Abstract
In recent years the focus of glaucoma research has shifted toward neuroprotection, as the traditional strategies of lowering intraocular pressure have been shown to be unable to prevent progressive vision loss in some glaucoma patients. As a result various neuroprotective drug-based approaches have been shown capable of reducing the death of retinal ganglion cells, which is the hallmark of glaucomatous optic neuropathy. There has been increasing evidence that glaucomatous neurodegeneration is analogous to other neurodegenerative diseases in the central nervous system, with recent work from our group elucidating a strong link between basic cellular processes in glaucoma and Alzheimer’s disease. Additionally, there is a growing trend for using existing neuroprotective strategies in central nervous system diseases for the treatment of glaucoma. In fact, a trial treating patients with primary open-angle glaucoma with memantine, a drug approved for the treatment of Alzheimer’s disease, has recently been completed. Results of this trial are not yet available. In this review, we will examine currently advocated neuroprotective drug-based strategies in the potential management of glaucoma.
Keywords: glaucoma, neuroprotection, retinal ganglion cell apoptosis
Glaucoma is a multifactorial, neurodegenerative disease characterized by optic neuropathy, visual field loss, and retinal degeneration with retinal ganglion cells (RGC) apoptosis being a recognized early phenomenon.1 It is the second major leading cause of irreversible blindness worldwide affecting approximately 2% of the population over the age of 40.2
There are many factors associated with an increased risk of developing glaucoma.3-6 Currently lowering intraocular pressure (IOP) is the only clinical therapy available in the treatment of glaucoma7,8 with elevated IOP having previously been implicated as a possible primary insult in the disease resulting in mechanical or ischemic conditions leading to the development of RGC death and glaucoma. Unfortunately, patients can continue to lose vision despite successful IOP control.9 It is becoming clear that a methodology which only focuses on the reduction of IOP in patients is not the answer. As IOP is no longer regarded as the only therapy target in glaucoma, the focus of research is now shifting toward other strategies, such as neuroprotection of RGCs and the central visual pathway neurons.
Neuroprotection may be defined as the use of therapeutic agents to prevent, hinder, and in some instances reverse neuronal cell death whatever the primary injury.10 Several neuroprotective treatments have been established in central nervous system (CNS) disease such as Alzheimer’s (AD), Parkinson’s (PD), and Huntington’s (HD) diseases.11-15
In recent years, there has been a growing trend for using existing neuroprotective drugs which have been found effective in other CNS disease for the treatment of glaucoma.16 Indeed our recent study has elucidated a strong link between mechanisms of cell death in glaucoma and AD.15 A good example of this trend is memantine, which is a drug that has Food and Drug Administration approval for the treatment of AD, and is currently in clinical trial phase IV in glaucoma patients.17-20 With the increasing association of glaucoma as a neurodegenerative disorder, it appears there may be a possibility of using and developing agents with applications to glaucoma and all neurodegenerative disease.
It should be noted that the notion of neuroprotective treatment for glaucoma has been around for a while, though there has been little significant breakthrough to date. Researchers have continued to focus on neuroprotection and as a result steady progress has been made giving rise to several different neuroprotective strategies. However, what is clearly lacking appears adequate clinical measures of neuroprotection, and this may account for the difficulty in assessing efficacy in patients. One of the obstacles for establishing neuroprotection in glaucoma is that glaucoma is a slow, progressive disease and the current method of measuring progression, a computerized visual field assessment, is highly variable. The advent of sophisticated optical instrumentation such as the Optical Coherence Tomography and new perimetric and electrophysiological techniques may lead to more meaningful assessment of disease. In particular, we believe that new methodologies for imaging cellular events, such as the in vivo detection of apoptosis such as detection of apoptosing retinal cells (DARC)15,21,22 should provide clinicians and researchers with new sensitive tools with which to assess neuroprotection.
In this review we will be focusing on neuroprotection strategies specifically aimed toward glaucoma, that are not gene or stem cell therapy-based, as these will be covered elsewhere in the journal. Fig. 1 briefly summaries current and potentially available approaches to neuroprotection in glaucoma. All these strategies have previously been advocated in CNS diseases.
FIGURE 1.
Summary of current research strategies employed to study neuroprotection in glaucoma as previously applied in CNS disease.
