Abstract
Phosphoinositide 3-OH kinases (PI3Ks) are a group of major intracellular signaling molecules. In our previous study, we found that inhibition of PI3K activity suppressed the androgen receptor (AR)-mediated gene expression in prostate cancer cells. The AR has been considered as a critical determinant for the development and progression of human prostate cancers. In this study, we sought to identify the PI3K isoforms involved in AR transactivation. Using a gene-specific small interference RNA (siRNA) approach, we determined that the regulatory isoform p85α and the catalytic isoform p110β, but not p110α, were required for androgen-stimulated AR trans-activation and cell proliferation in prostate cancer cells. Consistently, overexpression of wild-type p110β but not p110α gene led to androgen-independent AR transactivation. Silencing p110β gene in prostate cancer cells abolished tumor growth in nude mice. Of the dual (lipid and protein) kinase activities, p110β’s lipid kinase activity was required for AR transactivation. Further analysis by a chromatin immunoprecipitation assay showed that p110β is indispensable for androgen-induced AR–DNA interaction. Finally, gene expression analysis of clinical specimens showed that both p85α and p110β were highly expressed in malignant prostate tissues compared to the nonmalignant compartments, and their expression levels correlated significantly with disease progression. Taken together, our data demonstrated that p85α and p110β are essential for androgen-stimulated AR transactivation, and their aberrant expression or activation might play an important role in prostate cancer progression.
Keywords: prostate cancer, PI3K, androgen receptor, siRNA, tumor progression, mouse xenograft
Introduction
Phosphoinositide 3-OH kinases (PI3Ks) regulate multiple cellular signal pathways (reviewed in Bader et al., 2005; Engelman et al., 2006). In mammalian cells, there are three classes (I, II and III) of PI3K isoforms, and the class I PI3Ks are further divided into two subtypes (IA p110 α/β/δ and IB p110γ), of which IA catalytic isoforms are associated with p85 regulatory subfamily, while IB p110γ is associated with p101/p84 regulatory subunit. The classes I and III but not class II PI3Ks are dual functional kinases, namely lipid kinase and protein kinase (Engelman et al., 2006).
Androgens are essential not only in the physiological development of male sexual phenotype but also in the genesis of prostate cancer. Upon its entry into target cells, the androgenic hormone transmits its regulatory signal to the nucleus through its cognate androgen receptor (AR), which is a ligand-activated transcription factor. After androgen stimulation, the AR itself and its co-factors are modified by post-transcriptional mechanisms (that is, phosphorylation, acetylation and so on), and growth factor-induced intracellular signaling pathways were reported to activate the AR independent of the androgens or to sensitize the AR to reduced levels of hormones, leading to hormone refractory (or so-called castration-resistant) progression (reviewed in Scher and Sawyers, 2005).
Due to the inactivating mutation of PI3K signaling opponent PTEN gene (phosphatase and tensin homologue deleted on chromosome 10; Li et al., 1997; Steck et al. 1997), or through unknown mechanism after androgen withdrawal, elevated PI3K activity has been proposed as one of the major mechanisms for prostate cancer progression (Murillo et al., 2001; reviewed in Majumder and Sellers, 2005). Previous studies from our group (Liao et al., 2004) and others (Li et al., 2001; Sharma et al., 2002) have shown that PI3K activity is indispensable for androgen-induced AR transactivation in prostate cancer cells. However, it is not clear which PI3K isoforms participate in androgen-induced AR transactivation and castration-resistant progression in prostate cancers.
To understand the mechanisms involved in prostate cancer progression, in this study, we went on to determine the PI3K isoforms that are required for androgen-induced AR transactivation and gene expression. We determined that PI3K class IA p85α and p110β isoforms are essential for androgen-stimulated AR transactivation and cell proliferation. We also found that these two isoforms are highly expressed in malignant prostate tissues compared to the nonmalignant compartments, and their expression levels correlate significantly with disease progression of prostate cancers.
Results
PI3K p85α and p110β are essential for androgen-induced AR transactivation and cell proliferation
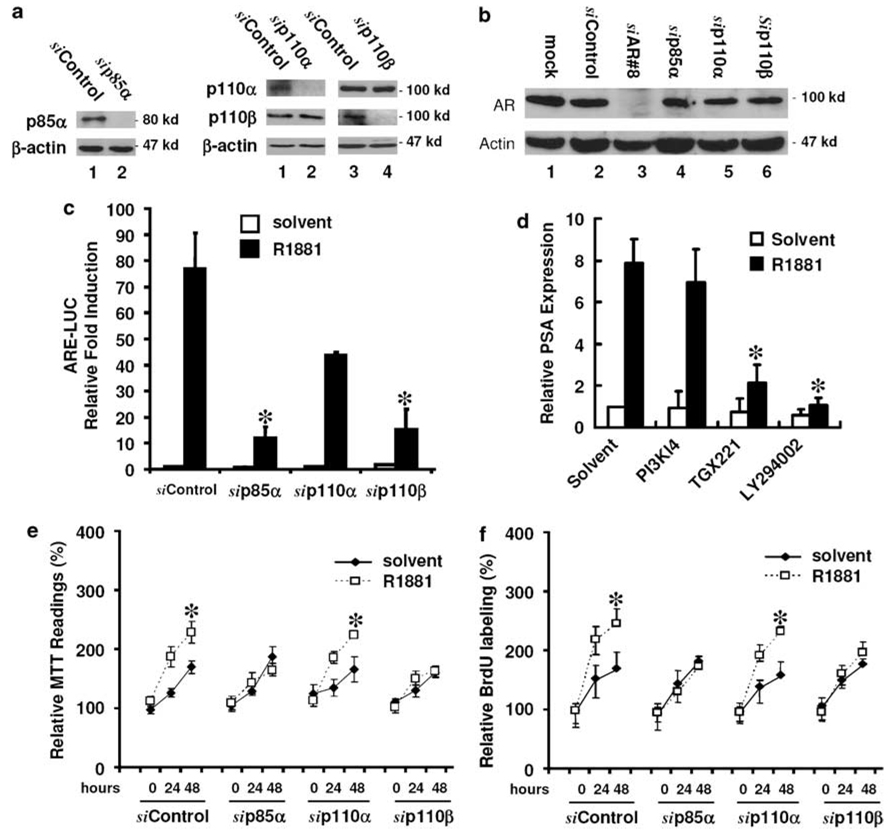
Although PI3K has been long considered as an important factor for prostate cancer progression (Murillo et al., 2001; reviewed in Majumder and Sellers, 2005) and PI3K activity is required for AR-mediated gene expression (Liao et al., 2004), the PI3K isoforms responsible for this activity were not determined. Therefore, in this study, we used the small interference RNA (siRNA) approach to determine which PI3K isoforms are involved in AR transactivation. We focused on three ubiquitously expressed class IA isoforms: p85α, p110α and p110β. The efficiency and the specificity of the siRNAs against the corresponding PI3K isoforms were verified in prostate cancer LNCaP cells (Figure 1a). Meanwhile, no obvious effect of these PI3K siRNAs on AR expression was noticed (Figure 1b). Then, an androgen-responsive reporter construct (ARE-LUC) was used to evaluate the effect of PI3K siRNAs on androgen-induced AR transactivation. As shown in Figure 1c, transfection with the siRNAs for p85α and p110β but not