Abstract
Gastric cancer is the second leading cause of cancer-related deaths worldwide. Treatment for Helicobacter pylori infection, the leading causal risk factor, can reduce disease progression, but the long-term impact on cancer incidence is uncertain. Using the best available data, we estimated the potential health benefits and economic consequences associated with H. pylori screening in a high-risk region of China. An empirically calibrated model of gastric cancer was used to project reduction in lifetime cancer risk, life-expectancy and costs associated with (i) single lifetime screening (age 20, 30 or 40); (ii) single lifetime screening followed by rescreening individuals with negative results and (iii) universal treatment for H. pylori (age 20, 30 or 40). Data were from the published literature and national and international databases. Screening and treatment for H. pylori at age 20 reduced the mean lifetime cancer risk by 14.5% (men) to 26.6% (women) and cost less than $1,500 per year of life saved (YLS) compared to no screening. Rescreening individuals with negative results and targeting older ages was less cost-effective. Universal treatment prevented an additional 1.5% to 2.3% of risk reduction, but incremental cost-effectiveness ratios exceeded $2,500 per YLS. Screening young adults for H. pylori could prevent one in every 4 to 6 cases of gastric cancer in China and would be considered cost-effective using the GDP per capita threshold. These results illustrate the potential promise of a gastric cancer screening program and provide rationale for urgent clinical studies to move the prevention agenda forward.
Keywords: simulation model, cost-effectiveness, Helicobacter pylori, gastric cancer
Helicobacter pylori (H. pylori) infection is classified as a human carcinogen by the International Agency for Research on Cancer (IARC) and is associated with an estimated 65–80% of gastric cancer.1-3 Gastric cancer is the second leading cause of cancer-related deaths worldwide and a major public health problem with over 40% of all cases occurring in China. On the basis of current age-specific rates of gastric cancer and projected demographic changes, the annual number of expected cases in the country alone will increase from 393,000 in 2002 to 795,700 in 2030.4
Over the past 30 years, the understanding of gastric carcinogenesis has advanced. Specifically, the role of H. pylori and the development of gastric cancer through a series of precancerous lesions have shifted the focus of gastric cancer research from palliative strategies to the development of preventive strategies, including screening for H. pylori, especially in China where nearly 60% are infected, the majority of whom are asymptomatic.5,6 Although the infection is relatively easy to detect and treat with a non-invasive diagnostic test and course of antibiotics, the effectiveness of treatment to prevent gastric cancer is uncertain and screening thus far has not been recommended. Ideally, the strongest evidence would come from randomized controlled trials that use gastric cancer incidence as the primary outcome. Several clinical trials are underway7 and results from the first of such studies showed that after 7.5 years, treatment for H. pylori reduces cancer incidence among individuals without preexisting precancerous lesions, defined as atrophy, intestinal metaplasia and dysplasia, at time of treatment.8 Studies measuring intermediate outcomes also provide indirect support for a benefit on cancer risk via reduced progression of precancerous lesions to cancer.
To estimate the public health benefits of a H. pylori screening program, several factors must be considered: the underlying natural history of H. pylori-associated disease; the heterogeneity of risk conferred by gender, age and country; the effectiveness of treatment for H. pylori in interrupting the pathway to cancer; and the feasibility of implementing a secondary prevention program at the population level. As no single empirical study can evaluate all possible H. pylori screening and treatment strategies, by integrating the best biologic, epidemiologic and economic data, mathematical simulation models can assist in decision making by leveraging available data on intermediate outcomes to estimate the avertable burden of disease expected with different strategies, identify influential factors for which better empirical data would be most valuable, and provide insight into the potential cost-effectiveness of different strategies.9
The feasibility of introducing a new public health intervention in any country, especially one that is resource-constrained, is complex given competing health priorities. Cost-effectiveness analysis when used in conjunction with other equally important information on affordability, political will and cultural preferences and distributional and equity considerations can provide useful information to decision makers considering alternative public health interventions and policies. By quantifying the relative health and economic consequences of one investment compared with another, cost-effectiveness analyses provide information on the investment’s “value for money.” To inform the dialogue and debate about the potential value of gastric cancer prevention programs, we explored the health benefits and economic consequences associated with screening for H. pylori in China.
Material and methods
Analytical overview
We used an empirically calibrated natural history model of noncardia intestinal gastric adenocarcinoma to estimate the benefits, costs and cost-effectiveness of multiple H. pylori screening strategies to prevent gastric cancer.10 The model was calibrated using a likelihood-based approach that ensures multiple model outputs are consistent with epidemiologic data on the prevalence of precancerous lesions and incidence of gastric cancer. We used a randomly-selected subset of good-fitting parameter sets identified in our model calibration to project the mean (and range) of lifetime risk of cancer, life expectancy and lifetime costs associated with different screening strategies. To assess the comparative performance of various screening strategies, we calculated incremental cost-effectiveness ratios, defined as the additional cost of a specific strategy divided by its additional clinical benefit, compared to the next least expensive strategy. We adopted a modified societal perspective in that we did not include patient time costs, and discounted all costs and clinical consequences at a rate of 3% per year as recommended by the U.S. Panel on Cost-Effectiveness in Health and Medicine and other guidelines.11-13 Costs are expressed in US 2005 dollars (US$ 1 = 8.18 Yuan).
Natural history model
We developed a state-transition simulation model of the natural history of noncardia intestinal gastric adenocarcinoma in which disease progression of a cohort entering the model is characterized as a sequence of monthly transitions between health states (Fig. 1).10 At the start of the simulation, a cohort representative of 20-year olds in the high-risk region of Linqu, China enters the model and is distributed among the health states defined by the precancerous process based on H. pylori seroprevalence, age-specific prevalence of precancerous lesions and the proportion of gastric cancers that are H. pylori-positive (Fig. 1). Although some H. pylori-negative individuals have normal gastric mucosa, all individuals infected with H. pylori have gastritis or more advanced precancerous lesions. Movement through the health states occurs in monthly increments according to probabilities that are dependent on sex and H. pylori status. Individuals are followed throughout their lifetime and the model is run separately for men and women.
Figure 1.
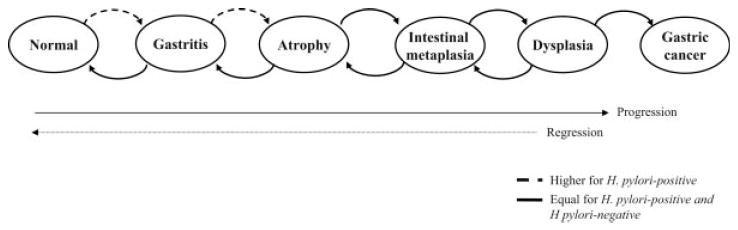
Model structure of gastric cancer natural history. The model simulates the natural history of gastric carcinogenesis through a series of health states (normal gastric mucosa, chronic nonatrophic gastritis, gastric atrophy, intestinal metaplasia, dysplasia and gastric cancer). Each month, individuals can progress and regress among the health states and face age-dependent risks of dying from other causes. H. pylori-infected individuals face higher probabilities of progressing to gastritis and atrophy. Not shown are unique health states, which were defined to distinguish individuals with H. pylori infection and gastric cancer detected through symptoms, and the dead state.
