Abstract
Invariant (i)NKT cells influence the response to viral infections, although the mechanisms are poorly defined. Here we show that these innate-like lymphocytes secrete IFN-γ upon culture with CpG oligodeoxynucleotide-stimulated dendritic cells (DCs) from mouse bone marrow. This requires TLR9 signaling and IL-12 secretion by the activated DCs, but does not require CD1d expression. iNKT cells also produce IFN-γ in response to mouse CMV (MCMV) infection. Their mechanism of MCMV detection is quite similar to that of CpG, requiring bothTLR9 signaling and IL-12 secretion, while the need for CD1d expression is relatively minor. Consequently, iNKT cells have the ability to respond to a variety of microbes, including viruses, in an antigen-independent manner, suggesting they may play a broad role in anti-pathogen defenses despite their limited TCR repertoire.
Keywords: viral, Natural Killer T cells, rodent, cytokines, cell activation
Introduction
Invariant Natural Killer T (iNKT) cells are a subpopulation of innate-like T lymphocytes that expresses an invariant T cell antigen receptor (TCR) α chain. iNKT cells rapidly mount a response to synthetic glycolipid antigens (Ags) presented by CD1d, characterized by the production of large amounts of interferon (IFN)-γ and interleukin (IL)-4 (1, 2). Additionally, iNKT cells respond to several bacteria that have glycolipids that engage their invariant TCR.iNKT cells probably also can be activated by microbes that do not encode Ags for their TCR (3). For example they have been reported to influence the course of some viral infections. In fact, several viruses down regulate human CD1d expression, suggesting a key role for iNKT cells in antiviral defenses (4, 5).
It is well established that stimulation of APC with bacterial products or the TLR ligand LPS activate iNKT cells in the absence of foreign Ag for their TCR (6, 7). Until recently, however, it was not known if other TLR ligands, such as those that detect viral danger signals, also activate iNKT cells. Therefore, in this study we investigated the response of iNKT cells to a TLR9 ligand, CpG oligodeoxynucleotides (ODN), and the response to virus infection with mouse CMV (MCMV) (8). MCMV is a β-herpes virus that is a widely studied model, with viral control dependent on innate immune responses by stromal cells, DCs and natural killer (NK) cells (9, 10). Despite studies of the innate and adaptive immune responses to MCMV (11), a thorough analysis of the role for TLR9 and IL-12 in iNKT cell activation following MCMV infection has not been performed. Here we show that the mechanism of iNKT cell activation by MCMV is similar to in vitro activation by CpG ODN stimulated APC.
Materials and Methods
Mice
C57BL/6 (B6), B6.129S1-Il12btm1Jm/J (IL-12p40-/-), and B6.129P2-Il18tm1Aki/J (IL-18-/-) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CD1d-/- mice were a gift from Dr. L. Van Kaer (Vanderbilt University, Nashville TN). Tlr9cpg1 mice were a gift from Dr. B. Beutler (The Scripps Research Institute, La Jolla CA)(8). All mice were housed in specific pathogen-free conditions.
Cell preparation
Liver lymphocytes were isolated as described previously (12). For preparation of DCs, bone marrow was cultured in medium supplemented with 100 ng/ml recombinant human fms-like tyrosine kinase-3 ligand (hFlt3L, Amgen, Inc., Thousand Oaks, CA) for 8 days. DCs were treated with 10μg/ml type B CpG ODN 1826 or control 1982 ODN (Alexis Biochemicals, San Diego, CA), infected with MCMV Smith strain at multiplicity of infection = 3, or control for 2 h. DCs were cultured with iNKT cells for 48 h before cytokine detection (7). iNKT cells were obtained from spleen by selecting for NK1.1+ TCRβ+ cells by flow cytometry as previously described (7). The purity of the cells collected, typically >98%, was assessed using CD1d tetramers loaded with αGalCer (provided by Kirin Pharma, Gunma, Japan).
ELISA
A standard sandwich ELISA was performed to measure mouse IFN-γ, TNF, and IL-4 following the manufacturer’s instructions (R&D Systems Inc.).
Viral infections
Salivary gland extract stocks of Smith strain MCMV were prepared as previously described (13). Mice were infected with 5 × 104 PFU of virus in 500 μl PBS via i.p. injection.
mAb treatment
To block CD1d in vitro, 30 μg of anti-mouse CD1d clone 1B1 mAb was added to cultures. For blocking in vivo, mice received 300 μg of mAb on day -1, 200 μg on day 0 and 100 μg on day +1 of 1B1 mAb or rat IgG controls (eBiosciences) in PBS via i.p. injection (14).