Excitotoxicity
Glutamate is an essential amino acid that is abundant in all cells and known to play an important role as the main excitatory neurotransmitter in the CNS and retina. Glutamate release has been implicated as a mechanism of RGC death in glaucoma3,22-24 and inhibition or blockade of glutamate activity—in particular, modulation of the N-methyl-d-aspartate (NMDA)-type receptor has been advocated to be an important strategy for neuroprotection in glaucoma17,18,22,25,26 although its exact role is controversial. Dreyer et al. first reported an increase of glutamate in vitreous in glaucoma patients and experimental monkey glaucoma in 199627 and a similar result was reported by Brooks et al. using dogs.28 Although these results recently have been called into question,29-32 there is also an appreciation of the inherent difficulties in measuring in vivo glutamate levels.24
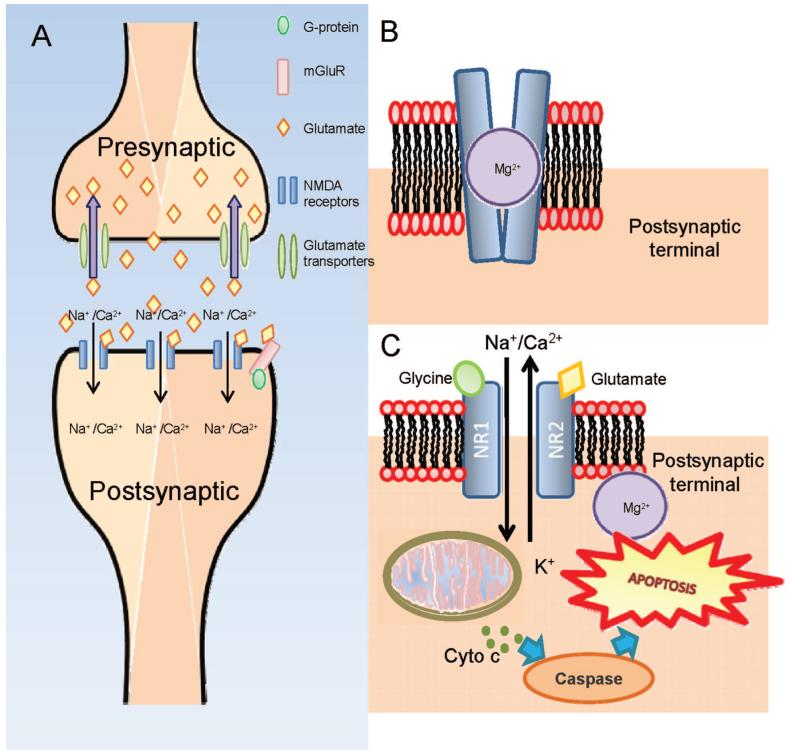
Glutamate is tightly regulated in the presynaptic cells as excessive expression of glutamate is potent and neurotoxic. Such is the importance of regulating glutamate; transporters are present in the membranes of neurons and glial cells to remove the excess glutamate from the synapses. Fig. 2A illustrates the induction of glutamate release from the presynaptic terminal by a nerve impulse and the binding of glutamate onto NMDA receptors located on the postsynaptic terminal. This leads to the influx of Ca2+ and Na+ ions. The term excititotoxicity refers to the phenomenon where cells die via apoptosis (programmed cell death) because of the presence of excessive amounts of glutamate.33-35 When the cells undergo apoptosis, intracellular glutamate is released from the dying cell and dispersed among neighboring cells in the vicinity causing secondary degeneration and triggering a cascade of excititoxicity events leading to further cell death.36-39
FIGURE 2.
A, Upon excitation by the nerve impulse, glutamate is released from the presynaptic terminal into the synaptic cleft where it binds onto the NMDA-receptors located on the postsynaptic terminal. Glutamate transporters located presynaptic terminal actively transport glutamate back into the terminal. B, Glutamate and glycine bind onto the receptors and the interaction causes transient conformational change in the channel and the depolarization of the cell resulting in the liberation of the Mg2+ which blocks the channel. The opening of the channel allows extracellular ionic molecules such as Ca2+ and Na+ to diffuse through the channel into the cell. Under normal physiological conditions, the NMDA channel is closed and is open transiently to enable the generation of a nerve impulse. C, When there is excessive glutamate present, the channel remains open causing a flood of Ca2+ and Na+ resulting in the depolarization of the mitochondrial membrane potential. Such events trigger off the release of cytochrome c which subsequently activates the caspase pathway leading toward apoptosis. A color version of this figure is available at www.optvissci.com.
The mechanism of excitotoxicity has been well researched. Several glutamate receptor systems have been identified, namely the ionotropic [NMDA, Kainate, and Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)] and metabotropic receptors, and disruption of their effects offer a promising strategy to preventing effects of neurodegeneration.22 Postsynaptic NMDA receptors are heteromeric ion channel complexes that are composed of several different subunits, two NR1 subunits and two NR2 subunits.40 Glutamate binds onto the target site on the NR1 subunit while glycine, which acts as a coagonist, binds onto the subunit NR2. Under normal physiological conditions, glutamate and glycine41 bind onto the receptors and the interaction causes transient conformational change in the channel and the depolarization of the cell resulting in the liberation of the Mg2+ ion which normally blocks the channel (Fig. 2B). The opening of the channel allows extracellular ionic molecules such as Ca2+ and Na+ to diffuse through the channel into the cell.42-45 Fig. 2C illustrates that in pathological conditions, the excess glutamate causes the channel to remain open for a long period of time thereby allowing a flood of extracellular Ca2+ into the cell triggering off the production of free radicals and the initiation of apoptosis.
