Abstract
Continued production of wear debris affects both initial osseointegration and subsequent bone remodeling of total joint replacements (TJRs). However, continuous delivery of clinically relevant particles using a viable, cost effective, quantitative animal model to simulate the scenario in humans has been a challenge for orthopaedic researchers. In this study, we successfully infused blue-dyed polystyrene particles, similar in size to wear debris in humans, to the intramedullary space of the mouse femur for four weeks using an osmotic pump. Approximately 40% of the original particle load (85 ul) was delivered into the intramedullary space, an estimate of 3 × 10 9 particles. The visible blue dye carried by the particles confirmed the delivery. This model demonstrated that continuous infusion of particles to the murine bone-implant interface is possible. In vivo biological processes associated using wear debris particles can be studied using this new animal model.
Keywords: Murine model, Continuous infusion, Particles
Introduction
Total joint replacement (TJR) is an effective treatment for advanced arthritis. However, the longevity of total joint replacements is often jeopardized by wear-debris associated aseptic loosening and osteolysis 1-3. Understanding the mechanisms of chronic inflammation and periprosthetic osteolysis, and developing prostheses with increased longevity have become paramount within the orthopedic community.
Soon after implanting a TJR, the bone-prosthesis interface is exposed to continuously generated wear debris over time. Analysis of retrieved implants showed a linear wear rate of 5 um per year after the first year of implantation4. These particles stimulate the differentiation, maturation and activation of osteoclasts, interfere with osteoblast function and eventually disturb the normal homeostatic balance between bone formation and degradation 5,6. Studies of the response of macrophages to clinically relevant particles have shown that particles with a mean size of 0.24 um stimulated bone resorption at a ratio of 10 um3 /cell, whereas particles of 0.45 and 1.71 um were active at a ratio of 100 um3/cell 7.
A number of animal models have attempted to simulate the biological processes of particle associated osteolysis in vivo 8-13. To date, none of the studies was performed on a murine model incorporating the presence of a stable intramedullary implant and the continuous delivery of particles. Because the accumulation of particles in the intramedullary environment over time is a major characteristics of failed TJR with bone loss in humans, developing a murine model simulating this feature becomes especially important to further our understanding of periprosthetic osteolysis at the cellular and molecular level 14-16.
In this study, we report that continuous delivery of particles, similar in size to clinically relevant wear debris, to the intramedullary space of mice is feasible. The model is based on previous in vitro studies using the same apparatus17. The method will facilitate future in vivo studies of wear debris-associated osteolysis using more clinically representative particles. Furthermore, because genetically manipulated variants of wild type mice are widely available, in-depth mechanistic experiments can be carried out using this model to further our understanding of periprosthetic osteolysis.
Materials and Methods
Animals
5 adult (9 weeks old) male C57BL/6 wild type mice were obtained from the university in-house breeding colony. C57BL/6 mice were chosen for these experiments because they are a popular, readily available strain and are the basis for many transgenic strains for our future studies.
Surgery
Institutional guidelines for the care and use of laboratory animals were strictly followed. Animals were anesthetized with 3% isoflurane in 100% oxygen at a flow rate of 1 L/min. Using sterile technique, the intercondylar notch of the distal femur was exposed through a medial parapatellar arthrotomy. A 27-gauge needle was used to manually drill through the intercondylar notch to access the medullary cavity. The hole was then expanded in size stepwise using the following series of needles: 25 gauge, 23 gauge and 21 gauge. An 18 gauge needle was used to ream the initial 2mm of the entrance to accommodate the wider diameter of the silicone tubing overlying the titanium tubing. Another incision was made posteriorly, between the scapulae. The subcutaneous connective tissue was bluntly dissected, creating a subcutaneous pathway to the knee joint.
An Alza osmotic pump (Model 2004, Durect Corporation, Cupertino CA) loaded with the particles was placed in the flank through the posterior incision, and connected to silicone tubing overlying a 6 mm long hollow titanium rod at the opposite end. The tubing and rod were directed through the subcutaneous tunnel, and the titanium rod was inserted into the distal femur until the end of the rod was flush with the end of the femur. The patellar tendon was repositioned and a single suture was placed to repair the quadriceps-patellar complex. The incisions were closed with surgical adhesive.
Particles and pumps
Commercially available blue-dyed polystyrene particles (Cat#: 15709, Polysciencs, Warrington, PA) were used in this study. The average diameter of the particles was 0.5 ± 0.015 μm according to the information provided by the manufacturer. The particles are microspheres according to the manufacturer and the shape was confirmed by SEM scans performed by our lab. When supplied, the particles were packaged as 2.5% (m/v) aqueous suspension. An appropriate dilution was made so that each pump will hold 6 × 109 particles.
