Abstract
5-Lipoxygenase initiates the biosynthesis of leukotrienes, lipid mediators involved in normal host defense and in inflammatory and allergic disorders. Despite an obvious gender bias in leukotriene-related diseases (e.g., asthma), gender aspects have been neglected in studies on leukotrienes and 5-lipoxygenase. Here, we show that leukotriene formation in stimulated whole blood or neutrophils from males is substantially lower compared with females, accompanied by changed 5-lipoxygenase trafficking. This is due to gender-specific differential activation of extracellular signal-regulated kinases (ERKs). The differences are directly related to variant male/female testosterone plus 5α-dihydrotestosterone levels, and addition of 5α-dihydrotestosterone to female blood or neutrophils reduced the high (female) LT biosynthesis capacity to low (male) levels. In conclusion, regulation of ERKs and leukotriene formation by androgens constitutes a molecular basis for gender differences in the inflammatory response, and in inflammatory diseases such as asthma.
Keywords: 5-lipoxygenase, arachidonic acid
Leukotrienes (LTs) are proinflammatory lipid mediators formed from arachidonic acid (AA) (1). The most established effects of LTs in the inflammatory response involve phagocytes and vascular endothelium, leading to leukocyte extravasation and migration, and increased vascular permeability. Thus, LTs are established within the innate immune system. However, effects of LTs on dendritic cells and on T cells suggest roles also in the adaptive immune response. The functions of LTs in normal inflammatory reactions imply LTs in inflammatory diseases (2). Associations are established for asthma and allergic rhinitis, and anti-LTs are used to treat these disorders. Ample support implies roles of LTs also in autoimmune diseases and atherosclerosis (2).
Sex hormones regulate sexual differentiation and reproduction, but also influence the immune system (3). Gender differences regarding susceptibility to certain autoimmune diseases are well known, for example rheumatoid arthritis and systemic lupus erythematosus (SLE) more often affect women, which has been associated with sex hormone levels, major histocompatibility complex genetic background, and cytokine production (3, 4). However, female sex can also protect against inflammatory disease. Thus, hepatocellular carcinoma, strongly linked to chronic liver inflammation, is less common among females because of estrogen inhibiting secretion of IL-6 from Kupffer cells (5). In asthma, sex differences are apparent with a male predominance in childhood, whereas after puberty females predominate, because of a drop in males (6), reflecting a protective function of androgens. However, the biochemical mechanisms for this gender disparity are unknown.
The capacity for LT biosynthesis depends on the availability of free AA and the subcellular compartmentalization of 5-LO (7, 8). In neutrophils, 5-LO resides in the cytoplasm of resting cells, and redistributes to the nuclear membrane upon activation by Ca2+ where it colocalizes with cPLA2 and the 5-LO-activating protein (FLAP) (7). The C-terminal catalytic domain can be phosphorylated by p38 mitogen-activated protein kinase (MAPK)-regulated MAPKAPK-2, extracellular signal-regulated kinases (ERKs), and protein kinase A, which modulate 5-LO nuclear localization and product synthesis (8–13). Moreover, coactosin-like protein (CLP) binds to 5-LO and promotes LT formation, apparently by functioning as chaperone or scaffold for 5-LO (14).
Despite an obvious gender bias in LT-related diseases [e.g., asthma (15)], gender aspects or effects of sex hormones have been neglected in studies on LT biosynthesis or 5-LO cell biology. Neutrophils are the major source of 5-LO products in blood (16) and these cells are important early effectors of the innate immune response (17). Only few studies have addressed gender differences in neutrophil biology, and effects of sex hormones on typical neutrophil functions are largely unknown (3). Here, we show that variant testosterone levels in males and females cause a differential activation status of ERKs in human neutrophils, which mediates sex differences in LT biosynthesis by regulating the subcellular localization of 5-LO.
Results
Gender-Dependent Capacities for 5-LO Product Formation.
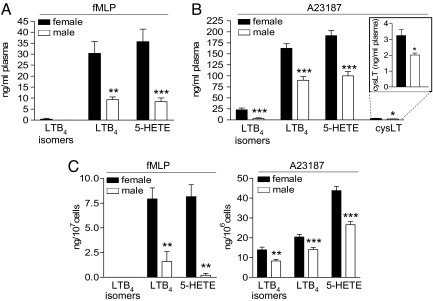
Formation of LTs and 5-H(P)ETE in female blood stimulated with lipopolysaccharide (LPS) plus N-formyl-methionyl-leucyl-phenylalanine (fMLP) (Fig. 1A) or with Ca2+-ionophore A23187 (Fig. 1B) was significantly higher than in blood from males. Only traces of 5-LO products were formed in unstimulated blood regardless of gender (data not shown) and generation of the cyclooxygenase (COX)-derived 12(S)-hydroxy-5-cis-8,10-transheptadecatrienoic acid (12-HHT) did not vary [see supporting information (SI) Fig. S1].
Fig. 1.