Model assumptions
We convened an expert panel to review our model assumptions and identify reasonable estimates for model parameters for which direct clinical data are unavailable. The following assumptions were made based on the expert consultation: (i) individuals acquire H. pylori infection during childhood and unless treated with antibiotics, remain infected;14 (ii) new infections and reinfection in adulthood are rare;15,16 (iii) all infected individuals develop gastritis and face a higher risk of developing atrophy;17 (iv) precancerous lesions may regress to less advanced lesions;18-20 (v) in the absence of other causes of death, all gastric cancers become clinically symptomatic within 2 years;21 and (vi) 95% of all gastric cancers are adenocarcinomas, 95% of adenocarcinomas are distal to the cardia and 40, 50 and 60% of noncardia adenocarcinomas are intestinal type for individuals younger than 44, between 45 and 65, and older than 65 years of age.22
Model parameterization and calibration
Details of model parameterization, including the calibration methods, have been described previously.10 Briefly, we first identified a plausible range for each parameter by conducting a literature review and selecting the highest and lowest values among all available studies. We then established targets for calibration using epidemiologic data on: (i) age-specific prevalence of gastritis, atrophy, intestinal metaplasia and dysplasia for 5-year age groups between the ages of 35 and 64 and (ii) age-specific incidence of gastric cancer for 5-year age groups between the ages of 25 and 84. Likelihood-based methods were used to identify good-fitting parameter sets, defined as those with goodness-of-fit scores statistically indistinguishable from the score of the best-fitting parameter set (α level = 0.05), which produced output consistent with these data. To explicitly incorporate the effect of parameter uncertainty, analyses were conducted with a random subset of 50 such good-fitting parameter sets, and results were reported as a mean and range of outcomes, whereas incremental cost-effectiveness ratios were reported as the ratio of the mean-costs divided by the mean-effects of all 50 parameter sets.
Screening strategies
For a cohort of 20-year olds, we assessed the health and economic outcomes associated with the baseline strategy of no screening or treatment for H. pylori and the following 3 strategies at ages 20, 30, 40, 50 and 60: (i) H. pylori screening once with a serology test and antibiotic treatment for positive test results, (ii) H. pylori screening once followed by rescreening individuals with negative results and (iii) universal treatment for H. pylori with antibiotics. We also evaluated the strategies for older cohorts between the ages of 30 and 60. For the screening strategy with rescreening, we varied the number of opportunities to repeat screening in individuals who originally tested negative (1 or 2 opportunities for rescreening) and assumed the next screening opportunity would occur during the subsequent 5-year interval. Although screening for H. pylori among asymptomatic individuals has been debated, proposals for universal treatment to our knowledge are not currently being considered.23 Because of the high prevalence of H. pylori in China and limited consequences associated with treatment, we evaluated this strategy to illustrate the relative benefits and costs associated with avoiding all screening costs and allocating resources to simply providing treatment.
We assumed that (i) all individuals without symptomatic gastric cancer are eligible for screening; (ii) diagnostic test characteristics do not vary by precancerous lesion; (iii) once screened, all test positive individuals receive treatment; (iv) treatment takes effect after 1 month;24 (v) H. pylori-positive individuals who are screened, test positive and treated will face a lower risk of progression to atrophy and a higher likelihood of regressing to gastritis than untreated H. pylori-positive individuals;25 (vi) all other individuals will face transition probabilities that reflect disease progression for their H. pylori status and (vii) individuals who progress to symptomatic gastric cancer during the month of screening will still receive H. pylori treatment.
Clinical data
Table I shows selected model variables and their plausible ranges.8,18,26-46 For H. pylori treatment, based on 3 epidemiologic studies which evaluated the effect on precancerous lesions or gastric cancer incidence after 5–7.5 years,8,18,36 we assumed that effectiveness depended on the absence of advanced precancerous lesions and reduced the probabilities of disease progression for individuals with gastritis and atrophy as described above. To estimate the magnitude of effect, we calibrated the transition probabilities between gastritis and atrophy to fit intention-to-treat post-treatment data on the prevalence of gastritis and atrophy from a clinical study in Linqu, China (see Supplementary Appendix for additional details).36 In sensitivity analysis, as the clinical evidence for atrophy regression after treatment is less conclusive,25 we limited treatment effect to individuals with gastritis. Because the estimate of treatment effectiveness was specific to the amoxicillin and omeprazole regimen used in the Linqu clinical study, we varied the relative risk of progression to atrophy over a wide range uniformly across all parameter sets to provide insight on other treatments for which no empirical data are available.
TABLE I.
MODEL VARIABLES: BASELINE VALUES AND RANGES USED IN SENSITIVITY ANALYSIS
| Variable | Base case | Range | Reference |
|---|---|---|---|
| H. pylori and precancerous lesions prevalence | |||
| H. pylori seroprevalence (%) | 70 | 30–80 | 26, 27 |
| Baseline prevalence at age 20 (%) | |||
| Gastritis | 65 | – | 28 |
| Atrophy | 18 | – | 28 |
| Intestinal metaplasia | 12 | – | 28 |
| Dysplasia | 5 | – | 28 |
| Clinical | |||
| Invasive cancer to symptomatic cancer1 | 0.25 | 0.2–0.3 | 2 |
| Five-year gastric cancer survival rate (%) | 20 | 18–23 | 29–31 |
| Monthly all-cause mortality rate | 0.0001–0.04693 | – | 32 |
| Screening and treatment | |||
| H. pylori diagnostic test characteristics (%) | |||
| Serology test | |||
| Sensitivity | 90 | 85–95 | 33–35 |
| Specificity | 90 | 79–98 | 33–35 |
| C13 urea breath test | |||
| Sensitivity | 95 | 92–98 | 35 |
| Specificity | 95 | 94–99 | 35 |
| Treatment effectiveness4 | |||
| Relative risk of progression to atrophy | 0.1–0.7 | 5 | 8, 18, 36 |
| Relative risk of regression to gastritis | 2.0–2.4 | 5 | 8, 18, 36 |
| Direct medical costs, US $ (2005)6 | |||
| Outpatient visit | 1.8 | 0.9–3.5 | 37 |
| H. pylori serology test | 1.6 | 0.7–3.8 | 33, 37–40 |
| H. pylori C13 urea breath test | 8.0 | 3.2–14.5 | 37, 39 |
| Antibiotic treatment for H. pylori | 4.3 | 2.0–12.0 | 8, 36, 41, 42 |
| Gastric cancer treatment | 2615 | 250–5230 | 33, 37, 43, 44 |
| Program costs (%)7 | 25.0 | 12.5–50.0 | 2 |
| Quality of life, weights | |||
| Normal gastric mucosa | 0.566–0.945 | 3 | 45 |
| Gastritis | 0.566–0.945 | 3 | 45 |
| Atrophy | 0.566–0.945 | 3 | 45 |
| Intestinal metaplasia | 0.566–0.945 | 3 | 45 |
| Dysplasia | 0.566–0.945 | 3 | 45 |
| Symptomatic gastric cancer | 0.49 | 0.17–0.79 | 46 |
Monthly probability.