Flow cytometry and intracellular cytokine staining
Lymphocytes were stained with αGalCer/CD1d tetramers labeled with streptavidin allophycocyanin, anti-NK1.1-PerCp PE cyanin (Cy) 5, anti-CD8-PECy7, anti-CD11b-PECy7, anti-TCRβ-allophycocyanin-AF750 and CD25-FITC. All antibodies and isotype controls, except anti-TCRβ-allophycocyanin-AF750 from eBiosciences, were purchased from BD Biosciences. As a positive control for iNKT cell responses, mice were injected with 2 μg of αGalCer. Cells were fixed and permeabilized using CytoFix/CytoPerm buffer and stained for intracellular IFN-γ with PE-labeled clone XMG1.2. The data were collected on a LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software, (Tree Star Inc.).
Results and Discussion
TLR9 stimulated DCs activate iNKT cells
Previous studies have demonstrated that mouse iNKT cells were activated following stimulation of DCs with the TLR4 ligand, LPS (7). To further investigate the potential role of iNKT cells in regulating the immune response to viral danger signals, we evaluated the ability of TLR9 stimulated or MCMV infected DCs to activate iNKT cells in vitro.
As triggering the TLR9 pathway with CpG-ODN serves as a model for innate recognition of viral DNA, we tested the ability of DCs stimulated with CpG ODN to activate iNKT cells in culture. DCs derived from bone marrow by culture with hFlt3L were exposed to CpG ODNs and co-cultured with highly purified splenic iNKT cells. High levels of IFN-γ, but no IL-4 or TNF, were detected in the stimulated co-cultures (Fig. 1A and data not shown). Similar results were obtained when the DCs were stimulated with the TLR7 agonists (data not shown). The production of IFN-γ in the absence of IL-4 was similar to previous results obtained from analyzing iNKT cell responses to LPS (7), but contrasts with the results obtained with iNKT cell activation by TCR agonists, which elicits all three cytokines. IFN-γ secretion by iNKT cells was dependent on TLR9 activation of the DCs, as non-stimulated DCs, or DCs stimulated with an inactive CpG ODN did not induce it. Additionally, hFlt3L derived DCs from TLR9CpG1 mutant mice were unable to induce IFN-γ secretion by iNKT cells (Fig. 1B). IFN-γ production in these cultures was dependent on iNKT cells, although TLR9 stimulated DCs in the absence of iNKT cells produced IL-12p70 (data not shown).
Figure 1.
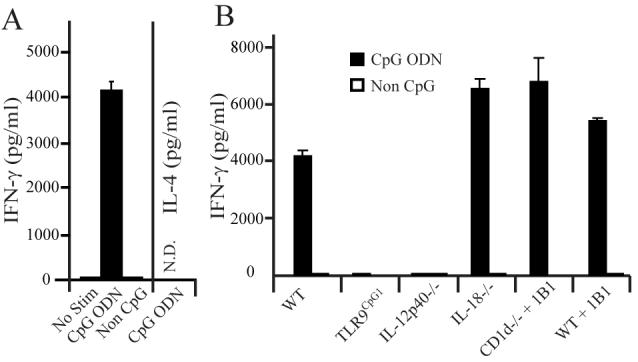
IFN-γ production by iNKT cells in response to CpG stimulated DCs. A, WT DCs were stimulated with CpG ODN or non-activating CpG ODN (Non CpG), co-cultured with purified splenic iNKT cells, and cytokine production was measured by ELISA. B, Purified iNKT cells were co-cultured with CpG or non-CpG ODN stimulated DCs derived from the indicated mouse strains, or DCs from CD1d-/- or WT mice in the presence of anti-CD1d blocking mAb. Cytokine production was measured in the supernatant by ELISA. Shown is a representative experiment out of 5 performed. Results represent the mean of 1 experiment with 3 replicate cultures measured in triplicate for ELISA. Error bars, SEM (n=9 for each set of DCs).
