Abstract
α-Lipoic acid (LA) has been widely studied as an agent for preventing and treating various diseases associated with oxidative disruption of mitochondrial functions. To investigate a related mitochondrial antioxidant, we compared the effects of lipoamide (LM), the neutral amide of LA, with LA for measures of oxidative damage and mitochondrial dysfunction in a human retinal pigment epithelial (RPE) cell line. Acrolein, a major component of cigarette smoke and a product of lipid peroxidation, was used to induce oxidative mitochondrial damage in RPE cells. Overall, using comparable concentrations, LM was more effective than LA at preventing acrolein-induced mitochondrial dysfunction and oxidative stress. Relative to LA, LM improved ATP levels, membrane potentials, and activities of mitochondrial complexes I, II, and V and dehydrogenases that had been decreased by acrolein exposure. LM reduced acrolein-induced oxidant generation, calcium levels, protein oxidation, and DNA damage to a greater degree than LA. And, total antioxidant capacity, glutathione content, glutathione S-transferase, and superoxide dismutase activities and expression of nuclear factor-E2-related factor 2 were increased by LM relative to LA. These results suggest that LM is a more potent mitochondrial-protective agent and antioxidant than LA in protecting RPE from oxidative damage.
Keywords: Mitochondrial complex, Protein oxidation, Oxidative DNA damage, Nuclear factor-E2-related factor 2, Glutathione, Free radicals
Smoking has been identified as a primary risk factor associated with the prevalence and the incidence of neovascular macular degeneration and geographic atrophy [1,2]. Increased exposure to numerous toxins, including aldehydes and NOx, markedly depletes both ascorbic acid levels and protein sulfhydryl concentrations and causes oxidation of lipids and proteins [3–5].
Acrolein is one of six aldehydic toxicants present in cigarette smoke. It is an in vivo lipid peroxidation product that has a high hazard index and causes oxidative stress by reacting with sulfhydryl groups [6]. Acrolein is about 10–1000 times more toxic than formaldehyde, acetaldehyde, or 4-hydroxynonenal [7] and can reach concentrations of 80 µM in the respiratory tract fluid of smokers [8]. In a recent study [9], we treated both human retinal pigment epithelial cell lines (ARPE-19) and primary cultures of human fetal RPE1 cells with acrolein and observed similar kinds of oxidative damage and mitochondrial dysfunction, suggesting that smoking may cause oxidative mitochondrial damage that could be a major cause of RPE cell damage. However, α-lipoic acid (LA), which is a mitochondrion-targeted antioxidant [10] and mitochondrial nutrient [11], effectively protected RPE cells from acrolein-induced toxicity [9,12]. These results suggest that treatment with mitochondrion-targeted antioxidants may be an effective strategy for reducing or preventing chronic RPE degeneration induced by oxidants in vivo from a variety of sources, including cigarette smoke.
Lipoamide (LM) is the neutral amide of LA. Unlike LA, LM does not occur naturally in either animals or plants. LM was first synthesized from LA based on the mixed carbonic–carboxylic anhydride method [13]. LM has been shown to be a better cofactor for α-oxo-acid dehydrogenase enzymes than free LA, and it is able to promote the recovery of postischemic myocardium in rats [14,15]. It also has been shown to be an antioxidant in vitro [16] and prevents Fenton-type chemistry and resultant oxidative damage and apoptosis more efficiently than LA in a lysosomal membrane system [17].
Based on these findings, we hypothesized that LM may be at least as protective as LA against RPE toxicity. In this study, we compared the protective effects of LM and LA in human ARPE-19 cells with acrolein-induced oxidative damage and mitochondrial dysfunction, focusing upon: (1) cell viability and mitochondrial function, (2) oxidant generation, (3) oxidative damage, and (4) the antioxidant mechanisms of activation on the phase 2 enzyme system.
Materials and methods
Chemicals
dl-α-LM was purchased from Sigma (Fluka). (R)-α-lipoic acid tris salt (LA)was a gift from KlausWessel (Viatris, Germany) and was used for all experiments. Acrolein was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All other reagents were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA).
Cell culture and treatments
The human ARPE-19 cell line was obtained from Dr. Nancy J. Philip (Thomas Jefferson University, Philadelphia, PA, USA) and cultured in DMEM-F12 medium supplemented with 10% fetal bovine serum, 2.438 g/L sodium bicarbonate, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. ARPE-19 cells were used within 10 generations. For all experiments, unless otherwise stated, cells were pretreated with LM or LA for 48 h. The medium was discarded and new medium, with or without 75 µM acrolein, was added to the cells for 24 h. Finally, the cells were washed with PBS and collected for assay.
Cell viability and morphology
Cell viability was measured with a crystal violet staining assay. Cells were cultured in 96-well plates. After treatment, cells were washed twice with PBS, and then fixed with 4% paraformaldehyde for 30 min. Crystal violet (0.1%) was used to stain the cells for 10 min. Excess stain was washed away and crystal violet bound to the cells was dissolved with 10% acetic acid. Optical densities were measured at 570 nm with a microplate spectrophotometer (Spectra Max 190; Molecular Devices, Sunnyvale, CA, USA). For cell morphology, cells were cultured in 100-mm dishes. Cell morphology was assessed using light microscopy at 10× magnification.
JC-1 assay for mitochondrial membrane potential
Mitochondrial membrane potential changes in live ARPE-19 cells cultured in 96-well plates were determined using the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethylbenzimidazolylcarbocyanine iodide (JC-1) with a fluorescence spectrometer (Flex Station II 384; Molecular Devices) [18]. The fluorescence ratio (590 to 530 nm) was used for quantitative analysis.
MTT assay for qualitative mitochondrial dehydrogenase activity
The MTT [3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction assay was used as a qualitative index of mitochondrial dehydrogenase with a microplate spectrophotometer [18].
Mitochondrial complexes I, II, III, IV, and V and pyruvate dehydrogenase activities
For the activity measurements of mitochondrial enzymes, cells were cultured on 100-mm plates. After various treatments, cells were harvested for enzyme activity measurement. For complexes I and II and pyruvate dehydrogenase (PDH), cells were fragmented by ultrasonication, and the crude homogenates were used to test enzyme activities. For complexes III, IV and V, cell mitochondria were isolated. NADH – CoQ oxidoreductase (complex I) ativity was assayed by Kumar’s method [19]. Assays of succinate–CoQ oxidoreductase (complex II), CoQ–cytochrome c reductase (complex III), and cytochrome c oxidase (complex IV) were performed as described [20–22]. Complex V activity was measured as oligomycin-sensitive Mg2+-ATPase activity [23]. The PDH assay was carried out according to Hinman’s method [24].
Intracellular adenosine 5′-triphosphate (ATP)
Cells were cultured in six-well plates. After various treatments, cells were lysed by 0.5% Triton X-100 in 100 mM glycine buffer, pH 7.4. Intracellular ATP levels were assayed with an ATP bio-luminescence assay kit (Sigma) based on the luciferase-catalyzed oxidation of d-luciferin [25].
Intracellular generation of reactive oxygen species (ROS)
Intracellular generation of ROS was assayed with the 2′,7′-dichlorofluorescein diacetate (DCFH-DA) method using a fluorescence spectrometer (Flex Station II 384; Molecular Devices) at 488 nm excitation wavelength and 530 nm emission wavelength [18].
Intracellular calcium levels
Intracellular calcium levels were measured with a commercial assay kit (Jiancheng Biochemical, Nanjing, China). In this assay, calcium is reacted with methyl thymol blue to form a blue complex. Optical density was measured at 610 nm with a microplate reader.
