Abstract
PURPOSE
To determine whether (R)-α-lipoic acid (LA) protects cultured human fetal retinal pigment epithelial (hfRPE) cells against oxidative injury and identify the pathways that may mediate protection.
METHODS
Cultured hfRPE cells were pretreated with various concentrations of LA for 14 to 16 hours followed by treatment with a chemical oxidant, tert-butylhydroperoxide (t-BuOOH; 0.8 mM, 3 hours). Reactive oxygen species (ROS) production and cell viability were measured using H2DCF and MTT assays, respectively. RPE cells were evaluated with fluorescent dyes (SYTOX Orange and SYTO Green; Molecular Probes, Eugene, OR), which differentiate between live and dead cells. Apoptosis was visualized by using the TUNEL assay. Changes in mitochondrial membrane potential were detected by JC-1 dye. Intracellular levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured by HPLC. Regulation of γ-glutamylcysteine ligase (GCL), the rate-controlling enzyme of GSH production, was assayed by RT-PCR.
RESULTS
Pretreatment of hfRPE cells with LA, 0.2 mM and 0.5 mM, significantly reduced the levels of t-BuOOH-induced intra-cellular ROS, by 23% and 49%, respectively. LA (0.5 mM) prevented oxidant-induced cell death and apoptosis and also increased the viability of oxidant-treated hfRPE cells from 38% to 90% of control. LA upregulated the mRNA expression of GCL, and was protective against t-BuOOH-induced decreases in both mitochondrial membrane potential and intracellular levels of GSH and GSH/GSSG.
CONCLUSIONS
The present study suggests that the protective effect of LA involves multiple pathways and that LA could be effective against age-associated increase in oxidative stress and mitochondrial dysfunction in RPE cells.
Age-related macular degeneration (AMD) is the major cause of severe visual impairment for elderly individuals in developed countries.1–3 Although the underlying pathogenic mechanisms and risk factors are not well understood,1–9 epidemiologic studies suggest that environmental factors associated with oxidative stress—for example, cigarette smoking, sunlight exposure, and low dietary intake of antioxidants—are contributing factors in the development of AMD.4–7 Recent results have shown that antioxidants and zinc protect against the progression of the disease.8
There is good evidence to suggest that the retinal pigment epithelium (RPE), choriocapillaris, and distal retina are all involved in this process, but the pathology associated with each can be difficult to distinguish, given their close anatomic, physiologic, and metabolic relationships.10 The RPE undergoes oxidative stress because it is metabolically very active,11–13 because there are large oxygen fluxes across its boundaries,14 and because it experiences exposure to sunlight.7,15 A significant oxidative load is associated with the RPE phagocytosis of photoreceptor outer segments,12,13 which, compared to all other body tissues, possess the highest amount of docosahexanoic acid and other polyunsaturated fatty acids.16 Cellular membranes with high polyunsaturated fatty acids are particularly susceptible to radical-induced chain reactions of lipid peroxidation.17 Age-associated decreases in antioxidant defense mechanisms throughout the body can further increase the levels of RPE exposure to oxidants. For example, the levels of plasma glutathione (GSH), one of the major water-soluble antioxidants, decrease with age.6
Several lines of evidence suggest that mitochondria are an important cellular target of oxidative stress in RPE and may play a role in RPE aging.18 Exposure of RPE cells to blue light and oxidants promotes mitochondrial (mt)DNA damage, changes in mitochondrial morphology, and a decrease in mitochondrial membrane potential and respiration.19–21 Oxidant-induced mitochondrial dysfunction and death of RPE cells may contribute to the onset of AMD.15,22 Because the integrity and normal functioning of the RPE layer are critical for the preservation of vision,23 several mechanisms that protect against oxidant-induced RPE injury have been studied. These mechanisms can be divided into three major groups; (1) mitochondrial protective mechanisms,20,24 (2) direct antioxidant protection, 25,26 and (3) enhancement of GSH-dependent intracellular defenses.27–29
α-Lipoic acid is a potent intracellular antioxidant that can induce all three cellular protective mechanisms.30 The physiologically relevant (R) form of lipoic acid (LA) is a coenzyme in mitochondria and has been shown to reverse the age-associated decline in mitochondrial function.31–34 In addition, LA can act as an indirect antioxidant by inducing γ-glutamylcysteine ligase (GCL), the rate-controlling enzyme in GSH synthesis, and other phase II enzymes that are involved in detoxification of xenobiotic compounds.35 Previous studies have shown that the pretreatment of cultured hepatocytes with LA protected against the decrease in cell viability induced by oxidant tert-butylhydroperoxide (t-BuOOH).36 t-BuOOH is a relatively stable alkyl peroxide that readily penetrates cell membranes37,38 and is known to promote cellular oxidation, loss of GSH, mitochondrial damage, and cell death.28,39 Inside the cell, it can be metabolized by reduction to t-butanol by GSH peroxidase promoting the depletion of intracellular GSH.39 Because of the higher stability and presence of the hydrophobic butyl moiety which allows easier membrane penetration, t-BuOOH provides a convenient alternative to the natural oxidants hydrogen peroxide and lipid hydroperoxide.
Confluent monolayers of human fetal RPE (hfRPE) primary cell cultures mimic many aspects of the physiology of native fetal and adult RPE, 40–44 (Maminishkis A, et al. IOVS. 2005; 46:ARVO E-Abstract 3035). This in vitro cell culture system thus provides an appropriate and convenient model to analyze cellular and molecular mechanisms of disease and to screen putative protective agents. Given that mitochondria are the source and also target of reactive oxygen species (ROS), we hypothesize that the antioxidant mitochondrial protector LA might prevent or repair oxidant-induced mitochondrial decay and oxidative damage or degeneration in RPE. In the present study, we used confluent monolayers of hfRPE to examine the effects of LA on t-BuOOH-induced RPE mitochondrial dysfunction and oxidative damage.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). (R)-α-lipoic acid (Tris salt) was a gift from Klaus Wessel, Viatris, Germany.
hfRPE Cell Preparation and Culture
The research adhered to the tenets of the Declaration of Helsinki. Research protocols were approved by the University of California Committee for the Protection of Human Subjects. Fetal eyes were obtained by an independent procurer (Advanced Bioscience Resources, Alameda, CA). Human fetal eyes of nominal gestation of 15 to 17 weeks were dissected within several hours after enucleation. The anterior of the eye and the vitreous were removed in Hanks’ buffered salt solution and the eyecups were incubated with 2 U/mL dispase (Invitrogen-Life Technology, Rockville, MD) at 37°C for 30 minutes in RPE culture medium (Eagle’s minimum essential medium, α modification [α-MEM]), supplemented with 5% heat-inactivated fetal bovine serum (Atlanta Biological, Norcross, GA), 20 µg/L hydrocortisone, 13 µg/L tridothyron (3,3′,5-triiodo-l-thyronine, sodium salt; Sigma-Aldrich), 250 mg/L taurine, N1 medium supplement (×100; no. N6530), MEM nonessential amino acid solution (×100; no. M7145), and l-glutamine-penicillin-streptomycin (×100; no. G1146), all from Sigma-Aldrich.