Based on expert opinion.
Values are age- and sex-specific.
Values are sex-specific and vary by natural history parameter set.
For sensitivity analysis, base case estimate was varied ±25%.
Unit costs, except where noted. See Supplementary Appendix for details on upper and lower bound estimations.
Percent of initial outpatient visit and serology test costs.
Other clinical data, including test characteristics of H. pylori diagnostic tests,33-35 5-year gastric cancer survival rate,29-31 all-cause mortality rates32 and health-related quality of life associated with symptomatic cancer46 were obtained from the published literature. Quality-adjusted life years (QALYs) were age- and sex-specific to China.45
Cost data
Direct medical costs were based on the treatment protocol of the clinical study in Linqu, China36 and estimated from a comprehensive review of published cost-effectiveness studies, cost studies and national and international databases (Table I). We utilized a quantity-and-price approach for costing, in which quantities of each input and cost per input were estimated and total cost was then computed by multiplying price per unit and number of units per service to calculate price per service. Direct medical costs included costs for outpatient visits,37 H. pylori diagnostic tests,33,37-40 anti-H. pylori antibiotic treatment8,36,41,42 and gastric cancer treatment.33,37,43,44 Costs were based on World Health Organization (WHO) regional estimates for outpatient visits,37 published estimates for diagnosis test and gastric cancer treatment costs,33 and an international drug price indicator guide,41 adjusted upwards to reflect transportation costs associated with tradable goods,42 for antibiotic costs.
We assumed that (i) screening entailed an initial outpatient visit and H. pylori serology test, anti-H. pylori antibiotic treatment for test positive results, a follow-up visit to evaluate treatment success with a C13 urea breath test, and re-treatment for test positive results; (ii) universal treatment included an initial outpatient visit and anti-H. pylori antibiotic treatment, a follow-up visit with a C13 urea breath test to confirm treatment success, and retreatment for test positive results and (iii) gastric cancer treatment consisted of an initial surgery with hospitalization and 2 years of outpatient care. On the basis of expert opinion, we assumed program-related costs for each strategy were equivalent to 25% of initial outpatient visit and serology test costs. Details on the plausible range used for each cost in sensitivity analyses are described in the Supplementary Appendix. Sensitivity analyses also explored the impact of alternative discount rates and inclusion of indirect patient time costs.
Because our analysis is intended to inform decision making within China and to primarily compare different strategic approaches to screening (as opposed to broad international comparison of the cost-effectiveness of gastric cancer prevention between countries), we do not express results in international dollars. Costs in Chinese Yuan were adjusted to 2005 for inflation using the country-specific consumer price index (CPI) and then converted to US dollars.47 Results in international dollars are available from the authors upon request.
Results
Calibration results
The majority of model output for men from the random subset of 50 good-fitting parameter sets fell within the 95% confidence intervals of the epidemiological data on age-specific prevalence of precancerous lesions and incidence of gastric cancer (Fig. 2). Overall results for women were similar although showed lower rates of dysplasia (see Supplementary Appendix).
Figure 2.
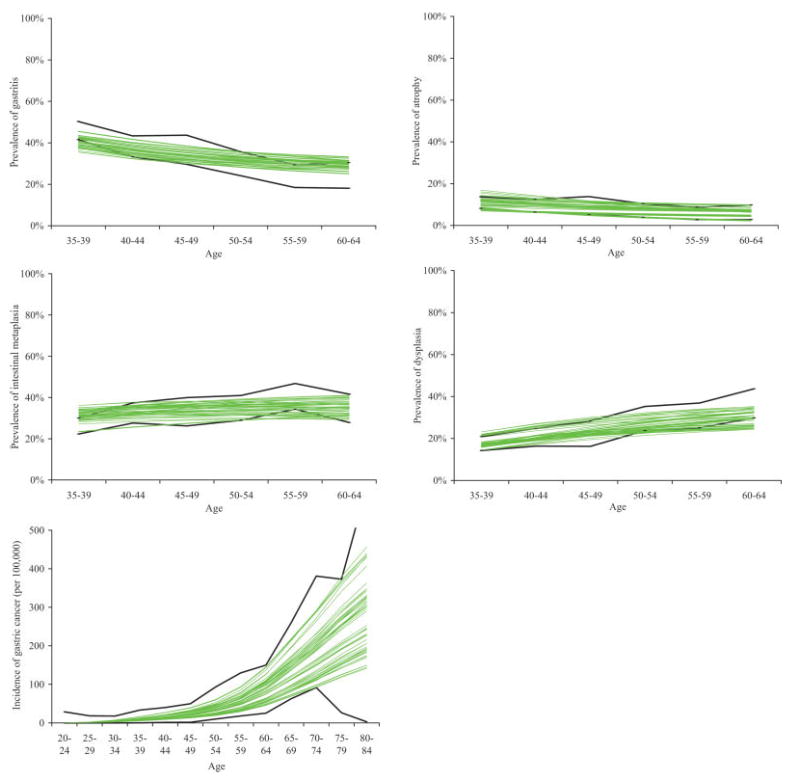
Comparison of model output to epidemiologic data on prevalence of precancerous lesions and gastric cancer incidence for 50 good-fitting parameter sets for men. Model output for precancerous lesions prevalence are depicted in the top rows and for gastric cancer incidence in the bottom row. Bold lines indicate 95% confidence intervals of age-specific prevalence or incidence data. Non-bold lines depict model output for 50 randomly-selected good-fitting parameter sets. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Reduction in lifetime risk of gastric cancer
Among a cohort of 20-year-old men with a H. pylori seroprevalence of 70%, a single screening at age 20 was estimated to reduce the lifetime risk for intestinal type gastric cancer by a mean of 14.5% (Table II). Strategies which employed 1 and 2 opportunities for rescreening individuals who tested negative during their initial screen provided little incremental benefit. Universal H. pylori treatment at age 20 reduced lifetime risk by 16.1%. Results for women were similar although the mean reduction was greater (26.6–29.5%), reflecting the higher proportion who had gastritis or atrophy and benefited from treatment.
TABLE II.
