Abstract
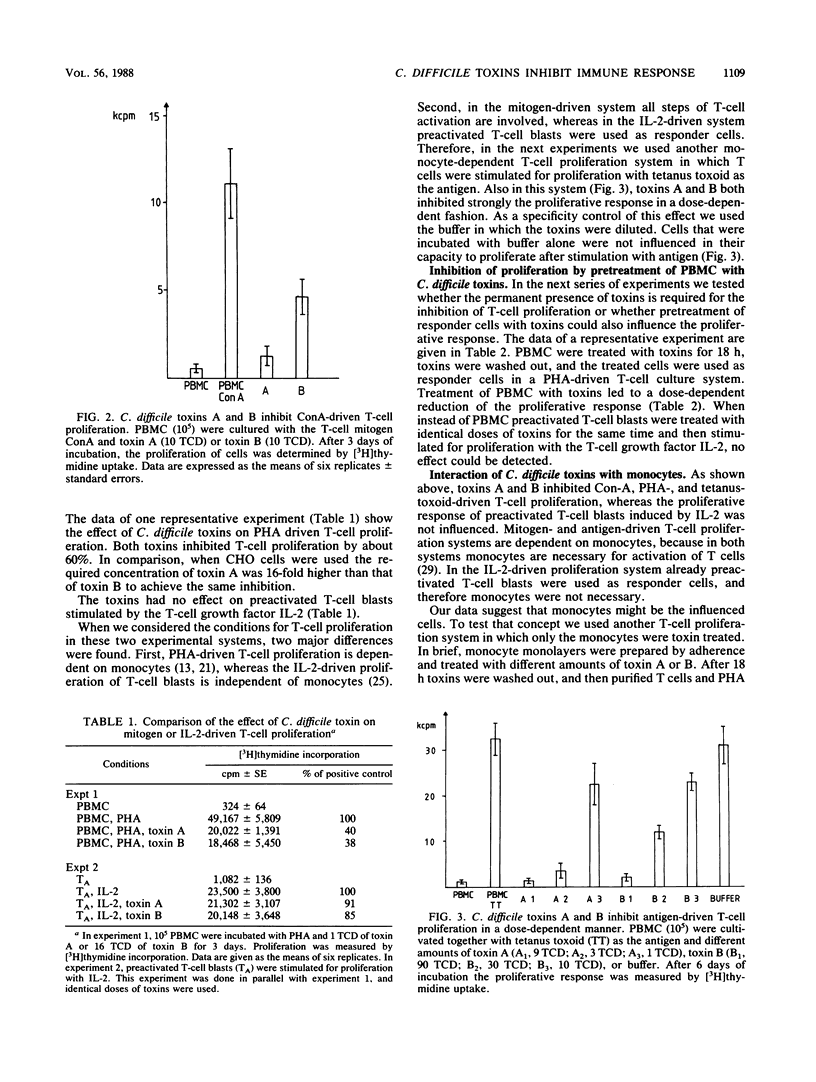
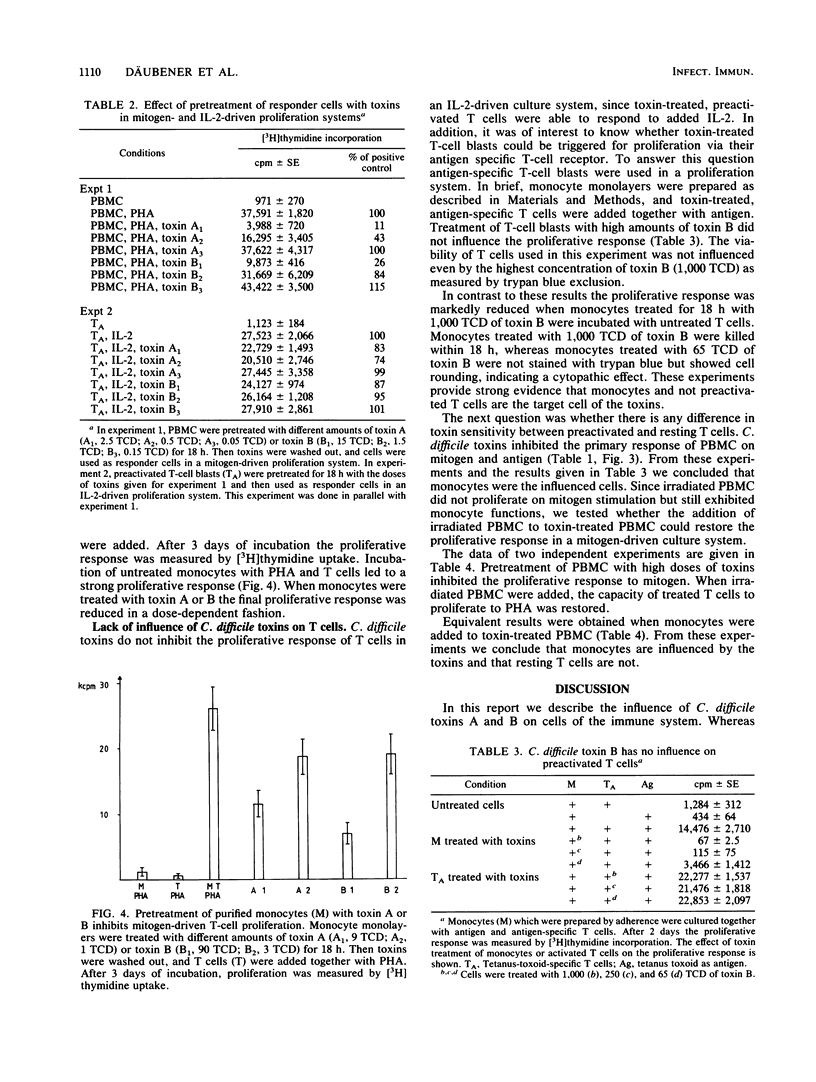
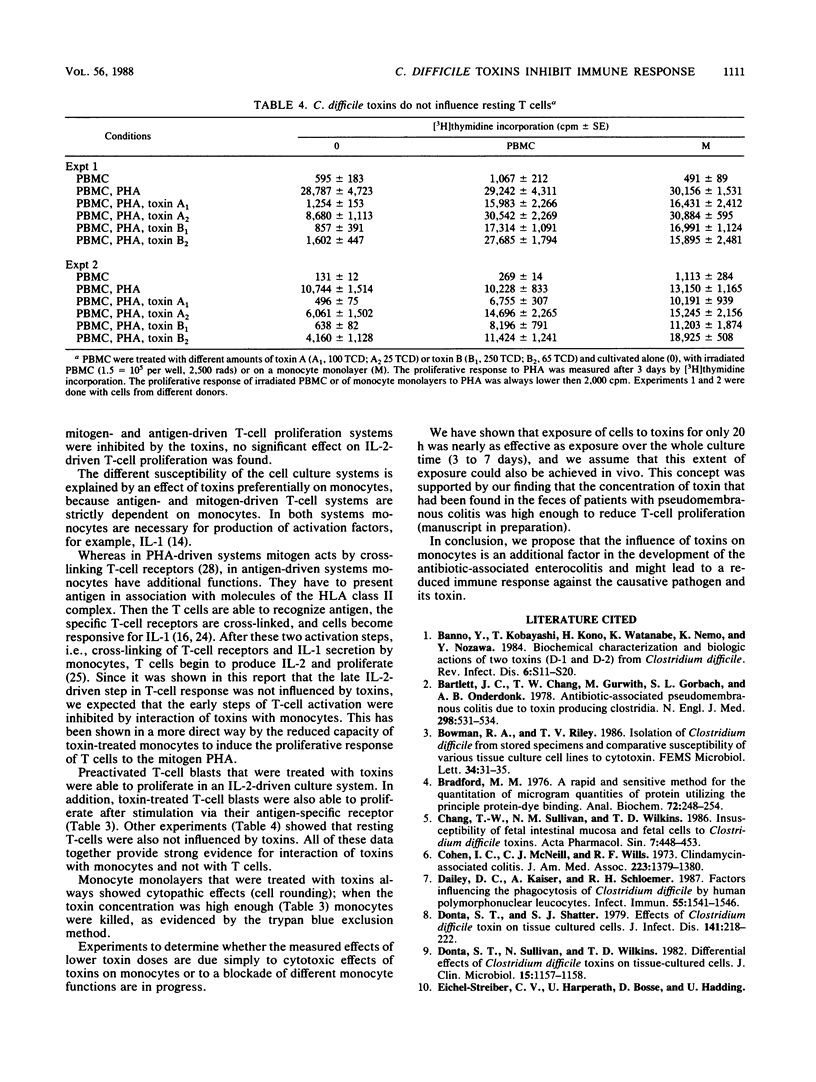
Two Clostridium difficile toxins isolated from strain VPI 10463 were tested for their effect on different human T-cell proliferation systems. In mitogen- and antigen-driven T-cell proliferation systems, toxins inhibited the proliferative response in a dose-dependent fashion. In interleukin-2-driven culture systems, no effect of toxins could be found on preactivated T cells. We suspected that monocytes were the influenced cells, since in antigen- and mitogen-driven systems monocytes were necessary for the proliferative response, whereas the interleukin-2-driven system was independent of monocytes. To prove this concept, purified monocytes were treated with toxins. The treatment was found to markedly reduce the capacity of monocytes to stimulate T-cell proliferation. No inhibition of the proliferative response was measured when, instead of monocytes, resting or preactivated T cells were treated with toxins. These experiments clearly show that C. difficile toxins interact with monocytes and not with T cells. The effect of toxins on cells of the immune system might be one factor in the development of pseudomembranous colitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Kobayashi T., Kono H., Watanabe K., Ueno K., Nozawa Y. Biochemical characterization and biologic actions of two toxins (D-1 and D-2) from Clostridium difficile. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S11–S20. doi: 10.1093/clinids/6.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Sullivan N. M., Wilkins T. D. Insusceptibility of fetal intestinal mucosa and fetal cells to Clostridium difficile toxins. Zhongguo Yao Li Xue Bao. 1986 Sep;7(5):448–453. [PubMed] [Google Scholar]