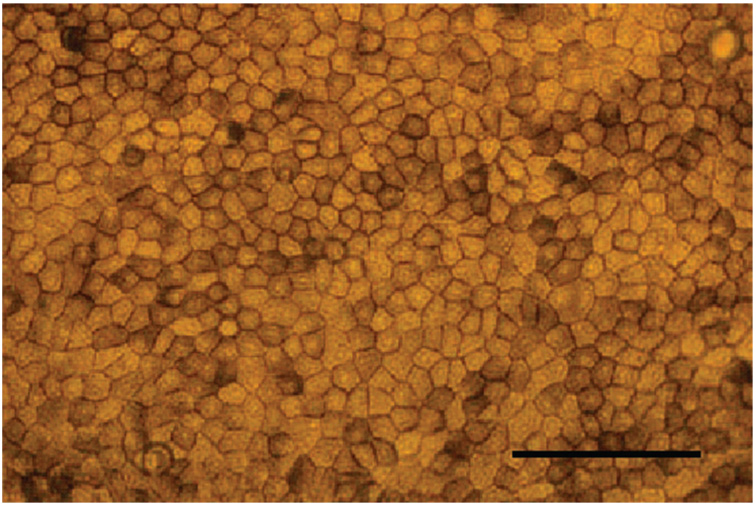
After the incubation, the dispase was discarded, and the posterior portion of the eye was cut in half through the optic nerve. The neutral retina was peeled away, and the sheets of RPE cells were carefully dissected off the choroid with fine forceps. The collected RPE cells were separated by repeated trituration in RPE cell culture medium, pelleted by centrifugation, and resuspended in RPE cell culture medium. All RPE cells isolated from a single fetal eye were plated in 5 mL of RPE cell culture medium on a 25-cm2 tissue culture flask (Corning-Costar, Corning, NY). After 3 to 5 weeks of growth the cells were dissociated with 0.25% trypsin (Invitrogen-Life Technologies) and passaged at a density of 1:9. The passaged cells were grown 4 to 5 weeks, trypsinized, and plated on 1-cm diameter cell migration filters (pore size 0.4 µm; Transwell; Corning-Costar) coated with 10 µg human extracellular matrix (ECM; BD Biosciences, Franklin Lakes, NJ). Each insert contained 0.5 mL of RPE cell culture medium and was seeded with 2 × 105 cells. One milliliter of RPE cell culture medium was added to the bottom of the cell migration assay plate. RPE cells of the second to fourth passages grown on ECM-coated filters were used in the studies. All studies were performed with confluent RPE cells that showed appropriate epithelial morphology (Fig. 1). These cells have a physiology that is closely comparable to that of native human fetal and adult mammalian RPE cells40–44 (Maminishkis A, et al. IOVS. 2005;46: ARVO E-Abstract 3035; and Miller S, unpublished observations, 2005).
FIGURE 1.
Bright-field photograph of confluent hfRPE cells with typical epithelial morphology grown on cell-migration filters for 6 weeks. Scale bar, 100 µm.
LA and t-BuOOH Treatments
RPE cells were treated with different concentrations of LA dissolved in cell culture medium for 14 to 16 hours at 37°C. After one washing with α-MEM, RPE cells were exposed to 0.8 mM of t-BuOOH (except for dose-dependent experiments with different t-BuOOH concentrations) in α-MEM for 3 hours at 37°C.
MTT Assay
The MTT reduction assay was used as a qualitative index of cell viability. Mitochondrial and cytosolic dehydrogenases of living cells reduce the yellow tetrazolium salt (MTT) to produce a purple formazan dye that can be detected spectrophotometrically.45 RPE cells were treated with different concentrations of LA and t-BuOOH, rinsed once, and incubated with serum-free medium containing 0.4 mg/mL MTT.
After 2 to 2.5 hours, MTT solution was aspirated, dimethylsulfoxide (0.3 mL/well) was added, and the plates were shaken for 10 minutes. The optical densities of the supernatant were read at 555 nm using a microplate spectrophotometer (Spectra Max 340; Molecular Devices, Sunnyvale, CA). Absorbances were normalized with respect to the untreated control cultures to calculate changes in cell viability.
JC-1 Staining
Mitochondrial membrane potential change (Δψ) was assessed in live RPE cells using the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine iodide (JC-1). JC-1 staining is a direct correlate of mitochondrial membrane potential, a fundamentally important determinant of cell function and health.46–48 In living cells, JC-1 exists as a green fluorescent monomer (490 nm excitation/530 nm emission) at depolarized mitochondrial membrane potentials. At normal and hyperpolarized Δψ, JC-1 is concentrated inside mitochondria and forms J aggregates, which shift the emission from 530 to 590 nm. Cell mitochondria demonstrate increasingly higher red fluorescence (590 nm) of J aggregates with increasingly negative mitochondrial membrane potential.46–48 For RPE tissue staining, JC-1 was dissolved in 37°C cell culture medium at a concentration of 10 µM, with concomitant vortexing. The JC-1 solution was immediately centrifuged at 7200g for 5 minutes, and the supernatant was used for staining within 10 to 20 minutes. After treatment with different concentrations of LA and t-BuOOH the apical surface of RPE cells on filters was exposed to JC-1 solution for 30 minutes at 37°C.
For quantitative fluorescence measurements RPE cells were kept in the cell-migration plates after JC-1 staining, rinsed once, and scanned with a multilabel counter (Wallac 1420; PerkinElmer Life Sciences, Wellesley, MA) at 485 nm excitation and 535 nm and 590 nm emission, to measure green and red JC-1 fluorescence, respectively. Each filter was scanned at 25 areas rectangularly arranged in a 5 × 5 pattern with 1-mm intervals and an approximate beam area of 1 mm2 (bottom scanning). The average fluorescence intensities for each well and red to green fluorescence ratios were calculated (Excel 5.0; Microsoft, Redmond, WA).
For microscopic observations of JC-1 staining RPE, the filters were excised, rinsed once, and mounted in RPE cell culture medium in Sykes-Moore chambers (Bellco Glass, Vineland, NJ). Images were collected with FITC and TRITC fluorescence filter cubes on a microscope (Axiovert25; Carl Zeiss Meditec, Inc., Thornwood, NY) equipped with a charge-coupled device (CCD) digital camera (Diagnostic Instruments, Sterling Heights, MI), and processed with image-management software (Photoshop ver. 7.0; Adobe Systems, Mountain View, CA).
