Abstract
Food interacts with sensors all along the alimentary canal to provide the brain with information regarding its composition, energy content, and beneficial effect. Vagal afferents innervating the gastrointestinal tract, pancreas, and liver provide a rapid and discrete account of digestible food in the alimentary canal, as well as circulating and stored fuels, while vagal efferents together with the sympathetic nervous system and hormonal mechanisms codetermine the rate of nutrient absorption, partitioning, storage, and mobilization. Although vagal sensory mechanisms play a crucial role in the neural mechanism of satiation, there is little evidence suggesting a significant role in long-term energy homeostasis. However, increasing recognition of vagal involvement in the putative mechanisms making bariatric surgeries the most effective treatment for obesity should greatly stimulate future research to uncover the many details regarding the specific transduction mechanisms in the periphery and the inter-and intra-neuronal signaling cascades disseminating vagal information across the neuraxis.
Keywords: Gut-brain axis, gut hormones, vagal afferents, obesity surgery, Roux-en-Y, gastric pacing
1. Ingested food interacts with sensors along the alimentary canal: importance of vagal afferents
1.1. Oral cavity: taste receptors and trigeminal mechanosensors
Gustatory input via taste receptor cells on the tongue and palate is considered most important for guiding food intake and selection (Fig. 1). Although only a minor portion of this information is mediated to the brain by the vagus nerve, the gustatory system is included here because it shares the nucleus tractus solitarius (NTS) and other central processing stations with vagal afferents from the gastrointestinal tract. The gustatory and trigeminal systems act as “gate keepers” at the entrance to the alimentary canal (Scott and Verhagen, 2000). According to this view, the classical four taste modalities represent innate detectors for acceptable foods (sweet), dangerous or toxic foods (bitter and sour), and special needs (salt, water).
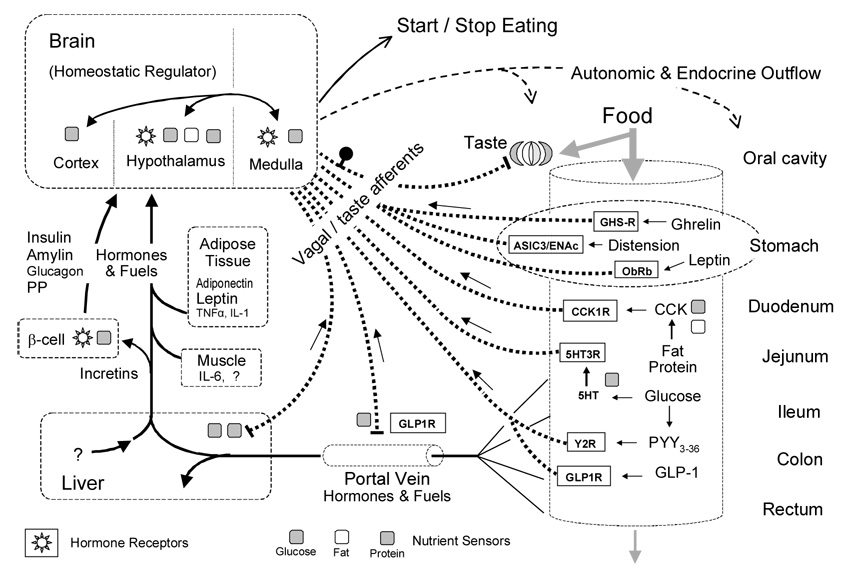
Fig. 1. Nutrient sensing in the alimentary canal and the control of food intake.
Simplified schematic diagram showing the major pre- and postabsorptive transduction sites and mechanisms for the detection of ingested food and its macronutrient components. Nutrient information is sent to the brain through vagal and taste afferents (heavy dotted lines) or through the blood circulation (full lines). Specific receptors expressed by vagal afferent neurons are shown in rectangular boxes. Specific sensor mechanisms demonstrated for glucose, amino acids/proteins, and lipids/fatty acids are shown by gray, striped, and white squares, respectively.
There was considerable recent progress in taste receptor physiology, with a number of receptor proteins and signaling mechanisms discovered (as reviewed by (Sugita, 2006)). There is also some progress in deciphering the neural encoding mechanism. The observation that in the mouse, each taste bud is innervated by about 5 primary afferent geniculate ganglion cells that only innervate that bud, suggests a labeled-line system, although each taste bud can contain receptor cells of different modality (Zaidi and Whitehead, 2006). In addition to receptors for sweet, bitter, sour, and salty substances, there may also be a taste for fat. The fatty acid transporter CD36 is co-expressed with α-gustducin in taste receptor cells and unlike wildtype mice, CD36-deficient mice are unable to develop a preference for fatty foods (Laugerette et al., 2005). The newly discovered tastes for fat and amino acids (Umami) suggest that the system may be capable of at least recognizing, if not metering, the macronutrient content of mixed foods.
The trigeminal somatosensory system with its mechano- and temperature sensors picks up important additional attributes of ingested foods, such as creaminess and crunchiness. These are thought as important dimensions of overall palatability of particular food items.
Understanding the transduction mechanisms of the gustatory system may have important implications for investigation of chemosensory processes in the small intestines as discussed below.
1.2. Stomach: stretch, tension, leptin, and ghrelin
Far from being a passive reservoir for ingested food, the stomach is a highly regulated organ with elaborate neural and hormonal control mechanisms. The presence of ingested food is detected by vagal afferent fibers in the mucosa sensitive to mechanical touch (Berthoud et al., 2001), and the volume of ingested food is detected by vagal afferents in the external muscle layers sensitive to stretch and tension (Phillips and Powley, 2000) (Fig. 2). Intraganglionic laminar vagal afferent endings are located in the connective tissue capsule of myenteric plexus ganglia, between the longitudinal (outer) and circular (inner) muscle layers (Berthoud and Neuhuber, 2000). They thus respond to muscle tension generated by both passive stretch and active contraction of the muscle layers (Zagorodnyuk et al., 2001). This type of vagal afferent ending is found in large numbers throughout the esophagus and gastrointestinal tract (Berthoud et al., 1997; Neuhuber et al., 1998). Intramuscular arrays are distinctly different from intraganglionic laminar endings and are almost exclusively located in the stomach longitudinal and circular muscle layers (Berthoud and Powley, 1992) (Fig. 2). Although IMAs were thought to represent in-series tension receptors long before the functional proof of mechanosensitivity for IGLEs (Zagorodnyuk et al., 2001), it is now unclear how they are functionally different from IGLEs.
Fig. 2. Vagal afferent mechano- and nutrient-sensors in the rat gastrointestinal tract.
Vagal afferent fibers and terminal structures were anterogradely traced with the fluorescent dye DiI (bright white) injected into nodose ganglia. A: Intramuscular array (IMA) in longitudinal muscle layer of gastric fundus. Arrow indicated parent axon entering the muscle layer from myenteric plexus. The inset shows vagal afferent fibers in intimate anatomical contact with Interstitial Cell of Cajal. B: Intraganglionic laminar endings (IGLE) in myenteric plexus of gastric fundus. Two different parent axons are indicated by arrows. Myenteric ganglion is indicated by arrowheads. C: Mucosal endings close to epithelium (e) in villous of proximal duodenum.
Vagal afferent fibers are also innervating the gastric mucosa, where they are likely to detect locally released hormones such as leptin and ghrelin. Leptin is produced in the mucosa of the stomach and rapidly mobilized by feeding and high doses of exogenous cholecystokinin (Bado et al., 1998), and it appears to activate vagal afferents (Wang et al., 1997), shown to express the long form of leptin receptor (Buyse et al., 2001; Burdyga et al., 2002). Small doses of leptin infused into the celiac artery significantly decreased sucrose intake and this effect was not observed in rats with subdiaphragmatic vagotomy or perivagal capsaicin-treatment aimed to selectively ablate vagal afferent fibers. It is unlikely that celiac artery leptin produced its effect by spilling into the systemic circulation because infusion of the same dose of leptin into the jugular vein did not reduce sucrose intake, and because the increases in circulating leptin were similar for both routes of infusion (Peters et al., 2006).