THE COSTS, LIFE EXPECTANCY, AND INCREMENTAL COST-EFFECTIVENESS OF H. pylori SCREENING STRATEGIES FOR A 20-YEAR-OLD COHORT
| Sex | Strategy | Mean for 50 good-fitting parameter sets (range)
|
ICERs1 |
||||
|---|---|---|---|---|---|---|---|
| Gastric cancer incidence reduction, % | Additional undiscounted life expectancy vs. no screen or treat, days | Additional discounted life expectancy vs. no screen or treat, days | Discounted lifetime costs, $ | $ per YLS | $ per QALY | ||
| Men | No screen or treatment2 | – | – | – | 18.50 (10.00–31.30) | – | – |
| Screen | 14.5 (6.5–30.2) | 14.1 (11.1–25.0) | 3.2 (2.5–5.8) | 30.30 (22.40–41.20) | $1,340 | $1,560 | |
| Screen + rescreen once | 15.6 (7.0–32.5) | 15.1 (11.9–26.6) | 3.4 (2.7–6.2) | 32.50 (24.60–43.30) | Dominated3 | Dominated3 | |
| Universal treatment | 16.1 (7.2–33.6) | 15.6 (12.3–27.5) | 3.6 (2.8–6.4) | 32.90 (25.10–43.70) | $2,720 | $3,250 | |
| Screen + rescreen twice | 15.7 (7.0–32.7) | 15.2 (12.0–26.8) | 3.4 (2.7–6.2) | 33.60 (25.70–44.30) | Dominated | Dominated | |
| Women | No screen or treatment2 | – | – | – | 10.70 (5.50–20.00) | – | – |
| Screen | 26.6 (12.9–40.0) | 17.0 (10.2–33.5) | 3.4 (2.0-6.8) | 22.30 (18.20–28.90) | $1,230 | $1,500 | |
| Screen + rescreen once | 28.8 (13.9–43.3) | 18.3 (11.0–36.0) | 3.7 (2.2–7.2) | 24.50 (20.50–30.90) | Dominated3 | Dominated3 | |
| Universal treatment | 29.5 (14.3–44.5) | 18.9 (11.4–37.2) | 3.8 (2.3–7.5) | 25.00 (20.90–31.30) | $2,510 | $3,060 | |
| Screen+ rescreen twice | 28.9 (14.0–43.6) | 18.4 (11.1–36.2) | 3.7 (2.2–7.3) | 25.60 (21.50–32.00) | Dominated | Dominated | |
Calculated as the ratio of the mean-costs divided by the mean-effects of the 50 good-fitting parameter sets for each strategy compared with the next-best strategy. ICER denotes incremental cost-effectiveness ratio. YLS denotes year of life saved. QALY denotes quality-adjusted life year.
Assumes no screening and cases identified only via symptoms.
Eliminated because of extended dominance: strategies with a higher incremental cost-effectiveness ratio than a more effective alternative strategy.
Cost-effectiveness of H. pylori screening and treatment
Cost-effectiveness results are shown in Table II for a 20-year-old cohort. In the absence of H. pylori screening or treatment, the discounted per-person average lifetime cost was $19 and the discounted average life expectancy was 25.8015 years. Screening once at age 20 provided a mean reduction of 14.5% in the lifetime risk of gastric cancer, providing an average increase in life expectancy of 3.2 days and an increase in lifetime costs of $12. The incremental cost-effectiveness ratio (ICER) was $1,340/YLS compared to no screening. Universal treatment dominated strategies that included rescreening in that they were less costly and less cost-effective (extended dominance), or more costly and less effective (strong dominance). Results in which life expectancy was quality-adjusted were similar, with an ICER of $1,560 per QALY for screening once and $3,250 per QALY for universal treatment. The ranking of strategies, incremental costs and ICERs (screening once = $1,230/LYS; universal treatment = $2,510/LYS) were comparable for women. There is no universal criterion that defines a threshold about which an intervention would be considered cost-effective (or good value for money). One heuristic that has evolved from the Commission on Macroeconomics and Health suggests that interventions with ICERs less than 3-times the GDP per capita ($5,400 in China) are “cost-effective” and less than 1-times GDP per capita ($1,700) to be cost-effective.48 At the 1-times GDP per capita threshold, screening 20-year olds for H. pylori, and treating those who test positive, would be considered very cost-effective for both men and women. (Of note, cost-effectiveness analysis does not provide insight into affordability. There are many interventions that would be considered good value for money but are not affordable).
Sensitivity analyses
Univariate sensitivity analyses showed that results were stable despite varying in the base case values for H. pylori serology test characteristics, medical costs for outpatient visits and gastric cancer treatment and program costs (Fig. 3). Results were most sensitive to H. pylori diagnostic test costs, antibiotic costs, H. pylori seroprevalence and treatment effectiveness. Rank ordering of strategies and general results were robust despite discount rates of 6% and inclusion of patient time costs. H. pylori screening was less effective and cost-effective for older cohorts ranging from 30 to 60 years of age (Table III). For all cohorts, screening or treatment at the youngest age was more cost-effective than all other strategies. When new infection and reinfection rates after treatment of 1% per year were included, results were similar for screening once ($1,540–1,600/LYS), although universal treatment was dominated by strategies that included rescreening.
Figure 3.
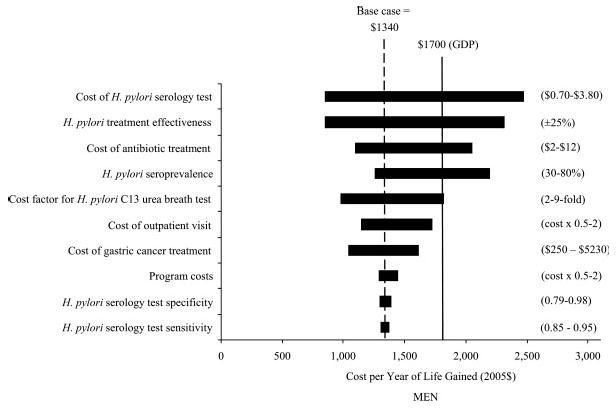
Sensitivity analysis on select variables for men. The X-axis shows the effect of changes in selected variables on the incremental cost-effectiveness ratio ($/YLS) for H. pylori screening once among men. The Y-axis shows the selected model variables. Values in parentheses are the upper and lower bounds used in the sensitivity analysis; the shaded bars indicate the variation in the cost-effectiveness ratio caused by changes in the value of the indicated variable while all other variables were held constant. The vertical dashed line indicates the incremental cost-effectiveness ratio for the base case. The solid line indicates an implied cost-effectiveness threshold using the gross domestic product (GDP) per capita in China.
TABLE III.
REDUCTION IN LIFETIME RISK OF GASTRIC CANCER AND COST-EFFECTIVENESS OF H. pylori SCREENING STRATEGIES FOR OLDER COHORTS
| Cohort age | Gastric cancer incidence reduction, %1 | Men–ICER ($/YLS)2 |
Gastric cancer incidence reduction, %1 | Women–ICER ($/YLS)2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screen | Screen + rescreen once | Screen + rescreen twice | Universal treatment | Screen | Screen + rescreen once | Screen + rescreen twice | Universal treatment | |||
| 20 | 14–16 | $1,340 | Dominated3 | Dominated | $2,720 | 27–30 | $1,230 | Dominated3 | Dominated | $2,510 |
| 30 | 9–10 | $2,050 | Dominated3 | Dominated | $4,030 | 18–20 | $1,710 | Dominated3 | Dominated | $3,420 |
| 40 | 5–6 | $3,940 | Dominated3 | Dominated | $7,530 | 11–12 | $2,790 | Dominated3 | Dominated | $5,460 |
| 50 | 2–3 | $9,420 | Dominated3 | Dominated | $19,020 | 6–7 | $5,430 | Dominated3 | Dominated | $10,560 |
| 60 | 1 | $30,030 | Dominated3 | Dominated | $60,360 | 3 | $13,680 | Dominated3 | Dominated | $24,290 |
Range of mean reductions calculated using all 50 good-fitting parameter sets among all H. pylori screening strategies.