Gender-dependent differences in 5-LO product formation. 5-LO product formation in human whole blood induced by (A) fMLP (1 μM; 15 min, 37°C) after 30 min priming with LPS (1 μg/ml) or by (B) A23187 (30 μM; 10 min, 37°C). Data are means + SEM; n = 11 (fMLP/LPS), n = 13 (A23187), duplicates, each. (C) 5-LO product formation in human neutrophils induced by fMLP (1 μM; 5 min, 37°C) after 30 min priming with LPS (1 μg/ml) plus Ada (0.3 U/ml), or with A23187 (2.5 μM; 10 min, 37°C). Data are means + SEM; n = 6 (LPS/Ada/fMLP), n = 14 (A23187), duplicates, each. *, P < 0.05; **, P < 0.01; ***, P < 0.001; male vs. female, Student t test.
Next, 5-LO product formation was analyzed in human neutrophils, which are the major 5-LO product-forming cells in blood (16). To preserve properties of blood neutrophils because of sex hormones we isolated neutrophils at 4°C. The low temperature should minimize cellular and biochemical alterations when the extracellular milieu is changed (blood to buffer). Generation of LTB4 and 5-H(P)ETE in female neutrophils after stimulation with fMLP (after priming with LPS/adenosine deaminase (Ada)) was 4.9- and 10.1-fold higher than in male cells, respectively. Similarly, A23187 caused the synthesis of 1.6-fold more 5-LO products in female versus male neutrophils (Fig. 1C).
5-LO protein levels and 5-LO activity in homogenates of blood or isolated neutrophils were not different between genders (Fig. S1). It appeared reasonable that the endogenous substrate supply for 5-LO could vary, and in fact, supplementation of whole blood or neutrophils with excess of exogenous AA abolished unequal 5-LO product synthesis (Fig. S1). However, the amounts of AA liberated from activated neutrophils were equal for male/female cells (Fig. S1), implying that instead the accessibility of endogenously released AA for 5-LO could differ between the genders, because of different subcellular localization of 5-LO.
Gender-Dependent Subcellular Localization and Trafficking of 5-LO.
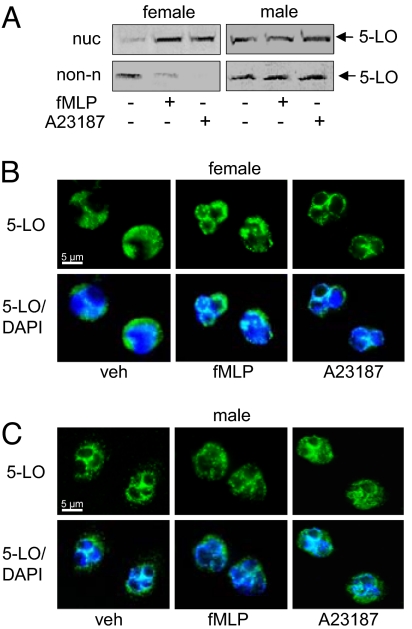
To assess 5-LO subcellular distribution, we used subcellular fractionation techniques [i.e., mild-detergent lysis (9) or sonication (18) combined with centrifugation steps] coupled to 5-LO immunodetection as well as indirect immunofluorescence (IF) microscopy (19). In neutrophils from females, 5-LO resided in the cytoplasm of resting cells and redistributed to the nucleus upon stimulation, in accordance with the well established model for 5-LO activation (9, 20). In contrast, in neutrophils from males, 5-LO was detected in both the cytosol and the nuclear compartment of resting cells, and upon stimulation, the compartmentalization of 5-LO was not significantly altered (Fig. 2A and Fig. S2). IF analysis of 5-LO confirmed cytoplasmic 5-LO in resting female neutrophils and, after cell activation 5-LO accumulated in the perinuclear region (Fig. 2B). However, for male neutrophils, a prevalent punctuate staining in the perinuclear region was observed in both resting and activated cells (Fig. 2C). Together, in female neutrophils, 5-LO translocates from the cytosol to the nucleus upon cell challenge, whereas in male cells, a fraction of 5-LO is associated with the nucleus in resting cells, and there is no obvious change in 5-LO distribution upon cell stimulation.
Fig. 2.
Subcellular localization and trafficking of 5-LO in neutrophils are regulated in a gender-dependent manner. 5-LO subcellular localization after activation of neutrophils with fMLP (1 μM; 5 min, 37°C) after 30 min priming with LPS (1 μg/ml) plus Ada (0.3 U/ml), or with A23187 (2.5 μM; 5 min, 37°C), analyzed by immunodetection of 5-LO in the nuclear (nuc) and non-nuclear (non-n) fractions of mild-detergent (0.1% Nonidet P-40)-lysed cells (A), or analyzed by IF microscopy (B and C). Results are representative of 6–9 independent experiments. In B and C, pictures with single staining for 5-LO (green) and merged with DNA-stain (diamidino-2-phenylindole, DAPI, blue), are shown.