The very recent discovery of ghrelin was particularly exciting because it is the first and only peripherally secreted hormone with a stimulatory effect on appetite. Ghrelin is mainly secreted from oxyntic gland cells in the mucosa of the empty stomach and secretion is rapidly suppressed upon the ingestion of food (Cummings et al., 2001; Cummings, 2006). There is evidence from one laboratory that ghrelin secreted from the gastric mucosa stimulates appetite by inhibiting activity of vagal afferents. Ghrelin’s appetite-stimulating effect was abolished in rats with subdiaphragmatic vagotomy and in rats with capsaicin-induced vagal deafferentation (Date et al., 2002). Ghrelin receptor is expressed by a subset of vagal afferent neurons in the nodose ganglia and appears to regulate expression of other peptide receptors in vagal afferents (Date et al., 2002; Burdyga et al., 2006). Furthermore, ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy (le Roux et al., 2005).
Thus, besides mechanosensory signals, the stomach appears to generate hormonal signals that are picked up by vagal afferents. This is interesting with regard to the question whether the stomach can sense only food volume or energy as well. Available behavioral data using pyloric cuffs suggest that gastric satiation is strictly volumetric and intestinal satiation is nutritive (Powley and Phillips, 2004). The finding that the two gastric hormones leptin and ghrelin can modulate appetite and satiation puts an interesting twist to this view. While the nutrient sensors responsible for the release appear to be located in the small intestine, the hormone producing cells themselves are located in the stomach.
1.3. Upper small intestine: CCK, GIP, and “taste in the gut”
Vagal afferent innervation of the duodenal mucosa was demonstrated anatomically using DiI tracing from the rat nodose ganglia (Berthoud et al., 1995; Williams et al., 1997) (Fig. 2). Labeled vagal afferent fibers were present in the lamina propria of duodenal and jejunal villi and crypts of Lieberkühn, but do not cross the basal membrane to innervate the epithelial layer. Thus, vagal afferents are not in a position to sense luminal nutrients directly. Double labeling with an antibody to CCK revealed the presence of close anatomical appositions between labeled vagal afferent fibers and CCK-ir enteroendocrine cells in the lamina propria of duodenum and jejunum (Berthoud and Patterson, 1996). Together with the demonstration that vagal afferents express CCK-1 receptor (Moriarty et al., 1997; Broberger et al., 1999; Patterson et al., 2002) and numerous functional studies, these findings strongly suggest that luminal nutrients, particularly lipids and proteins activate vagal afferents via release of CCK from adjacent enteroendocrine cells (Smith et al., 1985; Geary, 2004; Raybould et al., 2006).
Since glucose is a very poor stimulator of CCK release, it is not clear whether a similar mechanism exists for the sensing of glucose by vagal afferents. There is some limited evidence that 5-HT-secreting enteroendocrine cells and the 5-HT3 receptor might be involved (Freeman et al., 2006). The 5-HT3 receptor is expressed in vagal afferent neurons (Morales and Wang, 2002), and stimulation of c-Fos in nodose ganglion neurons by luminal administration of 5-HT or maltose was blocked by prior vagotomy or 5-HT3 receptor antagonist treatment (Wu et al., 2005).
Glucose-dependent Insulinotropic Polypeptide (GIP) also known as Gastric Inhibitory Polypeptide is secreted from K-cells primarily concentrated in the upper portions of the small intestine (Buchan et al., 1982). It is secreted in response to glucose ingestion and stimulates insulin secretion preferentially at high circulating glucose levels (Pederson et al., 1975; Andersen et al., 1978). Therefore, the GIP-cell can detect luminal glucose and could theoretically participate in the encoding of glucose-specific information to be transmitted to the brain. However, there is no evidence of receptors for this peptide on vagal afferent fibers and they are not activated by GIP (Nishizawa et al., 1996; Nakagawa et al., 2004). Furthermore, GIP release does not seem to be affected by vagotomy, direct efferent vagal stimulation, or indirect vagal stimulation through sham feeding (Berthoud et al., 1982; Ohneda et al., 1985).
Compared to the gustatory system, little is known about the encoding of nutritional information in the gut mucosa by enteroendocrine cells, primary afferent neurons of the enteric nervous system, and vagal afferents (Raybould, 2002). Some similarities are consistent with the possibility that the two systems evolved from a common precursor. One of the specialized cell types in mammalian gastrointestinal mucosa, the brush cell (also known as tufted or caveolated cell), shares distinct features with the chemosensory taste receptor cells of the tongue. They both have an apical tuft of microvilli and express α-gustducin, the α subunit of the heterotrimeric cGMP-coupled protein involved in bitter transduction in oral taste receptor cells (Hofer et al., 1999; Rozengurt, 2006). Furthermore they all share the NTS as the first central relay and place of integration.
1.4. Lower gut: The ileal brake hormones GLP-1 and PYY
Polypeptide YY and Glucagon-like peptide 1, two peptide hormones secreted mainly from the lower gut, particularly the ileum, colon, and rectum, seem to be most relevant for the control of appetite. Interestingly they are both secreted from the same enteroendocrine cells, the L-cells. Because only high concentrations of glucose and fatty acids directly infused into the vascularly perfused rat ileum where effective in stimulating PYY secretion, it is thought that this mechanism represents the so called ileal brake, that operates only in cases of malabsorption or maldigestion (Dumoulin et al., 1998). However, since hydrolyzed protein was even more potent in stimulating PYY release from the perfused rat ileum (Dumoulin et al., 1998), and compared to high fat and high carbohydrate diets, high protein diet produced the highest PYY plasma levels (Batterham et al., 2006), it is possible that digested protein levels in the distal small intestine are high enough to physiologically stimulate PYY release.
In addition to direct stimulation by local luminal contents, the PYY producing L-cells appear to be stimulated also indirectly through a neural mechanism originating in the proximal gut and depend on intact vagal afferents, because peak PYY secretion is reached long before a meal has reached the PYY-rich part of the ileum and is blunted by tetrodotoxin, hexamethonium, and sensory vagotomy (Fu-Cheng et al., 1997). It is not clear whether this neural pathway is contained entirely within the myenteric plexus of the enteric nervous system or uses an extrinsic route via vago-vagal and/or vago-spinal reflexes.
Once PYY is released into the circulation, the first two amino acids are rapidly cleaved by dipeptidyl peptidase IV, resulting in PYY3–36 (Eberlein et al., 1989). This truncated peptide has a high affinity for the Y2 receptor and has anorexigenic potency in both rats and humans (Larhammar, 1996; Batterham et al., 2002; Chelikani et al., 2005a; Batterham et al., 2006; Chelikani et al., 2006), whilst PYY1–36 has a higher affinity for the Y1 and Y5 receptors and is orexigenic if injected into the brain. A physiological role for PYY in satiation is supported by the observation that PYY null mice are slightly hyperphagic and develop obesity, both effects being reversible with PYY3-36 administration (Batterham et al., 2006). PYY thereby appears to selectively affect the satiating effects of proteins but not fats and carbohydrates (Batterham et al., 2006).
Because PYY3–36 can easily cross the blood brain barrier, and direct application of PYY3–36 to neurons in the arcuate nucleus inhibits their activity, it has been suggested that the anorectic effect of peripheral PYY3–36 is mediated by Y2 receptors in the arcuate nucleus (Batterham et al., 2002). However, vagal afferents may also be involved in the anorectic effects of PYY3–36, as abdominal vagotomy abolished both the anorectic effect and c-Fos expression in the arcuate nucleus following peripherally administered PYY3–36 (Koda et al., 2005). The Y2 receptor is expressed in at least some vagal afferents (Ghilardi et al., 1994; Zhang et al., 1997; Koda et al., 2005) and PYY stimulates firing of gastric vagal afferents (Koda et al., 2005).
Glucagon-like peptide-1 (GLP-1), also secreted from L-cells, is the site-specific splice product of the proglucagon gene also expressed in pancreatic islet cells where its major cleavage product is glucagon. Not unlike PYY, GLP-1 release also appears to be stimulated by all three macronutrients by both an indirect, partly neural reflex originating in the upper small intestine and by direct mucosal contact in the lower gut (Herrmann-Rinke et al., 1995; Herrmann et al., 1995; Anini et al., 2002). GLP-1 actions on pancreatic hormone secretion and gastric emptying make it a powerful regulator of glycemic homeostasis (D'Alessio et al., 1995; Ritzel et al., 1995; Schirra and Goke, 2005).