Detection of protein oxidation
Protein oxidation was determined using the Oxyblot kit (Cell Biolabs, San Diego, CA, USA). Protein carbonyls were labeled by 2,4-dinitrophenylhydrazine and detected by Western blot.
Comet assay for DNA damage
DNA damage was detected by the comet assay [26]. Cells were imaged using an Olympus BX61 microscope (40× objective) that was linked to an Olympus DP70 microcomputer imaging device. Nuclei stained with DAPI were excited with a UV laser (380 nm). Nuclei with tails were considered to be damaged.
Intracellular GSH levels
GSH levels were assayed with a commercial kit (Jiancheng Biochemical) based on a thiol-specific reagent, dithionitrobenzoic acid. The adduct was measured spectrophotometrically at 412 nm.
Glutathione S-transferase (GST) activity
Cells were cultured in six-well plates. After treatment, cells were lysed ultrasonically in 10 mM sodium phosphate buffer, pH 6.5. Protein content in the cell lysate was quantified by the BCA method. GST activity was measured in 5 mg protein in the presence of 1 mM GSH, 1 mM chloro-2,4-dinitrobenzene, 3 mg/ml BSA in 10 mM sodium phosphate buffer. The mixture was scanned at 340 nm for 5 min at 25°C as previously described [27].
Intracellular superoxide dismutase (SOD) activity
Intracellular SOD activity was assayed with a commercial kit (Jiancheng Biochemical) using a xanthine and xanthine oxidase system to produce superoxide. The superoxide oxidizes hydroxylamine to nitrite to form a carmine-colored reagent and the optical density at 550 nm was measured with a microplate reader.
Total antioxidant capability
The total antioxidant capability was assayed with a commercial kit (Jiancheng Biochemical) using a spectrometric method. Ferric ion was reduced by antioxidant reducing agents and the optical density was measured at 520 nm with a microplate reader.
Nuclear factor-E2-related factor 2 (Nrf2) in total and nuclear proteins
Cells were grown on 100-mm plates. Total protein and nuclear protein were isolated for Western blot analysis of Nrf2 level. Nrf2 was probed with anti-Nrf2 antibodies (Santa Cruz) at 1:1000 in both total and nuclear proteins. β-Actin and histone H1 were used as loading controls for total protein and nuclear protein, respectively. Anti-β-actin antibody (Sigma) was used at 1:10,000 and anti-histone H1 antibody (Upstate) was used at 1:1000. Chemiluminescence detection was done with an ECL Western blotting detection kit from Amersham Pharmacia [28].
Statistical analysis
Results are presented as means±SEM from at least three independent experiments. Group comparisons were made by one-way ANOVA, followed by determination of significant differences using post hoc comparisons with a Tukey HSD test. A p value <0.05 was considered significant.
Results
Dose-dependent effects of LM on cell viability and mitochondrial dehydrogenases and membrane potentials
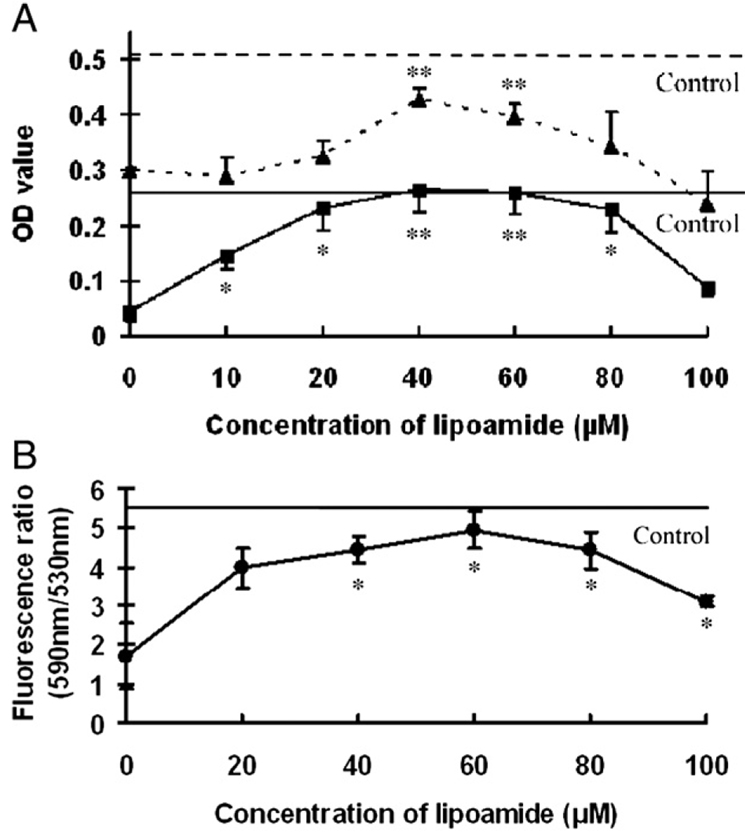
In order to study any protective effects by LM, AREP-19 cells were pretreated with varying concentrations of LM(10 to 100 µM) for 48 h and then treated with 75 µM acrolein for 24 h. Cell viability was measured with the crystal violet assay. In Fig. 1A, the solid horizontal line shows the optical density (OD) readings for viable control cells (OD ≈ 0.28). Without pretreatment (0 µM LM), acrolein caused a decline in cell viability to about 41.9% of the control (OD ≈ 0.12). In contrast, pretreatment with LM showed varying improvements to acrolein-induced declines in cell viability, with the maximal protective effect in the 40–60 µM range of LM pretreatment (Fig. 1A, solid squares and solid line).
Fig. 1.
Effects of LM on acrolein-induced damage to cell viability, mitochondrial dehydrogenase activity, and mitochondrial membrane potential. ARPE-19 cells were pretreated with the indicated concentrations of LM before acrolein exposure. (A) Cell viability: solid horizontal line is the optical density (OD) reading for untreated control cells stained with crystal violet. Filled squares and solid line are OD readings for cells pretreated with LM before acrolein exposure. Mitochondrial dehydrogenase activity: broken horizontal line is the OD reading for untreated control cells. Filled triangles and broken line are OD readings for cells pretreated with LM before acrolein exposure. (B) Mitochondrial membrane potential. Solid horizontal line is the 590 nm/530 nm fluorescence ratio for untreated control cells. Filled circles and solid line are the ratios for cells pretreated with LM before acrolein exposure. Values are means±SEM for three independent experiments in eight wells each, except for 100 µM (n=2). *p<0.05 and **p<0.01 vs 0 µM LM pretreatment.
Also in Fig. 1A, the broken horizontal line shows the OD readings for control cell mitochondrial dehydrogenase activity (OD ≈ 0.5). Acrolein at 75 µM for 24 h, without LM pretreatment, caused a significant decrease in mitochondrial dehydrogenase activity (OD ≈ 0.3). LM pretreatment showed varying, dose-dependent protective effects upon dehydrogenase activities in the 20–100 µM range (Fig. 1A, solid triangles and broken line). Fig. 1B shows similar effects of LM pretreatment for mitochondrial membrane potentials.
For the three measures related to cell viability and mitochondrial functions shown in Fig. 1, maximal protective effects were observed for pretreatment of cells with 40 µM LM. Therefore, 40 µM LM was used in the following assays and compared with the same concentration of LA.