NMDA receptors are expressed in many cell types of the retina. However, RGCs appear to be the most vulnerable in glutamate-mediated excitotoxicity.46 Selective loss of RGCs is a hallmark in glaucoma. Overexpression of NMDA receptors caused by increased glutamate release may at least partially explain selective RGC death in glaucoma. Glutamate induces a selective and dose-dependent loss of RGCs in cultured adult pig retina whereas amacrine cells and all other retinal neurons have been shown to be resistant.47 A similar result has been reported in the rabbit retina when NMDA was applied in vitro.48 The variable NMDA response by different retinal neurons may be attributed to discrepancy in NMDA receptor subunit nature, receptor density, and function properties.48 Furthermore, there is evidence that although RGCs express an abundant number of NMDA receptors,49 a large cohort of amacrine cells appear to have limited NMDA receptor function.49
In addition, reduced clearance of extracellular glutamate may also account for neuronal excitotoxicity.50 Muller cells in the retina play a major role in maintaining appropriate levels of glutamate and regulating synaptic transmission. Using high-affinity sodium-dependent transporters, Muller cells take up glutamate from the synaptic cleft and quickly convert it into glutamine which can be reused by neurons to synthesize glutamate for neurotransmission.50 Any disturbance in the pathway of glutamate uptake and recycling may cause increased extracellular levels of glutamate, as a result, not only prolonging synaptic transmission, but also triggering neuronal apoptosis and neuronal death. A significant reduction of the glutamate and aspartate transporters (GLAST) [Excitory amino acid transporter (EAAT1)] and Glutamate transporter (GLT-1) (EAAT2) has been observed in experimental rat glaucoma retina.51 Although other studies have failed to confirm the observation,52 a specific glutamate transporter GLT-1c, which is normally only expressed by photoreceptors, has recently been identified in RGCs in experimental glaucoma, and it may be indicative of an anomaly in glutamate homeostasis.53
MK801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine], an uncompetitive NMDA antagonist is perhaps the best known glutamate modifier.25 We and others have demonstrated that MK801 is neuroprotective in experimental glaucoma.22,25 However, as MK801 is neurotoxic and induces acute neuronal vacuolization histologically, it has not reached advanced stages of clinical trials.54,55 The problem is believed to occur due to its high affinity for the NMDA receptor channel and its long dwell time,56 resulting in its accumulating in the channels and blocking critical normal functions.
Memantine is currently the only available clinical glutamate modifier. It was first synthesized in the 1960s by Eli Lilly & Company and was patented in 1968. Memantine is a derivative of amantadine which was used as an anti-influenza compound. Memantine has been demonstrated to be effective in the treatment of AD and PD.57-60 Up to the 1980s, it was believed that memantine was anticholinergic or dopaminergic and it was only relatively recently shown to be an NMDA-receptor antagonist.61 Like MK801, memantine is an uncompetitive open-channel blocker but with a dwell time in the channel to only inhibit NMDA-receptor activity when glutamate is elevated to pathological levels, sparing normal physiological levels required for homeostatic synaptic activity.62
Memantine has been shown to be a highly effective neuroprotective agent in both acute and chronic animal modes of RGC death.11,18,19,26,63-69 Daily administration of memantine (5 to 10 mg/kg) enhanced survival of RGCs in a laser-induced rat and primate model of chronic ocular hypertension.17,18,20 A similar effect was documented by Schuettauf et al. using a Dilute brown non-agouti (DBA)/2J transgenic mouse model of glaucoma.70 Memantine currently is in a phase IV clinical trial assessing its efficiency in glaucoma patients, the results of which should be known in 2008.
Mitochondrial Dysfunction
Mitochondrial dysfunction has been implicated in neuronal apoptosis, and shown to occur in experimental glaucoma.71,72 A recent in vitro study has provided additional evidence that the mitochondrial dysfunction accompanying RGC death may be induced by glaucoma-related stimuli such as tumor necrosis factor (TNF)-α and hypoxia.73 Furthermore, mitochondrial dysfunction-associated oxidative stress has also been implicated as a risk factor in glaucoma patients.74
A mitochondrion is a membrane bound organelle and can be found in most eukaryotic cells. Mitochondria is responsible for the production of chemical energy, via the electron transport chain, that is required for normal cellular function such as cell proliferation and cell differentiation to name a few. External stress can trigger mitochondrial dysfunction which in turn can lead to the production of reactive oxygen species (ROS) that cause cell death at neurotoxic levels. Mitochondrial dysfunction can be triggered by hypoxia, treatment with hydrogen peroxide, and oxidative stress.75,76
Nuclear factor (NF)-κB is a dimeric transcription factor, composed of the p50 and p65 subunits, responsible for inflammation, autoimmune disease, viral infection, and linked with cancer. The inactive form of NF-κB is bound to the inhibitory IκBα protein and is retained in the cytosol.77 NF-κB has been implicated in both the induction and prevention of apoptosis; the outcome depends on the cell type and the stimuli. Zamora et al.78 demonstrated that overexpression of adenine nucleotide translocase (ANT)-1 recruits the IκBα/NF-κB complex into the mitochondria resulting in the down-regulation of the expression of antiapoptotic genes, Bcl-XL, MnSOD2, and c-IAP2.
Coenzyme Q10 (CoQ10) is a cofactor and plays a crucial role in energy production via the mitochondrial electron transport chain. CoQ10 is responsible for the transport of electrons from complex I and II to complex III.