Peripheral administration of GLP-1 or its stable analog exendin-4, the naturally occurring peptide from the Gila monster lizard, enhance satiation and reduce food intake in humans and rats (Gutzwiller et al., 1999; Chelikani et al., 2005b). Because of rapid breakdown by dipeptidyl peptidase-VI, endogenously secreted GLP-1 has a very short half-life in plasma. Thus, although endogenous GLP-1 may partly act as a true hormone through the circulation on feeding circuits in the brain, it could also act in a paracrine fashion on vagal afferent nerve fibers within the gut mucosa. This view is supported by observations that GLP-1 receptor is expressed in nodose ganglion and GLP-1 increases cytosolic Ca2+ and evokes action potentials in vagal afferent neurons (Kakei et al., 2002; Nakagawa et al., 2004). In addition, GLP-1 released from the ileum can also act through GLP-1 receptors expressed in the area postrema (Merchenthaler et al., 1999; Yamamoto et al., 2003) to activate catecholaminergic neurons with projections to the NTS, ventrolateral medulla, and parabrachial nucleus (Yamamoto et al., 2003). Studying the site of action for the anorectic action of GLP-1 is complicated by the fact that the peptide is also expressed in a small population of neurons in the NTS with projections to the hypothalamus (Tang-Christensen et al., 2001).
In summary, vagal afferent nerve fibers in the gastrointestinal tract are in an excellent position to pick up information regarding volume and composition of luminal contents. They can directly detect mechanical touch, distension, and stretch at any location. In addition, they can indirectly detect the presence and concentration of all three macronutrients through mediation by peptides and transmitters released from specialized endothelial cells. These peptides are also absorbed into the blood stream and can interact with receptors in specialized brain areas. There are still many unsolved issues regarding nutrition-related gut-to-brain communication. The relative contributions of the hormonal versus the paracrine, vagally mediated mode of action, to the control of satiation and appetite, as well as reflex control of gastrointestinal, pancreatic, and hepatic functions are uncertain. The relationship between individual vagal primary afferent neurons and the various populations of peptide-secreting enteroendocrine cells is ill defined.
2. Vagal and other autonomic hindbrain mechanisms involved in food intake and energy expenditure
2.1. Integration of vagal mechanisms within the hindbrain
The brainstem harbors an impressive array of neurons and circuits directly involved in ingestion, digestion, and absorption of food, as well as in utilization of metabolites and fuels. The neural circuits controlling most of these tasks are contained within the brainstem and do not require the forebrain for their execution. Just as for respiration and circulation, other body functions essential for survival, the regulation of nutrient supply is to a large extent autonomically organized within the brainstem.
The caudal brainstem contains the complete pathways necessary for mastication and swallowing, with all the accompanying autonomic responses such as saliva secretion (Jordan et al., 1992). Both mastication and deglutition are complex behaviors that involve cooperation between large numbers of muscles. Protection of the airways is of critical importance, so that certain muscles cannot be activated independently. Rhythmic, temporally fixed, and sequential patterns of muscle action are therefore organized within specialized pattern generator circuits.
Both the sensory and motor limbs of brainstem reflexes related to ingestion have been relatively well characterized because of easily available methodology such as anterograde and retrograde neuronal tracing and recording from nerve fibers (Fig. 3). However, it is much less understood how sensory information is processed and leads to meaningful motor action, as this analysis typically requires more challenging methodology. It should also be noted that gastrointestinal hormones released into the circulation could affect brainstem function independent of primary afferent nerves by acting directly on neurons in the area postrema and NTS. Both these structures have a weak or absent blood brain barrier (Gross et al., 1990) (Gross et al., 1991). Since dendrites of vagal motor neurons penetrate deep into the NTS, they could also be directly affected by circulating factors.
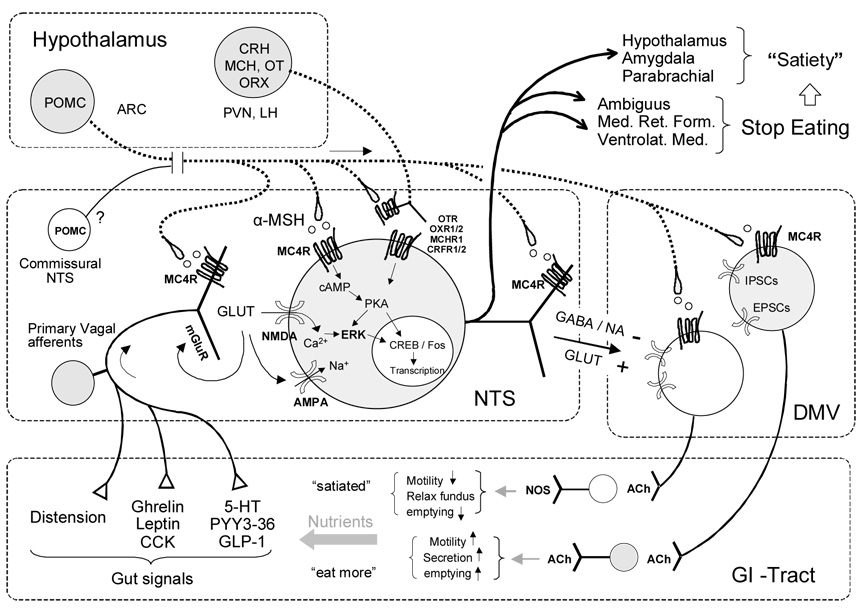
Fig. 3. Simplified schematic diagram showing the neural systems responsible for satiation and meal size control.
Hypothetical neuron in the nucleus tractus solitarius (NTS) receives information from gastrointestinal mechano- and chemo-sensors through vagal afferents and projects back to the gut via vago-vagal reflexes through the dorsal motor nucleus (DMV). Other outputs (heavy lines) of certain NTS neurons are directed towards the medullary reticular formation (Med. Ret. Form.) and eventually to brainstem motor nuclei responsible for oromotor control necessary to start and stop eating, as well as to forebrain areas, responsible for sustained satiety. Descending modulatory projections from hypothalamic areas are also shown (heavy dotted lines).
Based on experiments with the decerebrate rat, it appears that the isolated brainstem can terminate a meal and thus exhibit the basic behavior of satiety (for reviews see (Grill and Kaplan, 1990, 2002). This brainstem-based satiation process likely involves the activation of neurochemically specific NTS neurons such as the A2 catecholaminergic neurons (Rinaman et al., 1995; Rinaman, 2003), GLP-1 expressing neurons (Vrang et al., 2003)(ref), and POMC-expressing neurons in the commissural NTS (Fan et al., 2004).
Other areas in the hindbrain harbor sympathetic premotor neurons that ultimately control different steps involved in energy expenditure. These include neurons in the ventrolateral medulla and in the NTS innervating white adipose tissue responsible for fat mobilization (Bamshad et al., 1998; Bartness and Bamshad, 1998; Bamshad et al., 1999; Shi and Bartness, 2001) and neurons in the caudal raphé nuclei innervating brown adipose tissue responsible for thermogenesis (Morrison, 2001, 2003).
2.2. Integration of vagal mechanisms in the forebrain
The hypothalamic “center” hypothesis has dominated research on food intake during much of the last century. However, with the advent of neuronal tracing in the seventies, it became clear that the hypothalamus is well connected to most other areas of the brain and does not work in isolation. One of the emerging new views is that the neural system regulating energy balance is complex and distributed, involving specific areas of hindbrain, midbrain, and forebrain (Watts, 2000; Berthoud, 2002; Grill and Kaplan, 2002). Information from vagal sensors in the periphery arriving at the NTS is distributed widely in the CNS, including hypothalamus, amygdala, and cortex (Fig. 3). Some of these projections are direct, while others are indirect, via relay stations in the ventrolateral medulla, locus coeruleus, and parabrachial nucleus. There are particularly prominent direct ascending projections from the NTS to the paraventricular nucleus and weaker ones to the lateral hypothalamus and the dorsomedial and arcuate nuclei (Ricardo and Koh, 1978; Ter Horst et al., 1989; Cunningham et al., 1990). Thus, information from vagal afferents can be integrated with other nutritional information, as well as information pertaining to the time of day, reproductive cycle and state of arousal, all represented in the hypothalamus.