Calculated as the ratio of the mean-costs divided by the mean-effects of the 50 good-fitting parameter sets for each strategy compared with the next-best strategy. ICER denotes incremental cost-effectiveness ratio. YLS denotes year of life saved.
Eliminated because of extended dominance: strategies with a higher incremental cost-effectiveness ratio than a more effective alternative strategy.
We also found results were insensitive to assumptions about the proportion of gastric cancers related to H. pylori, screening participation rates and alternative H. pylori treatment and detecting protocols. For example, if a lower cost, lower specificity H. pylori stool test was used to confirm post treatment eradication, ICERs for screening once ($950–1,040/LYS) and universal treatment remained similar ($1,240–1,350/LYS). Results varied by time interval between rescreening opportunities and H. pylori diagnostic test characteristics. Shorter 1-year intervals increased the incremental cancer incidence reduction compared with screening once by 20%. Although our base case false negative rate (FNR) of 10% resulted in only a 10.3% increase in cancer reduction compared to a single screen, at a FNR of 30%, strategies that included rescreening increased the cancer reduction by 37.5%. Despite this greater benefit, these strategies were still more costly and less effective or less costly and less cost-effective than universal treatment, and the ICER associated with the preemptive strategy became more attractive ($1,480/YLS).
To reflect the geographical variation in H. pylori seroprevalence in China, we identified the optimal strategy for a given seroprevalence and cost-effectiveness threshold (Fig. 4). Given the 1-times the GDP per capita cost-effectiveness threshold, H. pylori screening or universal treatment was the preferred strategy for seroprevalence levels greater than 40%.
Figure 4.
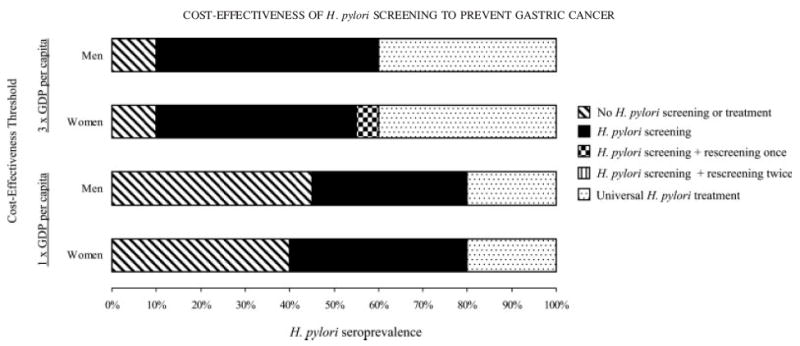
Optimal strategy by cost-effectiveness threshold and H. pylori seroprevalence. Top 2 graphs depict optimal strategy for men and women given the 3-times the GDP per capita cost-effectiveness threshold ($5,400). Lower 2 graphs depict optimal strategy given the 1-times GDP per capita threshold ($1,700).
To further access the uncertainty around treatment effectiveness among men, we conducted a series of scenario analyses in which H. pylori treatment affected disease progression for gastritis only (i.e. no effect on atrophy). Under this assumption, the mean reduction in gastric cancer incidence was 29% lower, and the ICER moderately increased to $1,990/LYS. When we varied the relative risk of progressing to atrophy uniformly for all parameter sets, the mean reduction in gastric cancer incidence ranged from 3% to 42% depending on whether treatment reduced the risk of progressing to atrophy by 20% (relative risk = 0.8) or halted progression entirely (relative risk = 0; Fig. 5). Figure 6 shows that if treatment reduced disease progression rate by 60–70% (relative risk = 0.3–0.4), screening once would be considered cost-effective given the 1-times GDP per capita threshold, even if antibiotic costs increased by 3-fold from $4 to $13.
Figure 5.
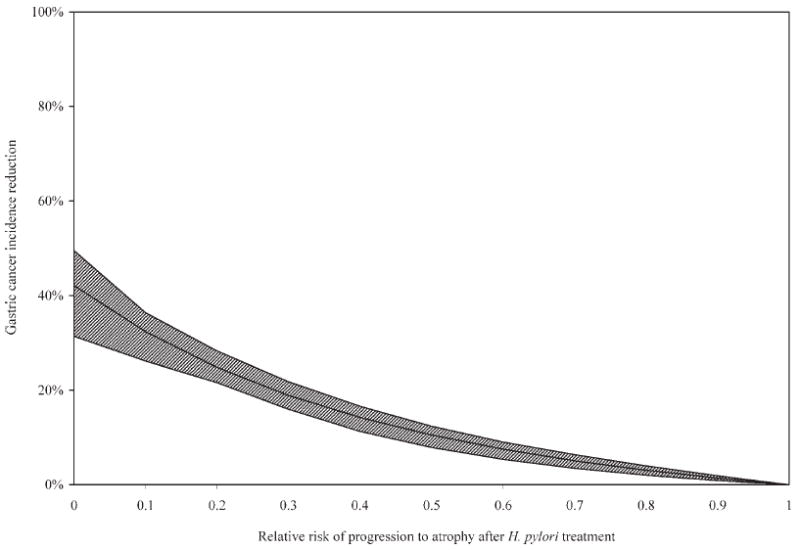
Sensitivity analysis on treatment effectiveness for gastritis. If treatment for H. pylori reduced disease progression for gastritis only (i.e. no effect on atrophy), the mean reduction in gastric cancer incidence ranged from 0% (RR = 1; no effect) to 42% (RR = 0; halt progression entirely). Solid line indicates the mean reduction among 50 good-fitting parameter sets. Shaded area indicates the range.
Figure 6.
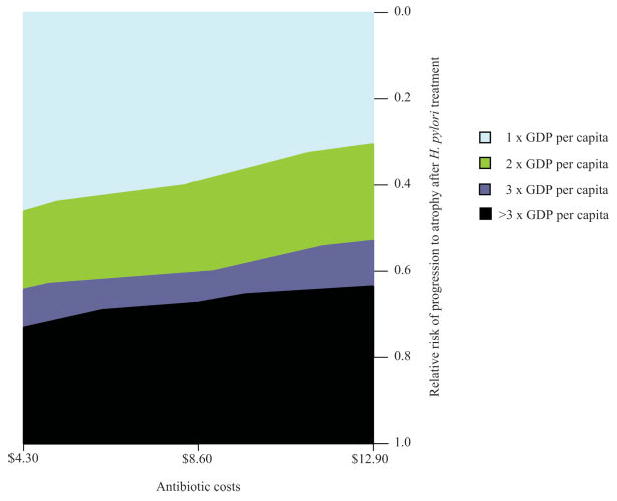
Two-way sensitivity analysis on treatment effectiveness and cost. H. pylori screening once would be considered cost-effective given commonly-used thresholds if treatment reduced disease progression to atrophy by more than 40% (RR = 0.6), even if antibiotic costs were 3-fold higher than base case estimates. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
Currently, clinical guidelines do not recommend screening for H. pylori in asymptomatic individuals.49-53 Our results suggest that H. pylori screening and treatment has the potential to significantly reduce gastric cancer incidence among both men and women, and there appear to be strategies that would be considered cost-effective in China. Reductions were greatest when screening occurred at age 20, suggesting that H. pylori prevention efforts should target younger age groups. This policy-relevant result presents an interesting contrast to the older age groups who are the focus of on-going H. pylori clinical trials and from which results are awaited.7 We also found that opportunities for rescreening did not provide substantial additional benefit provided the false-negative rate of serological screening was less than 15%. The reduction in gastric cancer risk was greater among women than men, reflecting the higher proportion with gastritis or atrophy who benefited from treatment.
