Abstract
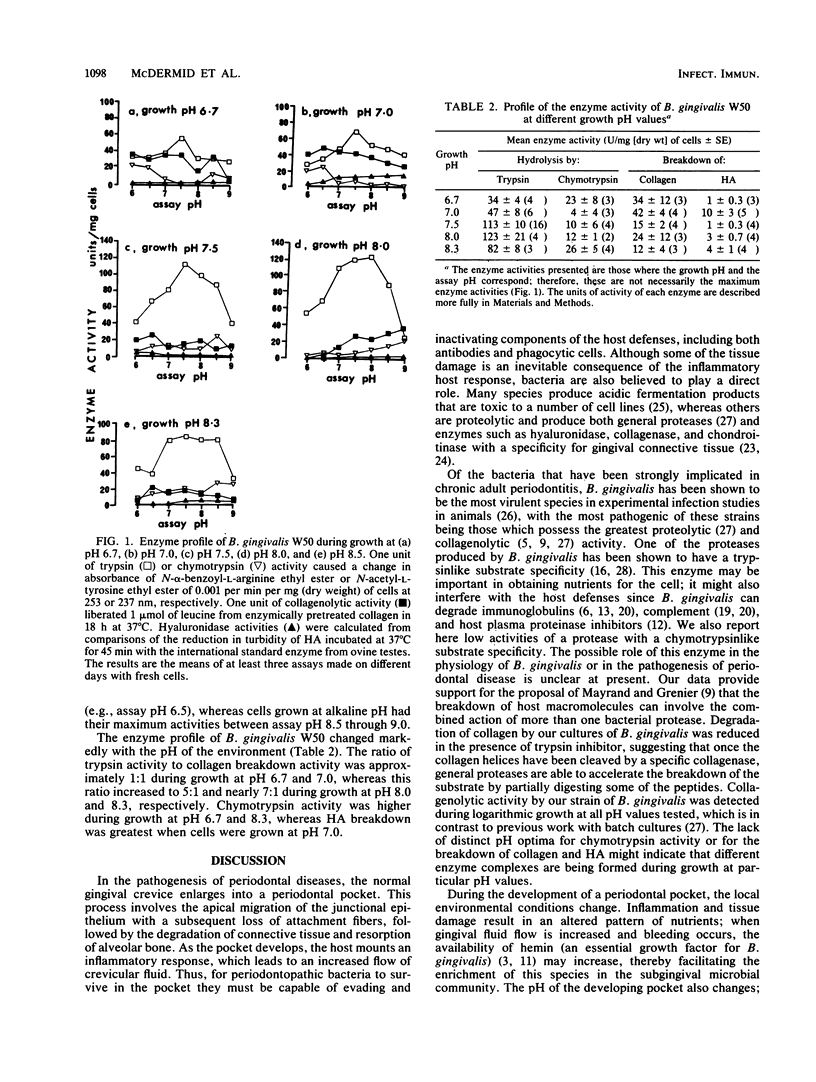
Since the pH of the gingival crevice increases from below neutrality in health to above pH 8 in disease, we decided to investigate the effect of environmental pH on the growth and enzyme activity of Bacteroides gingivalis W50. Cells were grown in a chemostat under hemin-excess conditions over a range of pH values; stable growth was observed only between pH 6.7 and 8.3, with the maximum yields obtained between pH 7.0 and 8.0. The enzyme profile of cells varied markedly with pH. Enzymes with a specificity for gingival connective tissue (collagenase, hyaluronidase) were produced optimally at or below neutral pH, whereas trypsinlike activity increased with the growth pH and was maximal at pH 8.0. Chymotrypsinlike activity was generally low, although its activity was highest at the extremes of growth pH, i.e., at pH 6.7 and 8.3. Inhibitor studies provided evidence that the breakdown of collagen involved the concerted action of both a collagenase and the trypsinlike enzyme. The ratio of trypsin to collagenolytic activity rose from 1:1 during growth at neutral pH and below to almost 7:1 during growth at pH 8.3. Thus B. gingivalis appears to be uniquely adapted as a periodontopathic organism in that under environmental conditions likely to prevail during the initial stages of pocket development it produces maximally those enzymes with a tissue-damaging potential. Then, as the pH of the pocket rises during the host inflammatory response, the activity of the trypsinlike enzyme increases markedly, which may enable cells to inactivate key components of the host defenses such as immunoglobulins and complement.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickel M., Cimasoni G. The pH of human crevicular fluid measured by a new microanalytical technique. J Periodontal Res. 1985 Jan;20(1):35–40. doi: 10.1111/j.1600-0765.1985.tb00408.x. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Degradation of collagenous substrates by Bacteroides melaninogenicus. J Bacteriol. 1961 Apr;81:614–621. doi: 10.1128/jb.81.4.614-621.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987 Apr;25(4):738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDL I., MACLENNAN J. D., HOWES E. L. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953 Dec;32(12):1323–1329. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Grenier D. Detection of collagenase activity in oral bacteria. Can J Microbiol. 1985 Feb;31(2):134–138. doi: 10.1139/m85-026. [DOI] [PubMed] [Google Scholar]