Effects on cell viability
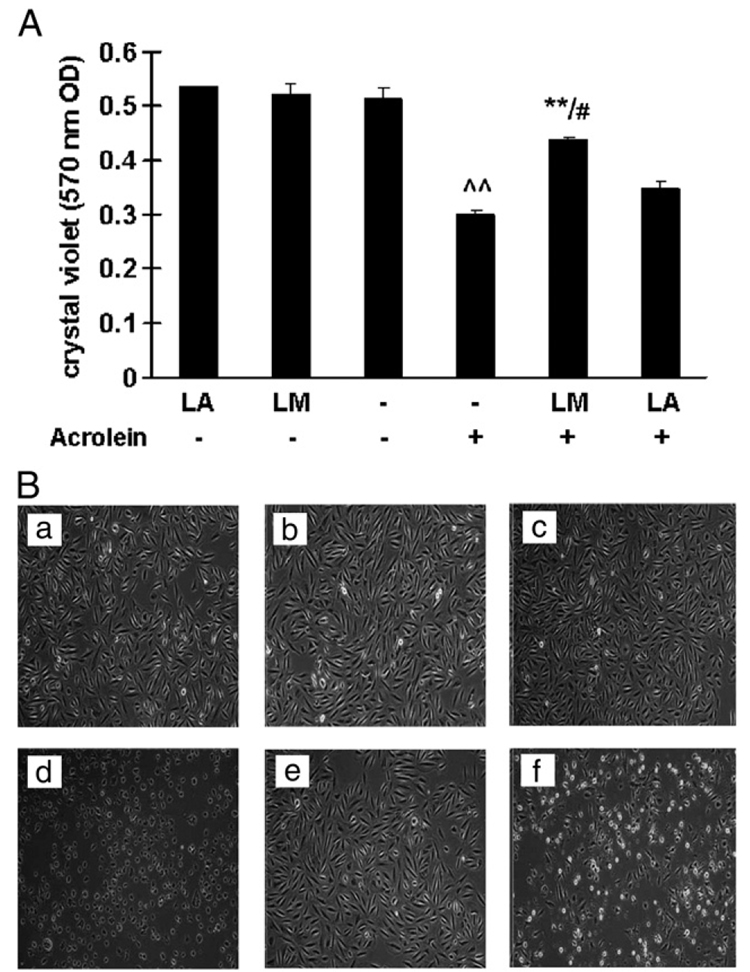
Fig. 2A shows the viability results (OD readings from crystal violet assay) for cells with or without acrolein exposure and with or without 48 h pretreatment with either 40 µM LA or 40 µM LM. Without pretreatment, ARPE-19 cell viability declined about 40% after treatment with 75 µM acrolein. Cells pretreated with 40 µM LA or LM, but without acrolein, showed the same viability as the untreated control cells.
Fig. 2.
Effects of LM and LA on acrolein-induced decreases in cell viability. (A) Cell viability was assayed with crystal violet. Values are means±SEM from three independent experiments, three wells each. ^^p<0.01 vs control; **p<0.01 vs acrolein; #p<0.05 vs LA + acrolein. (B) Representative images of cell morphology. (a) Control; (b) LM alone; (c) LA alone; (d) acrolein only; (e) LM + acrolein; (f) LA + acrolein. Images from light microscopy at 10× original magnification.
For cells exposed to acrolein, pretreatment with 40 µM LM showed a statistically significant improvement in cell viability compared to acrolein-exposed cells without pretreatment (Fig. 2A). However, pretreatment with the same concentration of LA showed no significant improvement (Fig. 2A). Also, based on percentage cell viability, LM pretreatment was more protective than LA (p <0.05).
Fig. 2B shows representative cell morphological images corresponding to the experimental conditions of Fig. 2A (images a–c, no acrolein exposure; d–f, acrolein exposure). Without pretreatment, cells exposed to acrolein (image d) were fewer in number and had significant morphological changes compared to unexposed control cells (image a). LA pretreatment before acrolein exposure (image f) showed some protective effects compared to LA pretreatment without acrolein exposure (image c). However, similar to the results in Fig. 2A, pretreatment with LM before acrolein exposure (image e) showed significant protective effects, with little change in cell numbers or morphological characteristics compared to cells not exposed to acrolein (image b).
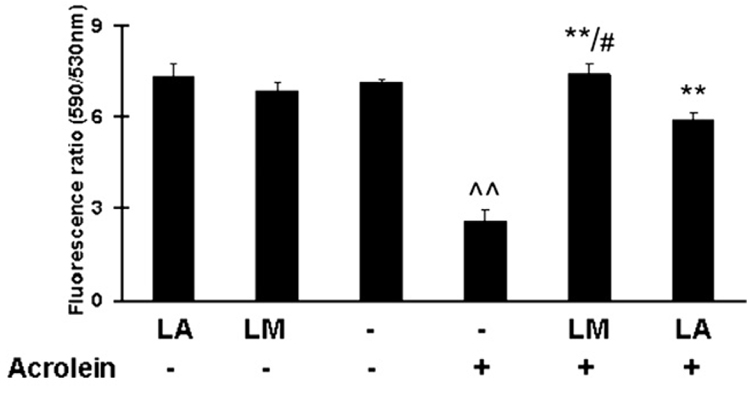
Effects on mitochondrial membrane potentials
LA or LM pretreatments alone had no obvious effects on the membrane potential measured with JC-1 fluorescence (Fig. 3). The fluorescence ratio of ARPE-19 cells decreased to 36.4% of the control after 75 µM acrolein treatment for 24 h. Pretreatment with 40 µM LA or LM for 48 h significantly protected cells from acrolein-induced decreases in membrane potential (Fig. 3). For LA pretreatment, the fluorescence ratio recovered 82.3% of acrolein-unchallenged levels, and for LM the improvement was 103.5%. LM had about a 20% greater improvement over LA (p <0.05).
Fig. 3.
Effects of LM and LA on acrolein-induced decreases in mitochondrial membrane potential. Data are red/green (590 nm/530 nm) fluorescence ratios. Values are means±SEM from three independent experiments, in eight wells each. ^^p<0.01 vs control; **p<0.01 vs acrolein; #p<0.05 vs LA + acrolein.
Effects on mitochondrial enzymes
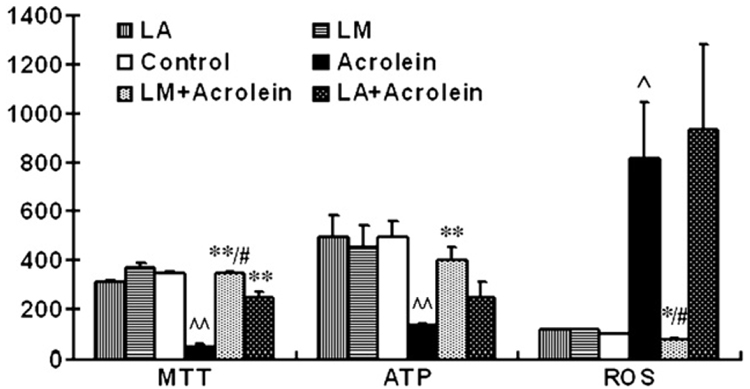
The MTT assay is a mitochondrial dehydrogenase activity-based test. Pretreatment with 40 µM LA or LM protected cells from the acrolein-induced decrease in cell mitochondrial dehydrogenase (Fig. 4, left). Protection was 72.3% for LA and 98.9% for LM. LM was more protective than LA (p < 0.05).
Fig. 4.