The particles were reconstituted in physiological buffered saline and approximately 6 × 109 particles were loaded into each pump. The mean volume of the pumps was 246 ul and the mean pumping rate was 0.2 ul / hr according to the manufacturer.
Assessment
Animals were allowed to ambulate in the cage after the initial implantation. At the end of the 4-week infusion, the mice were euthanized. The pump and the tubing were dissected and exposed carefully to ensure that no damage or disconnection occurred along the delivery system. The pump and tubing were then removed and femora harvested. The gross inspection of the femora for blue coloration was performed and recorded. The femora were then embedded in OCT, sectioned transversely, stained with Hematoxylin and Eosin and viewed under a light microscope.
Results
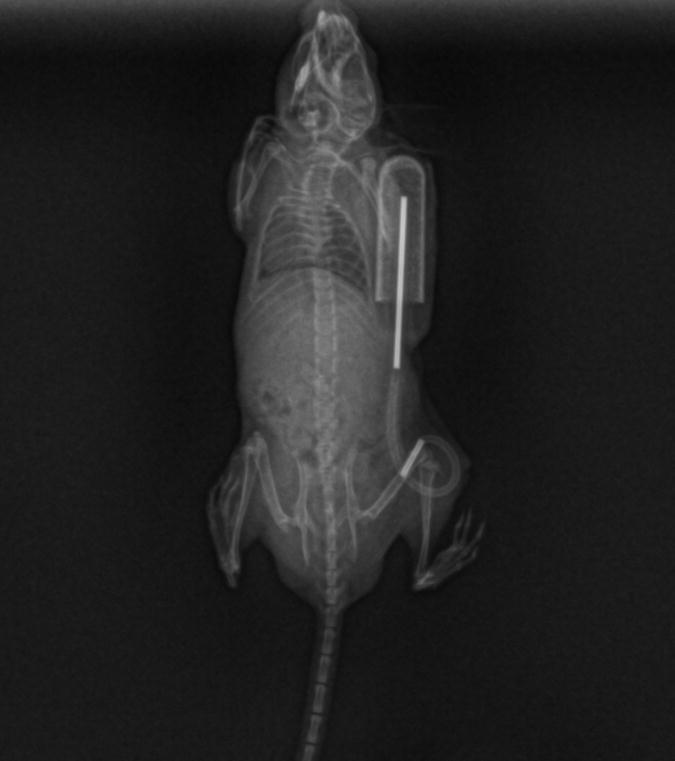
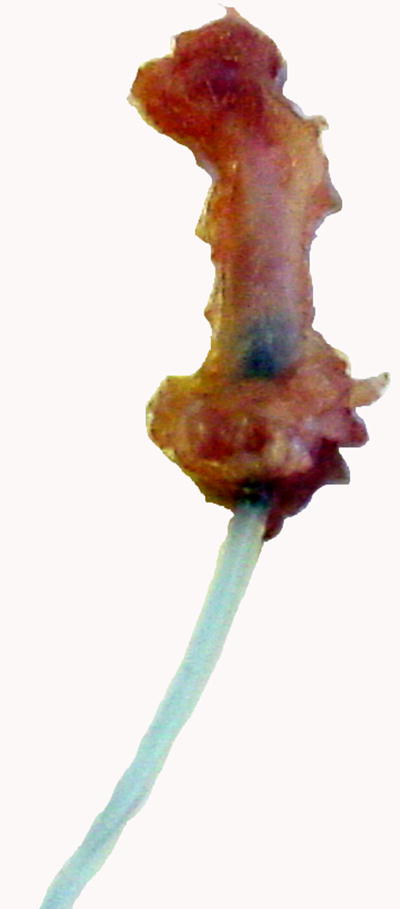
Radiographs were taken of the animals immediately after surgery and before euthanasia to verify the position of the implants. As shown on the radiographs (Fig. 1), the titanium rod was placed within the distal 2/5 of the intramedullary canal, parallel to the longitudinal axis of the femur. No subsequent change in position of the rod was observed on the x-rays taken after the 4-week infusion period. At harvest, blue staining was easily visible on gross inspection of the intact distal femur in 2 of the animals (Fig. 2). In two further animals whose femur did not initially appear blue on gross inspection, the femur was dissected and the intramedullary cavity exposed. Blue colored staining was then observed in the intramedullary canal of these femora. In one animal, the distal tubing-rod interface was found to have disconnected. In this animal, the soft tissues around the knee joint were stained with a blue coloration. This was not observed in any of the other animals.