FLAP was exclusively detected in the nuclear membrane fraction, irrespective of the gender or the cellular activation state (Fig. S2). cPLA2 colocalized with 5-LO in female neutrophils and the same subcellular distribution pattern was obvious in male neutrophils, implying that cPLA2 subcellular compartmentalization does not differ between genders (Fig. S2). Interestingly, the subcellular distribution of CLP was gender-dependent, as it consistently colocalized with 5-LO in female and in male neutrophils (Fig. S2).
Testosterone and 5α-Dihydrotestosterone Cause Nuclear Localization of 5-LO.
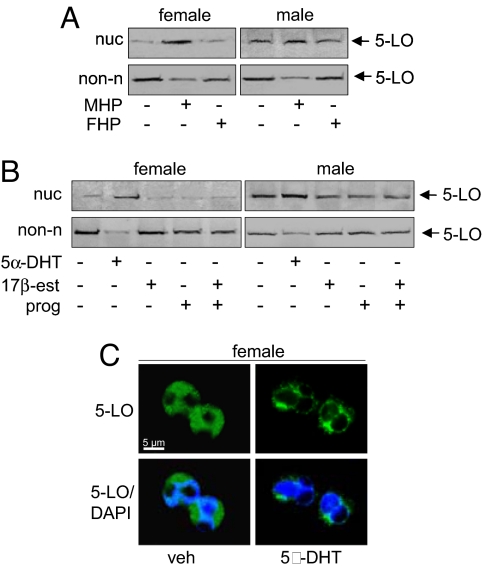
The gender-related difference in 5-LO regulation could be related to variant plasma levels of sex hormones in men and women. Exposure of female neutrophils to male plasma induced 5-LO redistribution to the nucleus, whereas for male neutrophils, addition of female plasma was essentially ineffective and male plasma induced only a moderate increase of 5-LO in the nuclear compartment (Fig. 3A). Treatment of female neutrophils with 5α-dihydrotestosterone (5α-DHT) caused translocation of 5-LO to the nuclear compartment in a rapid (within 5 min) and concentration-dependent manner (starting at 10 pM with maximum at 10 nM), whereas 17β-estradiol (100 nM) and progesterone (10 μM) had no effect (Fig. 3B and Fig. S3). Interestingly, 5α-DHT -treated neutrophils from females showed a prevalent punctuate staining of 5-LO in the perinuclear region, similar to that observed in resting cells from males (Fig. 3C). In male neutrophils, 17β-estradiol and/or progesterone had no influence on 5-LO subcellular localization, but 5α-DHT caused a slight increase of nuclear 5-LO (Fig. 3B). Conclusively, 5α-DHT causes a rapid redistribution of 5-LO from the cytosol to the nucleus, resulting in differential 5-LO subcellular localization in male and female neutrophils.
Fig. 3.
Androgens induce nuclear localization of 5-LO. (A) Neutrophils were incubated in male (MHP) or female (FHP) human plasma for 30 min at 37°C. 5-LO was analyzed by immunodetection in the nuclear (nuc) and non-nuclear (non-n) fractions. (B) Neutrophils were treated with 5α-DHT (10 nM; 30 min, 37°C), 17β-estradiol (100 nM; 30 min, 37°C) and/or progesterone (10 μM; 30 min, 37°C). 5-LO was analyzed by immunodetection in nuclear (nuc) and non-nuclear (non-n) fractions. (C) 5α-DHT (10 nM, 30 min, 37°C) induces association of 5-LO with the nucleus in neutrophils from females, as analyzed by IF microscopy. Pictures with single staining for 5-LO (green) and merged with DNA-stain (diamidino-2-phenylindole, DAPI, blue), are shown. Results are representative of n = 3–4 independent experiments.
After removal of 5α-DHT, the effect on 5-LO in female neutrophils was reversed (Fig. S3). Moreover, when neutrophils isolated from males were incubated at 37°C for 120 min (instead of keeping on ice), a prevalent cytosolic localization of 5-LO was observed and 5-LO was susceptible to A23187 and redistributed to the nucleus (Fig. S3), as observed for female cells. Hence, the effect of 5α-DHT on neutrophils is reversible, and constant cooling on ice during neutrophil isolation (and thus androgen removal) obviously preserves the influence of plasma androgens.
5α-DHT Suppresses Cellular 5-LO Product Formation.
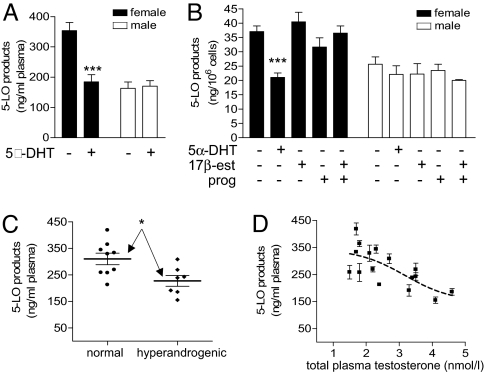
Preincubation of whole blood or neutrophils from males with sex hormones caused no significant change in 5-LO product synthesis upon A23187-stimulation (Fig. 4A and B). In contrast, treatment of blood or neutrophils from females with 5α-DHT suppressed 5-LO product synthesis to levels found for male-derived samples (Fig. 4 A and B). Exposure of female neutrophils to 5α-DHT did not affect 5-LO protein levels or agonist-evoked release of AA (data not shown). Interestingly, 5-LO activity in A23187-challenged whole blood from hyperandrogenic females (testosterone >2.6 nmol/l) was significantly lower compared with blood from females with normal testosterone levels (< 2.6 nmol/l) (Fig. 4C), and there was a correlation between androgen levels and 5-LO activity (Fig. 4D).