- Cohen L. E., McNeill C. J., Wells R. F. Clindamycin-associated colitis. JAMA. 1973 Mar 19;223(12):1379–1380. [PubMed] [Google Scholar]
- Dailey D. C., Kaiser A., Schloemer R. H. Factors influencing the phagocytosis of Clostridium difficile by human polymorphonuclear leukocytes. Infect Immun. 1987 Jul;55(7):1541–1546. doi: 10.1128/iai.55.7.1541-1546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Shaffer S. J. Effects of Clostridium difficile toxin on tissue-cultured cells. J Infect Dis. 1980 Feb;141(2):218–222. doi: 10.1093/infdis/141.2.218. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Sullivan N., Wilkins T. D. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J Clin Microbiol. 1982 Jun;15(6):1157–1158. doi: 10.1128/jcm.15.6.1157-1158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin I., Thelestam M. Internalization of Clostridium difficile cytotoxin into cultured human lung fibroblasts. Biochim Biophys Acta. 1983 Dec 19;763(4):383–392. doi: 10.1016/0167-4889(83)90100-3. [DOI] [PubMed] [Google Scholar]
- Gurwith M. J., Langston C., Dunsmore B. Morphologic and functional effects of Clostridium difficile enterotoxin in tissue culture. Can J Microbiol. 1982 Jan;28(1):100–105. doi: 10.1139/m82-009. [DOI] [PubMed] [Google Scholar]
- Hünig T., Loos M., Schimpl A. The role of accessory cells in polyclonal T cell activation. I. Both induction of interleukin 2 production and of interleukin 2 responsiveness by concanavalin A are accessory cell dependent. Eur J Immunol. 1983 Jan;13(1):1–6. doi: 10.1002/eji.1830130103. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Meyer zum Büschenfelde K. H. T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol. 1986 Jun 1;136(11):4106–4112. [PubMed] [Google Scholar]