Figure 1. The in vivo particle delivery system.
An Alza osmotic pump loaded with the particles was connected with silicone tubing overlying a 6 mm long hollow titanium rod at the end. The tubing was directed through the subcutaneous tunnel, and the titanium rod was inserted into the femur until the end of the rod was flush with the end of the femur.
Figure 2. The gross view of harvested femur after 4-week particle infusion.
After 4-week infusion, the femur was harvested and the picture of the femur was taken immediately using a digital camera. The blue dye carried by the particles was grossly visible in the distal 1/3 of the intact femur and in the silicone tubing.
The amount of particles delivered into the intramedullary space was estimated based on the volume loaded into the pumps before (200 ul) and present after (100 ul) the 4-week infusion period taking into consideration the volume of the silicone catheter (15 ul) that could potentially hold particles. The estimate on the volume contained in the tubing was a mathematical calculation based on the length and diameter of the tubing used. Approximately 85 ul (200ul – 100 ul – 15ul) of particle suspension was delivered into the intramedullary space. Given the initial concentration of the particles loaded into the pump, the number of particles delivered into the intramedullary space was approximately 3 × 109.
We obtained histology slides from the distal and middle portion of the femur after sacrifice. However, no particle was observed on the histology sections.
Discussion
Animal models for study of the biological mechanisms underlying wear debris induced osteolysis have primarily used large animals18. In particular, Jacobbs et al. demonstrated the adverse effects of spinal implant debris utilizing a rabbit osteolysis model 19, Bostrom et al and Kim et al studied particle induced osteolysis in a rat model 11,20, and Shanbhag et al studied the effects of particles in hip arthroplasty using a canine model 21.
Developing murine models is of particular interest because mice are hearty, the surgical procedures are less costly than with larger animals. Furthermore, the availability of genetically manipulated variants and molecular methods makes murine models even more advantageous in identifying underlying biological mechanisms.
Segregation of particles during the prolonged flow condition was studied in a separate experiment in vitro. Utilizing the same type of particles with the same delivery mechanism, the efficacy of such particle delivery was around 46% 17. Under the in vivo condition, particles are distributed randomly upon interacting with the local tissue. Thus, measuring the exact number of particles that reached the destination is extremely difficult. Our estimation based on the volume change of the fluid inside the pump yielded a 50% delivery efficiency, which agreed with the in vitro data.
In an effort to develop a more clinically relevant murine model, we recently reported a novel mouse model utilizing an intramedullary implant and particle injection in the form of a single bolus 22,23. This current study builds on the previous model and incorporates the continuous infusion of particles, which simulates the continuous production of particles over time seen in humans. The model showed for the first time that particles could be delivered continuously and quantitatively in mice over an extended period of time.
Histological processing of the harvested femoral specimens was performed using frozen, undecalcified specimens. There was no blue dyed particles visible on the histology slides using standard light microscopy. We suspect that the blue particles could have been dissolved or lost during the tissue processing, similar to the loss of polyethylene particles under similar processing conditions, or the small particles were not visible using the standard light microscopy techniques. Since the particles are composed of blue polystyrene, similar to commercially available dye particles, gross inspection of the retrieved femora verifies delivery by the blue coloration of the infused tissues.
Polystyrene particles used in this study were chosen because they are similar in size to clinically relevant wear debris and their blue color makes them easily visible for verification of delivery. The purpose of this current experiment was to validate the particle delivery system rather than examine specific biological mechanisms associated with the inflammatory reaction to particles. To elucidate the complicated process of particle associated osteolysis, more clinically relevant particles, such as ultra high molecular weight polyethelene particles, will be used in future studies.
Acknowledgments
This study was supported in part by NIH Grant # R21 AR053189-01. The pumps were a generous gift from Durect Corporation.