Effects of LM and LA on acrolein-induced changes in mitochondrial dehydrogenases, intracellular ATP levels, and reactive oxygen species level. (Left) Mitochondrial dehydrogenases assayed with MTT assay; expressed as milli-OD. Results are means±SEM of three independent experiments, in eight wells each. (Middle) Intracellular ATP levels expressed as luminescence value per milligram of protein. Values are means±SEM (n=4). (Right) ROS generation tested with DCF-DA staining expressed as ratio relative to controls. Values are means±SEM of five independent experiments. ^p<0.05, ^^p<0.01 vs control; *p<0.05, **p<0.01 vs acrolein; #p<0.05 vs LA + acrolein.
The activities of the mitochondrial electron transport complexes I – V are essential for mitochondrial functions, and PDH is a critical enzyme in the energy metabolism cycle. As shown in Table 1, compared to untreated control cells, acrolein exposure caused decreases of 27.9, 35.2, 19.6, and 48.2% for complexes I, II, and V and PDH activities, respectively. No significant decreases in activities for complexes III and IV were observed (data not shown).
Table 1.
Effects of pretreatment with lipoamide (LM) and lipoic acid (LA) on acrolein-induced decreases in activities of mitochondrial complexes I, II, and V and PDH
| Ca | A | LM | LM+A | LA | LA+A | |
|---|---|---|---|---|---|---|
| Complex I | 100b | 27.9±4.0† | 147.1±7.1 | 132.8±10.4**,# | 120.6±0.2 | 86.9±4.8** |
| Complex II | 100 | 35.2±12.1† | 150.8±15.8 | 131.0±20.8*,# | 99.3±10.7 | 61.0±11.9 |
| Complex V | 100 | 26.4±6.5† | 102.6±6.2 | 89.3±0.6**,## | 76.0±3.7 | 44.0±6.7 |
| PDH | 100 | 48.2±5.3† | 126.8±9.4 | 108.5±1.6**,## | 118.0±9.1 | 75.7±6.1* |
ARPE-19 control cells (C) were untreated. Acrolein-only cells (A) were exposed to 75 µM acrolein for 24 h. Pretreated cells, before acrolein exposure (+A), were exposed for 48 h to either 40 µM LM or 40 µM LA.
Control cell enzyme activities set to 100%. Results for treated cells are percentage of control. Values are means±SEM of three independent experiments performed in triplicate.
p<0.01 vs control.
p<0.05
p<0.01 vs acrolein.
p<0.05
p<0.01 vs LA + acrolein.
LA pretreatment was effective in protecting complex I and PDH activities (Table 1). By comparison, LM pretreatment was effective in protecting the activities of all four enzymes whose activities were diminished by acrolein exposure (complexes I, II, and V and PDH). Also, in these four cases, protection by LM was significantly greater than that of LA (Table 1).
Effects on intracellular ATP levels
Results of assays for ATP levels are shown in Fig. 4 (middle). Acrolein treatment significantly decreased intracellular ATP levels to about 27.0% of the untreated control level. LM pretreatment significantly inhibited the acrolein-induced decrease in ATP level (81.1% of control). LA pretreatment also inhibited the ATP decrease (50.8% of control), but this level was not statistically different from the group treated with acrolein alone. LM is more effective than LA in inhibiting the acrolein-induced ATP level decrease.
Effects on ROS generation
Results for intracellular generation of ROS are also shown in Fig. 4 (right). Acrolein treatment caused a significant increase in intracellular generation of ROS, 8.2-fold over that of the control. LM pretreatment inhibited the acrolein-induced ROS generation to normal cell levels. In contrast, LA pretreatment did not show any inhibition of ROS generated by acrolein.
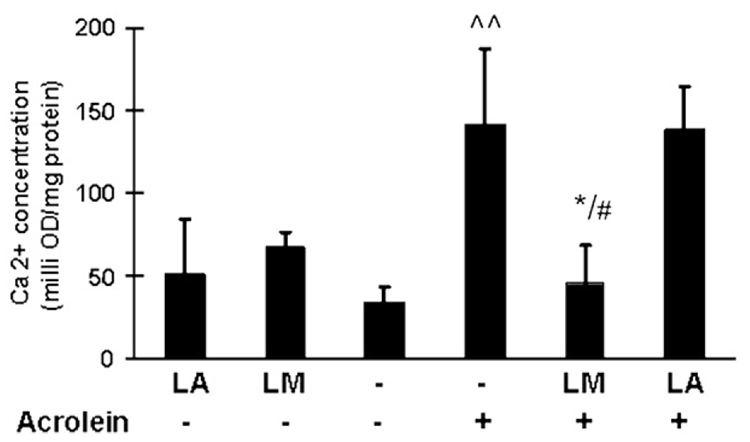
Effects on intracellular calcium levels
Oxidative stress and mitochondrial dysfunction may either result in or result from an increase in cytoplasmic Ca2+ levels. As shown in Fig. 5, treatment of ARPE-19 cells with 75 µM acrolein caused a significant increase in intracellular Ca2+ levels (3.9-fold of control). Similar to the results of oxidant generation shown above, LM pretreatment significantly inhibited the acrolein-induced calcium increase, but LA pretreatment did not show any inhibition.
Fig. 5.
Effects of LM and LA on acrolein-induced increases in intracellular calcium levels. Intracellular calcium measured by spectrophotometry method; results are milli-OD/mg protein. Values are means±SEM (n=3). ^^p<0.01 vs control; *p<0.05 vs acrolein; #p<0.05 vs LA + acrolein.
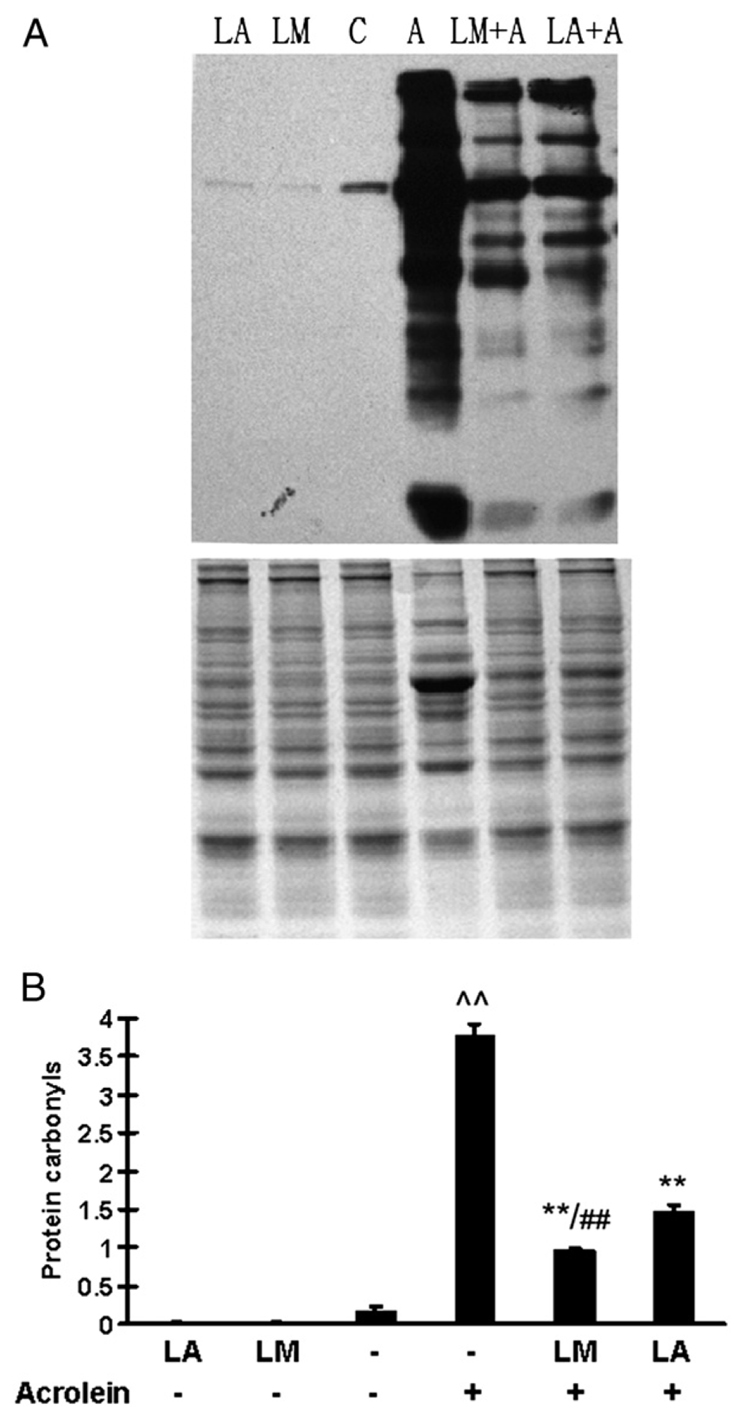
Effects on protein carbonyls
Fig. 6A shows representative Western blots for protein carbonyls, and Fig. 6B shows the quantitative results based upon OD scans. Acrolein treatment (75 µM, 24 h) caused a significant increase in protein carbonyls, 22-fold greater than that of the control. Both LM and LA pretreatments significantly inhibited the acrolein-induced formation of protein carbonyls; the decreased production due to LM pretreatment was significantly greater than that of LA pretreatment (p <0.01).
