cervical interneurons ipsi- and contralateral to the side of BDA injection and PhMN pool infection. This close approximation of VRC axons to PRV-positive cells suggests that some pre-phrenic interneurons are present in the rat, which are interposed within primary descending respiratory pathways (Hayashi et al., 2003). The relationship of VRC projections to pre-phrenic interneurons raises additional considerations for the neural substrates involved in respiratory plasticity and the spontaneous CPP.
Integrity of Pre-Phrenic Interneuron Connections after C2 Hemisection
In the context of spontaneous respiratory neuroplasticity, an immediately relevant question is whether any major changes occur in the relationship between cervical interneurons and PhMNs after high cervical hemisection. We selected the two-week post-C2HMx interval since prior studies have shown this to be the earliest onset of the spontaneous CPP (Fuller et al., 2008; Fuller et al., 2006; Fuller et al., 2003; Golder and Mitchell, 2005). Our present findings are admittedly indirect since it is presently unknown to what degree PRV infection may alter neuronal activity prior to cell lysis and thus negate PRV tracing and neurophysiological analyses in the same animal.
Quantitative analyses revealed a reduction in the raw number of labeled interneurons. One possibility is that this change could be due to variability in post-injury PhMN infection (Table 1). However, PhMN counts from C2HMx animals were not statistically different from uninjured rats. Any differences in PhMN labeling could nonetheless be due to peripheral changes such as remodelling of neuromuscular junctions (Mantilla and Sieck, 2003) among other possibilities. Normalized data, as described above, revealed a significant regional decrease in the proportion of contralateral laminae VII/X interneurons after C2HMx. However, caution needs to be exercised in interpreting this result because assumptions used for counts from normal animals may not apply after SCI.
The most conservative conclusion is that overall interneuronal labeling is not substantially altered after C2HMx. We know from other preliminary data (not shown), that interneuronal PRV infection still occurs twelve weeks post-C2HMx - well after PhMN neurons recover some function after C2HMx (Fuller et al., 2008; Nantwi et al., 1999). Use of PRV in a lesion setting has been very limited (Bareyre et al., 2004; Kim et al., 2002). Despite its potential for demonstrating circuitry alterations, improved approaches for quantitative analyses will be required. The application of PRV transneuronal tracing after SCI in the present work provides some technical insights in that regard.
Pre-Phrenic Interneurons & Implications for Respiratory Recovery after SCI
Little functional significance has been attributed to pre-phrenic interneurons in relation to rhythmic breathing. Regardless such cells may play other vital roles in modulating PhMN function under various behavioral and physiological conditions, including SCI. Accordingly, the stability of interneuronal relationships with PhMNs after C2HMx raises the possibility these cells are part of a neural substratum that can modulate the onset and degree of early and long-term PhMN recovery or be therapeutically recruited to augment respiratory outcomes. The onset of the CPP can be accelerated or its magnitude enhanced by various surgical, physiological, or pharmacological manipulations (Goshgarian, 1981; Zimmer and Goshgarian, 2006; 2007). For example, successive C3-C8 contralateral rhizotomies result in enhanced activation of the CPP, which implies removal of segmental inhibition of PhMN recovery (Goshgarian, 1981; Vinit et al., 2007). Such inhibition of PhMN activity may occur via GABAergic connections, although the specific cellular source of inhibition has not been determined (Zimmer and Goshgarian, 2007). Therefore, it is tempting to speculate from the post-C2HMx data discussed that reduced PhMN innervation by a subset of contralaterally located interneurons may lead to attenuated inhibition of PhMNs and emergence of the CPP. Consistent with this consideration are findings from studies of phrenic afferent projections in rats which have identified three terminal fields (Goshgarian and Roubal, 1986; Song et al., 1999) corresponding to the distribution of PRV-labeled neurons observed in the present study. Alternatively, pre-phrenic interneurons in the dorsal horn may be even more plausible sources of PhMN inhibition. While PhMNs appear to be relatively unresponsive to proprioceptive regulation normally, their sensitivity may change after SCI (Forster, 2003).