Determination of ROS
The intracellular level of ROS is an important biomarker for oxidative stress and increased ROS level generally indicates increased oxidative stress. Production of ROS was estimated with the fluorescent dye 2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes, Eugene, OR), a nonpolar compound that is converted by cellular esterases to polar and membrane impermeable derivative H2DCF. H2DCF is nonfluorescent but in the presence of intracellular ROS is oxidized to highly fluorescent 2′,7′-dichlorofluorescein (DCF). After treatment with different concentrations of LA and t-BuOOH, cells were washed once with α-MEM and loaded with 20 µM H2DCFDA. After cells were incubated at 37°C for 30 minutes the dye was removed, and cells were washed once with α-MEM and scanned with a plate reader (Wallac; PerkinElmer) at 485-nm excitation and 535-nm emission. The pattern of scanning was the same as described in JC-1 staining.
Fluorescent Staining
One consequence of oxidative damage to cells is cell death. To demonstrate the toxic effects of oxidant insult and the protective effects of LA in RPE cells we used double labeling with fluorescent dyes (SYTO Green and SYTOX Orange; Molecular Probes, Eugene, OR), to help distinguish live from dead cells. SYTOX Orange (Molecular Probes, Inc.) is a high-affinity nucleic acid stain that readily penetrates cells that have compromised plasma membranes, but it does not cross the intact membrane of live cells. SYTO Green (Molecular Probes, Inc.) is a cell-permeant nucleic acid stain that preferentially labels live cell nuclei. The RPE cells were incubated with 5 µM SYTOX Orange and 5 µM SYTO Green for 20 minutes at 37°C after treatment with different concentrations of LA and t-BuOOH. Live and dead cells were visualized with FITC and TRITC fluorescence filter cubes, respectively (Axiovert 25; Carl Zeiss Meditec, Inc.), equipped with a CCD digital camera (Diagnostic Instruments), and processed with image-management software (Photoshop 7.0; Adobe Systems).
Apoptosis Assay
DNA cleavage, which commonly occurs in apoptosis, was used to help specify the mechanism of cell death. Cleavage was measured by TdT-mediated dUTP nick-end labeling (TUNEL), with a cell-death detection kit (In Situ Cell Death Detection Kit, Fluorescein; Roche Diagnostics, Indianapolis, IN). Propidium iodide (1 µg/mL) was used for RPE nuclei counterstaining.
GSH and GSH/GSSG Measurements
GSH, the most important endogenous antioxidant, is oxidized to GSSG when reacting with ROS. Higher levels of GSH or the ratio of GSH/GSSG provide a stronger antioxidant defense system. GSH and GSSG concentrations were determined according to the methods of Fariss and Reed.49 RPE cells (1 × 106) were acidified in 10% (wt/vol 0.25 mL) perchloric acid containing 5 mM EDTA and centrifuged to precipitate the proteins. The supernatant (150 µL) containing internal standard (γ-glutamyl-glutamate; 100 µM final) was mixed with 50 µL of iodoacetic acid (100 mM dissolved in 0.2 mM m-cresol purple) and the pH adjusted to 10 by using KOH-KHCO3 buffer (2 M KOH:2.4 M KHCO3). Samples were placed in the dark at room temperature for 1 hour. After completion of S-carboxymethyl derivatization of free thiols, an equal volume of 1-fluoro-2,4-dinitrobenzene (1% vol/vol in absolute ethanol) was added and incubated overnight in the dark at room temperature. Samples (100 µL) were separated by HPLC and detected (Waters Alliance Systems, Milford, MA) with the absorbance set at 365 nm. Quantitation was obtained by integration relative to the internal standard.
GLC mRNA Expression
RNA Extraction
Total RNA from RPE cell cultures was extracted (RNeasy Mini Kit; Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions.
cDNA Synthesis
A kit (Advantage RT-for-PCR; BD Biosciences) was used for cDNA synthesis. The reactions were performed in a 20-µL volume, with 200 U MMLV reverse transcriptase, 1 µM oligo-(dT) primer, 0.5 mM dNTPs, 20 U recombinant RNase inhibitor, and 1 µg total RNA. The reaction was incubated at 42°C for 1 hour for reverse transcription of RNA, followed by 94°C for 5 minutes, to terminate the reaction.
Quantitative Real-Time Polymerase Chain Reaction
Gene expression assay mixes (TaqMan; for the modulatory subunit of γ-glutamyl-cysteine ligase [GCLM], the catalytic subunit of γ-glutamylcysteine ligase [GCLC], and β-actin) were purchased from Applied Biosystems, Inc. (ABI; Foster City, CA). RT-PCR amplification was performed on a sequence detection system (7900HT Prism; ABI) in a total volume of 18 µL containing 7 mL diluted cDNA (1:5 dilution in RNase-free water), 10 µL universal PCR master mix, and 1 µL gene expression assay mix (both TaqMan; ABI). The following thermal cycling conditions were used for PCR amplification: (1) one cycle, 50°C for 2 minutes; (2) one cycle, 95°C for 10 minutes; and (3) 40 cycles, 95°C for 15 seconds and 60°C for 1 minute.
Data Analysis
The manufacturer’s software (SDS ver. 2.1 ABI) was used for data collection. Quantitative analysis of GCLM and GCLC mRNA was performed by using the 2−ΔΔCt method according to the software instructions with normalization to β-actin mRNA.
Statistical Analysis
Statistical significance was calculated (Prism, ver. 4.0a; GraphPad, San Diego, CA) according to the unpaired two-tailed Student’s t-test. P <0.05 was considered significant.
RESULTS
Intracellular ROS levels
The dose–response relationship for t-BuOOH in the absence and presence of LA is shown in Figure 2 and Figure 3. The data summarized in Figure 2 show that treatment of RPE cells with t-BuOOH concentrations of 0.6 and 0.8 mM significantly increased the levels of intracellular ROS: 1.6-fold (P < 0.006, n = 6) and 2.6-fold (P < 0.001, n = 6), respectively. In another series of experiments (Fig. 3), RPE cells were incubated for 14 to 16 hours, with different concentrations of LA before t-BuOOH challenge. Pretreatments with 0.2 and 0.5 mM LA significantly reduced the level of ROS in RPE cells relative to t-BuOOH treatment by 23% (P < 0.04, n = 6) and 49% (P < 0.001, n = 6), respectively (Fig. 3A). There was no significant difference in the levels of intracellular ROS production between the control and 0.5 mM LA-treated RPE cells (P = 0.4, n = 4; Fig. 3B). These experiments suggest that the protective effects of LA are mediated, at least, in part by a direct antioxidant mechanism of scavenging ROS.