There is also ample anatomical and functional evidence for prominent descending projections from the hypothalamus to areas in the dorsal vagal complex and other feeding relevant areas in the caudal brainstem (Fig. 3). Hypothalamic neurons expressing the following peptides involved in food intake all project directly to the dorsal vagal complex and other brainstem areas: POMC/CART (Palkovits et al., 1987; Alessi et al., 1988), AGRP/NPY (Bagnol et al., 1999), oxytocin (Siaud et al., 1989; Rinaman, 1998), gastrin-releasing peptide (Bombesin) (Costello et al., 1991), orexin/dynorphin (Peyron et al., 1998), and MCH (Bittencourt et al., 1992). While AGRP, oxytocin, and MCH are exclusively expressed in the hypothalamus, POMC, NPY, and GRP are also expressed in the medulla (Joseph et al., 1985; Bronstein et al., 1992; Tohyama, 1998), and orexin is expressed in vagal afferent neurons (Kirchgessner, 2002). In addition, receptors for all these peptides have been identified in the dorsal vagal complex (Tohyama, 1998; Kishi et al., 2003).
There is also electrophysiological evidence suggesting that specific neurons in the dorsal vagal complex can be modulated by hypothalamic signals. Bereiter and coworkers (Bereiter et al., 1980) were the first to demonstrate that NTS neurons receiving oral input through the chorda tympany nerve are modulated by lateral hypothalamic stimulation. Such facilitation of gustatory responses in NTS neurons by lateral hypothalamic stimulation was recently confirmed in the hamster (Cho et al., 2002). Rogers and coworkers then demonstrated a similar effect of paraventricular nucleus stimulation on the activity of NTS neurons receiving gastrointestinal afferents through the vagus nerve (Rogers and Hermann, 1985). Most recently, Schwartz and Moran demonstrated opposing modulatory effects of intracerebroventricularly injected leptin and NPY on the electrical responsiveness of NTS neurons to gastric distension. Leptin increased distension-induced spike activity, whereas NPY decreased this activity (Schwartz and Moran, 2002). This study suggests that these peptides act at the level of the hypothalamus and via descending projections, but a direct action on the medulla cannot be excluded.
Finally, behavioral and functional evidence suggests that information from the hypothalamus can modulate the capacity of vagal satiety signals at the level of the dorsal vagal complex. Smith’s proposal (Smith, 1996) for a new classification of the factors controlling meal size was an important stimulus for studies addressing the integration of short-term signals such as CCK (direct controls), and longer-term signals such as leptin (indirect controls). He defined food stimuli contacting preabsorptive receptors along the surface of the gut from the tip of the tongue to the end of the small intestine as direct controls and everything else as indirect controls. Indirect controls that require the forebrain (metabolic, ecologic and rhythmic), change meal size by modulating the potency of direct controls.
Smith’s thesis was tested mainly by using c-Fos expression as an indicator of neural activity (Emond et al., 1999; Emond et al., 2001), analysis of food intake (McMinn et al., 2000; Matson et al., 2002) and meal structure (Eckel et al., 1998; Flynn et al., 1998; Schwartz et al., 1999; Azzara et al., 2002). The results of most of these studies show that leptin increases the potency of CCK to reduce meal size, supporting Smith’s hypothesis. The behavioral effects where also reflected by changes in c-Fos expression in the NTS/area postrema (and hypothalamus).
Behavioral tests of the Smith hypothesis focused mainly on potential modulation of satiation by descending melanocortin projections. Williams et al. reported that the MC4/3 receptor agonist MTII decreased, and the antagonist SHU9119 increased food intake when injected directly into the dorsal vagal complex at doses subthreshold for 4th-ventricular injections (Williams et al., 2000). Our own observations confirmed and extended these observations by demonstrating that DVC infusion of SHU9119 increases food intake by selectively increasing meal size, while not changing meal frequency. Also, with any exogenous injections, it is not clear whether the implicated endogenous ligand (α-MSH), originates from descending projections or local neurons, because POMC/α-MSH is expressed in a small population of neurons in the commissural NTS (Pilcher and Joseph, 1986; Palkovits et al., 1987; Bronstein et al., 1992) (Fig. 3).
3. The vagus and obesity
3.1. Evidence for vagal afferents in short- but not long-term control of food intake
Given this impressive breadth of sensory capacities of vagal afferents, one could expect that they play a crucial role in the control of food intake and possibly energy balance. Since vagal afferents transmit predominantly satiety signals from the gut to the brain, they seem important for keeping appetite under control and may help prevent development of obesity. However, total food intake and body weight are surprisingly impervious to manipulations of vagal integrity. Total abdominal vagotomies or selective vagal de-afferentations do not cause hyperphagia and obesity, and electrical stimulation of vagal afferents does not appear to decrease food intake and body weight (Koren and Holmes, 2006). Vagal deafferentations do produce subtle increases in meal size (Schwartz et al., 1999; Kelly et al., 2000; Powley et al., 2005), but because there is partial compensation by more frequent meals, it does not result in excessive body weight gain. From these observations it would appear that, compared to systems in the hypothalamus, vagal afferents play only a secondary role in the control of long-term food intake and energy balance. However, the methodology used to manipulate vagal afferents is rudimentary at best, as they do not allow selective ablation or stimulation of functionally specific neurons. For example, given that ghrelin (Date et al., 2002) and hyperglycemia (Niijima, 1982) can suppress, while gastric distension and CCK (Schwartz et al., 1993) can increase firing activity of specific populations of vagal afferents, simply cutting or stimulating both populations should theoretically lead to cancellation of their central effects. By learning more about the functional specificity of vagal afferents and the availability of more selective tools to manipulate them, they are still a worthwhile target for prevention or treatment of obesity. This view is enforced by emerging evidence that the impressive efficiency of bariatric surgery may, at least in part, be related to changes in gastrointestinal hormones acting on vagal afferents.
3.2. Vagal mechanisms affected by obesity surgeries
Bariatric surgery is currently the most effective treatment for morbid obesity and the number of procedures has dramatically increased over the last 10 years. The older malabsorptive jejunoileal bypass and the simple restrictive operations such as vertical and adjustable gastric banding techniques have been largely abandoned because they had either serious side effects and/or poor long-term efficacy. They have now been almost completely replaced by the more effective Roux en Y gastric bypass surgery (RYGB)(Cummings et al., 2004). This surgery consists of a small pouch of about 5% of the most proximal stomach, which is anastomosed with the upper end of the cut proximal to mid-jejunum (Fig. 4). The lower end of the cut jejunum is re-anastomosed to the distal jejunum. The result is a nutrient limb consisting of the small gastric pouch and the distal ileum, a bilio-pancreatic diversion limb consisting of the large stomach remnant, duodenum, and proximal jejunum, and a common piece consisting of mainly the ileum and all of the large intestines. Although this procedure results in approximately 1% mortality rate, it is reported as highly efficient for extended periods of time in reducing food intake, body weight, and indices of type-2 diabetes (Trostler et al., 1995; MacDonald et al., 1997).