Using a cost-effectiveness threshold of the GDP per capita ($1,700 in China), we found screening for H. pylori at age 20 would be considered very cost-effective for both men and women. For cohorts of all ages, screening or treatment at the youngest age was more cost-effective than all other strategies. For example, for a cohort of 20-year olds, delaying screening for 10 or 20 years versus beginning screening at age 20 was not only less effective, but more costly, as at older ages, a greater proportion of the cohort had progressed to more advanced precancerous lesions and did not benefit from H. pylori treatment as a result.
Given a specific cost-effectiveness threshold, we found that the optimal strategy was influenced by the underlying seroprevalence of H. pylori infection. This could be particularly important for establishing regional priorities within China because there is geographical variation of H. pylori seroprevalence.54,55 At the GDP per capita threshold, H. pylori screening once would be optimal in regions where H. pylori seroprevalence was between 40 and 80%; in regions where more than 80% were infected, universal treatment would be the preferred strategy. Because we did not include the consequences of antibiotic resistance and treatment side-effects, the threshold for universal treatment may be higher, although studies have consistently found rates of amoxicillin-resistant H. pylori equal to less than 1%56 and our results did not significantly change when we assumed treatment for side-effects increased antibiotic costs by 50%. As the emergence of resistant strains would adversely impact the efficacy of H. pylori treatment regimens, as well as antibiotic efficacy against other infections, efforts to minimize noncompliance should accompany all screening strategies.
From a public health perspective, screening for H. pylori is particularly attractive. Because reinfection in adulthood is rare, once treated, individuals do not need to be rescreened or retreated. This differs with prevention programs for other cancers such as breast, colon and cervical, which rely on routine screening to detect precancerous or cancerous growths or lifestyle changes that require continual modification of diet, exercise or smoking habits to achieve significant reductions in cancer risk. For example, H. pylori screening once would result in an average life expectancy gain of 17.0 days which is 2 to 4 times greater than the estimated 4.3–9.4 days achieved with biennial mammography screening to prevent breast cancer among Hong Kong Chinese women.57 In addition, H. pylori screening is appealing because of the low false-negative risk associated with the high sensitivity of serology tests available to detect the infection. Although test specificity may be lower, the consequences of false-positives are small given the low cost of antibiotic treatment and occurrence of adverse side-effects. For example, even at a 30% false positive rate, the ICER of H. pylori screening increased only to $1,440/YLS.
Adherence to the 14-day antibiotic regimen is critical to the effectiveness of an H. pylori screening program. More convenient and tolerable, shorter duration regimens with comparable eradication rates may improve patient adherence and minimize the emergence of antibiotic resistance from incomplete treatment. Although we reflected compliance rates of the clinical trial, real world rates are likely to be lower. By lowering treatment effectiveness by 25% to estimate the impact of lower compliance and adherence rates, we found that the cost-effectiveness of a single H. pylori screening would still be considered good value for resources given the GDP per capita threshold. However, if real world screening participation rates are lower than 70% or the per-person cost of H. pylori screening is $2 to $3 higher, universal treatment would be a strategy to seriously consider, even in medium-prevalence regions, although these thresholds are likely conservative as costs associated with consequences of treatment (i.e., antibiotic resistance and side-effects) were not included in our analyses.
Our findings are consistent with previous cost-effectiveness analyses that suggest H. pylori screening is cost-effective in both relatively low-risk populations in the US and UK ($10,000–$40,000/YLS)58-62 and high-risk populations in China or Taiwan ($200–$17,000/YLS).33,63 In contrast to previous models, we based treatment effectiveness on empirical data from an ongoing randomized clinical trial which showed H. pylori treatment reduced the prevalence precancerous gastric lesions.36 In addition, because we modeled gastric cancer development through a series of precancerous lesions, we were able to evaluate alternative assumptions on treatment impact. Although the ability of H. pylori treatment to heal gastritis and halt progression to precancerous lesions is well-supported by clinical evidence, the impact on regression is still debated.25 If treatment only reduced disease progression among individuals with gastritis, we found that screening once for H. pylori could still reduce the risk of gastric cancer among young adults by more than 10%. Specific to a low-efficacy dual therapy regimen no longer considered a choice of treatment,64 our estimate likely provides a lower bound estimate upon which higher-efficacy triple therapies can improve upon.
We also reflected in our model the impact of disease natural history uncertainty on outcomes by using an array of natural history parameters that provide a good fit to observed epidemiologic data. Although we estimated that screening once for H. pylori could significantly reduce cancer risk, we found that estimates of the absolute magnitude of benefit varied considerably when we explicitly considered the underlying uncertainty around disease progression and regression, highlighting the need for more data. As ongoing clinical trials are focused on older age groups, future clinical trials on younger adults are needed, with specific attention to the presence of precancerous lesions at time of treatment and high-efficacy, well-tolerated regimens that can achieve high compliance rates and minimize the emergence of antibiotic resistance. Given the need to follow large numbers of individuals for several years or decades, these trials will take many years to complete. In the short term, clinical trials with intermediate outcomes as their primary endpoints, such as the progression to atrophy or intestinal metaplasia, can provide valuable information on the effectiveness of H. pylori treatment. Our model suggests that if treatment reduces the risk of progression to atrophy by more than 40%, H. pylori screening once could be an effective and cost-effective gastric cancer prevention policy. Empirical data, especially on gastritis, from large, well-designed, randomized controlled trials should be high-priority. In addition, studies providing better estimates of disease progression rates for all precancerous lesions can reduce the uncertainty around disease natural history and provide insight into the management of advanced precancerous lesions, such as intestinal metaplasia, for which H. pylori treatment is unlikely to benefit and surveillance guidelines are currently unavailable.
Our analysis has several limitations. Data were combined from multiple sources with varied study designs, and many variables are uncertain. On the basis of the data from prospective cohort studies, we assumed that all lesions are reversible, though at some point in the precancerous process, this may no longer be biologically possible.20,65 Although a proportion of the regression observed in the prospective cohort studies may stem from low biopsy sensitivity for advanced lesions and histological misclassification, data on the precise proportion attributable are unavailable. Similarly, although some clinical studies suggest that H. pylori treatment may reduce disease progression among individuals with intestinal metaplasia, we conservatively assumed that lesions beyond atrophy do not benefit from treatment. As China-specific data on the proportion of intestinal type gastric cancers were unavailable, we also relied on data from Sweden. Because the incidence of intestinal type gastric cancers has declined more rapidly in developed countries, we may have therefore underestimated the proportion.66 All of these assumptions bias our results against treatment effect.