Fig. 4.
5α-DHT suppresses 5-LO product formation. (A) Whole blood from females was treated with 5α-DHT (100 nM; 30 min, 37°C) and stimulated with A23187 (30 μM, 10 min) for 5-LO product formation. Data are means + SEM; n = 10, duplicates. ***, P < 0.001 vs. vehicle (0.05% EtOH) control, Student's t test. (B) Effects of 5α-DHT (10 nM; 30 min, 37°C), 17β-estradiol (100 nM; 30 min, 37°C) and/or progesterone (10 μM; 30 min, 37°C) on 5-LO product formation in A23187 (2.5 μM, 10 min)-stimulated neutrophils. Data are means + SEM; n = 6, duplicates. ***, P < 0.001 vs. vehicle (0.05% EtOH) control, ANOVA + Tukey HSD post hoc tests. (C) Dot plot of 5-LO activity in whole blood from females with normal testosterone levels (total plasma testosterone <2.6 nmol/l) and hyperandrogenic females (total plasma testosterone >2.6 nmol/l) stimulated with 30 μM A23187 (10 min, 37°C). Bars show the group mean ± SEM. *, P < 0.05. (D) Correlation between plasma testosterone levels in females and 5-LO activity in A23187-challenged whole blood. Data are means ± SEM of duplicates. The broken line represents the fitted sigmoidal curve (R2 = 0.52).
Testosterone and 5α-DHT Cause Activation of ERK.
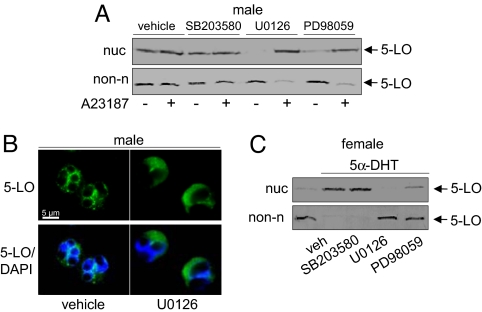
To elucidate the molecular mechanisms underlying the gender/5α-DHT -dependent regulation of 5-LO, crucial pathways and cofactors regulating 5-LO were examined. The p38 MAPK-regulated MAPKAPK-2 as well as ERKs phosphorylate and regulate 5-LO in leukocytes (10, 21). Pretreatment of male neutrophils with the ERK activation inhibitors U0126 and PD98059 gave cytosolic localization of 5-LO (Fig. 5 A and B), and upon A23187-challenge, 5-LO redistributed to the nuclear compartment (Fig. 5A). The p38 MAPK inhibitor SB203580 failed in this respect. Pharmacological suppression of ERKs in female neutrophils did not influence 5-LO (Fig. S4A). Accordingly, U0126 and PD98059 (but not SB203580) blocked 5α-DHT-induced 5-LO nuclear redistribution in female neutrophils (Fig. 5C and Fig. S4).
Fig. 5.
ERKs cause 5α-DHT/gender-dependent regulation of 5-LO subcellular localization. Effects of U0126 (3 μM), PD98059 (30 μM), and SB203580 (10 μM) on 5-LO subcellular localization. Neutrophils from males were pretreated with the kinase inhibitors (or 0.3% DMSO as vehicle) for 15 min at 37°C prior stimulation with A23187 (2.5 μM, 5 min, 37°C). 5-LO was analyzed by (A) immunodetection in the nuclear (nuc) and non-nuclear (non-n) fractions and (B) by IF microscopy. In B, pictures with single staining for 5-LO (green) and merged with DNA-stain (diamidino-2-phenylindole, DAPI, blue), are shown. (C) U0126 (3 μM) or PD98059 (30 μM), but not SB203580 (10 μM) block nuclear localization of 5-LO induced by 5α-DHT (10 nM; 30 min, 37°C) in neutrophils from females, as analyzed by immunodetection of 5-LO in the nuclear (nuc) and non-nuclear (non-n) fractions. Results are representative of at least three independent experiments.