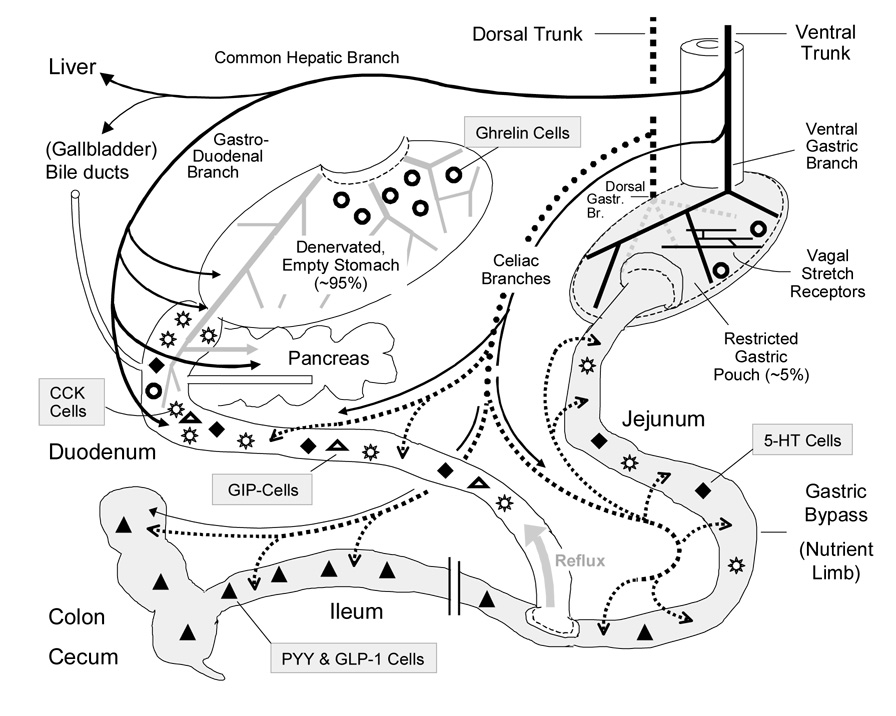
Fig. 4. Schematic diagram showing implications regarding vagal innervation and endocrine functions of gastric bypass surgery.
The nutrient limb of the Rou-xen-Y gastric bypass consisting of the small gastric pouch and the anastomosed jejunum is shown on the right (shaded). The bilio-pancreatic limb including the large gastric remnant with attached duodenum and proximal jejunum is shown on the left. Note that the stomach remnant is depicted at a much reduced size for clarity. The ventral (anterior) and dorsal (posterior) vagal trunks and their branches are shown as solid and dotted lines, respectively (the severed ventral gastric branches are in light gray). The relative density and distribution of enteroendocrine cells secreting peptide hormones or transmitters are depicted by different symbols as indicated.
With respect to gut innervation, both the ventral and dorsal gastric branches of the vagus nerve on the large gastric remnant are cut by the gastrostomy procedure (Fig. 4). Because some of these branches, traveling along the lesser curvature also cross the pyloric sphincter and reach the proximal duodenum and parts of the pancreas (Stavney et al., 1963; Berthoud et al., 1990; Berthoud and Powley, 1990; Berthoud et al., 1991b; Kressel et al., 1994), these areas are also partially vagotomized after RYGB surgery. Both vagal preganglionic efferent and afferent fibers are affected. As postganglionic sympathetic fibers and dorsal root afferents also innervate the stomach mainly via the left gastric artery entering the stomach near the cardia, they are likewise interrupted after RYGB.
In contrast, some vagal and sympathetic innervation of the distal part of the antrum, the pylorus, proximal duodenum, and pancreas should remain intact, as they are supplied by the gastroduodenal branch originating from the ventral trunk and traveling along the same named artery (Berthoud et al., 1991a; Berthoud et al., 2004). Similarly, the two celiac vagal branches traveling with the superior mesenteric artery and its subsidiaries and supplying most of the small and large intestines from the distal duodenum on downwards should remain intact. Special care has to be taken not to damage the dorsal celiac branch when carrying out the gastrostomy, as it exits the dorsal trunk very close to the gastric cardia. It would be helpful to carefully document the exact damage to nerves and to compare it with the outcome of each operation.
The other transection of nerve fibers in RYGB is associated with transection of the jejunum and affects mainly the enteric nervous system. Although this seems to have little impact on gut function, it may play some role in the communication between duodenal GIP and ileal GLP-1 secreting cells (Rocca and Brubaker, 1999), and may interrupt the migrating motor complex and normal peristalsis (Otterson and Sarr, 1993).
The potential mechanisms of hypophagia and weight loss have been discussed in an excellent review (Cummings et al., 2004). The most plausible explanation is reduced meal size because of the small capacity and accommodation of the gastric pouch. However, subjects could be expected to compensate for the smaller meal size by increasing meal frequency, and switching to energy dense foods, as has been seen after gastric banding. Two additional changes in RYGB patients, reduced ghrelin and increased PYY/GLP-1 secretion may prevent such compensatory behavior. Cummings and colleagues had hypothesized that ghrelin cells in the gastric mucosa may eventually cease to secrete ghrelin, as they are constantly stimulated by an empty stomach (override inhibition hypothesis) (Cummings et al., 2004). However, some doubt is cast on this explanation by reports of unchanged (Stoeckli et al., 2004), or even increased (Holdstock et al., 2003) ghrelin levels after RYGB, and by the observation in rats, that postprandial ghrelin suppression does not depend on nutrients in the stomach, but on nutrients in the duodenum and proximal jejunum (Williams et al., 2003a). Reflux of nutrients from the bypass limb into the proximal jejunum could thus still trigger prandial ghrelin suppression, rendering the override inhibition hypothesis doubtful.
As suggested by Cummings (Cummings et al., 2004), ghrelin secretion may be decreased after RYGB because of denervation of autonomic input to ghrelin cells in the stomach. Clearly, the entire gastric remnant containing most ghrelin cells is almost completely vagotomized and sympathectomized as discussed above (Fig. 4). Preliminary studies showed smaller fasting-induced increases of plasma ghrelin in subdiaphragmatic vagotomized rats, and an acute suppression with atropine treatment in intact rats (Williams et al., 2003b), suggesting that vagal efferents may be involved in the tonic increase of ghrelin in the empty stomach. However, more selective vagotomies, both in terms of vagal branches supplying specific targets and separating afferents from efferents, as well as vagal stimulation studies of the vascularly perfused stomach will be necessary to shed more light on the mechanisms involved. The need for more mechanistic studies is also indicated by disparate findings regarding vagal involvement of prandial ghrelin suppression. While in humans, vagal stimulation by means of modified sham feeding was able to enhance ghrelin suppression by oral fat intake (Heath et al., 2004) vagotomy was without any effect on basal levels or on re-feeding-induced suppression of ghrelin in rats (Williams et al., 2003b).
In light of the recent finding that electrical stimulation of sympathetic nerves supplying the upper abdominal viscera increases ghrelin concentration in portal blood, it is possible that the sympathetic nervous system may be more involved in the ghrelin spike during meal anticipation than the vagal system (Mundinger et al., 2006), and the complete sympathetic denervation of the stomach after RYGB surgery might explain low ghrelin levels observed by some. However, studies using a vascularly perfused stomach will be necessary to rule out indirect mediation of sympathetic stimulation-induced ghrelin secretion by other innervated targets in the stomach and adjacent organs.
Thus, lack of ghrelin secretion remains an attractive explanation for the success of RYGB in decreasing food intake and deserves further study. At this time it appears more likely that ghrelin suppression is due to interference with neural inputs to ghrelin cells rather than nutrient regulation.
Increased secretion of PYY3–36 and GLP-1 from ileal L-cells caused by dumping of nutrients after RYGB is the most plausible explanation for the beneficial effects on glucose homeostasis and suppression of food intake. Although considerable evidence suggests that these two peptides act directly on the brain, vagal afferents may also participate. Thus, the enhanced suppression of food intake after RYGB could be partly mediated by vagal afferents in the celiac and gastroduodenal abdominal branches. These branches should not be damaged by the surgery. A role for vagal efferents in the control of PYY and GLP-1 secretion has also been reported. The strongest evidence comes from experiments in pigs, where electrical vagal stimulation increased PYY secretion from the vascularly isolated ileum (Sheikh et al., 1989).The potential contributions of vagal afferents and efferents to the outcome of RYGB surgery could easily be tested in an animal model using selective abdominal vagotomies and chemical de-afferentations by means of locally applied capsaicin.
3.3. Vagal mechanisms affected by gastric pacing and vagal stimulation
Gastric pacing is the latest and least developed method considered for obesity therapy (Abell et al., 2006). It consists of electrically stimulating the stomach wall through a pair of serosal electrodes typically near the lesser curvature about halfway between esophagus and pylorus. Although it is assumed that mainly smooth muscle is stimulated, anything else such as vagal and non-vagal nerve fascicles, interstitial cells of Cajal pacemaker cells, and enteric neurons/fibers could also be stimulated, particularly when the pulse width of the electrical stimulation is increased. It is therefore not surprising that the effects are notoriously unreliable and irreproducible and that the mechanisms leading to anorexia and weight loss found in some reports are completely unknown. This approach is in desperate need of animal models that systematically investigate the underlying mechanisms, including a potential role for vagal afferents.