To define the mechanism of iNKT cell activation by CpG ODN treated DCs, DCs were generated from mice deficient for IL-12p40, IL-18, or CD1d. Similar to our previous results with LPS stimulated DCs, iNKT cells did not produce IFN-γ when the TLR9 stimulated DCs were made from IL-12p40-/- mice. Somewhat surprisingly, IL-18-/- DCs treated with CpG ODN did induce IFN-γ production by iNKT cells, contrasting with the results from LPS stimulation (7). DCs derived from CD1d-deficient mice were equally effective at causing IFNγ secretion, and similar results were obtained when a CD1d blocking mAb was added to co-cultures containing wild-type DCs, or CD1d-/- DCs, in order to block the contribution of CD1d expression by the iNKT cells themselves. These results indicate that iNKT cells can be activated in response to IL-12 induced by endosomal TLR-ligands, independently of either foreign or self-lipid Ag presentation by CD1d.
Our results are consistent with those from several recent reports showing iNKT cell activation following exposure to TLR9 agonists. In one of these, however, activation in vivo did not occur unless the CpG ODN was encapsulated in liposomes(15). In studies using human iNKT cells, activation marker expression was increased but cytokine release was not observed(16), while in another, TLR3, 7 and 9 agonists were shown to cause the in vitro expansion of human iNKT cells in cultures of PBMC (17). Furthermore, a recent report demonstrated that liver-derived mouse iNKT cells were activated by DCs exposed to TLR7 or TLR9 agonists. However, in that study iNKT cell activation was dependent upon type I interferon secretion, not IL-12, despite the production of both of these cytokines by the activated DCs (18). Furthermore, iNKT cell IFNγ secretion was found to be at least partially dependent upon CD1d expression and the presentation by CD1d of a charged glycosphingolipid autologous Ag of undefined structure (18). The reasons for this discrepancy are not known, but could be due either to differences in DCs generation, and/or the use of liver mononuclear cells as opposed to purified splenic iNKT cells. In our studies, Flt3L cultured DCs were used, while the previous study used 14-day cultured GM-CSF DCs. As described below, Flt3L cultured DCs may more appropriately model in vivo infection with virus.
MCMV infected DCs activate iNKT cells in vitro
The engagement of endosomally expressed TLR9 by CpG ODN in DCs is a model for some of the early events leading to innate immune activation following infection with a DNA virus. To test this, we evaluated the in vitro iNKT cell response to DCs infected with MCMV. Infected hFlt3L DCs were co-cultured with purified iNKT cells, and the supernatants were measured for cytokine production (Figs. 2A and B). MCMV infection induced rapid production of IFN-γ but not IL-4 (Fig. 2A). IFN-γ secretion required MCMV infection, as uninfected DCs (Fig. 2A) or infection with a mouse-adapted strain of Dengue virus (19) (data not shown) did not lead to IFN-γ production. MCMV infected DCs from TLR9CpG1 mutant mice or from IL-12p40-/- mice also did not induce IFN-γ production (Fig. 2B). By contrast, MCMV-infected GM-CSF cultured DCs did not activate iNKT cells (data not shown).
Figure 2.

Cytokine production by iNKT cells in response to MCMV infected DCs. A, DCs were infected with MCMV and cultured with sorted iNKT cells before cytokine measurement by ELISA. B, Sorted iNKT cells were co-cultured with MCMV or mock infected DCs from the indicated mouse strains or DCs from CD1d-/- or WT mice in the presence of an anti-CD1d blocking mAb. Cytokine production was measured by ELISA. Shown is a single experiment out of eight performed. Results show the mean of 1 experiment with 3 replicate cultures of DCs measured in triplicate for ELISA. Error bars, SEM (n=9 for each set of DCs).
MCMV has been shown to provoke innate immune responses that lead to IL-12 secretion and NK cell activation (9, 20). Our data suggest that recognition of MCMV by TLR9 induces IL-12 production by DCs, leading to IFN-γ production by iNKT cells. Unlike in vitro stimulation with CpG-ODN, however, optimal production of IFNγ by co-cultures with iNKT cells was dependent on the ability of the DCs to produce IL-18 (Fig. 2B). In no case did the absence of CD1d expression reduce the amount of IFN-γ produced by iNKT cells to the levels observed with DCs deficient for TLR9 or IL-12. In the experiment shown, IFN-γ release was reduced by approximately 30% when WT DCs were treated with an anti-CD1d mAb, or when mAb blockade was combined with the use of CD1d-/- DCs (Fig. 2B). Comparing multiple experiments, the reduction in IFN-γ when CD1d was blocked or deleted although variable, was generally minor and in several experiments there was little or no effect of inhibiting the interaction with CD1d (data not shown).