- McDermid A. S., McKee A. S., Ellwood D. C., Marsh P. D. The effect of lowering the pH on the composition and metabolism of a community of nine oral bacteria grown in a chemostat. J Gen Microbiol. 1986 May;132(5):1205–1214. doi: 10.1099/00221287-132-5-1205. [DOI] [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Baskerville A., Dowsett A. B., Ellwood D. C., Marsh P. D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986 May;52(2):349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Carlsson J., Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985 Nov;50(2):467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Sato M., Otsuka M., Maehara R., Endo J., Nakamura R. Degradation of human secretory immunoglobulin A by protease isolated from the anaerobic periodontopathogenic bacterium, Bacteroides gingivalis. Arch Oral Biol. 1987;32(4):235–238. doi: 10.1016/0003-9969(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. K., Carlsson J., Herrmann B. F., Höfling J. F., Vätäinen A. Degradation in vivo of the C3 protein of guinea-pig complement by a pathogenic strain of Bacteroides gingivalis. Scand J Dent Res. 1984 Feb;92(1):14–24. doi: 10.1111/j.1600-0722.1984.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Carlsson J., Herrmann B., Tärnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J Med Microbiol. 1985 Feb;19(1):85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- Svanberg M. Streptococcus mutans in plaque after mouth-rinsing with buffers of varying pH value. Scand J Dent Res. 1980 Feb;88(1):76–78. doi: 10.1111/j.1600-0722.1980.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Tam Y. C., Chan E. C. Purification and characterization of hyaluronidase from oral Peptostreptococcus species. Infect Immun. 1985 Feb;47(2):508–513. doi: 10.1128/iai.47.2.508-513.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Y. C., Harvey R. F., Chan E. C. Chondroitin sulfatase--producing and hyaluronidase--producing oral bacteria associated with periodontal disease. J Can Dent Assoc. 1982 Feb;48(2):115–120. [PubMed] [Google Scholar]
- Tipler L. S., Embery G. Glycosaminoglycan-depolymerizing enzymes produced by anaerobic bacteria isolated from the human mouth. Arch Oral Biol. 1985;30(5):391–396. doi: 10.1016/0003-9969(85)90065-2. [DOI] [PubMed] [Google Scholar]
- Touw J. J., van Steenbergen T. J., De Graaff J. Butyrate: a cytotoxin for Vero cells produced by Bacteroides gingivalis and Bacteroides asaccharolyticus. Antonie Van Leeuwenhoek. 1982;48(4):315–325. doi: 10.1007/BF00418285. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- van Steenbergen T. J., Kastelein P., Touw J. J., de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982 Jan;17(1):41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]