- Mitchell M. J., Laughon B. E., Lin S. Biochemical studies on the effect of Clostridium difficile toxin B on actin in vivo and in vitro. Infect Immun. 1987 Jul;55(7):1610–1615. doi: 10.1128/iai.55.7.1610-1615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. J., Ketley J. M., Burdon D. W., Candy D. C., Stephen J. Biological mode of action of Clostridium difficile toxin A: a novel enterotoxin. J Med Microbiol. 1987 May;23(3):211–219. doi: 10.1099/00222615-23-3-211. [DOI] [PubMed] [Google Scholar]
- Pothoulakis C., Barone L. M., Ely R., Faris B., Clark M. E., Franzblau C., LaMont J. T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986 Jan 25;261(3):1316–1321. [PubMed] [Google Scholar]
- Pothoulakis C., Triadafilopoulos G., Clark M., Franzblau C., LaMont J. T. Clostridium difficile cytotoxin inhibits protein synthesis in fibroblasts and intestinal mucosa. Gastroenterology. 1986 Nov;91(5):1147–1153. doi: 10.1016/s0016-5085(86)80010-5. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Rothman S. W., Brown J. E., Diecidue A., Foret D. A. Differential cytotoxic effects of toxins A and B isolated from Clostridium difficile. Infect Immun. 1984 Nov;46(2):324–331. doi: 10.1128/iai.46.2.324-331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Ucer U., Wrann M., Pfizenmaier K. Early events during primary activation of T cells: antigen receptor cross-linking and interleukin 1 initiate proliferative response of human T cells. Eur J Immunol. 1985 Nov;15(11):1091–1095. doi: 10.1002/eji.1830151105. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Brönnegård M. Interaction of cytopathogenic toxin from Clostridium difficile with cells in tissue culture. Scand J Infect Dis Suppl. 1980;(Suppl 22):16–29. [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wedel N., Toselli P., Pothoulakis C., Faris B., Oliver P., Franzblau C., LaMont T. Ultrastructural effects of Clostridium difficile toxin B on smooth muscle cells and fibroblasts. Exp Cell Res. 1983 Oct 15;148(2):413–422. doi: 10.1016/0014-4827(83)90163-5. [DOI] [PubMed] [Google Scholar]