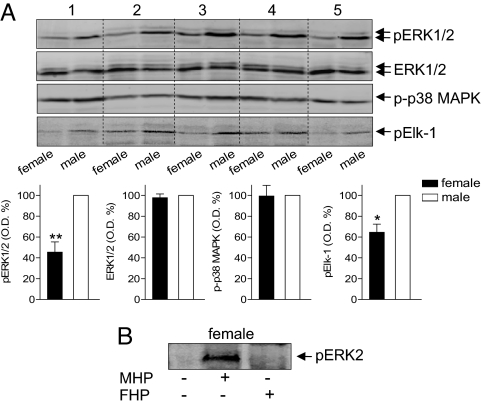
Our data suggest that male neutrophils should have higher levels of ERK activity versus female cells. The basal phosphorylation (= activation) status of ERK2 and of its substrate Elk-1 was significantly higher in male neutrophils versus female cells (Fig. 6A). ERK2 expression and phosphorylation of p38 MAPK did not vary between genders. 5α-DHT caused a rapid (within 0.5 min) and concentration-dependent (starting at 10 pM) phosphorylation of ERK2 in female neutrophils (Fig. S5), whereas 17β-estradiol and/or progesterone were not effective. Finally, incubation of female neutrophils with male (but not female) plasma caused activation of ERKs (Fig. 6B).
Fig. 6.
ERKs show a differential activation status in female and male neutrophils and are activated by androgens (A) Analysis of phosphorylated ERK1/2 (pERK1/2), total ERK1/2, phosphorylated p38 MAPK (p-p38 MAPK) and phosphorylated Elk-1 (pElk-1) in male and female neutrophils. In the pairwise analyses, optical densities measured for male samples were set as 100%, five representatives are shown. Data are means + SEM; n = 7; *, P < 0.05, **, P < 0.01 vs. male, Student t test. (B) Incubation of neutrophils from females in male human plasma (MHP), but not in female human plasma (FHP), for 1.5 min at 37°C, induce ERK2 activation, as analyzed by immunodetection of phosphorylated ERK2 (pERK2). Results are representative of n = 3 independent experiments.
Discussion
We show that in human neutrophils 5-LO product synthesis and 5-LO subcellular compartmentalization depend on gender, connected to a differential activation status of ERKs. Thus, stimulated blood or neutrophils from males generate less 5-LO products compared with females, accompanied by changed 5-LO subcellular trafficking. Androgens cause these gender differences, affecting 5-LO via ERKs. Because ERKs are central signaling kinases, regulating multiple neutrophil functions (17), these androgen actions may have profound effects on neutrophil biology.
Differences in 5-LO product formation between male and female blood or neutrophils were more pronounced in response to LPS/fMLP compared with A23187. LPS and fMLP use defined cellular signaling pathways to activate 5-LO (20, 21) and this is of pathophysiological relevance at sites of inflammation (16). In contrast, the extreme elevation of [Ca2+]i induced by A23187 may circumvent regulatory mechanisms of 5-LO. Hence, the striking differences in the capacities of LPS/fMLP-evoked 5-LO product synthesis between genders may better reflect pathophysiological conditions in the body.
Major factors governing 5-LO product synthesis, for example, levels of enzymatically active 5-LO and of FLAP and substrate availability (7, 9) were not different between the genders. However, 5-LO subcellular distribution clearly varied. Numerous studies have shown that 5-LO resides in the cytoplasm of resting neutrophils and redistributes to the nuclear envelope in response to fMLP (with or without LPS priming) or A23187 (9, 19, 20, 22). For female neutrophils, we confirmed this pattern of 5-LO subcellular redistribution. However, in male neutrophils, a substantial part of 5-LO is located to the perinuclear region already in resting cells, and 5-LO only marginally redistributed upon stimulation, accompanied by reduced 5-LO product synthesis. Previous findings suggest that intranuclear localization of 5-LO confers an increased capacity for subsequent LT biosynthesis (19), whereas 5-LO already associated with the nuclear envelope at the time of cell stimulation is less active (23). Thus, the perinuclear 5-LO localization induced by 5α-DHT may be a regulatory mechanism in males that attenuates LT formation at sites of inflammation.
5-LO subcellular compartmentalization is governed by ERKs (12) that phosphorylate 5-LO (21). We provide evidence that ERKs mediate the male pattern of 5-LO subcellular distribution caused by androgens. First, male (resting) neutrophils exhibit a higher ERK activation status versus females. Second, pharmacological suppression of ERK activity in male cells gave the 5-LO female pattern. Third, exposure of female neutrophils to male plasma or to 5α-DHT enhanced ERK activation, accompanied by the male pattern of 5-LO subcellular distribution, which was reversed by ERK inhibition.