Fig. 6.
Effects of LM and LA on acrolein-induced increases in protein carbonyls. (A) Western blots of protein carbonyls (top) and total protein as a loading control (bottom) stained with Coomassie blue. (B) Quantitative optical density results. Values are means±SEM of three independent experiments. ^^p<0.01 vs control; **p<0.01 vs acrolein; ##p<0.01 vs LA + acrolein.
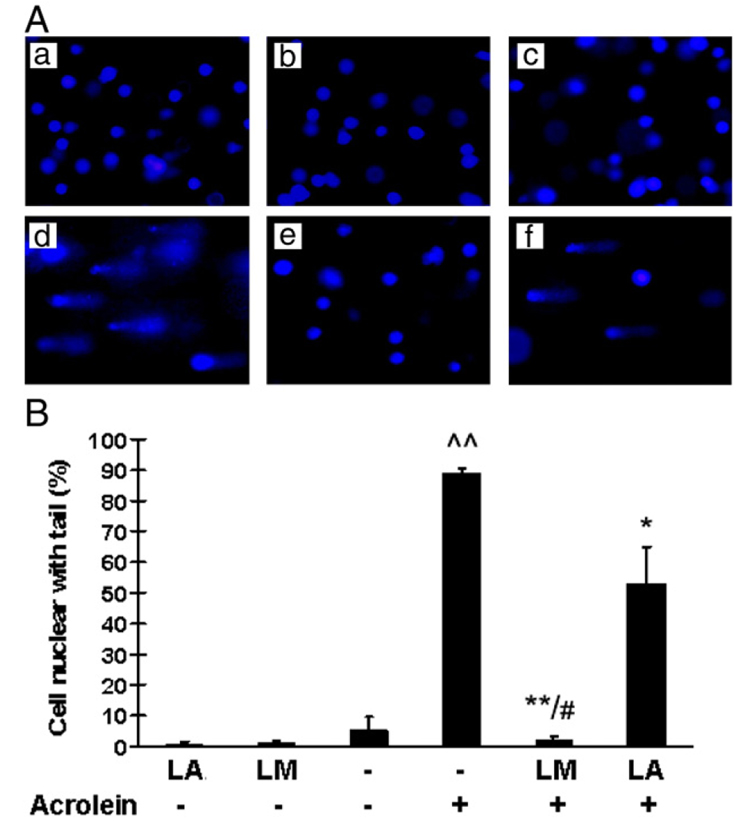
Effects on DNA damage
Fig. 7A shows representative images for the comet assay, which reflects DNA fragmentation, and Fig. 7B shows the percentages of cells with DNA damage based on this test. Acrolein treatment (75 µM, 24 h) caused 89% nuclear DNA fragmentation. LM pretreatment showed nearly complete protection (only 2% nuclear DNA fragmentation). This effect was much greater than that observed for LA pretreatment (53% nuclear DNA fragmentation).
Fig. 7.
Effects of LM and LA on acrolein-induced increases in DNA damage. (A) Representative images of comet assay results. (B) Percentage nuclear fragmentation from three independent experiments. More than 100 cells were analyzed in each experimental group. Values are means±SEM. ^^p<0.01 vs control; *p<0.05 and **p<0.01 vs acrolein; #p<0.01 vs LA + acrolein.
Effects on total antioxidant capacity, intracellular GSH level, and activities of GST and SOD
Table 2 shows the results from assays for intracellular GSH, SOD, total antioxidant capacity (T-AOC), and GST activity. Acrolein treatment (75 µM, 24 h) caused significant decreases in all of these antioxidant biomarkers. Compared to control levels, these were 26.9% for GSH level, 68.8% for SOD activity, 57.2% for T-AOC, and 13.8% for GST activity.
Table 2.
Effects of pretreatment with lipoamide (LM) and lipoic acid (LA) on acrolein-induced decreases in mitochondrial functions
| Ca | A | LM | LM+A | LA | LA + A | |
|---|---|---|---|---|---|---|
| GSH | 53.5±2.7b | 11.4±3.1† | 61.1±2.9 | 88.3±5.1** | 67.2±2.0 | 61.3±13.4** |
| SOD | 79.6±2.1 | 54.8±0.6† | 77.6±1.4 | 77.1±4.7* | 75.7±2.6 | 72.8±5.7* |
| T-AOC | 100 | 57.2±11.6† | 99.4±5.6 | 128.8±6.8** | 103.3±4.3 | 110.9±15.2* |
| GST | 88.1±14.9 | 12.1±1.4† | 94.7±13.1 | 78.7±13.9** | 82.8±10.3 | 44.2±17.5 |
Experimental groups as in Table 1.
Values are means±SEM for assay-specific results of three or four independent experiments performed in triplicate. Intracellular GSH levels (mg/g protein), SOD activity (unit/mg protein), total antioxidant capacity (T-AOC;%), and GST activity (milli-OD/min) are presented.
p<0.01 vs control.
p<0.05 and p<0.01 vs acrolein.
Without acrolein exposure, LM or LA pretreatment did not affect these antioxidant biomarkers (Table 2). However, with acrolein exposure, pretreatment with LM significantly protected against all these decreases. LA pretreatment protected against the decreases in GSH, T-AOC, and SOD, but not GST. The protection afforded by LM pretreatment seemed to be greater than that of LA; however, the differences between LM and LA pretreatment were not significant for these parameters (Table 2).