MCMV infection rapidly activates iNKT cells in vivo
Upon Ag recognition or exposure to the combination of IL-12 and IL-18, iNKT cells rapidly become activated in vivo (21). As early as 12 h post infection with MCMV, tetramer+ iNKT cells showed increased surface expression of CD25 (Fig. 3A). The increased expression peaked by 12 to 18 h, and rapidly declined by 36 h in both the liver and spleen (Fig. 3B). A similar temporal expression profile was observed when CD69 expression was analyzed (data not shown). This early activation of iNKT cells parallels very closely the induction kinetics of initial innate cytokine production by stromal cells upon MCMV infection (13). Liver and spleen iNKT cells produced IFN-γ 36 h post infection, when analyzed directly ex-vivo without re-stimulation (Fig. 3C and data not shown). No production of IL-4 or TNF was observed. A previous study demonstrated that a TCR+ /NK1.1+ population produced IFN-γ at this time point (8), but in the absence of tetramer staining, these data could not unequivocally show the activation of iNKT cells. A time course analysis revealed that iNKT cells were capable of producing IFN-γ as early as 24 h post infection (Fig. 3D), a time preceding viral spread within infected organs. Therefore, similar to results in vitro, the activation of iNKT cells after MCMV infection in vivo leads to an early CD25 and CD69 up-regulation, as well as IFN-γ production somewhat later.
Figure 3.
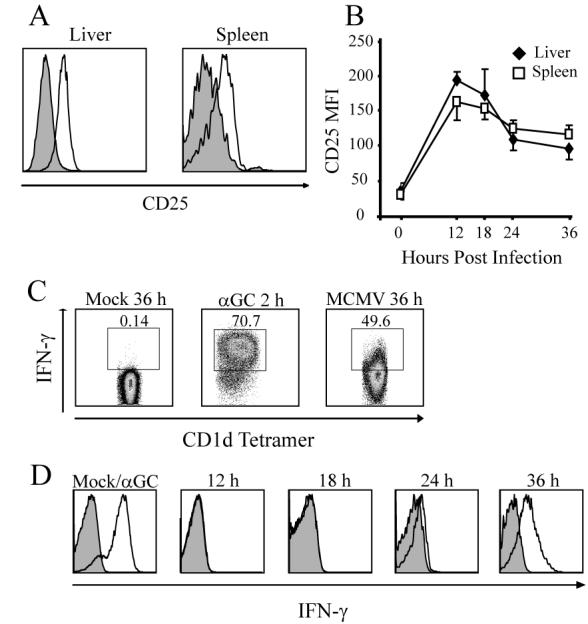
MCMV infection activates liver iNKT cells in vivo. A, WT mice were infected with MCMV i.p. and αGalCer/CD1d tetramer+ iNKT cells were analyzed for CD25 expression (open histograms) compared to PBS injected controls (shaded histograms) at12 h. Data are representative of 4 independent experiments of 2-3 mice per group. B, Time course of the mean fluorescence intensity (MFI) of CD25 expression after infection. Time 0 is the average MFI of PBS injected controls. Average MFI of iNKT cells in one of 4 independent experiments. Error bars, SEM (n=3 for each time point). C, At the indicated times, liver mononuclear cells were stained for intracellular IFN-γ and the data were gated for αGalCer/CD1d tetramer positive iNKT cells. Numbers represent the percentage of IFN-γ+ cells in the iNKT cell population. D, Time course of the MCMV activated iNKT cell IFNγ response. MFI of intracellular IFN-γ (open histograms) were compared to isotype controls (shaded histograms). Far left panels represent the iNKT cell response 2 h after i.p. injection of αGalCer. Results are representative of 3 independent experiments of 3-5 mice per group.
iNKT cell responses to MCMV infection require TLR9 and IL-12
Consistent with in vitro results, MCMV infection of TLR9CpG1 mice did not result in a marked activation of iNKT cells to increase CD25 expression at 18h or to secrete IFN-γ at 36 h after infection (Fig. 4). Also similar to the in vitro results, IL-12p40-/- (Fig. 4) or IL-12Rβ2-/- mice (data not shown) did not induce upregulation of CD25 in iNKT cells shortly after infection (18 h), or IFN-γ production at 36 h. IL-18-/- mice also had reduced CD25 expression, but IFN-γ production was not reduced, suggesting that in vivo IL-18 may be dispensable for the induction of iNKT cell effector function in response to MCMV.
Figure 4.