Inconsistent capacities of LT formation and 5-LO subcellular distribution were reported before and have been attributed to individual donor-related variability (16, 24), but gender aspects were never considered. One reason that the gender-difference was not observed before could be the reversibility of the androgen effects after neutrophil isolation from plasma at ambient temperature. We rapidly isolated neutrophils at 4°C, which preserved the plasma androgen effect on 5-LO and ERKs. ERKs have significant impact on numerous neutrophil functions (17) and, as found here for 5-LO, the different activation state of ERKs may lead to striking gender differences. Our results strongly suggest that reversible sex hormone effects should be considered in studies of neutrophil biology. Although activation of ERKs by exogenous androgens in nonhematopoietic cells was shown before [e.g., prostate cancer cells (25)], gender differences in basal ERK activation in humans are thus far unknown. Androgens classically mediate their effects through an intracellular androgen receptor (AR) via transcriptional pathways. Expression and functionality of the AR in neutrophils is not definite and studies investigating androgen effects on neutrophil functions (e.g., oxidative burst) yielded contradictory data (3). Our results suggest the existence of a high affinity receptor for 5α-DHT on neutrophils that rapidly causes activation of ERKs. For murine splenic T cells lacking the AR, the existence of a GPCR for testosterone in plasma membranes was demonstrated (26). The identification of the androgen receptor mediating ERK activation and thus neutrophil functions in response to 5α-DHT is a major future task.
In conclusion, LT formation in human blood and neutrophils depend on the gender, connected to a suppressive effect by androgens. These findings fit well with the reduction of asthma observed in males during puberty (6). Accordingly, in adolescence, LTs should be more important for asthma symptoms in afflicted females, than in males. This is supported by the better efficiency for the cysLT-1 antagonist montelukast as asthma treatment in girls reaching puberty, compared with boys at same age (27). Also, in a European multicenter study, females dominated among severe asthma patients (28). In patients with active SLE, LTs are strongly increased versus healthy controls, and pharmacological 5-LO inhibition was beneficial in patients with mild SLE (29). Interestingly, in a mouse model resembling human SLE (MRL-lpr/lpr) female mice had earlier mortality compared with male mice, but the male advantage was abolished after knockout of the 5-LO gene (30). The beneficial androgen effects in asthma and autoimmune diseases are certainly multifaceted, our data indicate that suppression of LTs is one contributing mechanism. Our findings also suggest that inflammatory responses in general, for which LTs are of relevance, may be more vigorous in females. LTs function in antimicrobial defense (2), in such context a high (female) capacity for LT production may be advantageous. For example, females resist bacterial pulmonary infections better than men (31). Finally, the sex-specific regulation of 5-LO implies that gender should be considered in the use of anti-LTs, to optimize pharmacological therapy, in men and women.
Methods
Preparation of Whole Blood and Plasma and Isolation of Neutrophils.
Venous blood was collected from fasted (12 h) adult male and female healthy volunteers, with consent (Blood Center, University Hospital, Tuebingen, Germany). The subjects had no apparent inflammatory conditions and had not taken sex hormones or anti-inflammatory drugs for at least 10 days before blood collection. Total serum testosterone was analyzed by an automated chemiluminescence immunoassay system (ADVIA Centaur, Siemens Medical Solution) according to the manufacturer's instructions. For isolation of plasma, blood was centrifuged at 600 × g/10 min/4°C, plasma was centrifuged again (800 × g/10 min/4°C), and the resulting supernatant was analyzed to confirm the absence of cellular contaminations.
For isolation of neutrophils, venous blood (see above) was subjected to centrifugation (4,000 × g/20 min/20°C) for preparation of leukocyte concentrates. Neutrophils were promptly isolated by dextran sedimentation, centrifugation on Nycoprep cushions, and hypotonic lysis of erythrocytes as described previously (11). The isolation procedure was strictly performed at 4°C. Neutrophils (purity >96–97%) were finally resuspended in ice-cold PBS plus 1 mg/ml glucose (PG buffer) or in PG buffer supplemented with 1 mM CaCl2 (PGC buffer).
Determination of 5-LO Product Synthesis and Formation of 12-HHT.
Freshly withdrawn blood (2 ml) was preincubated with test compounds at 37°C, and formation of 5-LO products and 12-HHT was started by addition of the respective stimuli. The reaction was stopped on ice, and the samples were centrifuged (600 × g/10 min/4°C). Aliquots of the resulting plasma (500 μl) were then mixed with 2 ml of methanol and 200 ng of prostaglandin B1 (internal standard). Samples were placed at −20°C for 2 h and centrifuged again (600 × g/15 min/4°C). The supernatants were collected and diluted with 2.5 ml of PBS and 75 μl of 1 N HCl.
For determination of cellular 5-LO product formation, neutrophils (5 × 106/ml PGC buffer) were preincubated with test compounds at 37°C, and the respective stimuli were added. The reaction was stopped with 1 ml of methanol and 30 μl of 1 N HCl, 200 ng of prostaglandin B1, and 500 μl of PBS were added. Formed 5-LO metabolites and 12-HHT were extracted and analyzed by HPLC as described (11). 5-LO product formation is expressed as ng of 5-LO products (LTB4 and its all-trans isomers, and 5-H(P)ETE) per ml of plasma or per 106 or per 107 cells. Formation of cysteinyl-LTs C4, D4 and E4 in whole blood was analyzed by an enzyme immunoassay kit (Assay Designs Inc.) after extraction of plasma, according to the manufacturer's instructions.