In addition to potential inhibitory influences, the possibility exists that some phrenic-associated interneurons also have an excitatory function. Vinit et al. (2007) suggest that ipsilateral phrenic afferent inputs facilitate PhMN activity following C2HMx. While sensory inputs to PhMNs have been primarily viewed as being monosynaptic (Vinit et al., 2007), the possibility exists of a polysynaptic substrate that may partially facilitate CPP-related PhMN recovery. Future studies involving neurotransmitter profiling of PRV-labeled interneurons in conjunction with primary afferent mapping may provide additional insights.
Anterograde VRC tracing demonstrated terminal arborizations in close proximity to the soma and dendrites of pre-phrenic interneurons. This raises the possibility that intact VRC projections from the contralateral spinal cord could influence PhMN activity via not only monosynaptic, but di- or polysynaptic routes as well. Since these initial observations were made in normal rats, what bearing these cells have on PhMN activity after C2HMx awaits further investigations in spinal-lesioned animals. Neither the precise distribution (apart from being at the level of the phrenic nucleus) nor relative number of crossed VRC fibers involved in the CPP is presently known. Conceivably, an inter- or intra-segmental relay could serve as an additional crossed pathway that may amplify inspiratory drive to ipsilateral PhMNs in the C2 lesion model or otherwise modulate PhMN excitability. Future studies are needed to determine whether any of these interneurons are among those which appear decreased after C2HMx, whether changes in VRC innervation of these cells post-injury can be demonstrated, and what other descending supraspinal inputs these interneurons receive.
While ipsilateral PhMN function spontaneously returns following C2HMx, the amplitude of phrenic output is often well below normal even after long post-lesion intervals (Fuller et al., 2008). One interpretation for this limited recovery is that synaptic efficacy is suboptimal. For example, various pharmacological approaches have been shown to promote enhanced PhMN excitability normally and after C2HMx (Fuller et al., 2003; Golder et al., 2008; Ling et al., 1995; Mitchell and Johnson, 2003; Nantwi and Goshgarian, 2002). The anatomical framework for such observations typically defaults to effects exclusively at the level of the PhMN. However, as exemplified by the discussion in a recent study (Golder et al., 2008), the possibility of pharmacological action within a spinal, polysynaptic pre-phrenic circuit cannot be dismissed. From that perspective, the present findings provide another important neural circuitry context for interpretation of important physiological/molecular observations which thus far have lacked a comprehensive neuroanatomical framework of any kind. Furthermore, Ling et al. (1995) reported neurophysiological evidence that polysynaptic inputs to PhMNs can be evoked by contralateral spinal cord stimulation after C2HMx. Under control conditions following C2HMx, electrical stimulation of the ventral funiculus contralateral to the lesion evoked a short-latency potential in the phrenic nerve that was entirely consistent with activation of a crossed-spinal, monosynaptic input. However, following systemic application of a serotonergic precursor (5-HTP), the identical stimulus paradigm produced complex, multi-peak evoked potentials in the ipsilateral phrenic nerve that were consistent with activation of polysynaptic inputs to the PhMNs. Under these experimental conditions, 5-HTP may have revealed a polysynaptic input to PhMNs in C2HMx rats.
Future demonstration of the various potential functions of pre-phrenic interneurons remains challenging. However, the present observations can guide the use of molecular, neurophysiological and neuropharmacological approaches to probe the functional relevance of pre-phrenic neurons in the normal and injured spinal cord of an animal model extensively employed in studies of respiratory neurophysiology and neuroplasticity.
Acknowledgements
PRV152 was generously provided by Lynn W. Enquist, Princeton University, as a service of the National Center for Experimental Neuroanatomy with Neurotropic Viruses: NCRR P40 RRO118604. Stocks of PRV614 and additional PRV152 were generously donated by Bruce Banfield, University of Colorado. Fig. 8B was kindly donated by Kation Scientific.
Thanks go to Drs. J. Patrick Card and Lynn Enquist, from the University of Pittsburgh and Princeton University respectively, for their valuable advice with PRV tracing and to Dr. Richard Johnson for his guidance with and medullary injections. The authors would also like to thank Dr. Ray Guillery for his advice with quantitative analysis. We also extend our appreciation to Barbara O’Steen, Kevin Siegel and Forest Hunsaker for their expert technical assistance with this work.