FIGURE 2.
Effect of different concentrations of t-BuOOH (3-hour treatment) on the levels of intracellular ROS. Changes in ROS levels were measured with the oxidant-sensitive dye H2DCFDA. Data are expressed as the mean ± SD of three separate experiments, each experiment performed in duplicate. **P < 0.01, ***P < 0.001 vs. control.
FIGURE 3.
t-BuOOH-induced changes in intracellular ROS levels and the protective effects of LA. (A) Pretreatment of RPE cells with different concentrations of LA. Data are expressed as mean ± SD of results in three separate experiments, each experiment performed in duplicate. (B) Treatment of RPE cells with 0.5 mM LA alone. Data are expressed as the mean ± SD of two separate experiments, each experiment performed in duplicate. ***P < 0.001 vs. control; #P < 0.05, ###P < 0.001 vs. t-BuOOH.
Cell Viability
Treatment of RPE cells with t-BuOOH at 0.8 mM for 3 hours caused approximately 62% loss of cell viability (P < 0.001, n = 6), as measured using a MTT colorimetric assay (Fig. 4). Pretreatment of RPE cells with LA resulted in a dose-dependent protection against t-BuOOH-induced toxicity. Viability of t-BuOOH-treated cells was restored to 70% of the control (P < 0.04, n = 6, versus t-BuOOH treatment) after pretreatment with 0.2 mM LA, and to 90% of the control (P < 0.001, n = 6, versus t-BuOOH treatment) after pretreatment with 0.5 mM LA. There was no statistically significant difference (n = 3) in viability between the control cells and cells that were pretreated with 0.5 mM LA, per se (results not shown).
FIGURE 4.
LA protection against t-BuOOH-induced decrease in RPE cell viability, as measured by the MTT assay. Data are expressed as mean ± SD of three separate experiments, each experiment performed in duplicate. ***P < 0.001 vs. control; #P < 0.05, ###P < 0.001 vs. t-BuOOH.
Cell Death
With SYTO Green and SYTOX Orange staining of live and dead cells, control RPE cells (Fig. 5A) showed only green nuclear staining characteristic of live cells, whereas RPE cells treated with 0.8 mM t-BuOOH (3 hours) demonstrated substantial red nuclear staining characteristic of dead cells (Fig. 5B). RPE cells pretreated with 0.5 mM LA (Fig. 5C) demonstrated only green staining of live cells, indicating that LA protected RPE cells against the t-BuOOH-induced RPE cell death. More specifically, the TUNEL assay was used to detect apoptosis. As shown in Fig. 6, t-BuOOH induced an apparent increase in the number of apoptotic cells, and the pretreatment with LA nearly completely protected the RPE cells from t-BuOOH-induced apoptosis. This result was obtained in three separate experiments (each in duplicate) on cells from three different donors.
FIGURE 5.
Representative images of SYTO Green (undamaged plasma membrane)–and SYTOX Orange (damaged plasma membrane)–labeled RPE nuclei. (A) Control RPE cells; (B) RPE cells treated with t-BuOOH; and (C) RPE cells treated as in (B) but pretreated with 0.5 mM LA. Each panel is representative of three separate experiments, each performed in duplicate, and involving RPE cell cultures from three different donors.
FIGURE 6.
Representative images of TUNEL staining of RPE nuclei. Fluorescein green signal indicates dUTP incorporation in apoptotic RPE nuclei (all RPE nuclei are counterstained with propidium iodide; red). (A) Control RPE cells; (B) RPE cells treated with t-BuOOH (C) RPE cells treated as in (B), but pretreated with 0.5 mM LA. Each micrograph is representative of RPE cultures from three different donors, with each experiment performed in duplicate.
Effects of t-BuOOH and LA on Mitochondrial Membrane Potential
We used both fluorescence microscopy and quantitative fluorescence measurements to evaluate the oxidant-induced decline in RPE mitochondrial membrane potential. Figure 7 shows a representative experiment, and Figure 8 summarizes the quantitative data. t-BuOOH treatment resulted in a strong decrease in red J-aggregate fluorescence, reflecting an oxidant-induced decrease in mitochondrial potential (Fig. 7B). Pretreatment with 0.5 mM LA prevented the oxidant-induced decrease in RPE mitochondrial membrane potential as demonstrated by red J-aggregate staining of LA-pretreated cells (Fig. 7C) similar to the control (Fig. 7A). t-BuOOH treatment decreased the 590:530 fluorescence ratio of JC-1 dye by 61% (P < 0.001, n = 5) and pretreatment of RPE cells with 0.2 and 0.5 mM LA resulted in 2-fold (P < 0.04, n = 5) and 2.9-fold (P < 0.001, n = 5) increases, respectively, in the red-green JC-1 fluorescence ratio relative to t-BuOOH-treated cells (Fig. 8). These results demonstrate that LA pretreatment protected RPE cells against the t-BuOOH-induced loss of mitochondrial membrane potential.
FIGURE 7.
Representative images of JC-1-labeled mitochondria. Loss of aggregate staining indicates a decrease in mitochondrial membrane potential. (A) Control RPE cells, (B) RPE cells treated with t-BuOOH, and (C) RPE cells treated as in (B) but pretreated with 0.5mMLA. Each panel is representative of three separate experiments, performed in duplicate and involving RPE cell cultures from three different donors.
FIGURE 8.
LA protection against oxidant-induced decrease in RPE mitochondrial potential, measured by quantitative analysis of JC-1 fluorescence. Data are expressed as the mean ± SD of two experiments involving RPE cells from two different donor eyes. The first experiment was performed in triplicate and the second in duplicate. ***P < 0.001 vs. control; #P < 0.05, ###P < 0.001 vs. t-BuOOH.