For determination of 5-LO product formation in homogenates, 1 mM EDTA was added to whole blood or freshly isolated neutrophils resuspended in ice-cold PBS. Samples were sonicated (3 × 10 sec) at 4°C, 1 mM ATP was added, and prewarmed for 30 sec at 37°C. After addition of 2 mM CaCl2 and AA, the reaction was stopped after 10 min at 37°C by addition of 1 ml of ice-cold methanol. Formed metabolites were analyzed by HPLC as described above.
Determination of 5-LO Protein Expression.
Neutrophils (3 × 107/ ml ice-cold PBS plus 1 mM EDTA) were sonicated (5 × 10 sec) at 4°C. Total cell lysates were centrifuged (12,000 × g/15 min/4°C), the supernatant was collected and mixed 1:1 with ice-cold 2 × SDS/PAGE sample loading buffer (SDS-b), heated for 6 min at 95°C, and analyzed for 5-LO protein by SDS/PAGE and immunoblotting.
Measurement of the Release of Arachidonic Acid.
Neutrophils (5 × 107 in 1 ml of PGC buffer) were preincubated (10 min) with the 12-LO inhibitor CDC (10 μM) and the 5-LO inhibitor BWA4C (1 μM) to avoid the conversion of released AA to LO metabolites. The reaction was started by addition of the indicated stimuli, and was stopped with 2 ml of methanol and 60 μl of 1 N HCl, and 2 ml of PBS were added together with 60 μg of margaric acid, used as internal standard. Released AA was extracted, coupled to dimethoxyaniline hydrochloride in presence of N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide and the derivatized AA was analyzed by RP-HPLC at 272 nm.
Analysis of Subcellular Redistribution of 5-LO, cPLA2, CLP, and FLAP by Cell Fractionation and Immunoblotting.
Neutrophils (3 × 107/ml PGC buffer or 3 × 107/500 μl of human plasma) were incubated with test compounds at 37°C. Samples were chilled on ice and centrifuged (200 × g/5 min/4°C). Subcellular fractionation was performed either by mild detergent (0.1% nonidet P-40) lysis, yielding a nuclear and a non-nuclear fraction (9), or by sonication of the cells and preparation of soluble (S100) and membrane (P100) fractions by 100,000 × g centrifugation (18). For more details, see SI Text. Nuclear and non-nuclear fractions and S100 and P100 fractions were analyzed by SDS/PAGE and Western blotting for 5-LO, CLP, FLAP, or cPLA2 as described in SI Text.
Indirect Immunofluorescence Microscopy.
Neutrophils (1.5 × 106/500 μl of PGC buffer) were incubated with test compounds at 37°C. Cells were centrifuged at 30 × g for 1 min onto polyL-lysine (MW 150,000–300,000; Sigma–Aldrich)-coated glass coverslips, and activated by addition of the stimuli for 3 min at 37°C. Cells were fixed in methanol (-20°C, 30 min) and permeabilized with 0.1% Tween 20 in PBS (RT, 10 min), followed by 3 washing steps with PBS. Samples were blocked with 10% non-immune goat serum (Invitrogen) for 10 min at RT, washed with PBS, and incubated with anti-5-LO serum (1551, affinity purified) for 30 min at RT. The coverslips were washed 10 times with PBS, incubated with Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) for 10 min at RT in the dark, and washed 10 times with PBS. The DNA was stained with 0.1 μg/ml diamidino-2-phenylindole (DAPI) in PBS for 3 min at RT in the dark. The coverslips were then washed 10 times and mounted on glass slides with Mowiol (Calbiochem) containing 2.5% n-propyl gallate (Sigma). The fluorescence was visualized with a Zeiss Axiovert 200M microscope.
Determination of MAPK Activation and Phosphorylation of Elk-1.
Neutrophils (107/100 μl of PGC buffer) were incubated with the indicated compounds at 37°C and the reaction was stopped by addition of 100 μl of ice-cold SDS-b and heated for 6 min at 95°C. When the effects of human plasma were analyzed, cells were resuspended in 100 μl of plasma and incubated at 37°C as indicated. The reaction was stopped on ice and cells were washed twice with ice-cold PBS. Total cell lysates (20 μl) were analyzed for ERK1/2, phosphorylated ERK1/2 (Thr-202/Tyr-204), phosphorylated p38 MAPK (Thr-180/Tyr-182), and phosphorylated Elk-1 (Ser-383) by SDS/PAGE and western blotting. Visualization of the immunocomplexes was carried out using an Ettan DIGE imaging system (GE-Healthcare), and densitometry was performed with ImageQuant TL image analysis software (GE Healthcare). For more technical details, see SI Text.
Statistics.
Results are expressed as mean ± standard error (SEM) of the mean of n observations, where n represents the number of experiments performed on different days in duplicates. Statistical evaluation of the data were performed by Student t test for paired observations. Where appropriate, one-way ANOVA for independent or correlated samples, followed by Tukey HSD post hoc tests, was applied. A P value <0.05 (*) was considered significant.
Supplementary Material
Acknowledgments.