p110α significantly suppressed R1881 (a synthetic androgen)-induced ARE-LUC activity. Since there was a reduction in ARE-LUC activity in p110α siRNA-transfected cells, we used PI3K isoform-specific inhibitors to verify if p110β is unique for AR-mediated gene expression. The isoform specificity of the inhibitors, PI3K Inhibitor 4 (PI3K-I4) for p110α and TGX-221 for p110β, was described previously (Jackson et al., 2005; Hayakawa et al., 2006). The mRNA expression level of the well-known AR target psa gene was chosen to evaluate the effect of these inhibitors on androgen-stimulated AR transactivation. Consistent with the data from the siRNA experiments (Figure 1c) and our previous report (Liao et al., 2004), pretreatment with LY294002 (a nonspecific PI3K inhibitor) or TGX-221 but not PI3K-I4 significantly suppressed R1881-stimulated prostate-specific antigen (PSA) expression (Figure 1d). These data indicated that type IA p85α and p110β but not p110α are required for androgen-induced AR transactivation.
Figure 1.
Phosphoinositide 3-OH kinases (PI3K) p85α and p110β isoforms are essential for androgen-induced androgen receptor (AR) transactivation and cell proliferation. (a) The efficiency of small interference RNA (siRNA)-induced gene silencing on PI3K isoforms and (b) the effect of PI3K siRNAs on AR expression in LNCaP cells. Equal amount of proteins from cells transfected with the siRNAs for individual PI3K isoforms, no. 8 AR siRNA or a negative control siRNA were subjected for western blot. Mock transfection was conducted by adding transfection reagent only. Anti-actin blot served as protein loading control. Molecular weight markers were positioned on the right side of each panel. (c) The effect of PI3K siRNA on AR transactivation. After transfection with the siRNAs as indicated for 2 days, LNCaP cells were co-transfected with a luciferase reporter construct controlled by synthetic androgen response elements (ARE-LUC) and CMV-secreted alkaline phosphatase (SEAP) reporter constructs. Cells were treated with the solvent or R1881 (1.0 nm) in a media supplied with 2% charcoal-stripped FBS (cFBS), and the media were collected for SEAP assay and cell lysates were subjected for luciferase assay. Average fold induction against control transfection was calculated after normalized against the corresponding SEAP activity and protein concentration. (d) The effect of PI3K isoform-specific inhibitors on androgen-stimulated prostate-specific antigen (PSA) expression. LNCaP cells were serum starved for 24 h and then pretreated with the inhibitors (PI3K-I4 at 100 nm, TGX-221 at 500 nm and LY294002 at 25 µm) as indicated for 45 min. After R1881 treatment for 8 h, cells were harvested for total RNA extraction. PSA expression at mRNA level was determined by real-time reverse transcription (RT)–PCR. The expression levels of epithelium-specific gene KRT18 was used to normalize psa expression values. Data were from three independent experiments. Error bars represent standard error (s.e.) and the asterisk (*) indicates a significant difference (P < 0.05, Student’s t-test). (e, f) After transfection with the siRNAs as indicated for 2 days, LNCaP cells were treated with the solvent or R1881 for up to 48 h in 5% cFBS-containing media. Cell proliferation rate was determined everyday with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (e) and bromodeoxyuridine (BrdU) incorporation (f) assays as described in the text. Data represent the average percentage of 24 and 48 h over 0 h from two independent experiments. Hour 0 indicates the starting date of treatment and the value was set as 100%. The asterisk (*) indicates a significant difference compared to the solvent control (P < 0.05, Student’s t-test).
To validate the biological significance of these PI3K isoforms’ involvement in androgen action, we utilized two different assays (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and bromodeoxyuridine (BrdU) incorporation) to determine the effect of PI3K siRNAs on androgen-stimulated LNCaP cell proliferation. Consistent with the results from ARE-LUC reporter gene assay (Figure 1c), transfection with the siRNAs for p85α and p110β but not for p110α significantly suppressed R1881-stimulated cell proliferation in LNCaP cells (Figures 1e and f). These data clearly demonstrated that p85α and p110β are required for androgen-stimulated cell proliferation of prostate cancer in vitro.
PI3K p110β is required for tumor growth in vivo
Then, we extended our in vitro cell-based studies into an in vivo system. LNCaP-derived mouse xenograft model was used to test if PI3K p110β is required for tumor growth. LNCaP subline LNCaP/p110βshRNA cells were established by stable transfection with a p110β siRNA-expressing construct (Czauderna et al., 2003) and were used to generate the xenografts in nude mice. The LNCaP/Neo subline cells transfected with a plasmid vector harboring the neomycin-resistant gene were used as the control. The efficiency of the p110β silencing construct on p110β expression was confirmed by western blot assay (Figure 2a), and no. 3 subline cells were then used for the xenograft experiments. As expected, when LNCaP/Neo cells were subcutaneously allocated into nude mice, an 80% tumor development rate (8 out of 10 allocations) was achieved after 6 weeks (Figure 2b; image data not shown), which was consistent with our recent report (Li et al., 2007b). In contrast, no tumor development was observed in LNCaP/p110βshRNA cells-allocated animals even after 8 weeks. These data indicated that p110β is essential for tumor growth of LNCaP xenograft in vivo.