In addition, we included only the benefit of treatment on intestinal type gastric cancer reduction. H. pylori treatment may also reduce the risk of MALT lymphomas,67 duodenal ulcers,68 and dyspepsia-related illness,69 and thus, our analysis does not reflect these health gains or treatment cost reductions that may result from the reduction of these diseases. Although H. pylori infection may protect against cardia or esophageal adenocarcinomas, the causal link is still uncertain and was not incorporated into our model.70 Finally, our results are based on data from one specific region in China and may not be generalized to other regions where disease progression may differ given the prevalence of other risk factors. Nonetheless, H. pylori is the leading risk factor for gastric cancer and the relative risk associated with the infection (5.9)2 is three- to four-fold higher than other important risk factors, including smoking (1.5-2.2).71 As such, our results likely provide reasonable estimates of the comparative benefits and economic consequences of H. pylori screening in other regions of China, as well as other countries, that share similar risk factor profiles to Linqu.
Screening young adults for H. pylori, followed by treatment in those who test positive, has the potential to prevent 1 in every 4 to 6 cases of gastric cancer in China, and would be considered cost-effective using the GDP per capita threshold. Although additional criteria such as affordability, capacity to deliver and equity are equally influential and important to consider, these results clearly illustrate the potential promise of gastric cancer prevention. Given the ease of detecting and treating H. pylori infection and the poor prognosis and limited treatment options for gastric cancer, better data on the effectiveness of treatment to reduce disease progression are needed while results from ongoing clinical trials are eagerly awaited.
Supplementary Material
Additional Supporting Information may be found in the online version of this article.
Acknowledgments
Grant sponsor: National Cancer Institute; Grant number: R25-CA92203.
Footnotes
Published online 29 September 2008 in Wiley InterScience (www.interscience.wiley.com).
References
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekstrom AM, Held M, Hansson LE, Engstrand L, Nyren O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784–91. doi: 10.1053/gast.2001.27999. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Bray F, Pisani P, Parkin DM. IARC cancer base no. 5 version 2.0. Lyon: IARC Press; 2004. GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide. [Google Scholar]
- 5.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1–15. doi: 10.1111/j.1523-5378.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 7.Forman D. Results of intervention trials in Helicobacter pylori-infected populations. In: Hunt R, Tytgat G, editors. Helicobacter pylori: basic mechanisms to clinical cure 2002. Dordrecht: Kluwer Academic Publishers; 2003. pp. 225–30. [Google Scholar]
- 8.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of. China: a randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Goldie SJ, Goldhaber-Fiebert JD, Garnett GP. Chapter 18: public health policy for cervical cancer prevention: the role of decision science, economic evaluation, and mathematical modeling. Vaccine. 2006;3(24Suppl):S155–S63. doi: 10.1016/j.vaccine.2006.05.112. [DOI] [PubMed] [Google Scholar]
- 10.Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY, Goldie SJ. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev. 2008;17:1179–87. doi: 10.1158/1055-9965.EPI-07-2539. [DOI] [PubMed] [Google Scholar]
- 11.Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 12.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Priorities in health. Washington, DC: The World Bank; 2006. Cost-effectiveness Analysis; pp. 39–57. [PubMed] [Google Scholar]
- 13.Edejer TT, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 14.Xia HH, Talley NJ. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol. 1997;92:1780–7. [PubMed] [Google Scholar]
- 15.Mitchell HM, Hu P, Chi Y, Chen MH, Li YY, Hazell SL. A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China) Gastroenterology. 1998;114:256–61. doi: 10.1016/s0016-5085(98)70475-5. [DOI] [PubMed] [Google Scholar]
- 16.Parsonnet J. What is the Helicobacter pylori global reinfection rate? Can J Gastroenterol. 2003;B(17Suppl):46B–48B. doi: 10.1155/2003/567816. [DOI] [PubMed] [Google Scholar]
- 17.Kuipers EJ, Uyterlinde AM, Pena AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–8. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 18.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–8. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 19.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–40. [PubMed] [Google Scholar]
- 20.You WC, Li JY, Blot WJ, Chang YS, Jin ML, Gail MH, Zhang L, Liu WD, Ma JL, Hu YR, Mark SD, Correa P, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer. 1999;83:615–19. doi: 10.1002/(sici)1097-0215(19991126)83:5<615::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Craanen ME, Dekker W, Ferwerda J, Blok P, Tytgat GN. Early gastric cancer: a clinicopathologic study. J Clin Gastroenterol. 1991;13:274–83. doi: 10.1097/00004836-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Lundegardh G, Lindgren A, Rohul A, Nyren O, Hansson LE, Bergstrom R, Adami HO. Intestinal and diffuse types of gastric cancer: secular trends in Sweden since 1951. Br J Cancer. 1991;64:1182–6. doi: 10.1038/bjc.1991.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T, Uemura N, Kim JG, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–65. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 24.Ley C, Mohar A, Guarner J, Herrera-Goepfert R, Figueroa LS, Halperin D, Johnstone I, Parsonnet J. Helicobacter pylori eradication and gastric preneoplastic conditions: a randomized, double-blind, placebo-controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:4–10. doi: 10.1158/1055-9965.epi-03-0124. [DOI] [PubMed] [Google Scholar]
- 25.Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Megraud F, Xiao SD, Sugano K, Nyren O. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100:2100–15. doi: 10.1111/j.1572-0241.2005.41688.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown LM, Thomas TL, Ma JL, Chang YS, You WC, Liu WD, Zhang L, Pee D, Gail MH. Helicobacter pylori infection in rural. China: demographic, lifestyle and environmental factors. Int J Epidemiol. 2002;31:638–45. doi: 10.1093/ije/31.3.638. [DOI] [PubMed] [Google Scholar]
- 27.Ma JL, You WC, Gail MH, Zhang L, Blot WJ, Chang YS, Jiang J, Liu WD, Hu YR, Brown LM, Xu GW, Fraumeni JF., Jr Helicobacter pylori infection and mode of transmission in a population at high risk of stomach cancer. Int J Epidemiol. 1998;27:570–3. doi: 10.1093/ije/27.4.570. [DOI] [PubMed] [Google Scholar]
- 28.You WC, Blot WJ, Li JY, Chang YS, Jin ML, Kneller R, Zhang L, Han ZX, Zeng XR, Liu WD, Zhao L, Correa P, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317–21. [PubMed] [Google Scholar]
- 29.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 30.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Fay MP, Feuer EJ, Edwards BK. SEER cancer statistics review, 1975-2000. Bethesda: National Cancer Institute; 2003. [Google Scholar]