LA and t-BuOOH-Mediated Changes in GSH and GSH/GSSG Ratio
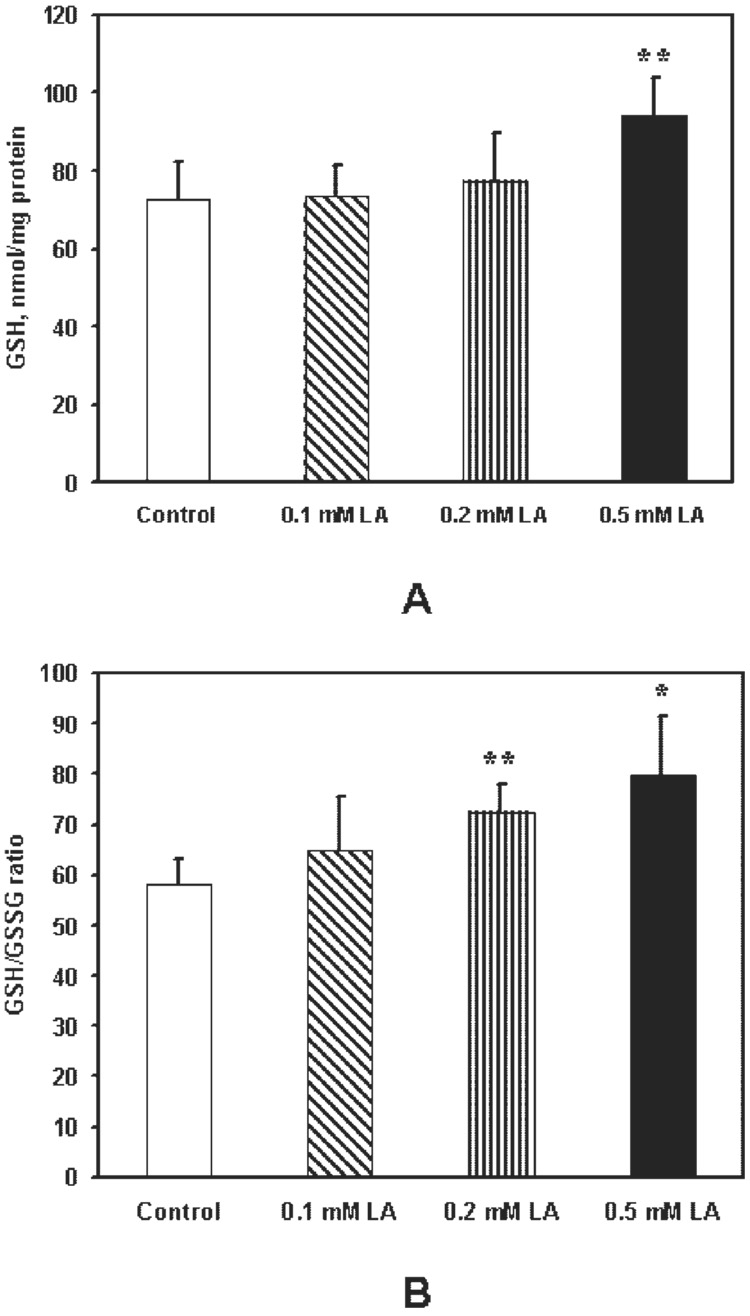
LA can increase the endogenous antioxidant GSH.35,50,51 We therefore measured GSH and its oxidized form GSSG. The GSH/GSSG ratio serves as an indicator of the intracellular balance between pro- and antioxidants.52 Figure 9A shows that GSH levels were increased by 30% (P < 0.003, n = 6) after treatment with 0.5 mM LA. In addition, LA, 0.2 mM and 0.5 mM, increased the GSH/GSSG ratio by 24% (P < 0.01, n = 4) and 37% (P < 0.02, n = 4), respectively (Fig. 9B).
FIGURE 9.
LA-induced increase in intracellular GSH and GSH/GSSG ratio measured by HPLC. (A) Dose-dependent response of GSH. Control and 0.5-mM LA treatments represent results of three independent experiments; 0.1- and 0.2-mM LA treatments represent results from two independent experiments, each performed in duplicate. (B) Dose-dependent response of the GSH/GSSG ratio. Data are the mean ± SD of two independent experiments, each performed in duplicate *P < 0.05, **P < 0.01 vs. control.
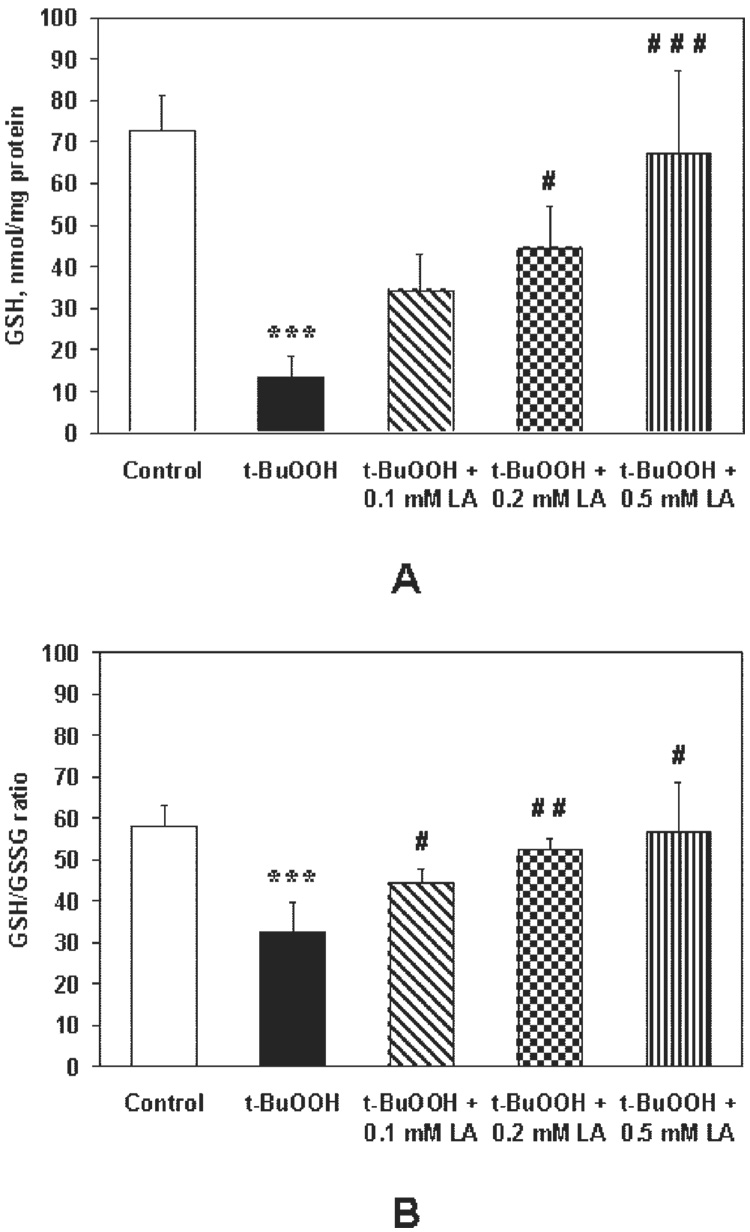
Treatment with t-BuOOH decreased intracellular GSH levels by 81% (P < 0.001, n = 6) and pretreatment with LA resulted in a dose-dependent increase of GSH levels, relative to t-BuOOH treatment, by 2.5-fold (P < 0.01, n = 4), 3.3-fold (P < 0.002, n = 4), and 4.9-fold (P < 0.001, n = 6; Fig. 10A). Similarly, t-BuOOH decreased the GSH/GSSG ratio by 44% (P < 0.002, n = 4), and pretreatment with LA resulted in 1.4-fold (P < 0.03), 1.6-fold (P < 0.003, n = 4), and 1.7-fold (P < 0.02, n = 4) increases in the GSH/GSSG ratio, relative to t-BuOOH treatment (Fig. 10B). Taken together, these results show that LA protected RPE cells against oxidant-induced decreases in GSH levels and a more oxidized cellular redox state.