Figure 2.
Knocking down phosphoinositide 3-OH kinases (PI3K) p110β expression abolishes tumor growth of LNCaP cell-derived xenograft in nude mice. (a) LNCaP cells were transfected with pU6+2/p110βshRNA vector, and stable clones (LNCaP/p110βshRNA) were selected in G418-containing media. An empty vector was used to establish the control subline (LNCaP/vector). The efficacy of p110β silencing in representative sublines was evaluated by western blot assay. (b) Single cell suspension of the LNCaP/vector and LNCaP/p110βshRNA no. 3 sublines at a dose of 2 × 106 cells per 0.1 ml media were inoculated subcutaneously into the rear flanks of 6- to 7-week-old male nude mice. Xenograft tumor growth was monitored every day. The percentage of tumor incidence was shown from each group.
PI3K p110β overexpression induces androgen-independent AR transactivation
Previous studies showed an association between increased PI3K activities and androgen-independent progression of prostate cancers (Murillo et al., 2001; reviewed in Majumder and Sellers, 2005). Thus, we tested if overexpression of p110α or p110β enhances androgen-stimulated AR transactivation or even induces androgen-independent AR transactivation. First, LNCaP cells were transfected with p110α- or p110β-expressing constructs together with the ARE-LUC reporter. Cells were then treated with increasing amount of R1881 or the solvent as control. As shown in Figure 3a, p110β overexpression not only significantly enhanced R1881-induced ARE-LUC reporter activity, but also activated ARE-LUC reporter in the absence of R1881. However, p110α overexpression did not affect R1881-induced ARE-LUC activity. A dose–response effect was clearly seen when increasing amounts of p110β constructs were used in the transfection experiments (Figure 3b). These data indicated again that p110β but not p110α enhances AR activity in the presence of castration-level androgens or even in the absence of androgens. It is plausible that elevated expression or overactivation of p110β isoform in prostate cancer cells may activate the AR after androgen withdrawal.
Figure 3.
Phosphoinositide 3-OH kinases (PI3K) p110β overexpression results in androgen-independent androgen receptor (AR) transactivation. LNCaP cells were transfected with wild-type p110α-or p110β-expressing constructs at 1.5 µg per well (a) or with increasing amount (0.25, 0.5, 1.0, 1.5 µg) of p110β constructs (b) together with the luciferase reporter construct controlled by synthetic androgen response elements (ARE-LUC) and CMV-secreted alkaline phosphatase (SEAP) reporters. Control cells received pcDNA3.1 empty vector (1.5 µg) for equalizing the DNA amount used. After serum starvation, cells were treated with the solvent, increasing doses of R1881 as indicated (a) or R1881 at 1.0 nm (b) for 24 h in 2% charcoal-stripped fetal bovine serum (cFBS)-containing media. Luciferase and SEAP activities were assessed and data were presented as described earlier. (c) LNCaP cells were transfected with wild-type and mutant p110β constructs (1.5 µg) together with the ARE-LUC and CMV-SEAP reporters. After serum starvation for 24 h, cells were treated and reporter assays were conducted as described above. The error bars represent standard error of the mean (s.e.m.) and the asterisk (*) indicates a significant difference (P < 0.05, Student’s t-test) between R1881 treatment and the solvent control. The # sign indicates a significant difference (P < 0.05) between wild-type p110β and the vector control in the absence of R1881.
It has been shown that p110β possesses both lipid and protein kinase activities (reviewed in Bader et al., 2005; Engelman et al., 2006). To determine which activity of p110β kinase is involved in AR transactivation, we tested the effect of different p110β mutants, a total kinase-dead mutant (K805R) and a protein kinase only mutant (PKO, lipid kinase deficient) on androgen-induced ARE-LUC activity. The efficacy of these p110β mutants was verified previously (Yart et al., 2002). As shown in Figure 3c, while the wild-type p110β construct significantly enhanced AR transactivation not only in the presence of androgen but also in the absence of androgen, overexpression of the K805R and PKO mutants both dramatically reduced androgen-induced ARE-LUC activity. These results suggested that the lipid kinase activity is responsible for p110β-mediated regulation of AR transactivation.
PI3K p110β is required for AR–DNA interaction
We previous showed that PI3K inhibitor suppressed AR-mediated gene expression but did not block AR nuclear translocation (Liao et al., 2004), indicating that PI3K signaling might be involved in the assembly of AR-mediated transcription complex including AR–DNA interaction. Therefore, we performed a chromatin immunoprecipitation (ChIP) assay to determine if p110β is required for AR interaction with chromatin DNA. The well-known AR-regulated gene psa promoter was used as the target sequence, and the primers were adapted from a recent publication (Shang et al., 2002). We first performed a pilot experiment to clarify the specificity of the procedure. As shown in Figure 4a, the antibodies for RNA polymerase II (Poly II) but not the normal serum pulled down the housekeeping glycer-aldehyde-3-phosphate dehydrogenase (GAPDH) gene promoter, as detected by the PCR reaction. Then, we conducted the anti-AR ChIP assay for psa promoter. As shown in Figure 4b, R1881 treatment induced AR interaction with the psa promoter, which is abolished by pretreatment of PI3K inhibitor Wortmannin. However, Wortmannin treatment did not affect Poly II binding to the GAPDH gene promoter, confirming the specificity of PI3K activity for AR transactivation. These data indicated that PI3K activity is required for AR–DNA interaction.
Figure 4.
Phosphoinositide 3-OH kinases (PI3K) signaling modulates androgen receptor (AR)–DNA binding. (a) Exponential grown LNCaP cells were fixed and harvested for a pilot chromatin immunoprecipitation (ChIP) assay with non-immunonized rabbit immunoglobulin G (IgG) and anti-RNA polymerase II antibodies as described in the text. The PCR reaction was performed with a primer pair for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene promoter. (b) Serum starved LNCaP cells were pretreated with the solvent or PI3K inhibitor Wortmannin (1.0 µm) for 45 min followed by R1881 (1.0 nm) as indicated for 12 h in 5% charcoal-stripped FBS (cFBS). Cells were fixed and harvested for anti-AR and anti-Poly II ChIP assay. PCR reaction was conducted with the primers for prostate-specific antigen (psa) gene promoter (anti-AR ChIP) or GAPDH gene promoter (anti-Poly II ChIP). (c) LNCaP cells were transfected with the small interference RNAs (siRNAs) as indicated for 3 days and mock transfection was included as a negative control. After treatment with the solvent or R1881 in 5% cFBS for 12 h, cells were fixed and harvested for anti-AR ChIP assay with the primer pairs for PSA gene promoter (PSA primer) and PSA gene coding region (rt-PSA primer) as described in the text. For all the panels, 1% of the input chromatin was used as template in PCR reaction as the positive control of the primers used. Data represent two independent experiments.