Financial Support
Studies supported by NIH/NINDS RO1 NS054025, the Anne and Oscar Lackner Endowed Chair (PJR) and the Craig H. Neilsen Foundation (MAL)
References
- Aoki M, Mori S, Kawahara K, Watanabe H, Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res. 1980;202(1):51–63. [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23(8):1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J Virol. 2003;77(18):10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82(3):1224–1232. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci. 2000;20(19):7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Hartge K, Card JP, Yates BJ. Transneuronal tracing of neural pathways controlling abdominal musculature in the ferret. Brain Res. 2001;912(1):24–32. doi: 10.1016/s0006-8993(01)02597-5. [DOI] [PubMed] [Google Scholar]
- Bluechardt MH, Wiens M, Thomas SG, Plyley MJ. Repeated measurements of pulmonary function following spinal cord injury. Paraplegia. 1992;30(11):768–774. doi: 10.1038/sc.1992.148. [DOI] [PubMed] [Google Scholar]
- Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol. 2007;159(2):115–126. doi: 10.1016/j.resp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471(4):462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- Card JP, Enquist LW, et al. Transneuronal Circuit Analysis With Pseudorabies Viruses. Current protocols in neuroscience. 1999;S9:1.5.1–1.5.28. doi: 10.1002/0471142301.ns0105s68. editorial board, Jacqueline N Crawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Enquist LW, Moore RY. Neuroinvasiveness of pseudorabies virus injected intracerebrally is dependent on viral concentration and terminal field density. J Comp Neurol. 1999;407(3):438–452. doi: 10.1002/(sici)1096-9861(19990510)407:3<438::aid-cne11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Card JP, Levitt P, Enquist LW. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol. 1998;72(5):4434–4441. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, Enquist LW. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13(6):2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, Enquist LW. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10(6):1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Whealy ME, Robbins AK, Moore RY, Enquist LW. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6(6):957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Getting PA. Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4-C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res. 1993;93(2):307–311. doi: 10.1007/BF00228399. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14(1):69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JG, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Troyer A. The canine phrenic-to-intercostal reflex. J Physiol. 1998;508:919–927. doi: 10.1111/j.1469-7793.1998.919bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature. 1967;214(5085):312–313. doi: 10.1038/214312a0. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand. 1969;75(4):568–579. [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347(1):64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res. 1996;112(1):35–40. doi: 10.1007/BF00227175. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Moller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000;299(3):335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, De Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop T. Spinal inhibition of phrenic motoneurones by stimulation of afferents from leg muscle in the cat: blockade by strychnine. J Physiol. 1987;389:137–146. doi: 10.1113/jphysiol.1987.sp016650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988;269(1):47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol. 1990;302(4):707–714. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Merrill EG, Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983;43(2-3):285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94(2):784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pearson K. Restoring walking after spinal cord injury. Prog Neurobiol. 2004;73(2):107–126. doi: 10.1016/j.pneurobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25(5):1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211(1):97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr., Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100(3):800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr., Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23(7):2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25(11):2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28(9):2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001;91(6):2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Goshgarian H. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72(1):211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111(1):135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201(3):441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Roubal PJ. Origin and distribution of phrenic primary afferent nerve fibers in the spinal cord of the adult rat. Exp Neurol. 1986;92(3):624–638. doi: 10.1016/0014-4886(86)90304-3. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev. 2008;57(1):255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Hultborn H, Jankowska E, Katz R, Storai B, Zytnicki D. Labelling of interneurones by retrograde transsynaptic transport of horseradish peroxidase from motoneurones in rats and cats. Neurosci Lett. 1984;45(1):15–19. doi: 10.1016/0304-3940(84)90322-7. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Fukuda Y. Electrophysiological properties of phrenic motoneurons in adult rats. Jpn J Physiol. 1995;45(1):69–83. doi: 10.2170/jjphysiol.45.69. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol. 2003;94(4):1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R. Brainstem and spinal control of respiratory muscles during breathing. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural control of the respiratory muscles. CRC; New York: 1997. pp. 91–105. [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26(28):7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S, Duffin J. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic motoneurones in cats. J Physiol. 1996;497(Pt 3):803–812. doi: 10.1113/jphysiol.1996.sp021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75:270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Juvin L, Morin D. Descending respiratory polysynaptic inputs to cervical and thoracic motoneurons diminish during early postnatal maturation in rat spinal cord. Eur J Neurosci. 2005;21(3):808–813. doi: 10.1111/j.1460-9568.2005.03910.x. [DOI] [PubMed] [Google Scholar]
- Kim ES, Kim GM, Lu X, Hsu CY, Xu XM. Neural circuitry of the adult rat central nervous system after spinal cord injury: a study using fast blue and the Bartha strain of pseudorabies virus. J Neurotrauma. 2002;19(6):787–800. doi: 10.1089/08977150260139156. [DOI] [PubMed] [Google Scholar]
- Kim JS, Enquist LW, Card JP. Circuit-specific coinfection of neurons in the rat central nervous system with two pseudorabies virus recombinants. J Virol. 1999;73(11):9521–9531. doi: 10.1128/jvi.73.11.9521-9531.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Treuttner J, Brunschwig J-P, Gomez A, Bunge MB, Dietrich WD, Ek CJ, Dziegielewska KM, VandeBerg JL, Saunders NR. Age-related differences in the local cellular and molecular responses to injury in developing spinal cord of the opossum, Monodelphis domestica. Eur J Neurosci. 2007;25(6):1725–1742. doi: 10.1111/j.1460-9568.2007.05439.x. [DOI] [PubMed] [Google Scholar]
- Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. The American review of respiratory disease. 1981;124(1):41–44. doi: 10.1164/arrd.1981.124.1.41. [DOI] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Phrenic responses to contralateral spinal stimulation in rats: effects of old age or chronic spinal hemisection. Neurosci Lett. 1995;188(1):25–28. doi: 10.1016/0304-3940(95)95690-d. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res. 1993;95(3):477–487. doi: 10.1007/BF00227141. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640(1-2):171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Invited review: Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94(3):1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94(1):358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116(3):219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG. Invited review: Neural network plasticity in respiratory control. J Appl Physiol. 2003;94(3):1242–1252. doi: 10.1152/japplphysiol.00715.2002. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian H. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29(10):915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Palisses R, Persegol L, Viala D. Evidence for respiratory interneurons in the cervical spinal cord in decorticate rabbit. Exp Brain Res. 1989;78(3):624–632. doi: 10.1007/BF00230250. [DOI] [PubMed] [Google Scholar]
- Palisses R, Persegol L, Viala D, Viala G. Reflex modulation of phrenic activity through hindlimb passive motion in decorticate and spinal rabbit preparation. Neuroscience. 1988;24(2):719–728. doi: 10.1016/0306-4522(88)90364-8. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Could enhanced reflex function contribute to improving locomotion after spinal cord repair? J Physiol. 2001;533(Pt 1):75–81. doi: 10.1111/j.1469-7793.2001.0075b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Song A, Tracey DJ, Ashwell KW. Development of the rat phrenic nerve and the terminal distribution of phrenic afferents in the cervical cord. Anat Embryol (Berl) 1999;200(6):625–643. doi: 10.1007/s004290050310. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;111(2):178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Viala D. Evidence for direct reciprocal interactions between the central rhythm generators for spinal “respiratory” and locomotor activites in the rabbit. Exp Brain Res. 1986;63(2):225–232. doi: 10.1007/BF00236841. [DOI] [PubMed] [Google Scholar]
- Viala D, Vidal C, Freton E. Coordinated rhythmic bursting in respiratory and locomotor muscle nerves in the spinal rabbit. Neurosci Lett. 1979;11(2):155–159. doi: 10.1016/0304-3940(79)90119-8. [DOI] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci. 2007;25(12):3551–3560. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98(6):3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2003;82(10):803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience. 1999;90(4):1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29(2):147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. GABA, not glycine, mediates inhibition of latent respiratory motor pathways after spinal cord injury. Exp Neurol. 2007;203(2):493–501. doi: 10.1016/j.expneurol.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]