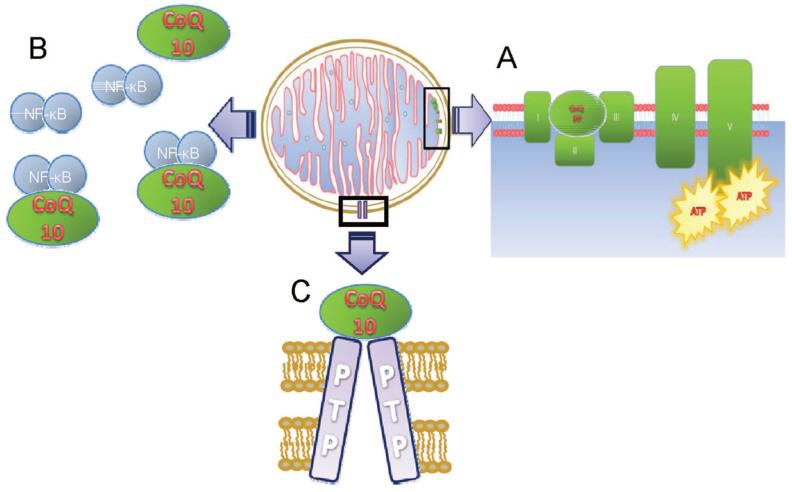
There are three potential mechanisms suggested for CoQ10 to exert its neuroprotective effects: first, as shown in Fig. 3A, it causes the augmentation of complex I in the electron transport chain; second, it inhibits the action of NF-κB, a transcription factor responsible for inflammation, autoimmune disease, viral infection, and linked with cancer (Fig. 3B); and finally, it inhibits the opening of the mitochondrial permeability transition pore79,80 (Fig. 3C). Our group recently showed topical CoQ10 to be effective in experimental glaucoma,81 and clinical trials are currently planned.
FIGURE 3.
Schematic diagram illustrating the role of CoQ10 in energy production in the mitochondria and its neuroprotective effects. CoQ10 transports electrons between complexes I, II, and III, and causes the augmentation of complex I in the electron transport chain (A). It is also believed to inhibit the action of NF-κB, a transcription factor responsible for inflammation, autoimmune disease, viral infection and linked with cancer (B); and finally, it inhibits the opening of the mitochondrial permeability transition pore (C). A color version of this figure is available at www.optvissci.com
Protein Misfolding
Protein aggregation is a prominent feature of many neurodegenerative diseases, such as AD, Huntington disease, and PD. These diseases are also called protein misfolding diseases as the proteins or peptides implicated are all self-assembled to form similar fibrillar structures. One such peptide is amyloid-β (Aβ), a 40 to 42 residue peptide and the primary component of the senile plaques found in AD brains.
Amyloid-β is derived from abnormal processing of amyloid precursor protein (APP) and is intricately involved in Alzheimer neuropathology.82 Aβ has recently been reported to be implicated in the development of RGC apoptosis in glaucoma, with evidence of caspase-3-mediated abnormal APP processing and increased expression of Aβ in RGCs in experimental glaucoma,83 and decreased vitreous Aβ levels (consistent with retinal Aβ deposition) in patients with glaucoma.84 Further evidence of a link between glaucoma and AD has emerged from studies showing that patients with AD have RGC loss associated with typical glaucomatous changes, such as optic neuropathy and visual functional impairment,85-88 as is also the case in PD.89 In addition, both diseases are chronic neurodegenerative conditions with a strong age-related incidence.90,91 This is further supported by increasing evidence of similar pathological mechanisms involving Aβ leading to RGC loss as implicated in the brain.90,92-94
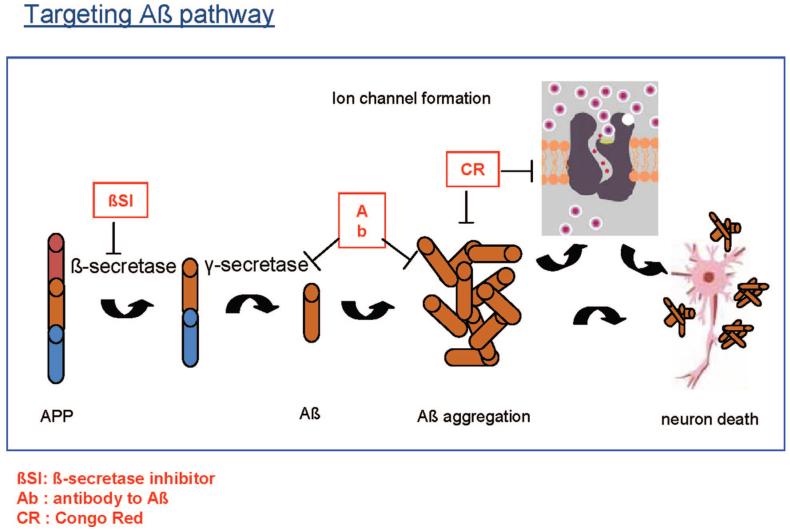
We have recently provided further strong evidence from experimental glaucoma supporting the involvement of Aβ in development of glaucomatous RGC apoptosis.15 Using our recently established novel imaging technique, DARC, we have further shown that exogenous Aβ peptide induces significant RGC apoptosis in vivo in a dose- and time-dependent manner.15 We next investigated the effects of targeting Aβ formation and aggregation in experimental glaucoma, using three different agents including a β-secretase inhibitor, an anti-Aβ antibody (Aβab), and Congo red.15 Fig. 4 illustrates the Aβ pathway, from the formation of Aβ from APP leading to Aβ aggregation and ultimately resulting in neuronal death. It also shows the target for each of the three inhibitors. All three treatments altered the profile of RGC apoptosis, but the anti-Aβ antibody appeared the most effective, with prolonged effects after a single application up to 16 weeks after IOP elevation. Perhaps the most exciting finding was the demonstration of combination therapy of all three strategies produced a maximal reduction of RGC apoptosis (>80%).