Next, we examined the effect of p110β siRNAs on AR–DNA interaction. In these experiments, we included a primer pair (rtPSA) that was used in our previous report for reverse transcription (RT)–PCR experiments (Liao et al., 2005) as a negative control. As shown in Figure 4c; no PCR products were obtained when the rtPSA primers were used, confirming the assay’s specificity for AR–DNA (psa promoter) binding. Transfection of p110β siRNAs but not the control siRNA abolished androgen-induced AR interaction with psa promoter. Taken together, these data clearly demonstrated that p110β-derived signaling is essential for androgen-induced AR–DNA binding.
PI3K p110β expression increases along with prostate cancer progression
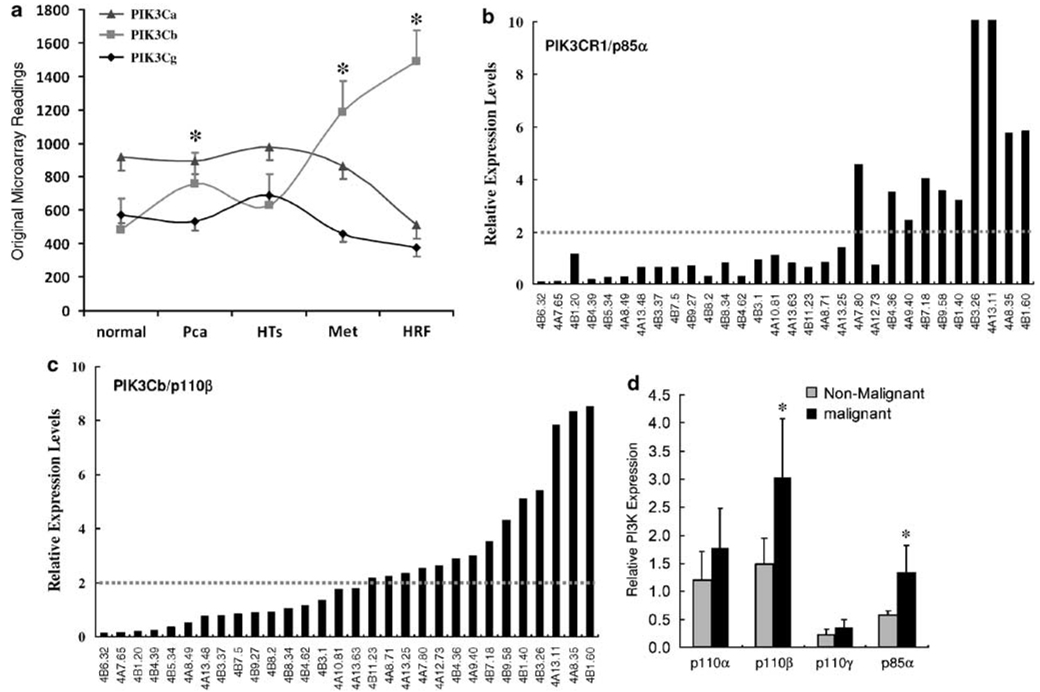
To establish the clinical relevance of p110β involvement in AR activation and prostate cancer progression, we examined the expression levels of PI3K isoforms in human prostate cancers. First, we re-analysed a published cDNA microarray data set (Holzbeierlein et al., 2004), of which the cDNA microarray experiments were conducted on an Affymetrix HG-U95 chip. There were five groups of prostate specimens from normal prostates, primary cancers, tumors after hormone therapy, metastatic tumors and hormone-refractory tumors. As shown in Figure 5a, the expression levels of p110β (PIK3Cb) but not p110α and p110γ was dramatically increased in prostate cancers, including the aggressive metastatic and hormone-refractory tumors compared to that in normal tissues. Most significantly, these data also showed a positive correlation between p110β expression and disease progression.
Figure 5.
Phosphoinositide 3-OH kinases (PI3K) p85α and p110β isoforms are overexpressed in human prostate cancers. (a) Raw data (mean) from a published cDNA microarray data set were graphed based on different patient groups, including normal prostate specimens (normal, n = 5), primary tumors (Pca, n = 23), tumors after hormone therapy (HTs, n = 17), metastatic tumors (Met, n = 9) and castration-resistant tumors (HRF, n = 3). Error bar represents the standard error of mean (s.e.m.). The asterisk (*) indicates a significant difference compared to the ‘normal’ group (P < 0.05, Student’s t-test). PIK3Ca, p110α; PIK3Cb, p110β; PIK3Cg, p110γ. (b–d) Quantitative analysis of PI3K isoforms in prostate cancers. Total RNAs were extracted using a TriZol-based protocol from frozen tumor specimens and the individually matched nonmalignant compartments. Gene expression at mRNA level was assessed by real-time reverse transcription (RT)–PCR. The expression levels of PI3K genes were normalized against the epithelium-specific gene KRT18 before the relative values were calculated. The relative ratio of gene expression level in malignant compared to nonmalignant tissues in each case was presented in (b) (p85α) and (c) (p110β). The average levels of PI3K genes were summarized in (d). Error bar represents the standard error of mean (s.e.m.), and the asterisk (*) indicate a significant difference (P < 0.05, Student’s t-test) compared to nonmalignant tissues.