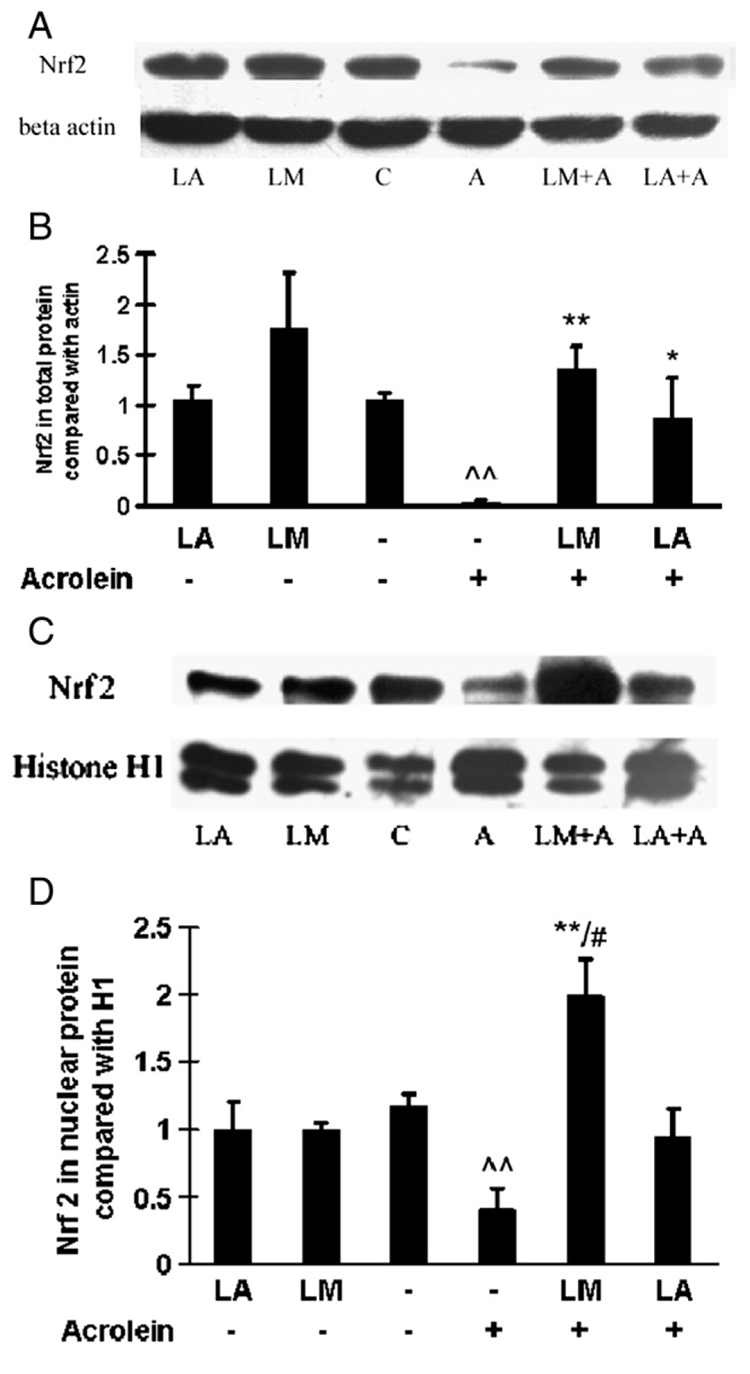
Effects on Nrf2 expression
Fig. 8 shows Western blot results for Nrf2 expression in total (Figs. 8A and 8B) and nuclear (Figs. 8C and 8D) proteins. Representative blots are shown in Figs. 8A and 8C, and their quantitative results after densitometric scanning are in Figs. 8B and 8D. Acrolein treatment (75 µM, 24 h) caused appreciable losses of Nrf2 expression in both total protein (Figs. 8A and 8B) and nuclear protein (Figs. 8C and 8D). Without acrolein exposure, LM or LA pretreatments did not significantly change Nrf2 expression.
Fig. 8.
Effects of LM and LA on Nrf2 expression in total and nuclear protein. Representative Western blots for Nr2f expression in (A) total protein and (C) nuclear protein. Optical density was analyzed by Quantity One software and normalized with (B) β-actin for total protein or (D) histone H1 for nuclear protein. Values are means±SEM of four independent Western blots. ^^p<0.01 vs control; *p<0.05 and **p<0.01 vs acrolein; #p<0.05 vs LA + acrolein.
For total protein, both LM and LA pretreatment significantly inhibited the acrolein-induced decrease in Nrf2 expression; LM pretreatment was more effective than LA pretreatment (Figs. 8A and 8B). For nuclear protein, LM pretreatment significantly inhibited the acrolein-induced decrease in Nrf2 expression. In contrast, LA pretreatment did not significantly inhibit the acrolein-induced decrease in Nrf2 expression (Figs. 8C and 8D).
Discussion
Mitochondria are the main generation sites and targets of oxidants [29]. Mitochondrial decay is a key factor in aging and age-related diseases. Increasing evidence shows that aldehydes, including acrolein [30], malondialdehyde [31,32], and 4-hydroxynoneanol [32,33], are reactive oxidants and that these toxins inactivate mitochondrial enzymes and cross-link proteins that are important in the aging process and age-related diseases.
Acrolein, as a major component of cigarette smoke and a by-product of lipid peroxidation, has received special attention as it is a cytotoxic and genotoxic environmental pollutant [6]. Mechanisms of acrolein toxicity have been reported for mitochondrial dysfunction in rat brain [7,23,34], heart [35], and RPE cells [18]. Thus, acrolein-induced toxicity is a useful model for finding antioxidants and protective agents to prevent and to treat diseases associated with increased oxidative damage and mitochondrial dysfunction.
Consistent with previous results [18], as shown here, acrolein treatment induced decreases in cell viability and measures of mitochondrial function, including decreased membrane potentials, activities of complexes I, II, and V and pyruvate dehydrogenase, and total ATP levels. Accompanying the mitochondrial damage, acrolein treatment induced changes in biomarkers of oxidative stress, including oxidant generation, calcium levels, protein oxidation, DNA damage, glutathione levels, expression of the antioxidant response regulator Nrf2, and total antioxidant capacity, and activities of GST and SOD. The acrolein-induced loss of enzyme activity may be attributed to the covalent binding to the enzyme active site to form various adducts, such as acrolein – lysine adducts [36,37].
Both in vitro and in vivo studies have shown that acrolein-induced toxicity is mediated by increased oxidant production and oxidative damage [38], suggesting that acrolein acts not only as a direct oxidant, but also as a generator of oxidants [39]. These newly generated oxidants may cause further loss of enzyme activity. To possibly counteract these effects, we have previously shown that LA, a known mitochondrial antioxidant and nutrient, protected RPE cells from acrolein-induced oxidative stress and mitochondrial dysfunction [9,18]. However, these protective effects were not absolute, thus making searches for more potent antioxidants a priority.
In this study, we evaluated the protective effects of LM, the neutral amide of LA, which does not occur naturally in animals or plants, using human ARPE-19 as a model. We compared the effects of LM with those of LA for the following measures of acrolein-induced RPE toxicity: (1) cell viability and mitochondrial function, (2) oxidant generation, (3) oxidative damage, and (4) the antioxidant mechanisms of activation on the phase 2 enzyme system.
As shown in Fig. 1A and Fig. 2A, acrolein induced a significant decrease in cell viability measured with the crystal violet assay. LM inhibited the acrolein-induced toxicity, with an optimal effect in the 40–60 µM range (Fig. 1A). At 40 µM concentration, LM showed greater protective effects than LA for acrolein-induced cellular toxicity (Fig. 2A). These results suggest that LM is an effective protective agent against acrolein-induced ARPE-19 cell toxicity.