FIGURE 4.
Schematic diagram illustrating the formation of Aβ aggregates which ultimately leads to neuronal death. The red boxes represent three different agents which act on three different stages of Aβ pathway to block Aβ formation, deposition, and aggregation, respectively. A color version of this figure is available at www.optvissci.com.
Another mechanism implicated in neurodegeneration is that involving molecular chaperones such as heat shock proteins (HSPs). HSPs are a family of highly conserved stress proteins which are constitutively expressed in most cells under normal and stress-induced conditions. The function of the HSPs and heat shock cognate proteins is to prevent the aggregation of denatured proteins and also acts as a molecular chaperone to facilitate nascent protein folding, protein unfolding, restoring the conformation of misfolded proteins, and translocation across membranes.
HSPs are classified according to their molecular weight expressed in kilodaltons (kDa). There are numerous families of HSPs including HSP-90, HSP-70, HSP-60, and HSP-25. Tezel et al.95 reported that the intensity of immunostaining for HSP-60 and HSP-27 was significantly greater in glaucomatous eyes compared with age-matched controls. A study using cultured human optic nerve head astrocytes exposed to increased hydrostatic pressure revealed increased expression of HSP-27. This idea was supported by the finding that glaucoma patients have increased titers of HSPs autoantibodies in their blood. Ishii et al.96 has reported that the use of geranylgeraylacetone, an acyclic polyisoprenoid, induced the upregulation of HSP-72 expression in RGCs and protected them from glaucomatous damage in a rat glaucoma model. Although this drug has not yet been administered to glaucoma patients, it is promising as it is active orally with very low toxicity.97
Oxidative Stress
The term “oxidative stress” refers to when the production of ROS reaches a pathological level and the cell’s antioxidant capacity is insufficient in offering protection against oxidative damage. It is hypothesized that oxidative stress can cause RGC death by damaging the trabecular meshwork, the optic nerve head, and the retina.98 The main source of ROS is mitochondrial production as a byproduct of cellular aerobic metabolism and as second messengers in signal transduction pathways.
It is believed that oxidative stress plays a role in RGC death in glaucoma. Evidence comes from studies that have shown significant increase in the levels of ROS and lipid peroxides in experimental glaucoma eyes together with changes in the activities of antioxidant enzymes.99-101 In addition, reduced levels of antioxidant glutathione and increased serum lipid peroxidation products have been identified in primary open-angle glaucoma patients.102-104
Vitamin E (α-tocopherol) is the major source of lipid-soluble antioxidant in cells and acts as a scavenger of peroxyl radicals. Some studies have suggested that glaucoma patients receiving vitamin E have displayed improved visual fields,102,105-107 but long-term studies have been less convincing.
The compound Extract Ginkgo biloba (EGb) 761 is an extract from leaves of Ginkgo biloba and has demonstrated to increase the survival of RGCs in the episcleral vein cauterization model of experimental glaucoma.108,109 EGb 761 is a potent antioxidant and scavenger of free radicals. It has also shown to interfere with glutamatergic NMDA receptor. The precise mode of action of EGb 761 is still not fully understood.
Inflammation
Recently researchers have investigated the immune system as a means of providing neuroprotection against neuronal damage. Autoimmunity is traditionally regarded as an attack on the host cell by activated T cells resulting in pathological autoimmune-mediated disease. Studies have revealed that autoimmune responses increased the survival of RGCs after optic nerve injury,110,111 where the immune response was mediated by T cells directed against a CNS-associated self-antigen such as a myelin oligodendrocyte protein, myelin basic protein, and proteolipid protein.110,112 It was next demonstrated that the response could be achieved by either active immunization with the protein or by passive transfer of activated T cells.113 Interestingly, T cells which were derived from cryptic (non-encephalitogenic) myelin-associated peptides displayed the same effectiveness as T cells derived from encephalitogenic cryptic epitopes with the former inducing no autoimmune disease. Schwartz114-116 revealed that autoimmunity was not an artificial experimental phenomena but in fact a physiological endogenous response to stressful situations such as neuronal damage.116 In support, rats devoid of mature T cells had an increased number of RGCs dying following optic nerve crush, and by contrast, a prior unrelated CNS injury to the optic nerve crush protected RGCs from loss.117,118 Therefore, boosting autoimmunity by vaccination appeared to be a promising therapy for glaucoma acting to enhance the protective effects of T cells.