To verify the cDNA microarray data, we quantitatively examined the expression levels of PI3K genes using real-time RT–PCR approach. A total of 30 primary tumor samples plus the individually matched nonmalignant compartments in each case were obtained from our tissue repository. These prostate specimens were obtained from patients with primary cancers at surgery (radical prostectomy). The pathology of each tissue sample was examined by haematoxylin and eosin (H&E) staining on a representative frozen section. Since the heterogeneous feature of prostate cancer tissue, we used the expression levels of an epithelial cell-specific gene KRT18, as described (Latil et al. 2001), to normalize the expression levels of PI3K isoform genes. In 33.3% (10 out of 30) of cases, their p85α expression levels were more than twofold higher in tumor samples compared to that in the nonmalignant compartments (Figure 5b). For 46.7% (14 out of 30) of cases, p110β expression showed a twofold or more increase in malignant tissues compared to the nonmalignant compartments (Figure 5c). The differences in the average levels of p85α and p110β were significant between the malignant tissues and their nonmalignant compartments (Figure 5d). However, there was not significant difference for the expression levels of p110α and p110γ between malignant tissues and nonmalignant compartments. Meanwhile, p110γ exhibited the lowest expression levels among the PI3K isoforms examined, which was consistent to previous reports (reviewed in Bader et al., 2005; Engelman et al., 2006).
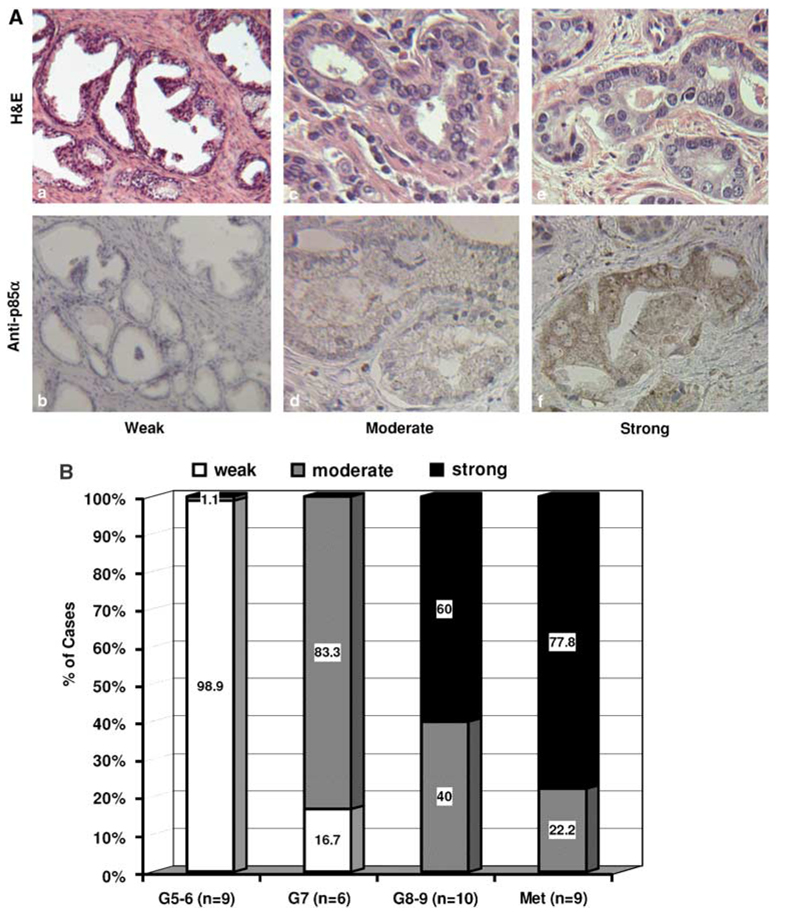
To test if p85α expression correlates with disease progression of prostate cancers, we examined p85α protein expression using immunohistochemical (IHC) staining approach. A total of 34 paraffin-embedded prostate cancer specimens from primary tumors with various grades (Gleason score) and metastatic tumors were used in this experiment. The immunosignal intensities were scored as weak, moderate and strong, and the representative images for this scoring system were presented in Figure 6A. On the basis of the sum of Gleason score, the primary tumors were divided into three groups; low (G5–6), medium (G7) and high (G8–9). As shown in Figure 6B, almost all cases (8 out of 9) of low-grade tumors showed a weak signal of p85α expression, while 60% (6 out of 10) of high-grade tumors showed strong signals for p85α expression. In metastatic tumors, 77.8% (7 out of 9) of the cases showed a strong signal for p85α expression. The difference in p85α expression was significant (P < 0.05, χ2-test) between the higher-grade tumors (G8–9 and Met) and the lower grade tumors (G5–6 and G7). These data suggested that elevated expression of p85α is associated with prostate cancer progression.
Figure 6.
Increased p85α expression correlates with prostate cancer progression. (A) Representative micrographics for anti-p85α immunostaining and haematoxylin and eosin (H&E) staining in benign tissues (a, b) and malignant tissues (c–f). Positive staining was defined as a defused pattern of brown color. Magnification, (a, b) × 100; (c–f) × 200. (B) Case distribution of p85α immunostaining intensities in primary tumors and metastatic tumors (Met). Semiquantitative scoring of immunosignals was conducted as described in our recent report (Sun et al., 2007b). Labeled values on each column represent the percentage of positive cases in the corresponding category as marked. The n number indicates the case number in each group.
Discussion
Although PI3K activity has been considered as an important factor in AR-mediated gene expression and prostate cancer progression, it is not clear which isoforms are involved in this process. In this study, we provided convincing evidence for the first time that PI3K isoforms p85α and p110β are essential for androgen-induced AR transactivation, cell proliferation and tumor growth. Knocking down PI3K p110α did not result in any significant effect on AR transactivation and cell proliferation, which is supported by a previous report (Czauderna et al., 2003). Most importantly, we demonstrated the involvement of p110β in androgen-induced AR-interaction with chromatin DNA, a key step for AR regulation of gene expression. We also determined that p110β overexpression led to androgen-independent AR transactivation, and that the expression levels of p85α and p110β genes are significantly higher in malignant prostate tissues compared to their nonmalignant compartments. Our studies suggest that aberrant expression of p85α /p110β isoforms might play an important role in androgen-independent (or so-called castration-resistant) progression of prostate cancers.