Vagal afferent stimulation is increasingly used to treat epilepsy, depression, and pain in humans (Groves and Brown, 2005). Electrodes placed near the intact cervical vagus are stimulated to preferentially activate thin myelinated vagal afferents. In a preliminary study in 21 patients without a non-stimulated control group, vagal nerve stimulation did not decrease body weight over an average of 20 months (Koren and Holmes, 2006).
3.4. Role of vagal innervation of pancreas, liver, and other abdominal organs
The vagal efferent innervation of the pancreas was extensively investigated in the late 70s and 80s following the observation that subdiaphragmatic vagotomy prevented or reversed development of VMH-induced obesity (Inoue and Bray, 1977; Cox and Powley, 1981; Sclafani et al., 1981). Because plasma insulin levels rose in a vagal-dependent fashion immediately after placing electrolytic VMH lesions, vagally mediated hyperinsulinemia was considered one of the primary mechanisms leading to hyperphagia and fat accumulation (Berthoud and Jeanrenaud, 1979). The vagal preganglionic neurons projecting to interlobular pancreatic ganglia were identified by tracing (Berthoud and Powley, 1991), and functional studies in the rat demonstrated that they reach the pancreas mainly via the gastric and hepatic-gastroduodenal vagal branches (Berthoud et al., 1981; Berthoud et al., 1990; Berthoud and Powley, 1990; Wang et al., 1999). Because cephalic-vagal release of insulin is important for normal glucose tolerance (Siegel et al., 1980; Trimble et al., 1981), damage to the gastric vagal branches innervating the pancreas could be expected to have a negative role on glucose homeostasis. Since improvement of glucose homeostasis is one of the hallmarks of RYGB surgery, there must be rapid compensation through vagal input to the pancreas via the gastroduodenal branch or the increased incretin release.
Although the pancreas is innervated by sparse vagal afferent fibers (Neuhuber, 1989), it appears to use the hormonal route to signal metabolic information to the brain. The pancreatic hormones insulin, amylin, and glucagon, each encodes some aspect of nutrient status and signals directly or via the liver to specific brain areas. Only glucagon’s suppressive effect on food intake is mediated by vagal afferents, apparently picking up increased glucose production produced by glucagons in the liver (Woods et al., 2006).
In the liver it is mainly the vagal afferents implicated in nutrient sensing that could play a potential role in the development of obesity. Sensors for glucose levels and total available energy derived from fat and glucose oxidation with vagal afferent signaling to the brain and effects on short-term food intake have been described (Friedman, 1997; Horn et al., 2001; Langhans, 2003). Vagal afferent terminals potentially serving these functions have been traced to mainly the portal vein and the periportal space in the liver (Berthoud et al., 1992). Thus, ablation of vagal sensory fibers to the liver could be expected to increase food intake and predispose to obesity. Signals from the liver may also lead to metabolic changes independent of food intake. In a mouse model with hepatic steatosis induced by adenoviral expression of peroxisome-proliferator-activated receptor (PPAR)-γ2 in hepatocytes, metabolic rate increased, resulting in improved insulin sensitivity and loss of body weight (Uno et al., 2006). Capsaicin-induced lesion of vagal afferents in the common hepatic branch prevented these effects, suggesting that vagal afferent information from the liver may protect against metabolic consequences induced by excessive energy storage in the liver (Uno et al., 2006). Since vagal efferents in the common hepatic branch are important for the suppression of hepatic glucose production when the hypothalamic nutrient sensor is stimulated by nutrient repletion signals such as insulin or fatty acid oxidation (Pocai et al., 2005b; Pocai et al., 2005a), ablation of vagal efferents innervating the liver could play an additional supportive role in the development of obesity. In support of this, rats with selective common hepatic branch vagotomies where slightly hyperphagic and gained weight more rapidly when fed sweet milk (Kraly et al., 1986).
Vagal innervation of adipose tissue is controversial. One group reported vagal efferent innervation of abdominal and subcutaneous white adipose tissue from the dorsal motor nucleus based on viral transneuronal and conventional retrograde tracing (Kreier et al., 2002; Kreier et al., 2006). In a carefully controlled subsequent study no evidence of such vagal efferent innervation was found (Giordano et al., 2006). As earlier anterograde studies also did not find evidence for innervation of fat tissue by vagal preganglionic fibers, it is highly likely that the positive findings of Kreier et al were due to tracer leakage (Berthoud et al., 2006; Fox and Powley, 1989).
4. Conclusions
Vagal pathways innervating the gastrointestinal tract, pancreas, and liver are intimately involved in the control of assimilation, storage, mobilization, conversion, and oxidation of macronutrients. Although much is already known about vagal afferent nutrient sensors informing the brain about the short term availability of energy, many details regarding the specific transduction mechanisms in the periphery and the inter-and intra-neuronal signaling cascades disseminating the sensory information across the neuraxis are still missing. Vagal sensory information plays a crucial role in the mechanism of satiation but the underlying circuitry in the caudal brainstem and higher up in the brain is still ill defined. Given this central role between the brain and nutrient-handling periphery, it could be expected that the integrity of the vagal system is directly linked to the development of obesity, but this has not yet been demonstrated. Vagal lesions have surprisingly small effects on energy balance. However this could be misleading, as typically used vagal lesions are not selective enough and the adequate challenges may not have been uncovered. The recent success with bariatric surgeries to reverse obesity highlights the importance of gut-brain and brain-gut communication. The development of animal models will clarify the role of vagal mechanisms in this presently most efficacious obesity treatment.