Cop-1 (glatiramer acetate; copolymer-1; copax-1) is a synthetic, random oligopeptide comprising the amino acids, tyrosine, glutamate, lysine, and alanine residues. Cop-1 is Food and Drug Administration-approved drug for the treatment of multiple sclerosis patients. It is a low affinity antigen and can evoke both active and passive T-cell-mediated response at various sites of injury. It has been demonstrated that Cop-1 reduces the damage caused by mechanical injury to the optic nerve or by intravitreally administered glutamate.119-121 Another study has reported that vaccination with Cop-1 leads to a significant reduction in elevated IOP-induced RGC death in a rat model of ocular hypertension.117
TNF-α is a potent proinflammatory cytokine and its production is up-regulated during ischemic and excitotoxic brain injury.121,122 It has been implicated as a mediator of RGC death in glaucomatous retina because of its up-regulation.123,124 TNF-α binds onto the death receptor, TNF receptor-1 (TNF-R1) and can induce both the caspase-dependent and the caspase-independent components of the mitochondrial cell death pathway. A dopaminergic and antiglaucoma drug, GLC756 has been recently shown to Inhibit TNF-α release from activated rat mast cells and suggested a potential of the compound on neuroprotection in glaucoma management.125,126
TNF-α may also be neuroprotective, because of the up-regulation of NF-κB, a redox-sensitive transcription factor. NF-κB is involved in the expression of a wide range of genes that regulate cellular differentiation, proliferation, apoptosis, oxidative response, and inflammation. Activation of NF-κB mediates the expression of mitogen-activated protein kinases which is known to regulate the response to proinflammatory and other stress signals.
Neurotrophin Deprivation
The family of neurotrophins consists of four members: brainderived nerve factor, nerve growth factor, neurotrophin-3, and neurotrophin-4/5. Neurotrophins promote the development, survival, and differentiation of neurons by binding onto either the Trk receptor or the p75 receptor.127-129
Most of the work in this area has been associated with molecular-based therapies, and will be covered elsewhere in this journal.
CONCLUSIONS
Currently the only form of treatment for glaucoma patients is to reduce their IOP either surgically or therapeutically. However, it is known that reducing IOP is in some instances inadequate as patients with low IOP continue to suffer vision loss. In this review, we have addressed several potential neuroprotective strategies. Many of the approaches employ and manipulate the cell’s endogenous mechanism to promote the survival of RCGs. Unfortunately except memantine, none of them have yet been adequately assessed in randomized clinical trials. One of the reasons for this is that currently recognized clinical end points in glaucoma, such as IOP and visual field, are not sufficient to adequately and swiftly assess the effects of neuroprotection. We believe that developments in perimetric, electrophysiological, and imaging techniques such as DARC technology and adaptive optics will provide a much needed objective measure in this field. As more and more understanding is gained into mechanisms of neuroprotection, it is likely that new neuroprotective agents will emerge, and be useful in the treatment of glaucoma.
REFERENCES
- 1.Cordeiro MF, Guo L, Luong V, Harding G, Wang W, Jones HE, Moss SE, Sillito AM, Fitzke FW. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci USA. 2004;101:13352–6. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv Ophthalmol. 2003;48(suppl 1):S21–S24. doi: 10.1016/s0039-6257(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg I. Is this neuroprotective drug good for my glaucoma patients? Some key factors in clinical decision-making. Can J Ophthalmol. 2007;42:418–20. [PubMed] [Google Scholar]
- 5.Wein FB, Levin LA. Current understanding of neuroprotection in glaucoma. Curr Opin Ophthalmol. 2002;13:61–7. doi: 10.1097/00055735-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb RN. Glaucoma neuroprotection: what is it? why is it needed? Can J Ophthalmol. 2007;42:396–8. [PubMed] [Google Scholar]
- 7.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DR, Drance SM, Schulzer M. Factors that predict the benefit of lowering intraocular pressure in normal tension glaucoma. Am J Ophthalmol. 2003;136:820–9. doi: 10.1016/s0002-9394(03)00478-1. [DOI] [PubMed] [Google Scholar]
- 9.Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 10.Chidlow G, Wood JP, Casson RJ. Pharmacological neuroprotection for glaucoma. Drugs. 2007;67:725–59. doi: 10.2165/00003495-200767050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by betaamyloid(1-40) Brain Res. 2002;958:210–21. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 12.Standridge JB. Pharmacotherapeutic approaches to the treatment of Alzheimer’s disease. Clin Ther. 2004;26:615–30. doi: 10.1016/s0149-2918(04)90064-1. [DOI] [PubMed] [Google Scholar]
- 13.Inestrosa NC, Urra S, Colombres M. Acetylcholinesterase (AChE)-amyloid-beta-peptide complexes in Alzheimer’s disease. The Wnt signaling pathway. Curr Alzheimer Res. 2004;1:249–54. doi: 10.2174/1567205043332063. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res. 2007;32:757–73. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 15.Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci USA. 2007;104:13444–9. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton SA. Retinal ganglion cells, glaucoma and neuroprotection. Prog Brain Res. 2001;131:712–18. [PubMed] [Google Scholar]
- 17.Hare WA, WoldeMussie E, Weinreb RN, Ton H, Ruiz G, Wijono M, Feldmann B, Zangwill L, Wheeler L. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, II: Structural measures. Invest Ophthalmol Vis Sci. 2004;45:2640–51. doi: 10.1167/iovs.03-0567. [DOI] [PubMed] [Google Scholar]