There are two major steps for hormone-bound AR to exert its genomic effect as a transcription factor; nuclear translocation and chromatin DNA binding. Currently, it is not clear how precisely androgen induces AR nuclear translocation. Once the AR is in the nucleus, it will assemble a mega-protein transcription complex, which is a dynamic process including transient recruitment and then dissociation of co-factors, chromatin remodeling molecules, proteasome subunits and general transcription machinery (reviewed in Heinlein and Chang, 2004). We recently showed that PI3K inhibitors could not block AR nuclear translocation but suppressed AR-mediated gene expression (Liao et al., 2004), indicating that a PI3K-dependent mechanism is involved in AR–DNA binding, or the assembly of AR-mediated transcription complex. Indeed, in this study, we found that PI3K inhibitor abolished androgen-induced AR–DNA binding. Knocking down p110β expression also resulted in loss of AR–DNA binding. A similar effect was also reported by another group using Her2 inhibitor (Mellinghoff et al., 2004). Our studies indicate that p110β signaling, most likely coupling with Her2 kinase, is required for AR–DNA interaction or the dynamic assembly of AR transcription complex.
Recent emerging evidences indicate that PI3K isoforms have oncogenic potential through gain-of-function mutations or gene amplification (Zhao et al., 2005; reviewed in Bader et al., 2005). Because of their important roles in cell signaling, elevated activities of PI3K pathway due to PTEN inactive mutation has long been considered as a key player in cancer pathogenesis including prostate cancers (Li et al., 1997; Steck et al., 1997; Murillo et al., 2001; reviewed in Engelman et al., 2006). However, no information is currently available about the genetic phenotype and expression pattern of PI3K isoforms in human prostate cancers. Only one report showed that siRNA-mediated p110β silencing suppressed cancer cell growth in vitro and tumor metastasis in vivo (Czauderna et al., 2003). In this study, we showed that both p85α and p110β are highly expressed in prostate cancers at the mRNA and protein levels, respectively. Their expression levels are associated with disease progression, poor differentiation and metastasis. Although the patient population was limited, our findings are of clinical significant because it opens a novel area for further investigation in prostate cancers.
Usually, type IA PI3K is activated by membrane-associated tyrosine kinase-based mechanisms through p85 regulatory isoforms. On the other hand, Gα subunits-mediated p110β activation was also reported (Murga et al., 1998; reviewed in Bader et al., 2005). A recent report showed that androgen induces PI3K activation via AR interaction with p85α and Src kinase in prostate cancer cells (Sun et al., 2003). However, we (Liao et al., 2004) and other (Mellinghoff et al., 2004) found that Src kinase inhibitor did not suppress androgen-induced gene expression, indicating that Src kinase is not involved in androgen-induced PI3K activation or AR genomic effect. In addition, recent studies showed that after androgen stimulation the AR was transiently translocated to the lipid rafts on the plasma membrane (Lu et al., 2001; Cinar et al., 2007), where multiple signaling molecules such as G proteins are located (reviewed in Chini and Parenti, 2004). In fact, G protein α-subunit has been shown to activate the AR in the absence or presence of androgens (Kasbohm et al., 2005). Collectively, it is plausible that p85α and/or G proteins are involved in androgen-stimulated p110β activation and that in turn induces AR transactivation.
In conclusion, we demonstrated that PI3K p85α and p110β are the essential signaling components in androgen-induced AR transactivation and are required for cell proliferation and tumor growth of prostate cancer. It is plausible that these PI3K isoforms or other signaling molecules, such as Her2 kinases (Mellinghoff et al., 2004), are working in concert to regulate AR–DNA interaction or assembly of AR-based transcriptional complex on the promoter region of target genes. Further investigation is desirable for dissecting this complicated process in detail.
Materials and methods
Cell culture and reagents
The human prostate cancer LNCaP cell line and human embryonic kidney cell line HEK293 were described previously (Liao et al., 2004). The synthetic androgen R1881 was obtained from ICN (Aurora, OH, USA). TGX-221 was purchased from Cayman Chemical (Ann Arbor, MI, USA). LY294002 and p110α inhibitor 4 (PI3K-I4) were purchased from Calbiochem (San Diego, CA, USA). Antibodies for PI3K isoforms and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-AR antibodies (Clone PG21) were obtained from Upstate (Charlottesville, VA, USA). Charcoal-stripped fetal bovine serum (cFBS, steroid-depleted) was obtained from Atlanta Biologicals (Norcross, GA, USA). The mammalian expression constructs for wild-type p110β and its mutants were described previously (Yart et al., 2002), and p110α construct was obtained from OriGene (Rockville, MD, USA).
Western blot analysis
For western blot analysis, cells were washed in phosphate-buffered saline and lysed in a radioimmunoprecipitation assay buffer supplied with a cocktail of protease inhibitors (CytoSignal, Irvine, CA, USA). Equal amount of proteins was separated on an 8–15% SDS–polyacrylamide gel and blotted onto a polyvinyl difluoride membrane (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked in a Tris-buffered saline solution with 5% nonfat dry milk containing 0.1% Tween-20 and incubated with antibodies overnight at 4 °C. Immunoreactive signals were detected by incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) followed by chemiluminescent detection using SuperSignal substrate (Pierce Chemical Co., Rockford, IL, USA).
siRNA transfection
The control siRNAs with scrambled sequences were purchased from Ambion (Austin, TX, USA) and the predefined siRNAs for PI3K isoforms were purchased from Santa Cruz Biotechnology. The no. 8 siRNA for human AR gene was described previously (Liao et al., 2005). Transfection was carried out with OligoFectamine reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. For the experiments involving a reporter gene assay, 3 days after transfection with the siRNAs, cells were transfected with the reporter constructs as described below. To determine the efficiency of the siRNAs, preliminary experiments were conducted in HEK293 and LNCaP cells by transfection of the siRNAs at various concentrations (1–100 nm), and a dramatic reduction was observed when the siRNAs were used at a final concentration of 100 nm in culture media. The scrambled siRNA duplex was used as a negative control.