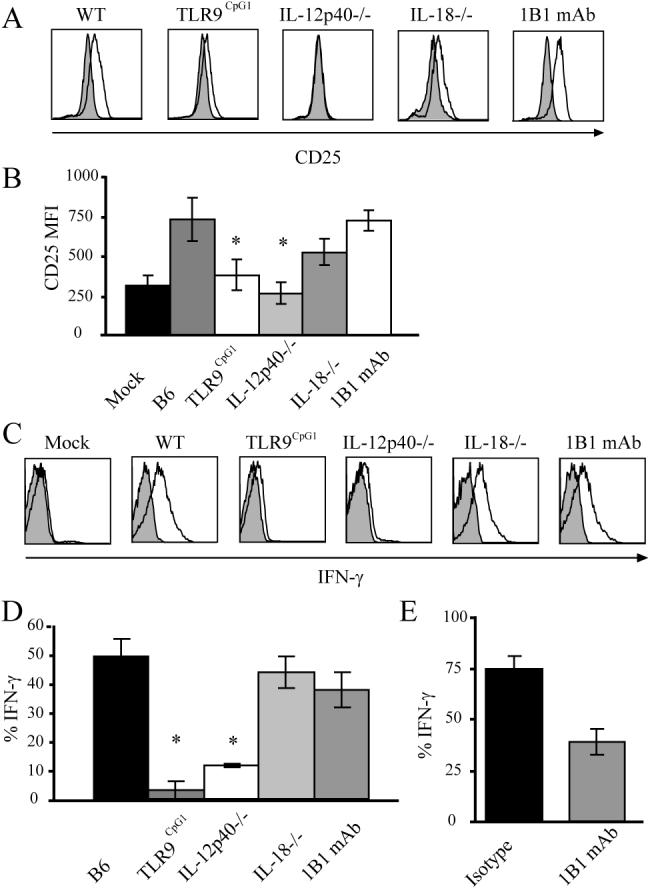
iNKT cell activation in vivo following MCMV infection. A, CD25 expression on iNKT cells from the indicated mouse strains 18 h post infection with MCMV (open histograms) compared to PBS controls (shaded histograms). Histograms are representative plots of 3 independent experiments of 3-8 mice per group. WT mice treated with a CD1d mAb (far right panels in A and C) are also indicated. B, Mean CD25 MFI of 3-8 mice per group 18 h post infection with MCMV from a minimum of 3 independent experiments. Statistically significant differences using the equal variance Student t test are indicated with * (p ≤ 0.01) comparing WT to either mutant strains or anti-CD1d mAb treated mice. C, IFN-γ production by iNKT cells measured directly exvivo 36 h post infection with MCMV (open histograms) compared to isotype controls (shaded histograms). Histograms are representative plots of a minimum of 3 independent experiments of 3-8 mice per group. D, Average percent of IFN-γ+ iNKT cells 36 h post MCMV infection. Values plotted are the mean of 3-8 mice per group from a minimum of 3 independent experiments. Error bars, SEM (n=3-8 for each group). Statistically significant differences using Student’s t test are indicated with * (p ≤ 0.01). E, Average percent of IFN-γ+ iNKT cells 2 h post αGalCer injection in isotype control or CD1d mAb treated mice. Values plotted are the mean of 3 mice per group from 2 independent experiments.
To determine if CD1d was required in vivo for viral activation of iNKT cells, WT mice were treated with a CD1d blocking mAb and infected with MCMV. As a control for the efficacy of blocking, the mAb was administered to WT mice that received the potent agonist αGalCer, and IFN-γ synthesis by liver and splenic iNKT cells was measured directly ex vivo 2 h later. Anti-CD1d treated mice showed a 50% reduction in iNKT cell IFN-γ production (Fig. 4E). Treatment with the CD1d blocking mAb did not alter the induction of iNKT cell CD25 expression following MCMV infection, however, and although the treated mice trended lower for IFN-γ production, this decrease was not statistically significant (p=0.063). Self-Ags presented by CD1d during viral infection are likely to be much less potent than αGalCer, and so the response to these Ags should be more effectively inhibited. Therefore, we conclude that CD1d-mediated Ag presentation is not likely to be a key player in MCMV induced activation of iNKT cells in vivo.