- 18.Hare WA, WoldeMussie E, Lai RK, Ton H, Ruiz G, Chun T, Wheeler L. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Invest Ophthalmol Vis Sci. 2004;45:2625–39. doi: 10.1167/iovs.03-0566. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield DS, Girkin C, Kwon YH. Memantine and progressive glaucoma. J Glaucoma. 2005;14:84–6. doi: 10.1097/01.ijg.0000146372.74366.e4. [DOI] [PubMed] [Google Scholar]
- 20.Yucel YH, Gupta N, Zhang Q, Mizisin AP, Kalichman MW, Weinreb RN. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch Ophthalmol. 2006;124:217–25. doi: 10.1001/archopht.124.2.217. [DOI] [PubMed] [Google Scholar]
- 21.Maass A, von Leithner PL, Luong V, Guo L, Salt TE, Fitzke FW, Cordeiro MF. Assessment of rat and mouse RGC apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr Eye Res. 2007;32:851–61. doi: 10.1080/02713680701585872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, Cordeiro MF. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–33. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(suppl 1):S102–S128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 24.Salt TE, Cordeiro MF. Glutamate excitotoxicity in glaucoma: throwing the baby out with the bathwater? Eye. 2006;20:730–1. doi: 10.1038/sj.eye.6701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhary P, Ahmed F, Sharma SC. MK801-a neuroprotectant in rat hypertensive eyes. Brain Res. 1998;792:154–8. doi: 10.1016/s0006-8993(98)00212-1. [DOI] [PubMed] [Google Scholar]
- 26.WoldeMussie E, Yoles E, Schwartz M, Ruiz G, Wheeler LA. Neuroprotective effect of memantine in different retinal injury models in rats. J Glaucoma. 2002;11:474–80. doi: 10.1097/00061198-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 28.Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE. Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res. 1997;58:864–7. [PubMed] [Google Scholar]
- 29.Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL, III, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002;21:91–125. doi: 10.1016/s1350-9462(01)00022-2. [DOI] [PubMed] [Google Scholar]
- 30.Honkanen RA, Baruah S, Zimmerman MB, Khanna CL, Weaver YK, Narkiewicz J, Waziri R, Gehrs KM, Weingeist TA, Boldt HC, Folk JC, Russell SR, Kwon YH. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Arch Ophthalmol. 2003;121:183–8. doi: 10.1001/archopht.121.2.183. [DOI] [PubMed] [Google Scholar]
- 31.Levkovitch-Verbin H, Martin KR, Quigley HA, Baumrind LA, Pease ME, Valenta D. Measurement of amino acid levels in the vitreous humor of rats after chronic intraocular pressure elevation or optic nerve transection. J Glaucoma. 2002;11:396–405. doi: 10.1097/00061198-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Wamsley S, Gabelt BT, Dahl DB, Case GL, Sherwood RW, May CA, Hernandez MR, Kaufman PL. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch Ophthalmol. 2005;123:64–70. doi: 10.1001/archopht.123.1.64. [DOI] [PubMed] [Google Scholar]
- 33.Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 34.Olney JW. Glutaate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropathol Exp Neurol. 1969;28:455–74. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Olney JW, Ho OL. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature. 1970;227:609–11. doi: 10.1038/227609b0. [DOI] [PubMed] [Google Scholar]
- 36.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 37.Lipton SA, Nicotera P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium. 1998;23:165–71. doi: 10.1016/s0143-4160(98)90115-4. [DOI] [PubMed] [Google Scholar]
- 38.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 39.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–87. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. [PubMed] [Google Scholar]
- 41.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 42.Tenneti L, D’Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-d-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998;71:946–59. doi: 10.1046/j.1471-4159.1998.71030946.x. [DOI] [PubMed] [Google Scholar]
- 43.Budd SL, Tenneti L, Lishnak T, Lipton SA. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc Natl Acad Sci USA. 2000;97:6161–6. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto S, Li Z, Ju C, Scholzke MN, Mathews E, Cui J, Salvesen GS, Bossy-Wetzel E, Lipton SA. Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2002;99:3974–9. doi: 10.1073/pnas.022036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci. 2004;24:10963–73. doi: 10.1523/JNEUROSCI.3461-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y, Liu XL, Yang XL. N-methyl-D-aspartate receptors in the retina. Mol Neurobiol. 2006;34:163–79. doi: 10.1385/MN:34:3:163. [DOI] [PubMed] [Google Scholar]
- 47.Luo X, Heidinger V, Picaud S, Lambrou G, Dreyfus H, Sahel J, Hicks D. Selective excitotoxic degeneration of adult pig retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1096–106. [PubMed] [Google Scholar]
- 48.Sun D, Rait JL, Kalloniatis M. Inner retinal neurons display differential responses to N-methyl-d-aspartate receptor activation. J Comp Neurol. 2003;465:38–56. doi: 10.1002/cne.10830. [DOI] [PubMed] [Google Scholar]
- 49.Brandstatter JH, Hartveit E, Sassoe-Pognetto M, Wassle H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci. 1994;6:1100–12. doi: 10.1111/j.1460-9568.1994.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 50.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–53. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002;43:2236–43. [PubMed] [Google Scholar]
- 52.Woldemussie E, Wijono M, Ruiz G. Muller cell response to laserinduced increase in intraocular pressure in rats. Glia. 2004;47:109–19. doi: 10.1002/glia.20000. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan RK, Woldemussie E, Macnab L, Ruiz G, Pow DV. Evoked expression of the glutamate transporter GLT-1c in retinal ganglion cells in human glaucoma and in a rat model. Invest Ophthalmol Vis Sci. 2006;47:3853–9. doi: 10.1167/iovs.06-0231. [DOI] [PubMed] [Google Scholar]