Luciferase and SEAP reporter assay
A luciferase reporter construct controlled by synthetic androgen response elements (ARE-LUC) and the control reporter construct CMV–SEAP, expressing secreted alkaline phosphatase (SEAP) under the control of the cytomegalovirus (CMV) promoter, were described previously (Lee et al., 2003; Shanmugam et al., 2007). For PI3K overexpression experiments, cells were plated in six-well tissue culture plates and co-transfected on the following day with 1.0 µg ARE-LUC, 0.5 µg CMV-SEAP and various amount of PI3K constructs as indicated in the figure using the LipoFectamine reagent (Invitrogen). A pcDNA3.0 empty vector was used to balance the DNA amounts used in each transfection. After 24 h, cells were serum starved for another 24 h and then treated with the solvent (ethanol) or R1881 (1.0 nm) in 2% cFBS. After 24 h, culture supernatants were collected and assayed for SEAP activity as described previously (Liao et al., 2004). Cells were lysed with a luciferase assay lysis buffer (Promega Corp., Madison, WI, USA). Protein concentration of cellular lysate was measured by the bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL, USA). Equal amount of proteins from each cell lysate was assayed in triplicate for luciferase enzyme activity by using the luciferase assay system (Promega Corp). The luciferase activity of each sample was normalized against the corresponding SEAP activity and the protein concentration before the fold induction value relative to control cells was calculated (Liao et al., 2004).
Frozen tissue and real-time RT–PCR
A total of 30 primary prostate cancer specimens and the individually matched, microscopically confirmed nonmalignant compartments were obtained from patients who received surgery at the KU Hospital. A research protocol with the exemption of human subject was approved by the Institutional Research Board. After H&E staining, each tumor specimen was examined by an experienced surgical pathologist for the diagnosis (grade and stage) following the criteria of the American joint committee on cancer, 1988 edition. Tumor samples that contain at least 70% or more malignant compartments were selected for further experiments. Specimens (80–100mg per sample) were snap frozen and stored at −80 °C before use. There were 24 cases with Gleason score of 7, 1 case with Gleason score of 6 and others were Gleason score 8–9. All patients were naive for hormonal therapy and the average of patient age was 62.5 years (57–78 years).
Total RNAs from frozen tissues and LNCaP cells after treatment with PI3K inhibitors were extracted using TriZol reagent (Invitrogen). The first-strand cDNA was synthesized using Omniscript-RT Kit (QiaGen, Valencia, CA, USA). The primers for the genes of PIK3Ca (p110α; 5′-ATCTTTTCTCAATGATGCTTGGCT-3′ and 5′-CTAGGGTGTTTCGAATGTATG-3′), PIK3Cb (p110β; 5′-CCCTTCTGAACTGGCTTAAAGA-3′ and 5′-GGACAGTGTAAATTCCTCAATGG-3′), PIK3R1 (p85α; 5′-AATGAACGACAGCCTGCAC-3′ and 5′-CCGTTGTTGGCTACAGTAGTAGG-3′), as well as for psa (5′-ACCAGAGGAGTTCTTGACCCCAAA-3′ and 5′-CCCCAGAATCACCCGAGCAG-3′) were synthesized by IDT Inc (Coralville, IA, USA). The real-time PCR was performed using SYBR Green Supermix kit with the Bio-Rad iQ5 system. Denaturation at 95 °C for 15 s, and annealing/extension (temperature varied due to different primer sequence composition) for 1 min was then performed for 30 cycles. The amplification value of the target genes was normalized against the epithelial cell-specific gene KRT18 as described (Latil et al., 2001).
Cell proliferation assay
Cells were seeded in 96-well microtiter plate overnight. Thereafter, cells were transfected with the siRNAs and then incubated for additional 24 and 48 h in the presence or absence of R1881. Cell proliferation rates in each cell line were analysed using MTT assay (Cell Proliferation kit I, Roche Molecular Biochemicals, Indianapolis, IN, USA). Formazan crystals were solubilized overnight, and the product was quantified spectrophotometrically at 570 nm.
For BrdU incorporation assay, cells were incubated with BrdU (BrdU labeling and detection kit III; Roche Molecular Biochemicals) for the last 18 h of the experiments. Cells were subsequently fixed with 0.5m ethanol/HCl and were then incubated with nucleases to partially digest DNA. Monoclonal anti-BrdU antibodies conjugated to peroxidase were subsequently added and detected with 2,2′-azino-bis(3-ethyl-benzthiazoline-6-sulfonic acid) as the substrate. Quantitation was performed colorimetrically at 405–490nm acording to the manufacutrer’s protocol.
ChIP assay
ChIP assay was conducted basically as described in our recent publications (Li et al., 2007a; Sun et al., 2007a). Cells were maintained in 10-cm dishes in medium without serum for at least 16 h. Following PI3K siRNA transfection for 3 days or addition of the PI3K inhibitor Wortmannin, cells were treated with or without 1.0 nm R1881 for 12 h in the presence of 5% cFBS. The ChIP assay kit and the polyclonal antibody against AR were obtained from Upstate. Normal rabbit serum was used as a negative control. An anti-RNA polymerase II antibody (Santa Cruz Biotechnology) was used as a positive control. GAPDH primers were supplied by the ChIP assay kit. The primers for psa gene in the PCR reactions were listed as follows: 5′-tctgcctttgtcccctagat-3′ and 5′-aaccttcattccccaggact-3′ (adapted from Shang et al., 2002), which amplify a 210-bp fragment corresponding to human PSA gene promoter sequence between −250 and −39 from the transcription start site. The PCR products were run on 1% agarose gel and stained with ethidium bromide for visualization.
LNCaP stable cell lines and mouse xenograft model
LNCaP cells were transfected with the pU6+2 vector bearing a hairpin-structured siRNA cassette for p110β silencing (pU6+2-p110β.shRNA), as described previously (Czauderna et al., 2003). Stable clones were selected in G418-containing media, and the control sublines were established as described (Liao et al., 2004; Shanmugam et al., 2007). The efficacy of p110β silencing was validated by western blot (Figure 2a). All animal studies were conducted under an approved institutional animal care and use committee protocol. Single cell suspension of 2 × 106 cells in 0.1 ml of RPMI 1640 medium plus MatriGel supplied with 10% FBS were inoculated subcutaneously into the rear flanks (two sites per mouse) of 6- to 7-week-old male nude mice (Charles River, Wilmington, MA, USA). Tumor development was monitored every other day.