Acknowledgments
Supported by NIH grant DK47348 and DK52257
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell TL, Minocha A, Abidi N. Looking to the future: electrical stimulation for obesity. Am J Med Sci. 2006;331:226–232. doi: 10.1097/00000441-200604000-00010. [DOI] [PubMed] [Google Scholar]
- Alessi NE, Quinlan P, Khachaturian H. MSG effects on beta-endorphin and alpha-MSH in the hypothalamus and caudal medulla. Peptides. 1988;9:689–695. doi: 10.1016/0196-9781(88)90108-8. [DOI] [PubMed] [Google Scholar]
- Andersen DK, Elahi D, Brown JC, Tobin JD, Andres R. Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest. 1978;62:152–161. doi: 10.1172/JCI109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagons-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002;143:2420–2426. doi: 10.1210/endo.143.6.8840. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. doi: 10.1016/s0031-9384(02)00883-1. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti- related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Bereiter D, Berthoud HR, Jeanrenaud B. Hypothalamic input to brain stem neurons responsive to oropharyngeal stimulation. Exp Brain Res. 1980;39:33–39. doi: 10.1007/BF00237067. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Fox EA, Neuhuber WL. Vagaries of adipose tissue innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1240–R1242. doi: 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Jeanrenaud B. Acute hyperinsulinemia and its reversal by vagotomy after lesions of the ventromedial hypothalamus in anesthetized rats. Endocrinology. 1979;105:146–151. doi: 10.1210/endo-105-1-146. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol. 1990;258:R523–R530. doi: 10.1152/ajpregu.1990.258.2.R523. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of DiI. Brain Res. 1991;553:336–341. doi: 10.1016/0006-8993(91)90846-n. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:117. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Trimble ER, Moody AJ. Lack of gastric inhibitory polypeptide (GIP) response to vagal stimulation in the rat. Peptides. 1982;3:907–912. doi: 10.1016/0196-9781(82)90059-6. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Fox EA, Powley TL. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol. 1990;258:R160–R168. doi: 10.1152/ajpregu.1990.258.1.R160. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Fox EA, Powley TL. Abdominal pathways and central origin of rat vagal fibers that stimulate gastric acid. Gastroenterology. 1991a;100:627–637. doi: 10.1016/0016-5085(91)80006-u. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991b;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol (Berl) 1992;186:431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Lynn PA, Blackshaw LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1371–R1381. doi: 10.1152/ajpregu.2001.280.5.R1371. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16 Suppl 1:28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B. Cephalic phase, reflex insulin secretion. Neuroanatomical and physiological characterization. Diabetologia. 1981;20 Suppl:393–401. [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: A putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci U S A. 1999;96:13506–13511. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein DM, Schafer MK, Watson SJ, Akil H. Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Ingman-Baker J, Levy J, Brown JC. A comparison of the ability of serum and monoclonal antibodies to gastric inhibitory polypeptide to detect immunoreactive cells in the gastroenteropancreatic system of mammals and reptiles. Histochemistry. 1982;76:341–349. doi: 10.1007/BF00543956. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, Dimaline R, Varro A, Dockray GJ. Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience. 2002;109:339–347. doi: 10.1016/s0306-4522(01)00474-2. [DOI] [PubMed] [Google Scholar]
- Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, Walker F, Lewin MJ, Meister B, Bado A. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology. 2005a;146:879–888. doi: 10.1210/en.2004-1138. [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2005b;288:R1695–R1706. doi: 10.1152/ajpregu.00870.2004. [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3–36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R298–R305. doi: 10.1152/ajpregu.00674.2005. [DOI] [PubMed] [Google Scholar]
- Cho YK, Li CS, Smith DV. Taste responses of neurons of the hamster solitary nucleus are enhanced by lateral hypothalamic stimulation. J Neurophysiol. 2002;87:1981–1992. doi: 10.1152/jn.00765.2001. [DOI] [PubMed] [Google Scholar]
- Costello JF, Brown MR, Gray TS. Bombesin immunoreactive neurons in the hypothalamic paraventricular nucleus innervate the dorsal vagal complex in the rat. Brain Res. 1991;542:77–82. doi: 10.1016/0006-8993(91)91000-q. [DOI] [PubMed] [Google Scholar]
- Cox JE, Powley TL. Prior vagotomy blocks VMH obesity in pair-fed rats. Am J Physiol. 1981;240:E573–E583. doi: 10.1152/ajpendo.1981.240.5.E573. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the shortand long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Prigeon RL, Ensinck JW. Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes. 1995;44:1433–1437. doi: 10.2337/diab.44.12.1433. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780–3786. doi: 10.1210/endo.139.9.6202. [DOI] [PubMed] [Google Scholar]
- Eberlein GA, Eysselein VE, Schaeffer M, Layer P, Grandt D, Goebell H, Niebel W, Davis M, Lee TD, Shively JE, et al. A new molecular form of PYY: structural characterization of human PYY(3–36) and PYY(1–36) Peptides. 1989;10:797–803. doi: 10.1016/0196-9781(89)90116-2. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;275:R186–R193. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276:R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav. 2001;72:123–128. doi: 10.1016/s0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR. Mode of action of OB protein (leptin) on feeding. Am J Physiol. 1998;275:R174–R179. doi: 10.1152/ajpregu.1998.275.1.R174. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL. False-positive artifacts of tracer strategies distort autonomic connectivity maps. Brain Res Brain Res Rev. 1989;14:53–77. doi: 10.1016/0165-0173(89)90009-x. [DOI] [PubMed] [Google Scholar]
- Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- Friedman MI. An energy sensor for control of energy intake. Proc Nutr Soc. 1997;56:41–50. doi: 10.1079/pns19970008. [DOI] [PubMed] [Google Scholar]
- Fu-Cheng X, Anini Y, Chariot J, Castex N, Galmiche JP, Roze C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: neural regulation by proximal gut. Pflugers Arch. 1997;433:571–579. doi: 10.1007/s004240050316. [DOI] [PubMed] [Google Scholar]
- Geary N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav. 2004;81:719–733. doi: 10.1016/j.physbeh.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- Ghilardi JR, Allen CJ, Vigna SR, McVey DC, Mantyh PW. Cholecystokinin and neuropeptide Y receptors on single rabbit vagal afferent ganglion neurons: site of prejunctional modulation of visceral sensory neurons. Brain Res. 1994;633:33–40. doi: 10.1016/0006-8993(94)91519-9. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM. Caudal Brainstem Participates in the Distributed Neural Control of Feeding. In: Stricker EM, editor. Handbook of Behavioral Neurobiology. New York: Plenum Press; 1990. pp. 125–149. [Google Scholar]
- Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Wainman DS, Shaver SW. Subregional topography of capillaries in the dorsal vagal complex of rats: II. Physiological properties. J Comp Neurol. 1991;306:83–94. doi: 10.1002/cne.903060107. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol. 2004;180:273–281. doi: 10.1677/joe.0.1800273. [DOI] [PubMed] [Google Scholar]
- Herrmann-Rinke C, Voge A, Hess M, Goke B. Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol. 1995;147:25–31. doi: 10.1677/joe.0.1470025. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- Hofer D, Asan E, Drenckhahn D. Chemosensory Perception in the Gut. News Physiol Sci. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- Horn CC, Tordoff MG, Friedman MI. Role of vagal afferent innervation in feeding and brain Fos expression produced by metabolic inhibitors. Brain Res. 2001;919:198–206. doi: 10.1016/s0006-8993(01)02963-8. [DOI] [PubMed] [Google Scholar]
- Inoue S, Bray GA. The effects of subdiaphragmatic vagotomy in rats with ventromedial hypothalamic obesity. Endocrinology. 1977;100:108–114. doi: 10.1210/endo-100-1-108. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Brownstone RM, Noga BR. Control of functional systems in the brainstem and spinal cord. Curr Opin Neurobiol. 1992;2:794–801. doi: 10.1016/0959-4388(92)90136-9. [DOI] [PubMed] [Google Scholar]
- Joseph SA, Pilcher WH, Knigge KM. Anatomy of the corticotrophin-releasing factor and opiomelanocortin systems of the brain. Fed Proc. 1985;44:100–107. [PubMed] [Google Scholar]
- Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102:39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Kelly L, Morales S, Smith BK, Berthoud HR. Capsaicin-treated rats permanently overingest low- but not high-concentration sucrose solutions. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1805–R1812. doi: 10.1152/ajpregu.2000.279.5.R1805. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL. Orexins in the brain-gut axis. Endocr Rev. 2002;23:1–15. doi: 10.1210/edrv.23.1.0454. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- Koren MS, Holmes MD. Vagus nerve stimulation does not lead to significant changes in body weight in patients with epilepsy. Epilepsy Behav. 2006;8:246–249. doi: 10.1016/j.yebeh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite. 1986;7:1–17. doi: 10.1016/s0195-6663(86)80038-1. [DOI] [PubMed] [Google Scholar]
- Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressel M, Berthoud HR, Neuhuber WL. Vagal innervation of the rat pylorus: an anterograde tracing study using carbocyanine dyes and laser scanning confocal microscopy. Cell Tissue Res. 1994;275:109–123. doi: 10.1007/BF00305379. [DOI] [PubMed] [Google Scholar]