Although iNKT cells are early responders to LPS, they are not the first responders, as they depend on IL-12 and IL-18 produced by other cell types. Likewise, the kinetics of the iNKT cell response indicates that these cells also are not the first responders to MCMV infection. Previous work has shown that the initial IFNαβ response to i.p. infection with MCMV peaks at approximately 8 h post infection in the spleen, and is dependent upon B cells expressing lymphotoxin that cross-talk with type I IFN-producing stromal cells (13). A second wave of TLR-dependent activation occurs at approximately 36 h post infection in the spleen, shortly after MCMV completes it’s first round of replication (13, 22). Given the time course of increased activation marker expression and IFN-γ production by iNKT cells, we propose a two-stage activation process, with the initial response to viral danger signals being sufficient to induce increased surface marker expression by iNKT cells. However, a second TLR-dependent wave of activation and cytokines may be necessary to induce the relatively high levels of IFNγ secretion needed to detect cytokine production by iNKT cells directly ex vivo. This hypothesis is consistent with data demonstrating that iNKT cells express slightly increased CD69 and CD25 expression in TLR9CpG1 mice 12 h post infection, but this was reduced by 18 h, and we were unable to detect iNKT cell IFN-γ production at later times (Fig. 4 and data not shown).
There are multiple pathways for the foreign Ag-independent activation of iNKT cells, which requires to varying extents IL-12, IL-18 and type I interferon, as well as CD1d presentation of self-Ags. We speculate that the pathway utilized will depend on the nature of the inciting stimulus and the type(s) of APC. Our findings indicate, however, that the mechanism of iNKT cell activation following MCMV infection in vivo is similar to that we observed with TLR9 ligands in vitro or with virus infection in vitro using Flt3L DCs. The data suggest that the iNKT cell response to viral danger signals in vivo from MCMV infection is mediated by TLR9 engagement leading to IL-12 production, and that this response is not highly dependent upon CD1d Ag presentation. Clearly, this pathway, along with the others described that do not depend on foreign Ag, endow innate-like iNKT cells with the ability to respond to diverse pathogens despite a highly constricted TCR repertoire.
Acknowledgements
We acknowledge A. Khurana for assistance with the generation of CD1d tetramers. We acknowledge Dr. Sujan Shresta for help with experiments and advice.
Footnotes
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
Disclosures
The authors have no financial conflict of interest.
This work was supported by National Institute of Health Grants AI 69296, AI 71922 (M.K.), and F32 AI 80087 (A.J.T.).
- DCs
- dendritic cells
- hFlt3L
- human fms-like tyrosine kinase-3 ligand
- ICCS
- intracellular cytokine staining
- i
- invariant
- MCMV
- mouse cytomegalovirus
- NK
- natural killer
- ODN
- oligodeoxynucleotide
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Kinjo Y, Kronenberg M. Valpha14i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol. 2005;25:522–533. doi: 10.1007/s10875-005-8064-5. [DOI] [PubMed] [Google Scholar]
- 4.Van Kaer L, Joyce S. Viral evasion of antigen presentation: not just for peptides anymore. Nat Immunol. 2006;7:795–797. doi: 10.1038/ni0806-795. [DOI] [PubMed] [Google Scholar]
- 5.Raftery MJ, Hitzler M, Winau F, Giese T, Plachter B, Kaufmann SH, Schonrich G. Inhibition of CD1 antigen presentation by human cytomegalovirus. J Virol. 2008;82:4308–4319. doi: 10.1128/JVI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 7.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 8.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 10.Vidal SM, Lanier LL. NK cell recognition of mouse cytomegalovirus-infected cells. Curr Top Microbiol Immunol. 2006;298:183–206. doi: 10.1007/3-540-27743-9_10. [DOI] [PubMed] [Google Scholar]
- 11.van Dommelen SL, Tabarias HA, Smyth MJ, Degli-Esposti MA. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J Virol. 2003;77:1877–1884. doi: 10.1128/JVI.77.3.1877-1884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tupin E, Kronenberg M. Activation of natural killer T cells by glycolipids. Methods Enzymol. 2006;417:185–201. doi: 10.1016/S0076-6879(06)17014-7. [DOI] [PubMed] [Google Scholar]
- 13.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, Nishimura T. Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res. 2004;64:8754–8760. doi: 10.1158/0008-5472.CAN-04-1691. [DOI] [PubMed] [Google Scholar]
- 16.Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, Krieg AM, Landay AL, Wilson SB. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated crosstalk with myeloid dendritic cells. J Immunol. 2006;177:1028–1039. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 17.Raftery MJ, Winau F, Giese T, Kaufmann SH, Schaible UE, Schonrich G. Viral danger signals control CD1d de novo synthesis and NKT cell activation. Eur J Immunol. 2008;38:668–679. doi: 10.1002/eji.200737233. [DOI] [PubMed] [Google Scholar]
- 18.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 22.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]


