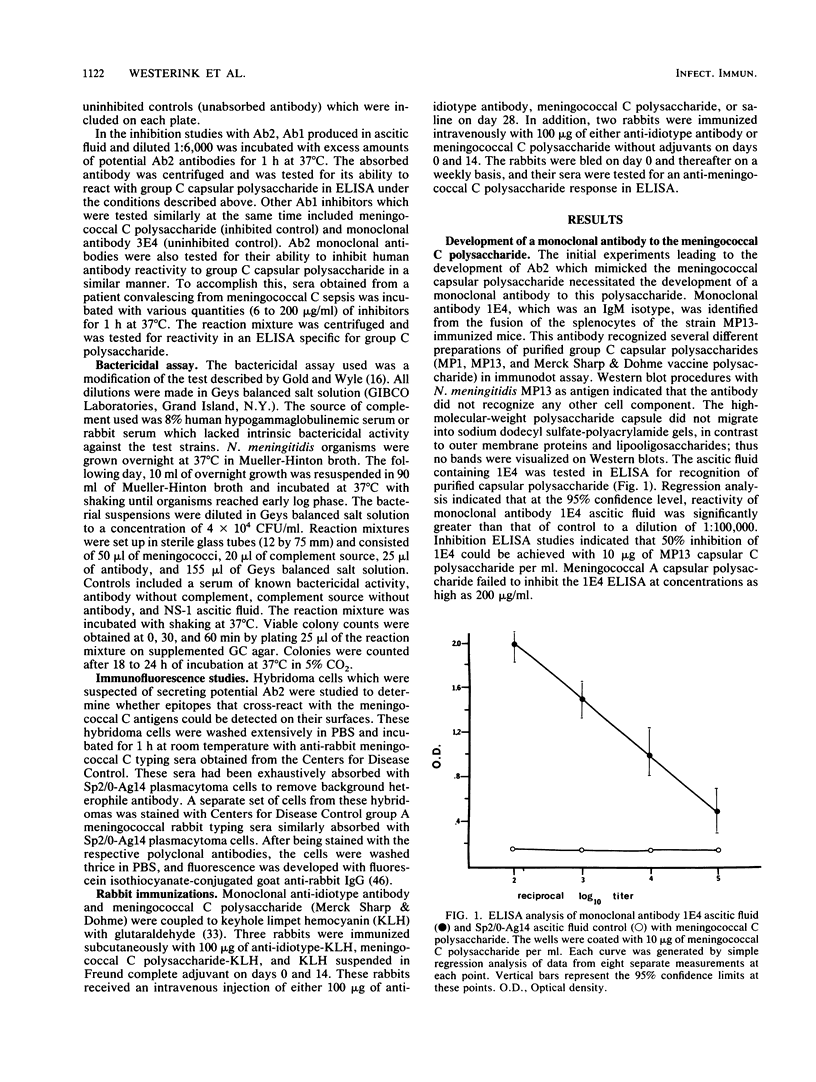
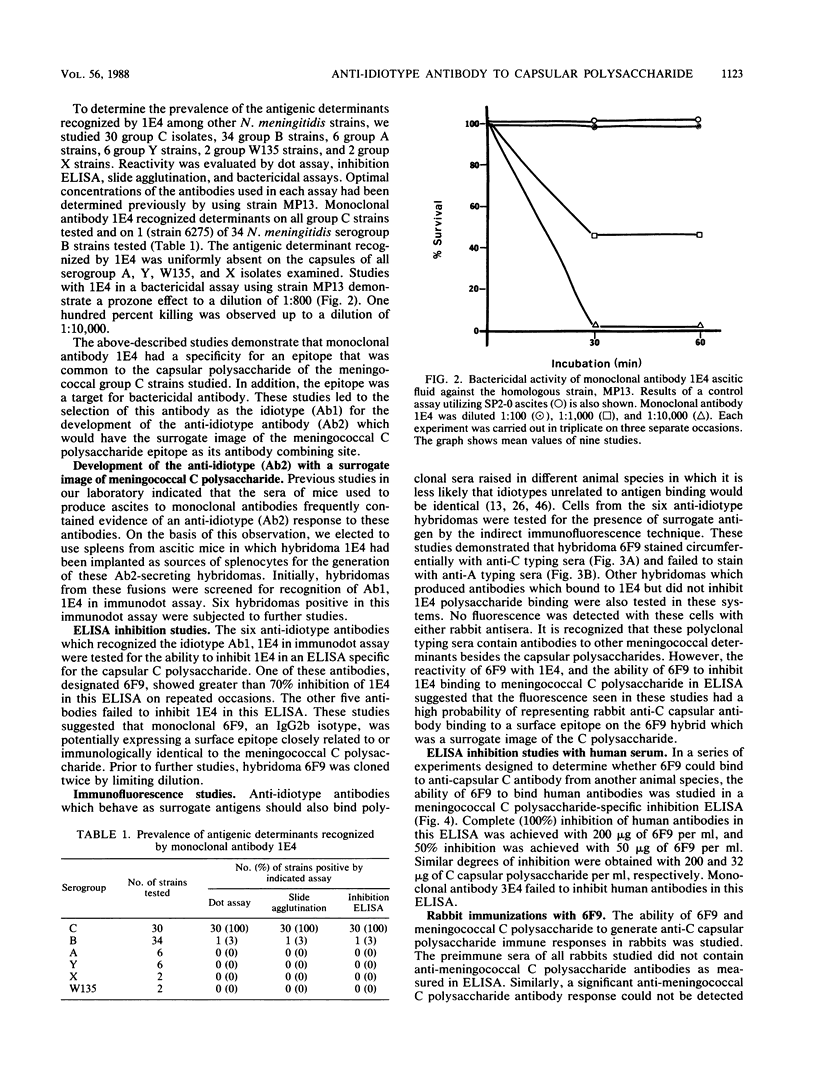
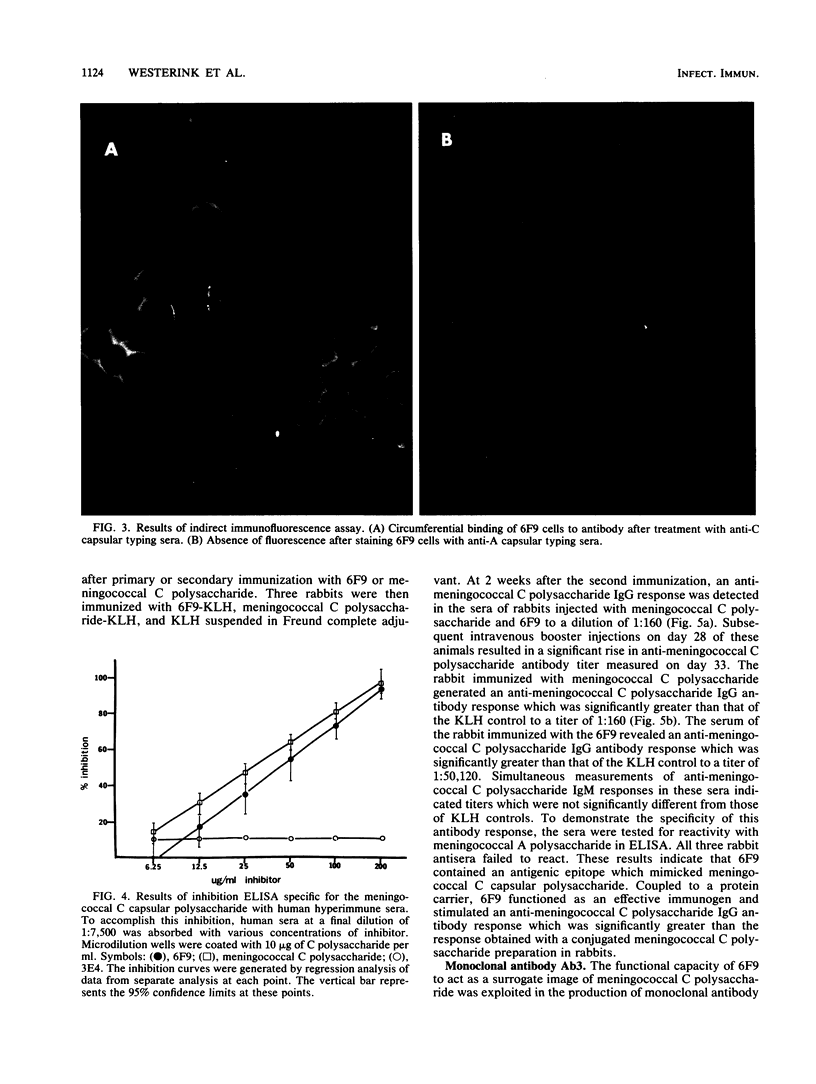
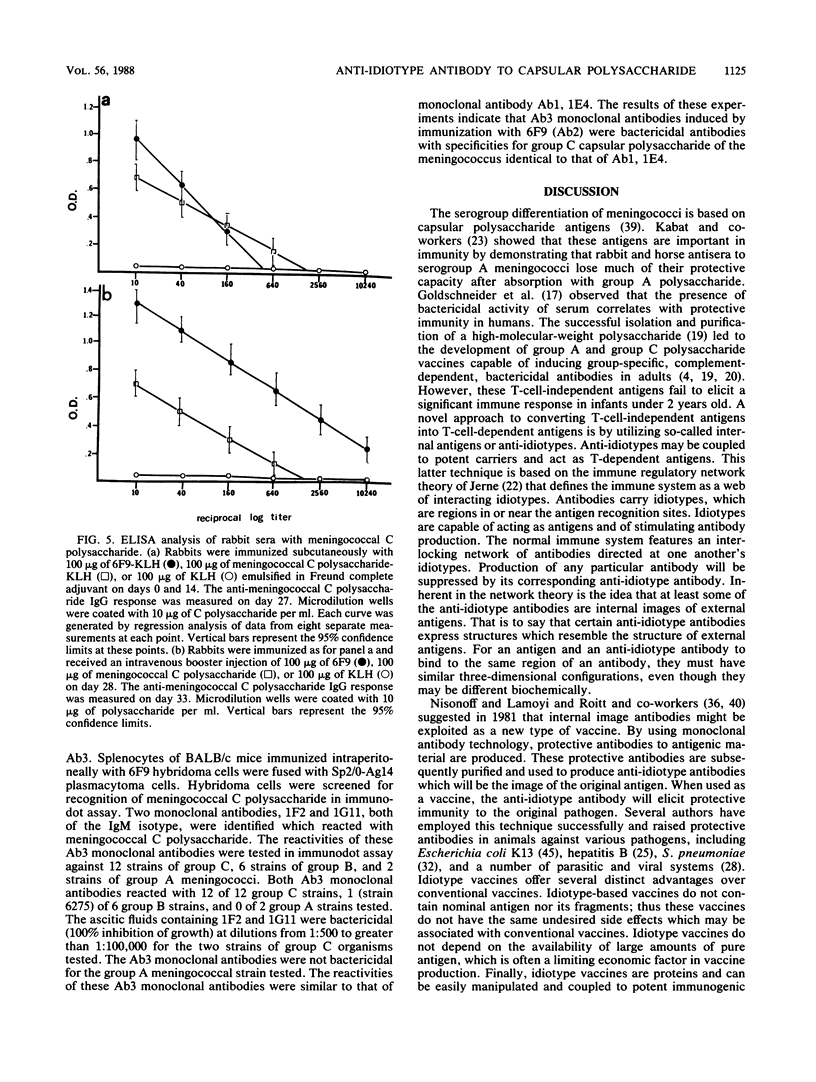
Abstract
A monoclonal anti-idiotypic antibody (Ab2) whose antibody combining site contained a surrogate image of the meningococcal group C capsular polysaccharide was developed. To accomplish this, a monoclonal antibody against the group C capsular polysaccharide was developed by the fusion of splenocytes from mice immunized with Neisseria meningitidis group C strain MP13 with Sp2/0-Ag14 plasmacytoma cells. Monoclonal antibody 1E4, an immunoglobulin M isotype, demonstrated binding to the serogroup C polysaccharide in enzyme-linked immunosorbent assay (ELISA). Monoclonal antibody 1E4 reacted with 30 of 30 group C strains and 1 of 36 group B strains in immunodot assay, slide agglutination, inhibition ELISA, and bactericidal assay. This monoclonal antibody was selected as idiotype (Ab1) for the development of hybridomas producing an anti-idiotype antibody. One of the hybridomas developed, designated 6F9, was capable of over 70% inhibition of 1E4 in binding in the meningococcal C polysaccharide-specific ELISA. Studies with convalescent human serum demonstrated 100% inhibition of a serogroup C-specific ELISA with 200 micrograms of 6F9 per ml and 50% inhibition of this ELISA was achieved with 50 micrograms of 6F9 per ml. Monoclonal anti-idiotype antibodies (Ab3) with specificities similar to Ab1, 1E4 were generated from BALB/c mice immunized with the Ab2 (6F9). Immunization of rabbits with 6F9 resulted in an immunoglobulin G response which was significantly greater than that of control to a titer of 1:160. These studies indicate that monoclonal 6F9 contained a surrogate image on the combining antibody site which mimicked meningococcal C polysaccharide. This surrogate image is capable of evoking antibodies to the meningococcal C polysaccharide in syngenic and xenogenic species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. M. Mortality in meningococcal infections. Scand J Infect Dis. 1978;10(4):277–282. doi: 10.3109/inf.1978.10.issue-4.04. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A. Identification of a subgroup antigen on the Neisseria meningitidis group C capsular polysaccharide. J Infect Dis. 1974 Feb;129(2):147–153. doi: 10.1093/infdis/129.2.147. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Branche W. C., Jr, Harkins C. Cutaneous reactions and antibody response to meningococcal group C polysaccharide vaccines in man. J Infect Dis. 1970 Apr;121(4):372–377. doi: 10.1093/infdis/121.4.372. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Band J. D., Chamberland M. E., Platt T., Weaver R. E., Thornsberry C., Fraser D. W. Trends in meningococcal disease in the United States, 1975-1980. J Infect Dis. 1983 Oct;148(4):754–758. doi: 10.1093/infdis/148.4.754. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Antigen requirements for priming of IgG producing B memory cells specific for Type III pneumococcal polysaccharide. Immunology. 1980 Aug;40(4):521–527. [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Ammann A. J., Wara D. W., Howie V. M., Schultz L., Doyle N., Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978 Nov;62(5):721–727. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Sharpe A. H., Chang D. W., Fields B. N., Greene M. I. Syngeneic monoclonal internal image anti-idiotopes as prophylactic vaccines. J Immunol. 1986 Nov 1;137(9):2930–2936. [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. L., Gotschlich E. C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Invest. 1975 Dec;56(6):1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Wyle F. A. New Classification of Neisseria meningitidis by Means of Bactericidal Reactions. Infect Immun. 