One possible mechanism for the cytotoxicity of acrolein may be associated with mitochondrial dysfunction, as we have previously shown that acrolein is a mitochondrial toxin [30]. LM showed dose-dependent protection against acrolein-induced decreases in mitochondrial membrane potentials (measured by JC-1 staining; Fig. 1B), with an optimal dose (40–60 µM) similar to that observed for protection of cell viability (Fig. 1A). The protection of mitochondrial membrane potential by LM, similar to its effect on cell viability, was more potent than that of LA at 40 µM concentration, as shown by JC-1 fluorescence ratios (Fig. 3).
We used the MTT assay for measuring total mitochondrial dehydrogenase activity, as this assay depends on mitochondrial dehydrogenases for MTT conversion. The results (Fig. 1A and Fig. 4, left) are similar to those of the JC-1 assay, providing additional evidence for mitochondrial function involvement in acrolein-induced cellular injury. All results from the JC-1 and MTT assays suggest that LM, like LA, is able to protect mitochondria from acrolein-induced injury and that LM is a mitochondrial-protective agent.
Decreased mitochondrial membrane potential leads to dysfunction of mitochondria, such as decreased activities of mitochondrial dehydrogenase and ox-phos enzyme complexes and in ATP synthesis [40]. As with previously discussed results, LM showed significant protective effects against acrolein-induced decreases in the activities of complexes I, II, and V (Table 1); dehydrogenases (Fig. 4, left); and PDH (Table 1) and in the levels of ATP (Fig. 4, middle). In addition, as measured by these mitochondrial parameters, the protective effect of LM was greater than that of LA for the same concentration.
Acrolein is an oxidant that causes damage, such as protein cross-linkage. It can also induce the generation of other reactive oxygen species and cause increases in intracellular calcium [30]. As an antioxidant, LA inhibits oxidative damage by reducing oxidant generation and by increased scavenging of ROS. We compared the effects of LM and LA on the inhibition of acrolein-induced increases in ROS and calcium. As shown in Fig. 4 (right) and Fig. 5, LM showed a greater inhibition of ROS and calcium increases than LA. These results suggest that LM, like LA, is also an effective antioxidant and that this antioxidant activity may account for its protective effects on cell viability and mitochondrial function.
Increased ROS, if not scavenged immediately, will cause oxidative damage to macromolecules, such as proteins/enzymes and DNA, and weaken antioxidant defenses. As LM could inhibit ROS increases in ARPE-19 cells, it should protect proteins/enzymes and DNA from oxidative damage. As expected, LM showed significant protection against acrolein-induced increases in protein oxidation (measured as protein carbonyls; Fig. 6) and oxidative DNA damage (Fig. 7) and decreases in total antioxidant capacity and SOD activity (Table 2). All the protective effects of LM were more potent than those of LA at the same concentration (40 µM). These results suggest again that LM is a more potent antioxidant than LA.
LA is an inducer of phase 2 enzymes [41]. Nrf2 is a known a key regulator of antioxidant response element-mediated gene expression and inducer of phase 2 enzymes. In previous studies, LA was an effective Nrf2 activator in ARPE-19 cells [18] that enhanced the expression of γ-glutamylcysteine ligase mRNA in human fetal RPE cells [9]. We examined whether LM could also activate the Keap1/Nrf2 system. As shown in Fig. 8, LM increased Nrf2 expression, and the effect was greater than that of LA at the same concentration. Like LA, LM is a potent inducer of Nrf2 expression.
A consequence of Nrf2 activation is the activation of phase 2 enzymes (GST, heme oxygenase-1, NAD(P)H quinine oxidoreductase-1, γ-glutamylcysteine ligase) that lead to an increase in antioxidants such as GSH and bilirubin [41,42]. In our study, we measured the activity of one phase 2 enzyme, total GST, and the level of one antioxidant, GSH, as examples of phase 2 system enhancement. LM treatment increased total GST activity and GSH levels, consistent with its ability to stimulate Nrf2 expression. Further studies of other parameters of the Keap1/Nrf2 system are warranted. For example, it is important to study the aldehyde-specific isoenzymes of the superfamily GSTs [43,44]. A recent study showed that RNAi knockdown of certain GSTs sensitized the nematode to electrophilic stress induced by exposure to 4-hydroxynonenol and that interference with the expression of certain GST genes significantly shortened the life span of the nematode [45].
The positive effects of LA can be attributed to several factors. First, the reduced form of LA, dihydrolipoic acid, is a powerful mitochondrial antioxidant [10,46–48]. It recycles other cellular antioxidants, including CoQ, vitamins C and E, and glutathione, and chelates iron and copper [10,46–48]. LA readily crosses the blood – brain barrier and is reduced to dihydrolipoic acid by NADH-dependent mitochondrial dihydrolipoamide dehydrogenase [46]. Second, dihydrolipoic acid is an effective transition metal chelator [49]. Third, LA is a redox regulator of proteins such as myoglobin, prolactin, thioredoxin, and transcription factor NF-κB [48,50,51]. In addition, LA is a cofactor of PDH and α-ketoglutarate dehydrogenase.
It has been suggested that increasing enzyme cofactor concentration may be an effective strategy to protect enzymes from oxidant attack or to stimulate defective enzyme activity [52,53]. Therefore, administration of LA is beneficial to a number of diseases associated with oxidative stress and mitochondrial dysfunction, such as diabetes, cataracts, HIV activation, neurodegeneration, and radiation injury in animals [11,18,54,55]. We clearly showed that LM is more potent than LA at the same concentration for protecting ARPE-19 cells from acrolein-induced oxidative stress and mitochondrial dysfunction. All protective effects of LA are attributable to its dithiol group. As LM has the same dithiol group, it is quite possible that it may also be beneficial in treating these diseases.
The reason for the greater protection by LM over LA is not known, but could be attributed to the substitution of – CONH2 for – COOH. The substitution makes LM, compared with LA, relatively more stable than LA. This is attributed to resonance stabilization of CO-N, which is much less acidic at physiological pH and more lipid soluble, leading to better cellular availability. In addition, the amine group may have other physiological reactions. Further studies are needed for investigating the greater potency of LM compared to LA.
In conclusion, LM, the neutral amide of LM, is more potent than LA in protecting against acrolein-induced oxidative stress and mitochondrial dysfunction in ARPE-19 cells. The strong protective effects of LM against acrolein-induced cytotoxicity suggest that LM may be useful for the prevention or treatment of smoking- and age-related degenerative diseases, such as ageassociated macular degeneration.
Acknowledgments
We are grateful to Dr. Edward Sharman at the University of California at Irvine for his critical and careful reading and editing of the manuscript. This work was supported by the National Eye Institute (NIH Grant EY0160101), Macular Degeneration Research (MDR Grant 2005-038), the Chinese Academy of Sciences (Grant 05PG14104), and the Pujiang Talent Award from the Shanghai Science and Technology Committee, Shanghai, China.
Abbreviations
- ATP
adenosine 5′-triphosphate
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- GST
glutathione S-transferase
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- LA
α-lipoic acid
- LM
lipoamide
- MTT
3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Nrf2
nuclear factor-E2-related factor 2
- OD
optical density
- PDH
pyruvate dehydrogenase
- ROS
reactive oxygen species
- RPE
retinal pigment epithelial
- SOD
superoxide dismutase
- T-AOC
total antioxidant capacity.