FIGURE 10.
(A) LA pretreatment reversed the t-BuOOH-induced decrease in intracellular GSH measured by HPLC. Control, t-BuOOH and t-BuOOH0.5 mM LA pretreatments represent data from three independent experiments, the others from two independent experiments, each performed in duplicate. (B) LA protection against an oxidant-induced decrease in the GSH/GSSG ratio measured by HPLC. Measurements from two independent experiments, each performed in duplicate. ***P < 0.01 vs. control and #P < 0.05; ##P < 0.01; ###P < 0.001 vs. t-BuOOH.
GCL mRNA Expression
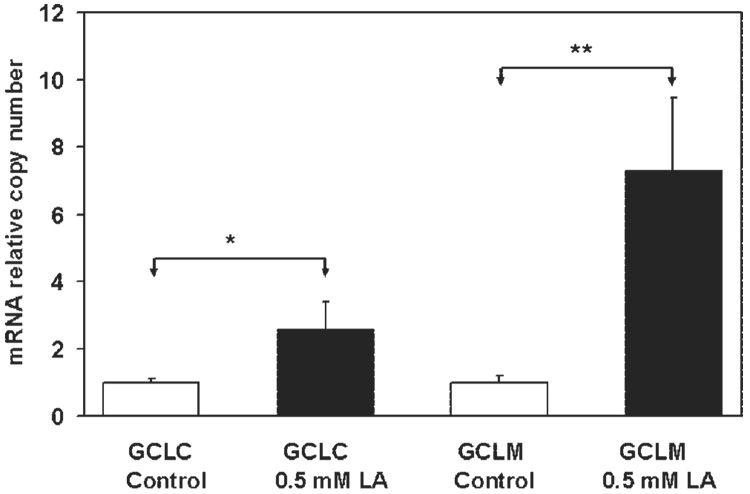
Glutathione is synthesized by GCL and glutathione synthase. GCL is the rate-limiting enzyme. As shown in Figure 11, LA treatment significantly upregulated mRNA expression of both GCLC (P < 0.03, n = 3) and GCLM (P < 0.01, n = 3) subunits, consistent with our observation that LA increased GSH levels.
FIGURE 11.
Quantification of LA-induced upregulation of GCL mRNA expression. RPE cells were incubated with 0.5 mM LA for 14 to 16 hours, and GCL mRNA expression was examined by quantitative RT-PCR. PCR amplifications were performed with a thermocycler. CGCL, catalytic GCL subunit; GCLM, modulatory GCL subunit. Data are the mean ± SD of results from three separate experiments, each performed in duplicate. *P < 0.05; **P < 0.01.
DISCUSSION
The causes and development of AMD are not yet understood, although multiple factors have been implicated.1,9 One such factor is oxidative stress.15,37,53 In RPE, mitochondria are particularly prone to oxidative damage,20 and mitochondrial dysfunction resulting from oxidant injury may be important in the development of RPE aging and AMD.18,37
A significant number of in vitro studies have used RPE-derived cells and cell lines at subconfluent densities to study a variety of RPE functions.20,21,25,28,53 In the present study, a model of fetal human RPE cells in confluent culture was used. Preliminary data (Csaky K, personal communication, 2005) indicate that our fetal primary cultures have increased resistance to oxidant-induced injury compared with ARPE-19 cells. This suggests that primary cultures of hfRPE may have more active or extensive protective mechanisms compared with transformed cell lines, a possibility that remains to be more fully explored. However, in terms of cell physiological properties, this model closely mimics native human fetal and adult mammalian RPE tissues. For example, these cultures generate a significant transepithelial potential (mean TEP ≈ 2.6 mV) and transepithelial resistance (mean TER, ≈500 Ω · cm2). These confluent monolayers also exhibit apical and basolateral membrane and fluid transport properties similar to native human fetal and adult mammalian RPE cells40–44 (Maminishkis A, IOVS 2005;46:ARVO E-Abstract 3035).
In contrast, the TER of ARPE-19 cell line monolayers only reached a maximum of 50 to 100 Ω · cm2 after several weeks in culture54 and the TEP apparently has not been measured. In addition, the ARPE-19 cell line was established from a population of cells with strong growth potential. Several studies have indicated that the mitochondrial bioenergetics of different cells (e.g., the balance between glycolytic and mitochondrial ATP production) is strongly influenced by the processes of proliferation and differentiation.55,56
We investigated whether treatment of cultured hfRPE cells with LA, a disulfide compound found naturally in plants and animals, can protect against t-BuOOH-induced oxidative injury and cell death. LA may protect mitochondrial function because it is a cofactor for the mitochondrial enzymes, pyruvate, and α-ketoglutarate dehydrogenases, and thus could improve mitochondrial metabolism.57 We have shown previously that LA fed to old rats significantly restores mitochondrial oxygen consumption, membrane potential, and cellular ascorbate level, but not when fed to young rats.31,32,58 (R)-α-lipoic acid, as opposed to the (S)-form, has been shown to decrease the susceptibility of hepatocytes to t-BuOOH-induced oxidative stress and cell death in vitro.36
In our experiments, pretreatment of RPE cells with LA showed an effective protection against the t-BuOOH-induced decrease of mitochondrial membrane potential. Mitochondria are one of the primary targets of oxidant-induced RPE injury18 and may have a central role in RPE cell survival.37,59 A recent clinical study suggested that a combination of mitochondrial protective compounds may improve visual function in patients with early AMD,60 but the improvements did not reach statistical significance, and no large-scale, randomized, controlled clinical study investigating the effect of LA on the prevention or treatment of AMD has been reported.
Studies in several different systems have demonstrated significant antioxidant properties of LA.30,50,61 The mitochondrial E3 enzyme, dihydrolipoyl dehydrogenase, reduces lipoate to dihydrolipoate at the expense of NADH. Dihydrolipoic acid reacts directly with various oxidants and regenerates different antioxidants, such as vitamin C, glutathione, and α-tocopherol. 62–64 LA has an unusual property of being a free radical scavenger, not only in reduced, but also in oxidized form. Its oxidized form has been shown to quench hydroxyl radicals, singlet oxygen, and hydrogen peroxide.65 In our experiments, pretreatment of RPE cells with LA resulted in significantly lower levels of t-BuOOH-stimulated intracellular ROS production, consistent with its ability to decrease oxidative damage and subsequent RPE cell death.