1970 May;1(5):479–484. doi: 10.1128/iai.1.5.479-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kabat E. A., Miller C. P., Kaiser H., Foster A. Z. CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION : VII. A QUANTITATIVE STUDY OF THE TYPE SPECIFIC AND GROUP SPECIFIC ANTIBODIES IN ANTIMENINGOCOCCAL SERA OF VARIOUS SPECIES AND THEIR RELATION TO MOUSE PROTECTION. J Exp Med. 1945 Jan 1;81(1):1–8. doi: 10.1084/jem.81.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Adler-Storthz K., Henkel R. D., Sanchez Y., Melnick J. L., Dreesman G. R. Immune response to hepatitis B surface antigen: enhancement by prior injection of antibodies to the idiotype. Science. 1983 Aug 26;221(4613):853–855. doi: 10.1126/science.6603657. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Eichberg J. W., Lanford R. E., Dreesman G. R. Anti-idiotypic antibody vaccine for type B viral hepatitis in chimpanzees. Science. 1986 Apr 11;232(4747):220–223. doi: 10.1126/science.3952505. [DOI] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Kieber-Emmons T., Ward R. E., Raychaudhuri S., Rein R., Kohler H. Rational design and application of idiotope vaccines. Int Rev Immunol. 1986 Jan;1(1):1–26. doi: 10.3109/08830188609056598. [DOI] [PubMed] [Google Scholar]
- Kohler H., Muller S., Bona C. Internal antigen and immune network. Proc Soc Exp Biol Med. 1985 Feb;178(2):189–195. doi: 10.3181/00379727-178-41996a. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Mäkelä P. H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984 Nov;74(5):857–865. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Brandt B. L., Artenstein M. S. Response of children to Neisseria meningitidis polysaccharide vaccines. J Infect Dis. 1973 Apr;127(4):394–400. doi: 10.1093/infdis/127.4.394. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Zaldivar N. M., Goldings E., Mond J., Scher I., Paul W. E. Formation of antibody in the newborn mouse: study of T-cell-independent antibody response. J Infect Dis. 1977 Aug;136 (Suppl):S14–S19. doi: 10.1093/infdis/136.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Nisonoff A., Lamoyi E. Implications of the presence of an internal image of the antigen in anti-idiotypic antibodies: possible application to vaccine production. Clin Immunol Immunopathol. 1981 Dec;21(3):397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983 Jan-Feb;5(1):71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- Rake G., Scherp H. W. STUDIES ON MENINGOCOCCUS INFECTION : III. THE ANTIGENIC COMPLEX OF THE MENINGOCOCCUS-A TYPE-SPECIFIC SUBSTANCE. J Exp Med. 1933 Aug 31;58(3):341–360. doi: 10.1084/jem.58.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I. M., Cooke A., Male D. K., Hay F. C., Guarnotta G., Lydyard P. M., de Carvalho L. P., Thanavala Y., Ivanyi J. Idiotypic networks and their possible exploitation for manipulation of the immune response. Lancet. 1981 May 9;1(8228):1041–1045. doi: 10.1016/s0140-6736(81)92199-1. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel J. E., Voller A. Detection of Neisseria meningitidis cell envelope antigen by enzyme-linked immunosorbent assay in patients with meningococcal disease. Trans R Soc Trop Med Hyg. 1980;74(5):644–648. doi: 10.1016/0035-9203(80)90156-x. [DOI] [PubMed] [Google Scholar]
- Sjögren-Jansson E., Jeansson S. Large-scale production of monoclonal antibodies in dialysis tubing. J Immunol Methods. 1985 Nov 28;84(1-2):359–364. doi: 10.1016/0022-1759(85)90442-9. [DOI] [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanavala Y. M., Roitt I. M. Monoclonal anti-idiotypic antibodies as surrogates for hepatitis B surface antigen. Int Rev Immunol. 1986 Jan;1(1):27–39. doi: 10.3109/08830188609056599. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]