References
- 1.Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye. 2005;19:935–944. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 2.Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br. J. Ophthalmol. 2006;90:75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000;71:530–536. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill CA, Halliwell B, van der Vliet A, Davis PA, Packer L, Tritschler H, Strohman WJ, Rieland T, Cross CE, Reznick AZ. Aldehyde-induced protein modifications in human plasma: protection by glutathione and dihydrolipoic acid. J. Lab. Clin. Med. 1994;124:359–370. [PubMed] [Google Scholar]
- 5.Cross CE, O’Neill CA, Reznick AZ, Hu ML, Marcocci L, Packer L, Frei B. Cigarette smoke oxidation of human plasma constituents. Ann. N.Y. Acad. Sci. 1993;686:72–89. doi: 10.1111/j.1749-6632.1993.tb39157.x. discussion 89–90. [DOI] [PubMed] [Google Scholar]
- 6.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen E, Picklo MJ., Sr Inhibition of succinic semialdehyde dehydrogenase activity by alkenal products of lipid peroxidation. Biochim. Biophys. Acta. 2003;1637:107–112. doi: 10.1016/s0925-4439(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am. J. Clin. Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- 9.Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. R)-Alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest. Ophthalmol. Visual Sci. 2005;46:4302–4310. doi: 10.1167/iovs.04-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv. Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr. Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Sun L, Zhu L, Jia X, Li X, Jia H, Wang Y, Weber P, Long J, Liu J. Hydroxytyrosol protects retinal pigment epithelial cells from acrolein-induced oxidative stress and mitochondrial dysfunction. J. Neurochem. 2007;103:2690–2700. doi: 10.1111/j.1471-4159.2007.04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed LJ, Koike M, Levitch ME, Leach FR. Studies on the nature and reactions of protein-bound lipoic acid. J. Biol. Chem. 1958;232:143–158. [PubMed] [Google Scholar]
- 14.Sumegi B, Butwell NB, Malloy CR, Sherry AD. LM influences substrate selection in post-ischaemic perfused rat hearts. Biochem. J. 1994;297(Pt 1):109–113. doi: 10.1042/bj2970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabados E, Fischer GM, Gallyas F, Jr, Kispal G, Sumegi B. Enhanced ADP-ribosylation and its diminution by LM after ischemia-reperfusion in perfused rat heart. Free Radic. Biol. Med. 1999;27:1103–1113. doi: 10.1016/s0891-5849(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 16.Bisby RH, Parker AW. Antioxidant reactions of dihydrolipoic acid and LM with triplet duroquinone. Biochem. Biophys. Res. Commun. 1998;244:263–267. doi: 10.1006/bbrc.1998.8245. [DOI] [PubMed] [Google Scholar]
- 17.Persson HL, Svensson AI, Brunk UT. Alpha-lipoic acid and alpha-LM prevent oxidant-induced lysosomal rupture and apoptosis. Redox Rep. 2001;6:327–334. doi: 10.1179/135100001101536472. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Zhu ZR, Ding HQ, Qian Z, Zhu L, Ke Y. Vulnerability of neurons with mitochondrial dysfunction to oxidative stress is associated with down-regulation of thioredoxin. Neurochem. Int. 2007;50:379–385. doi: 10.1016/j.neuint.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Kumar MJ, Nicholls DG, Andersen JK. Oxidative alpha-ketoglutarate dehydrogenase inhibition via subtle elevations in monoamine oxidase B levels results in loss of spare respiratory capacity: implications for Parkinson’s disease. J. Biol. Chem. 2003;278:46432–46439. doi: 10.1074/jbc.M306378200. [DOI] [PubMed] [Google Scholar]
- 20.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 21.Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J. Neurochem. 1999;72:1617–1624. doi: 10.1046/j.1471-4159.1999.721617.x. [DOI] [PubMed] [Google Scholar]
- 22.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picklo MJ, Montine TJ. Acrolein inhibits respiration in isolated brain mitochondria. Biochim. Biophys. Acta. 2001;1535:145–152. doi: 10.1016/s0925-4439(00)00093-4. [DOI] [PubMed] [Google Scholar]
- 24.Hinman LM, Blass JP. An NADH-linked spectrophotometric assay for pyruvate dehydrogenase complex in crude tissue homogenates. J. Biol. Chem. 1981;256:6583–6586. [PubMed] [Google Scholar]
- 25.Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes. 2002;51:2749–2756. doi: 10.2337/diabetes.51.9.2749. [DOI] [PubMed] [Google Scholar]
- 26.Kwong LK, Mockett RJ, Bayne AC, Orr WC, Sohal RS. Decreased mitochondrial hydrogen peroxide release in transgenic Drosophila melanogaster expressing intramitochondrial catalase. Arch. Biochem. Biophys. 2000;383:303–308. doi: 10.1006/abbi.2000.2093. [DOI] [PubMed] [Google Scholar]
- 27.Pabst MJ, Habig WH, Jakoby WB. Glutathione S-transferase A: a novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J. Biol. Chem. 1974;249:7140–7147. [PubMed] [Google Scholar]
- 28.Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 29.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Luo C, Long J, Wei D, Liu J. Acrolein is a mitochondrial toxin: effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion. 2006;6:136–142. doi: 10.1016/j.mito.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Long J, Wang X, Gao H, Liu Z, Liu C, Miao M, Liu J. Malonaldehyde acts as a mitochondrial toxin: inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci. 2006;79:1466–1472. doi: 10.1016/j.lfs.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-l-carnitine and/or R-alpha-lipoic acid. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphries KM, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem. Int. 2005;46:243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Biagini RE, Toraason MA, Lynch DW, Winston GW. Inhibition of rat heart mitochondrial electron transport in vitro: implications for the cardiotoxic action of allylamine or its primary metabolite, acrolein. Toxicology. 1990;62:95–106. doi: 10.1016/0300-483x(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 36.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction: formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potentialmarkers for oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 39.Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic. Biol. Med. 1993;15:187–193. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- 40.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem. Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 42.Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc. Natl. Acad. Sci. U. S. A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laisney V, Nguyen Van C, Gross MS, Frezal J. Human genes for glutathione S-transferases. Hum. Genet. 1984;68:221–227. doi: 10.1007/BF00418392. [DOI] [PubMed] [Google Scholar]
- 44.Vorachek WR, Pearson WR, Rule GS. Cloning, expression, and characterization of a class-Mu glutathione transferase from human muscle, the product of the GST4 locus. Proc. Natl. Acad. Sci.U. S. A. 1991;88:4443–4447. doi: 10.1073/pnas.88.10.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayyadevara S, Dandapat A, Singh SP, Siegel ER, Shmookler Reis RJ, Zimniak L, Zimniak P. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007;128:196–205. doi: 10.1016/j.mad.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol. Appl. Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 47.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic. Biol. Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 48.Packer L, Witt EH, Tritschler HJ. a-Lipoic acid as a biological antioxiant. Free Radic. Biol. Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 49.Ou P, Tritschler HJ, Wolff SP. hioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem. Pharmacol. 1995;50:123–126. doi: 10.1016/0006-2952(95)00116-h. [DOI] [PubMed] [Google Scholar]
- 50.Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998;24:1023–1039. doi: 10.1016/s0891-5849(97)00371-7. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs J, Packer L, Zimmer G. Lipoic acid in health and disease. New York: Dekker; 1997. [Google Scholar]
- 52.Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am. J. Clin. Nutr. 2002;75:616–658. doi: 10.1093/ajcn/75.4.616. [DOI] [PubMed] [Google Scholar]
- 53.Ames BN, Liu J, Suh JH. Enzymes lose binding affinity (increase Km) for coenzymes and substrates with age: a strategy for remediation. In: Kaput J, Rodriguez RL, editors. Nutritional genomics: discovering the path to personalized nutrition. Hoboken, NJ: Wiley; 2006. pp. 277–291. [Google Scholar]
- 54.Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888–895. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- 55.Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Munch G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol. Ther. 2007;113:154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]