The protective effects of LA against oxidant-induced damage may be associated with its metal chelating activity.66 In cultured lens epithelial cells, LA decreases iron uptake from transferrin-bound iron, increases iron deposition into ferritin, increases concentration of ferritin, and results in diminished size of the cytosolic highly reactive labile iron pool.67 A recent study reports a significant increase in the total iron level in AMD-affected maculas compared to healthy maculas.68 Oxidative cell death in the presence of iron involves Fenton-type reactions and peroxidative damage to lysosomal membranes and could be prevented by LA and lipoamide which prevent lysosomal rupture.69,70
Cellular GSH has been shown to be a critical factor in protecting RPE cells against oxidative stress.27,28,71 For example, glutathione S-transferase, one of several detoxification enzymes, protects RPE cells from oxidative damage and may play an important role in the development of age-related macular degeneration.72 LA has been suggested to recycle the GSSG and elevate the reduced form of antioxidant GSH.64 In vitro studies in other systems have demonstrated that LA can induce cysteine uptake and thus increase the concentration of this limiting component in GSH synthesis73 and can increase the expression levels of GCL, the rate-controlling enzyme in GSH synthesis.35
In our studies, LA increased GSH level in RPE cells (Fig. 9A) and reversed oxidant-induced decline in intracellular GSH levels (Fig. 10A). Treatment with LA also increased the GSH/GSSG ratio (Fig. 9B) and prevented the oxidant-promoted decrease of this indicator of the cellular redox state (Fig. 10B). These results are consistent with previous studies in other cell types,35,51,73 demonstrating that LA can increase levels of GSH-associated protective mechanisms. GSH is synthesized in two consecutive steps by two cytosolic enzymes: GCL and glutathione synthase. GCL appears to be the rate-limiting enzyme in the production of GSH.74 In rat liver,35 LA has been shown to increase GCL activity. The data summarized in Fig. 11 show that LA upregulates the mRNA expression of GCL, complementing the results of GSH measurement. Therefore, the protective effects of LA are at least partially attributable to the induction of GSH-related mechanisms.
In this study we found that concentrations of LA greater than 200 µM are necessary for protection. This requirement raises the question of whether such a high concentration may be difficult to achieve in vivo. Although the doses of LA needed to protect RPE cells from the oxidant insults are rather high, the treatment period is relatively short (14 –16 hours), given the high concentrations of t-BuOOH (0.8 mM for 3 hours) required for these acute experiments. In preliminary experiments (not shown), chronic treatment with t-BuOOH allowed for LA rescue at lower LA concentrations (1–10 µM). Additional work, planned and in progress, will help resolve this question.
The present results show that LA protected hfRPE cells against t-BuOOH-induced oxidative stress, as measured by cell viability, mitochondrial function, apoptosis, GSH, and the GSH/GSSG ratio. LA-mediated protection can be conferred by one or more of the following mechanisms: (1) direct scavenging of ROS, (2) chelating transition metals to inhibit ROS generation, (3) activation of antioxidant defenses via phase 2 enzymes, and (4) acting as a cofactor to protect, repair, and stimulate mitochondrial enzymes.57 LA may be an attractive candidate in preventing the slow visual impairment caused by AMD, because its antioxidant effects could be therapeutically effective on multiple mechanisms/pathways in the cytosol and mitochondria and in all the cell types in and around the macula. Indeed, the antioxidant effects of LA have been studied in clinical trials, including cataracts, glaucoma, neuropathy of type2 diabetes, cardiovascular disease, Wilson’s disease, HIV/AIDS and depression,51 and can be easily tested in animal models of AMD as well.
Acknowledgments
The authors thank Arvydas Maminishkis for developing the hfRPE cell culture preparation, Heather Elliot and Aria Ashraghi for expert technical help with the RT-PCR experiments, David Killilea for helpful advice and comments, and Teresa Klask for editorial assistance.
Supported by National Institute of Environmental Health Sciences Grant P30-ES01896; National Eye Institute Grants EY02205 and EY03176; Grant K05-AT001323 from the National Center of Complementary and Alternative Medicine, and Grant SS-0422-99 from the Ellison Medical Foundation.
Footnotes
Disclosure: L.A. Voloboueva, None; J. Liu, None; J.H. Suh, None; B.N. Ames, None; S.S. Miller, None
References
- 1.Bird AC. The Bowman lecture. Towards an understanding of age-related macular disease. Eye. 2003;17:457–466. doi: 10.1038/sj.eye.6700562. [DOI] [PubMed] [Google Scholar]
- 2.Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology. 2003;49:1–11. doi: 10.1159/000066507. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins BS, Bird A, Klein R, West SK. Epidemiology of age-related macular degeneration. Mol Vis. 1999;5:26. [PubMed] [Google Scholar]
- 4.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 5.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 6.Samiec PS, Drews-Botsch C, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshanks KJ, Klein R, Klein BE, Nondahl DM. Sunlight and the 5-year incidence of early age-related maculopathy: the beaver dam eye study. Arch Ophthalmol. 2001;119:246–250. [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 10.Marmor MW, Wolfensberger TJ. The Retinal Pigment Epithelium: Function and Disease. New York: Oxford University Press; 1998. [Google Scholar]
- 11.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62 suppl 6:1448S–161S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 12.Miceli MV, Liles MR, Newsome DA. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp Cell Res. 1994;214:242–249. doi: 10.1006/excr.1994.1254. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye. 1995;9:763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- 14.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 15.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 16.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- 18.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 19.Pautler EL, Morita M, Beezley D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem Photobiol. 1990;51:599–605. doi: 10.1111/j.1751-1097.1990.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 20.Godley BF, Jin GF, Guo YS, Hurst JS. Bcl-2 overexpression increases survival in human retinal pigment epithelial cells exposed to HO. Exp Eye Res. 2002;74:663–669. doi: 10.1006/exer.2001.1146. [DOI] [PubMed] [Google Scholar]
- 21.Cai J, Wu M, Nelson KC, Sternberg P, Jr, Jones DP. Oxidant-induced apoptosis in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:959–966. [PubMed] [Google Scholar]
- 22.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1988;1366:139–149. doi: 10.1016/s0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 23.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 24.King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem Photobiol. 2004;79:470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 25.Sparrow JR, Vollmer-Snarr HR, Zhou J, et al. A2E-epoxides damage DNA in retinal pigment epithelial cells: vitamin E and other anti-oxidants inhibit A2E-epoxide formation. J Biol Chem. 2003;278:18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 26.Castillo M, Bellot JL, Garcia-Cabanes C, Miquel J, Orts A, Palmero M. Effects of hypoxia on retinal pigmented epithelium cells: protection by antioxidants. Ophthalmic Res. 2002;34:338–342. doi: 10.1159/000067050. [DOI] [PubMed] [Google Scholar]