We thank Bianca Jazzar for expert technical assistance and Dr. Joachim Schultz for critically reading the manuscript and helpful discussions. This work was supported by Deutsche Forschungsgemeinschaft, Swedish Research Council Grant 03X-217, and European Union Grant LSHM-CT-2004-00533. C.P. received a stipend from the Carl-Zeiss-Stiftung.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809120105/DCSupplemental.
References
- 1.Samuelsson B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 2.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 3.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 4.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 5.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 6.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: A GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 7.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc Natl Acad Sci USA. 2003;100:12165–12170. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werz O, Klemm J, Samuelsson B, Rådmark O. Phorbol ester up-regulates capacities for nuclear translocation and phosphorylation of 5-lipoxygenase in Mono Mac 6 cells and human polymorphonuclear leukocytes. Blood. 2001;97:2487–2495. doi: 10.1182/blood.v97.8.2487. [DOI] [PubMed] [Google Scholar]
- 10.Werz O, Klemm J, Samuelsson B, Rådmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc Natl Acad Sci USA. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werz O, Burkert E, Samuelsson B, Rådmark O, Steinhilber D. Activation of 5-lipoxygenase by cell stress is calcium independent in human polymorphonuclear leukocytes. Blood. 2002;99:1044–1052. doi: 10.1182/blood.v99.3.1044. [DOI] [PubMed] [Google Scholar]
- 12.Flamand N, Lefebvre J, Surette ME, Picard S, Borgeat P. Arachidonic acid regulates the translocation of 5-lipoxygenase to the nuclear membranes in human neutrophils. J Biol Chem. 2006;281:129–136. doi: 10.1074/jbc.M506513200. [DOI] [PubMed] [Google Scholar]
- 13.Luo M, Jones SM, Phare SM, Coffey MJ, Peters-Golden M, Brock TG. Protein kinase A inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J Biol Chem. 2004;279:41512–41520. doi: 10.1074/jbc.M312568200. [DOI] [PubMed] [Google Scholar]
- 14.Rakonjac M, et al. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci USA. 2006;103:13150–13155. doi: 10.1073/pnas.0605150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surette ME, Palmantier R, Gosselin J, Borgeat P. Lipopolysaccharides Prime Whole Human Blood and Isolated Neutrophils for the Increased Synthesis of 5-Lipoxygenase Products by Enhancing Arachidonic Acid Availability - Involvement of the CD14 Antigen. J Exp Med. 1993;178:1347–1355. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burg ND, Pillinger MH. The neutrophil: Function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 18.Pouliot M, et al. Colocalization of cytosolic phospholipase A2, 5-lipoxygenase, and 5-lipoxygenase-activating protein at the nuclear membrane of A23187-stimulated human neutrophils. Eur J Biochem. 1996;238:250–258. doi: 10.1111/j.1432-1033.1996.0250q.x. [DOI] [PubMed] [Google Scholar]
- 19.Brock TG, McNish RW, Bailie MB, Peters-Golden M. Rapid import of cytosolic 5-lipoxygenase into the nucleus of neutrophils after in vivo recruitment and in vitro adherence. J Biol Chem. 1997;272:8276–8280. doi: 10.1074/jbc.272.13.8276. [DOI] [PubMed] [Google Scholar]
- 20.Surette ME, Dallaire N, Jean N, Picard S, Borgeat P. Mechanisms of the priming effect of lipopolysaccharides on the biosynthesis of leukotriene B4 in chemotactic peptide-stimulated human neutrophils. FASEB J. 1998;12:1521–1531. doi: 10.1096/fasebj.12.14.1521. [DOI] [PubMed] [Google Scholar]
- 21.Werz O, et al. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- 22.Rouzer CA, Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem. 1988;263:10980–10988. [PubMed] [Google Scholar]
- 23.Brock TG, McNish RW, Peters-Golden M. Capacity for repeatable leukotriene generation after transient stimulation of mast cells and macrophages. Biochem J. 1998;329(Pt 3):519–525. doi: 10.1042/bj3290519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgeat P, Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: Effects of ionophore A23187. Proc Natl Acad Sci USA. 1979;76:2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 26.Benten WP, et al. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13:123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 27.Johnston NW, et al. Attenuation of the September epidemic of asthma exacerbations in children: A randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120:e702–e712. doi: 10.1542/peds.2006-3317. [DOI] [PubMed] [Google Scholar]
- 28.Abraham B, et al. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Resp J. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 29.Hackshaw KV, Shi Y, Brandwein SR, Jones K, Westcott JY. A pilot study of zileuton, a novel selective 5-lipoxygenase inhibitor, in patients with systemic lupus erythematosus. J Rheumatol. 1995;22:462–468. [PubMed] [Google Scholar]
- 30.Goulet JL, et al. Deficiency of 5-lipoxygenase abolishes sex-related survival differences in MRL-lpr/lpr mice. J Immunol. 1999;163:359–366. [PubMed] [Google Scholar]
- 31.Mikerov AN, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.