- 31.Tian J, Wang XD, Chen ZC. Survival of patients with stomach cancer in Changle city of China. World J Gastroenterol. 2004;10:1543–6. doi: 10.3748/wjg.v10.i11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez AD, Salomon J, Ahmad O, Murray CJL, Mafat D. GPE discussion paper series no. 9. Geneva: World Health Organization; 2000. Life tables for 191 countries for 2000: data, methods, results. [Google Scholar]
- 33.Wang Q, Jin PH, Lin GW, Xu SR, Chen J. Cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: Markov decision analysis. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:135–9. [PubMed] [Google Scholar]
- 34.Loy CT, Irwig LM, Katelaris PH, Talley NJ. Do commercial serological kits for Helicobacter pylori infection differ in accuracy? A metaanalysis. Am J Gastroenterol. 1996;91:1138–44. [PubMed] [Google Scholar]
- 35.Vaira D, Malfertheiner P, Megraud F, Axon AT, Deltenre M, Hirschl AM, Gasbarrini G, O’Morain C, Garcia JM, Quina M, Tytgat GN. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet. 1999;354:30–3. doi: 10.1016/s0140-6736(98)08103-3. [DOI] [PubMed] [Google Scholar]
- 36.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, Ma JL, Pan KF, Liu WD, Hu Y, Crystal-Mansour S, Pee D, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 37.Adam T, Evans D, Murray CJL. Econometric estimation of country-specific hospital costs. Cost Effectiveness Resour Allocation. 2003;1:3. doi: 10.1186/1478-7547-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Medical Association. Medicare fee calculator. Chicago: American Medical Association; 2004. [Google Scholar]
- 39.UK Health Protection Agency Primary Care Unit. [July 23, 2006];Test & Treat Helicobacter Management of Dyspepsia: Cost Comparison of Serology to Stool Antigen & Breath Test, 2005. http://www.hpa.org.uk/
- 40.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–10. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 41.McFadyen JE, editor. International drug price indicator guide. Cambridge: Management Sciences for Health; 2005. [Google Scholar]
- 42.Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health interventions. Cost Effectiveness Resour Allocation. 2003;1:1. doi: 10.1186/1478-7547-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark CG, Boulos PB, Ward MW. Cost effectiveness in the treatment of gastric cancer. Clin Oncol. 1980;6:303–7. [PubMed] [Google Scholar]
- 44.D’Amico D, Bassi N, Ranzato R. Cost-benefit of follow-up after total gastrectomy. Hepatogastroenterology. 1989;36:266–72. [PubMed] [Google Scholar]
- 45.Mathers CD, Murray CJL, Lopez A, Salomon JA, Sadana R, Tandon A, Ustün TB, Chatterji S. GPE discussion paper no. 38. Geneva: World Health Organization; 2000. Estimates of healthy life expectancy for 191 countries in the year 2000: methods and results. [Google Scholar]
- 46.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 47.EIU. Country Data. Economist Intelligence Unit; London: [August 1, 2006]. http://www.eiu.com/ [Google Scholar]
- 48.Report of the commission on macroeconomics and health. Geneva: World Health Organization; 2001. Macroeconomics and Health: Investing in Health for Economic Development. [Google Scholar]
- 49.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–9. [PubMed] [Google Scholar]
- 50.Hunt R, Thomson AB. Canadian Helicobacter pylori consensus conference. Canadian association of gastroenterology. Can J Gastroenterol. 1998;12:31–41. doi: 10.1155/1998/170180. [DOI] [PubMed] [Google Scholar]
- 51.Malfertheiner P, Megraud F, O’Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection–the Maastricht 2-2000 consensus report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 52.Chinese Society of Gastroenterology, Chinese Medical Association. Consensus on some issues regarding Helicobacter pylori infection. Chin J Dig Dis. 2001;2:53–6. [Google Scholar]
- 53.Chinese Society of Gastroenterology, Chinese Medical Association. Consensus on the management of Helicobacter pylori infection: Tong-cheng, Anhui Province, 2003. Chin J Dig Dis. 2004;5:186–8. doi: 10.1111/j.1443-9573.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 54.Forman D, Sitas F, Newell DG, Stacey AR, Boreham J, Peto R, Campbell TC, Li J, Chen J. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990;46:608–11. doi: 10.1002/ijc.2910460410. [DOI] [PubMed] [Google Scholar]
- 55.Junshi C, Campbell TC, Junyao L, Peto R. A study of the characteristics of 65 Chinese counties. Oxford: Oxford University Press; 1990. Diet, life-style and mortality in China. [Google Scholar]
- 56.Megraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374–84. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong IO, Kuntz KM, Cowling BJ, Lam CL, Leung GM. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110:885–95. doi: 10.1002/cncr.22848. [DOI] [PubMed] [Google Scholar]
- 58.Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150–4. doi: 10.1016/s0140-6736(96)01501-2. [DOI] [PubMed] [Google Scholar]
- 59.Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Patel P, Bhandari P. Cost-effectiveness of population screening for Helicobacter pylori in preventing gastric cancer and peptic ulcer disease, using simulation. J Med Screen. 2003;10:148–56. doi: 10.1177/096914130301000310. [DOI] [PubMed] [Google Scholar]
- 60.Mason J, Axon AT, Forman D, Duffett S, Drummond M, Crocombe W, Feltbower R, Mason S, Brown J, Moayyedi P. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther. 2002;16:559–68. doi: 10.1046/j.1365-2036.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 61.Harris RA, Owens DK, Witherell H, Parsonnet J. Helicobacter pylori and gastric cancer: what are the benefits of screening only for the CagA phenotype of H. pylori? Helicobacter. 1999;4:69–76. doi: 10.1046/j.1523-5378.1999.98057.x. [DOI] [PubMed] [Google Scholar]
- 62.Fendrick AM, Chernew ME, Hirth RA, Bloom BS, Bandekar RR, Scheiman JM. Clinical and economic effects of population-based Helicobacter pylori screening to prevent gastric cancer. Arch Intern Med. 1999;159:142–8. doi: 10.1001/archinte.159.2.142. [DOI] [PubMed] [Google Scholar]
- 63.Lee YC, Lin JT, Wu HM, Liu TY, Yen MF, Chiu HM, Wang HP, Wu MS, Hsiu-Hsi Chen T. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:875–85. doi: 10.1158/1055-9965.EPI-06-0758. [DOI] [PubMed] [Google Scholar]
- 64.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cross-sectional studies. Cancer Res. 1990;50:4731–6. [PubMed] [Google Scholar]
- 66.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765–70. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 67.Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3:97–104. doi: 10.1016/s1470-2045(02)00651-4. [DOI] [PubMed] [Google Scholar]
- 68.Ford A, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2004:CD003840. doi: 10.1002/14651858.CD003840.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. A community screening program for Helicobacter pylori saves money: 10-year follow-up of a randomized controlled trial. Gastroenterology. 2005;129:1910–17. doi: 10.1053/j.gastro.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 70.Nyren O, Blot WJ. Helicobacter pylori infection: mainly foe but also friend? J Natl Cancer Inst. 2006;98:1432–4. doi: 10.1093/jnci/djj422. [DOI] [PubMed] [Google Scholar]
- 71.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–71. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.