- 27.Nelson KC, Carlson JL, Newman ML, et al. Effect of dietary inducer dimethylfumarate on glutathione in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:1927–1935. [PubMed] [Google Scholar]
- 28.Nelson KC, Armstrong JS, Moriarty S, et al. Protection of retinal pigment epithelial cells from oxidative damage by oltipraz, a cancer chemopreventive agent. Invest Ophthalmol Vis Sci. 2002;43:3550–3554. [PubMed] [Google Scholar]
- 29.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci USA. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs J, Packer L, Zimmer G. Lipoic Acid in Health and Disease. New York: Marcel Dekker; 1997. [Google Scholar]
- 31.Hagen TM, Yowe DL, Bartholomew JC, et al. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagen TM, Ingersoll RT, Lykkesfeldt J, et al. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- 33.Hagen TM, Liu J, Lykkesfeldt J, et al. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci USA. 2002;99:1870–1875. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci USA. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. doi: 10.1073/pnas.0400282101. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagen TM, Vinarsky V, Wehr CM, Ames BN. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Anti-oxid Redox Signal. 2000;2:473–483. doi: 10.1089/15230860050192251. [DOI] [PubMed] [Google Scholar]
- 37.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 38.Moore GA, Jewell SA, Bellomo G, Orrenius S. On the relationship between Ca2+ efflux and membrane damage during t-butylhy-droperoxide metabolism by liver mitochondria. FEBS Lett. 1983;153:289–292. doi: 10.1016/0014-5793(83)80626-7. [DOI] [PubMed] [Google Scholar]
- 39.Comporti M. Glutathione depleting agents and lipid peroxidation. Chem Phys Lipids. 1987;45:143–169. doi: 10.1016/0009-3084(87)90064-8. [DOI] [PubMed] [Google Scholar]
- 40.Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3513–3527. [PubMed] [Google Scholar]
- 41.Quinn RH, Quong JN, Miller SS. Adrenergic receptor activated ion transport in human fetal retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:255–264. [PubMed] [Google Scholar]
- 42.Lin H, Kenyon E, Miller SS. Na-dependent pHi regulatory mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3528–3538. [PubMed] [Google Scholar]
- 43.Maminishkis A, Jalickee S, Blaug SA, et al. The P2Y receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci. 2002;43:3555–3566. [PubMed] [Google Scholar]
- 44.Hughes B, Gallemore R, Miller S. Transport mechanisms in the retinal pigment epithelium. In: Marmor M, Wolfensberger T, editors. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. pp. 103–134. [Google Scholar]
- 45.Bernas T, Dobrucki J. Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry. 2002;47:236–242. doi: 10.1002/cyto.10080. [DOI] [PubMed] [Google Scholar]
- 46.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 47.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6 or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 48.Nuydens R, Novalbos J, Dispersyn G, Weber C, Borgers M, Geerts H. A rapid method for the evaluation of compounds with mitochondria- protective properties. J Neurosci Methods. 1999;92:153–159. doi: 10.1016/s0165-0270(99)00107-7. [DOI] [PubMed] [Google Scholar]
- 49.Fariss MW, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- 50.Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann NY Acad Sci. 2002;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 52.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 53.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 54.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 55.Brand K. Aerobic glycolysis by proliferating cells: protection against oxidative stress at the expense of energy yield. J Bioenerg Biomembr. 1997;29:355–364. doi: 10.1023/a:1022498714522. [DOI] [PubMed] [Google Scholar]
- 56.Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol. 2000;279:C1220–C1229. doi: 10.1152/ajpcell.2000.279.4.C1220. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Ames B. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease and Parkinson’s disease. Nutr Neurosci. doi: 10.1080/10284150500047161. In press. [DOI] [PubMed] [Google Scholar]
- 58.Lykkesfeldt J, Hagen TM, Vinarsky V, Ames BN. Age-associated decline in ascorbic acid concentration, recycling, and biosynthesis in rat hepatocytes: reversal with (R)-alpha-lipoic acid supplementation. FASEB J. 1998;12:1183–1189. doi: 10.1096/fasebj.12.12.1183. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 60.Feher J, Papale A, Mannino G, Gualdi L, Balacco Gabrieli C. Mitotropic compounds for the treatment of age-related macular degeneration: the metabolic approach and a pilot study. Ophthalmologica. 2003;217:351–357. doi: 10.1159/000071351. [DOI] [PubMed] [Google Scholar]
- 61.Sen CK, Roy S, Khanna S, Packer L. Determination of oxidized and reduced lipoic acid using high-performance liquid chromatography and coulometric detection. Methods Enzymol. 1999;299:239–246. doi: 10.1016/s0076-6879(99)99023-7. [DOI] [PubMed] [Google Scholar]
- 62.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 63.Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 64.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 65.Biewenga GP, Haenen GR, Bast A. The pharmacology of the anti-oxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 66.Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol. 1995;50:123–126. doi: 10.1016/0006-2952(95)00116-h. [DOI] [PubMed] [Google Scholar]
- 67.Goralska M, Dackor R, Holley B, McGahan MC. Alpha lipoic acid changes iron uptake and storage in lens epithelial cells. Exp Eye Res. 2003;76:241–248. doi: 10.1016/s0014-4835(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 68.Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch Ophthalmol. 2003;121:1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- 69.Persson HL, Svensson AI, Brunk UT. Alpha-lipoic acid and alpha-lipoamide prevent oxidant-induced lysosomal rupture and apoptosis. Redox Rep. 2001;6:327–334. doi: 10.1179/135100001101536472. [DOI] [PubMed] [Google Scholar]
- 70.Persson HL, Yu Z, Tirosh O, Eaton JW, Brunk UT. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic Biol Med. 2003;34:1295–1305. doi: 10.1016/s0891-5849(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 71.Sternberg P, Jr, Davidson PC, Jones DP, Hagen TM, Reed RL, Drews-Botsch C. Protection of retinal pigment epithelium from oxidative injury by glutathione and precursors. Invest Ophthalmol Vis Sci. 1993;34:3661–3668. [PubMed] [Google Scholar]
- 72.Maeda A, Crabb JW, Palczewski K. Microsomal glutathione S-transferase 1 in the retinal pigment epithelium: protection against oxidative stress and a potential role in aging. Biochemistry. 2005;44:480–489. doi: 10.1021/bi048016f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han D, Handelman G, Marcocci L, et al. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 74.Lu SC. Regulation of glutathione synthesis. Curr Top Cell Regul. 2000;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]