References
- 1.Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85-A(6):1095–9. doi: 10.2106/00004623-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Santavirta S, Konttinen YT, Bergroth V, Eskola A, Tallroth K, Lindholm TS. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J Bone Joint Surg Am. 1990;72(2):252–8. [PubMed] [Google Scholar]
- 3.Harris WH, Schiller AL, Scholler JM, Freiberg RA, Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg Am. 1976;58(5):612–8. [PubMed] [Google Scholar]
- 4.Grubl A, Marker M, Brodner W, Giurea A, Heinze G, Meisinger V, Zehetgruber H, Kotz R. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841–8. doi: 10.1002/jor.20381. [DOI] [PubMed] [Google Scholar]
- 5.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, Sibley R. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80(3):531–9. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 6.Fang HW, Yang CB, Chang CH, Huang CH, Liu HL, Fang SB. The potential role of phagocytic capacity in the osteolytic process induced by polyethylene wear particles. J Int Med Res. 2006;34(6):655–64. doi: 10.1177/147323000603400611. [DOI] [PubMed] [Google Scholar]
- 7.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271–86. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Ren W, Wu B, Mayton L, Wooley PH. Polyethylene and methyl methacrylate particle-stimulated inflammatory tissue and macrophages up-regulate bone resorption in a murine neonatal calvaria in vitro organ system. J Orthop Res. 2002;20(5):1031–7. doi: 10.1016/S0736-0266(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 9.Wooley PH, Morren R, Andary J, Sud S, Yang SY, Mayton L, Markel D, Sieving A, Nasser S. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials. 2002;23(2):517–26. doi: 10.1016/s0142-9612(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 10.Iwase M, Kim KJ, Kobayashi Y, Itoh M, Itoh T. A novel bisphosphonate inhibits inflammatory bone resorption in a rat osteolysis model with continuous infusion of polyethylene particles. J Orthop Res. 2002;20(3):499–505. doi: 10.1016/S0736-0266(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 11.Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236–49. doi: 10.2106/00004623-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Sundfeldt M, Widmark M, Johansson CB, Campbell P, Carlsson LV. Effect of submicron polyethylene particles on an osseointegrated implant: an experimental study with a rabbit patello-femoral prosthesis. Acta Orthop Scand. 2002;73(4):416–24. doi: 10.1080/00016470216314. [DOI] [PubMed] [Google Scholar]
- 13.Im GI, Kwon BC, Lee KB. The effect of COX-2 inhibitors on periprosthetic osteolysis. Biomaterials. 2004;25(2):269–75. doi: 10.1016/s0142-9612(03)00523-4. [DOI] [PubMed] [Google Scholar]
- 14.Spanogle JP, Miyanishi K, Ma T, Epstein NJ, Smith RL, Goodman SB. Comparison of VEGF-producing cells in periprosthetic osteolysis. Biomaterials. 2006;27(21):3882–7. doi: 10.1016/j.biomaterials.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 15.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;(311):46–53. [PubMed] [Google Scholar]
- 16.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004;(429):188–92. doi: 10.1097/01.blo.0000150126.73024.42. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz SG, Ma T, Epstein NJ, Smith RL, Goodman SB. Validation and quantification of an in vitro model of continuous infusion of submicron-sized particles. J Biomed Mater Res B Appl Biomater. 2007 doi: 10.1002/jbm.b.30875. [DOI] [PubMed] [Google Scholar]
- 18.Warme BA, Epstein NJ, Trindade MC, Miyanishi K, Ma T, Saket RR, Regula D, Goodman SB, Smith RL. Proinflammatory mediator expression in a novel murine model of titanium-particle-induced intramedullary inflammation. J Biomed Mater Res B Appl Biomater. 2004;71(2):360–6. doi: 10.1002/jbm.b.30120. [DOI] [PubMed] [Google Scholar]
- 19.Hallab NJ, Cunningham BW, Jacobs JJ. Spinal implant debris-induced osteolysis. Spine. 2003;28(20):S125–38. doi: 10.1097/00007632-200310151-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kim KJ, Kobayashi Y, Itoh T. Osteolysis model with continuous infusion of polyethylene particles. Clin Orthop Relat Res. 1998;(352):46–52. [PubMed] [Google Scholar]
- 21.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;(344):33–43. [PubMed] [Google Scholar]
- 22.Epstein NJ, Bragg WE, Ma T, Spanogle J, Smith RL, Goodman SB. UHMWPE wear debris upregulates mononuclear cell proinflammatory gene expression in a novel murine model of intramedullary particle disease. Acta Orthop. 2005;76(3):412–20. [PubMed] [Google Scholar]
- 23.Epstein NJ, Warme BA, Spanogle J, Ma T, Bragg B, Smith RL, Goodman SB. Interleukin-1 modulates periprosthetic tissue formation in an intramedullary model of particle-induced inflammation. J Orthop Res. 2005;23(3):501–10. doi: 10.1016/j.orthres.2004.10.004. [DOI] [PubMed] [Google Scholar]