Tissue samples and IHC staining
A total of 34 paraffin-embedded specimens from 25 primary cancers with different tumor grades (Gleason Score) and 9 metastasis tumors were selected for the IHC study, following a protocol approved by our Institutional Research Board. The Clinical data was collected at the time of selection. The histopathological data included the diagnosis at surgery (stage and grade, surgical margin, lymph note status), tumor volume, cell proliferation marker Ki-67, DNA content (ploidy) and p53 status. Patients were followed up for survival and disease recurrence. Mean patient age was 58.6 years and the average Gleason score was 7.4.
The IHC staining was conducted as described in our recent publication (Sun et al., 2007b). Briefly, the sections were deparaffinized and rehydrated, followed by antigen retrieval and endogenous peroxidase blocking. The primary antibodies for p85α (clone B-9, catalog no. SC1637) were purchased from Santa Cruz Biotechnology. Multiple dilutions of the primary antibodies were utilized for individual specimens to achieve optimal immunosignals. A negative control was set up for each case by omitting the primary antibody. The specificity of the primary antibodies was verified by western blot. Immunosignals were visualized with a DAKO LSAB kit (Dako, Carpenteria, CA, USA). The intensity of the immuno-signals was scored as weak, moderate and strong.
Statistical analysis
All experiments were repeated two or three times except animal studies. Western blot and IHC staining results are presented from a representative experiment. The mean and standard error of the mean from multiple experiments for reporter gene assays and cell proliferation assays are shown. The significance of the differences between treatment and control was analysed using the SPSS computer software (SPSS Inc., Chicago, IL, USA).
Acknowledgements
We thank Dr Alen Gao at Roswell Park Cancer Institute (Buffalo, NY) for the construct of ARE-LUC reporter, Dr David Spencer (Baylor College of Medicine) for the construct of CMV-SEAP reporter and Dr Jorg Kaufmann for the pU6+2p110beta.shRNA construct. We also thank Mrs Marsha Danley (Department of Pathology, KUMC Hospital) and Dr Ilanchezhian Shanmugam for excellent technical assistance in IHC staining and ChIP assay, respectively. This study was supported by KU William L Valk Endowment and Kansas Masonic Foundation through KU Cancer Center pilot grant. This work was also partially supported by a KU-NIH COBRE grant (1P20RR15563) from the National Center for Research Resources (NIH-NCRR), Department of Defense New Investigator Award (DAMD17-03-1-0121) and Idea Development Award (W81XWH-04-1-0214) to Dr Benyi Li.
Abbreviations
- AR
androgen receptor
- ARE
androgen response element
- BrdU
Bromodeoxyuridine
- ChIP
chromatin IP
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PAGE
polyacrylamide
- PI3K
phosphoinositide 3-OH kinase
- PKO
protein kinase only
- PSA
prostate-specific antigen
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- RT
reverse-transcription
- SEAP
secreted alkaline phosphatase
- s.e.m.
standard error of mean
- siRNA
small interference RNA.
References
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft micro-domains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- Czauderna F, Santel A, Hinz M, Fechtner M, Durieux B, Fisch G, et al. Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res. 2003;31:e127. doi: 10.1093/nar/gng127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Kaizawa H, Moritomo H, Koizumi T, Ohishi T, Okada M, et al. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem. 2006;14:6847–6858. doi: 10.1016/j.bmc.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- Kasbohm EA, Guo R, Yowell CW, Bagchi G, Kelly P, Arora P, et al. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Biol Chem. 2005;280:11583–11589. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
- Latil A, Bieche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, et al. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–1926. [PubMed] [Google Scholar]
- Lee SO, Lou W, Hou M, Onate SA, Gao AC. Interleukin-4 enhances prostate-specific antigen expression by activation of the androgen receptor and Akt pathway. Oncogene. 2003;22:7981–7988. doi: 10.1038/sj.onc.1206735. [DOI] [PubMed] [Google Scholar]
- Li B, Liao XB, Fujito A, Thrasher JB, Shen FY, Xu PY. Dual androgen-response elements mediate androgen regulation of MMP-2 expression in prostate cancer cells. Asian J Androl. 2007a;9:41–50. doi: 10.1111/J.1745-7262.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- Li B, Sun A, Youn H, Hong Y, Terranova PF, Thrasher JB, et al. Conditional Akt activation promotes androgen-independent progression of prostate cancer. Carcinogenesis. 2007b;28:572–583. doi: 10.1093/carcin/bgl193. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li P, Nicosia SV, Bai W. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J Biol Chem. 2001;276:20444–20450. doi: 10.1074/jbc.M010226200. [DOI] [PubMed] [Google Scholar]
- Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- Liao X, Thrasher JB, Holzbeierlein J, Stanley S, Li B. Glycogen synthase kinase-3beta activity is required for androgen-stimulated gene expression in prostate cancer. Endocrinology. 2004;145:2941–2949. doi: 10.1210/en.2003-1519. [DOI] [PubMed] [Google Scholar]
- Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinasegamma. J Biol Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- Shanmugam I, Cheng G, Terranova PF, Thrasher JB, Thomas CP, Li B. Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptor-dependent cell survival. Cell Death Differ. 2007;14:2085–2094. doi: 10.1038/sj.cdd.4402227. [DOI] [PubMed] [Google Scholar]
- Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Sun A, Shanmugam I, Song J, Terranova PF, Thrasher JB, Li B. Lithium suppresses cell proliferation by interrupting E2F-DNA interaction and subsequently reducing S-phase gene expression in prostate cancer. Prostate. 2007a;67:976–988. doi: 10.1002/pros.20586. [DOI] [PubMed] [Google Scholar]
- Sun A, Tawfik O, Gayed B, Thrasher JB, Hoestje S, Li C, et al. Aberrant expression of SWI/SNF catalytic subunits BRG1/BRM is associated with tumor development and increased invasiveness in prostate cancers. Prostate. 2007b;67:203–213. doi: 10.1002/pros.20521. [DOI] [PubMed] [Google Scholar]
- Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, et al. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- Yart A, Roche S, Wetzker R, Laffargue M, Tonks N, Mayeux P, et al. A function for phosphoinositide 3-kinase beta lipid products in coupling beta gamma to Ras activation in response to lyso-phosphatidic acid. J Biol Chem. 2002;277:21167–21178. doi: 10.1074/jbc.M110411200. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci USA. 2005;102:18443–18448. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]