- Langhans W. Role of the liver in the control of glucose-lipid utilization and body weight. Curr Opin Clin Nutr Metab Care. 2003;6:449–455. doi: 10.1097/01.mco.0000078993.96795.16. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA, Bloom SR. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90:4521–4524. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- MacDonald KG, Jr, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ. The Gastric Bypass Operation Reduces the Progression and Mortality of Non-Insulin-Dependent Diabetes Mellitus. J Gastrointest Surg. 1997;1:213–220. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
- Matson CA, Reid DF, Ritter RC. Daily CCK injection enhances reduction of body weight by chronic intracerebroventricular leptin infusion. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1368–R1373. doi: 10.1152/ajpregu.00080.2001. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology. 2000;141:4442–4448. doi: 10.1210/endo.141.12.7815. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty P, Dimaline R, Thompson DG, Dockray GJ. Characterization of cholecystokininA and cholecystokininB receptors expressed by vagal afferent neurons. Neuroscience. 1997;79:905–913. doi: 10.1016/s0306-4522(96)00675-6. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential regulation of brown adipose and splanchnic sympathetic outflows in rat: roles of raphe and rostral ventrolateral medulla neurons. Clin Exp Pharmacol Physiol. 2001;28:138–143. doi: 10.1046/j.1440-1681.2001.03406.x. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience. 2003;121:17–24. doi: 10.1016/s0306-4522(03)00363-4. [DOI] [PubMed] [Google Scholar]
- Mundinger TO, Cummings DE, Taborsky GJ., Jr Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Vagal afferent fibers almost exclusively innervate islets in the rat pancreas as demonstrated by anterograde tracing. J Auton Nerv Syst. 1989;29:13–18. doi: 10.1016/0165-1838(89)90015-5. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Kressel M, Stark A, Berthoud HR. Vagal efferent and afferent innervation of the rat esophagus as demonstrated by anterograde DiI and DiA tracing: focus on myenteric ganglia. J Auton Nerv Syst. 1998;70:92–102. doi: 10.1016/s0165-1838(98)00034-4. [DOI] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J Physiol. 1982;332:315–323. doi: 10.1113/jphysiol.1982.sp014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst. 1996;61:149–154. doi: 10.1016/s0165-1838(96)00071-9. [DOI] [PubMed] [Google Scholar]
- Ohneda A, Kobayashi T, Nihei J, Imamura M, Naito H, Tsuchiya T. Role of vagus nerve in secretion of gastric inhibitory polypeptide in dogs. Tohoku J Exp Med. 1985;147:183–190. doi: 10.1620/tjem.147.183. [DOI] [PubMed] [Google Scholar]
- Otterson MF, Sarr MG. Normal physiology of small intestinal motility. Surg Clin North Am. 1993;73:1173–1192. doi: 10.1016/s0039-6109(16)46186-4. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res. 1987;436:323–338. doi: 10.1016/0006-8993(87)91676-3. [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Berthoud HR. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat Rec. 2002;266:10–20. doi: 10.1002/ar.10026. [DOI] [PubMed] [Google Scholar]
- Pederson RA, Schubert HE, Brown JC. Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes. 1975;24:1050–1056. doi: 10.2337/diab.24.12.1050. [DOI] [PubMed] [Google Scholar]
- Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–R1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Pilcher WH, Joseph SA. Differential sensitivity of hypothalamic and medullary opiocortin and tyrosine hydroxylase neurons to the neurotoxic effects of monosodium glutamate (MSG) Peptides. 1986;7:783–789. doi: 10.1016/0196-9781(86)90096-3. [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005a;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005b;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol Behav. 2004;82:69–74. doi: 10.1016/j.physbeh.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Powley TL, Chi MM, Baronowsky EA, Phillips RJ. Gastrointestinal tract innervation of the mouse: afferent regeneration and meal patterning after vagotomy. Am J Physiol Regul Integr Comp Physiol. 2005;289:R563–R574. doi: 10.1152/ajpregu.00167.2005. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Visceral perception: sensory transduction in visceral afferents and nutrients. Gut. 2002;51 Suppl 1:i11–i14. doi: 10.1136/gut.51.suppl_1.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Freeman SL, Whited K, Darcel N, Liou A, Bohan D. Detection of macronutrients in the intestinal wall. Auton Neurosci. 2006;125:28–33. doi: 10.1016/j.autneu.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- Ritzel R, Orskov C, Holst JJ, Nauck MA. Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP-1 [7-36 amide] after subcutaneous injection in healthy volunteers. Dose-response-relationships. Diabetologia. 1995;38:720–725. doi: 10.1007/BF00401846. [DOI] [PubMed] [Google Scholar]
- Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Gastric-vagal solitary neurons excited by paraventricular nucleus microstimulation. J Auton Nerv Syst. 1985;14:351–362. doi: 10.1016/0165-1838(85)90081-5. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- Schirra J, Goke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005;128:109–115. doi: 10.1016/j.regpep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH. Leptin and neuropeptide y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–3784. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol. 1993;265:R872–R876. doi: 10.1152/ajpregu.1993.265.4.R872. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999;276:R1623–R1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Aravich PF, Landman M. Vagotomy blocks hypothalamic hyperphagia in rats on a chow diet and sucrose solution, but not on a palatable mixed diet. J Comp Physiol Psychol. 1981;95:720–734. doi: 10.1037/h0077830. [DOI] [PubMed] [Google Scholar]
- Scott TR, Verhagen JV. Taste as a factor in the management of nutrition. Nutrition. 2000;16:874–885. doi: 10.1016/s0899-9007(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Sheikh SP, Holst JJ, Orskov C, Ekman R, Schwartz TW. Release of PYY from pig intestinal mucosa; luminal and neural regulation. Regul Pept. 1989;26:253–266. doi: 10.1016/0167-0115(89)90193-6. [DOI] [PubMed] [Google Scholar]
- Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull. 2001;54:375–385. doi: 10.1016/s0361-9230(00)00455-x. [DOI] [PubMed] [Google Scholar]
- Siaud P, Denoroy L, Assenmacher I, Alonso G. Comparative immunocytochemical study of the catecholaminergic and peptidergic afferent innervation to the dorsal vagal complex in rat and guinea pig. J Comp Neurol. 1989;290:323–335. doi: 10.1002/cne.902900302. [DOI] [PubMed] [Google Scholar]
- Siegel EG, Trimble ER, Renold AE, Berthoud HR. Importance of preabsorptive insulin release on oral glucose tolerance: studies in pancreatic islet transplanted rats. Gut. 1980;21:1002–1009. doi: 10.1136/gut.21.11.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- Stavney LS, Kato T, Griffith CA, Nyhus LM, Harkins HN. A Physiologic Study of Motility Changes Following Selective Gastric Vagotomy. J Surg Res. 1963;23:390–394. doi: 10.1016/s0022-4804(63)80064-5. [DOI] [PubMed] [Google Scholar]
- Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- Sugita M. Taste perception and coding in the periphery. Cell Mol Life Sci. 2006;63:2000–2015. doi: 10.1007/s00018-006-6100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;25 Suppl 5:S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Tohyama M, Takatsuji K. Atlas of Neuroactive Substances and Their receptors in the Rat. Oxford: Oxford University Press; 1998. [Google Scholar]
- Trimble ER, Berthoud HR, Siegel EG, Jeanrenaud B, Renold AE. Importance of cholinergic innervation of the pancreas for glucose tolerance in the rat. Am J Physiol. 1981;241:E337–E341. doi: 10.1152/ajpendo.1981.241.5.E337. [DOI] [PubMed] [Google Scholar]
- Trostler N, Mann A, Zilberbush N, Avinoach E, Charuzi II. Weight Loss and Food Intake 18 Months following Vertical Banded Gastroplasty or Gastric Bypass for Severe Obesity. Obes Surg. 1995;5:39–51. doi: 10.1381/096089295765558141. [DOI] [PubMed] [Google Scholar]
- Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, Hasegawa Y, Gao J, Kaneko K, Iwasaki H, Ishihara H, Sasano H, Inukai K, Mizuguchi H, Asano T, Shiota M, Nakazato M, Oka Y. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP1/2 containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003 doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Berthoud HR. Functional vagal input to chemically identified neurons in pancreatic ganglia as revealed by Fos expression. Am J Physiol. 1999;277:E958–E964. doi: 10.1152/ajpendo.1999.277.5.E958. [DOI] [PubMed] [Google Scholar]
- Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol. 1997;273:R833–R837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003a;144:2765–2767. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003b;144:5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- Williams RM, Berthoud HR, Stead RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation. 1997;4:266–270. doi: 10.1159/000097346. [DOI] [PubMed] [Google Scholar]
- Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XY, Zhu JX, Gao J, Owyang C, Li Y. Neurochemical phenotype of vagal afferent neurons activated to express C-FOS in response to luminal stimulation in the rat. Neuroscience. 2005;130:757–767. doi: 10.1016/j.neuroscience.2004.09.060. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi FN, Whitehead MC. Discrete innervation of murine taste buds by peripheral taste neurons. J Neurosci. 2006;26:8243–8253. doi: 10.1523/JNEUROSCI.5142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi T, Holmberg K, Landry M, Huang W, Xiao H, Ju G, Hokfelt T. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc Natl Acad Sci U S A. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]