- 54.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–2. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 55.Fix AS, Horn JW, Wightman KA, Johnson CA, Long GG, Storts RW, Farber N, Wozniak DF, Olney JW. Neuronal vacuolization and necrosis induced by the noncompetitive N-methyl-d-aspartate (NMDA) antagonist MK(+)801 (dizocilpine maleate): a light and electron microscopic evaluation of the rat retrosplenial cortex. Exp Neurol. 1993;123:204–15. doi: 10.1006/exnr.1993.1153. [DOI] [PubMed] [Google Scholar]
- 56.Lipton SA. Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993;16:527–32. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- 57.Schneider E, Fischer PA, Clemens R, Balzereit F, Funfgeld EW, Haase HJ. Effects of oral memantine administration on Parkinson symptoms. Results of a placebo-controlled multicenter study. Dtsch Med Wochenschr. 1984;109:987–90. doi: 10.1055/s-2008-1069311. [DOI] [PubMed] [Google Scholar]
- 58.Rabey JM, Nissipeanu P, Korczyn AD. Efficacy of memantine, an NMDA receptor antagonist, in the treatment of Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1992;4:277–82. doi: 10.1007/BF02260076. [DOI] [PubMed] [Google Scholar]
- 59.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002;17:297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33:1834–9. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 61.Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur J Pharmacol. 1989;166:591–2. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 62.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 2004;566:261–9. doi: 10.1016/j.febslet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 64.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-d-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–36. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farlow MR. NMDA receptor antagonists. A new therapeutic approach for Alzheimer’s disease. Geriatrics. 2004;59:22–7. [PubMed] [Google Scholar]
- 66.Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol. 2003;48(suppl 1):S38–S46. doi: 10.1016/s0039-6257(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 67.Kim TW, Kim DM, Park KH, Kim H. Neuroprotective effect of memantine in a rabbit model of optic nerve ischemia. Korean J Ophthalmol. 2002;16:1–7. doi: 10.3341/kjo.2002.16.1.1. [DOI] [PubMed] [Google Scholar]
- 68.Lagreze WA, Knorle R, Bach M, Feuerstein TJ. Memantine is neuroprotective in a rat model of pressure-induced retinal ischemia. Invest Ophthalmol Vis Sci. 1998;39:1063–6. [PubMed] [Google Scholar]
- 69.Osborne NN. Memantine reduces alterations to the mammalian retina, in situ, induced by ischemia. Vis Neurosci. 1999;16:45–52. doi: 10.1017/s0952523899161017. [DOI] [PubMed] [Google Scholar]
- 70.Schuettauf F, Quinto K, Naskar R, Zurakowski D. Effects of antiglaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vision Res. 2002;42:2333–7. doi: 10.1016/s0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- 71.Mittag TW, Danias J, Pohorenec G, Yuan HM, Burakgazi E, Chalmers-Redman R, Podos SM, Tatton WG. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2000;41:3451–9. [PubMed] [Google Scholar]
- 72.Tatton WG, Chalmers-Redman RM, Sud A, Podos SM, Mittag TW. Maintaining mitochondrial membrane impermeability. an opportunity for new therapy in glaucoma? Surv Ophthalmol. 2001;45(suppl 3):S277–S283. doi: 10.1016/s0039-6257(01)00207-7. [DOI] [PubMed] [Google Scholar]
- 73.Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–59. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–41. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- 75.McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 76.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 78.Zamora M, Merono C, Vinas O, Mampel T. Recruitment of NF-kappaB into mitochondria is involved in adenine nucleotide translocase 1-induced apoptosis. J Biol Chem. 2004;279:38415–23. doi: 10.1074/jbc.M404928200. [DOI] [PubMed] [Google Scholar]
- 79.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–8. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 80.Nucci C, Tartaglione R, Cerulli A, Mancino R, Spano A, Cavaliere F, Rombola L, Bagetta G, Corasaniti MT, Morrone LA. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int Rev Neurobiol. 2007;82:397–406. doi: 10.1016/S0074-7742(07)82022-8. [DOI] [PubMed] [Google Scholar]
- 81.Cordeiro MF, Guo L, Cheung W, Wood N, Salt TE. Topical CoQ10 is neuroprotective in experimental glaucoma[abstract] Invest Ophthalmol Vis Sci. 2007;48 E-Abstract 4369. [Google Scholar]
- 82.Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223–41. doi: 10.1146/annurev.med.57.121304.131243. [DOI] [PubMed] [Google Scholar]
- 83.McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H, Quigley HA, Zack DJ. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002;43:1077–87. [PubMed] [Google Scholar]
- 84.Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49:106–8. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 85.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112:1860–7. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 86.Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 87.Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. 1996;17:385–95. doi: 10.1016/0197-4580(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 88.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–84. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 89.Bayer AU, Keller ON, Ferrari F, Maag KP. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am J Ophthalmol. 2002;133:135–7. doi: 10.1016/s0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 90.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:11830–5. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wostyn P. Can chronic increased intracranial pressure or exposure to repetitive intermittent intracranial pressure elevations raise your risk for Alzheimer’s disease? Med Hypotheses. 2004;62:925–30. doi: 10.1016/j.mehy.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Archer S, Hirano J, Diss JK, Fraser SP, Djamgoz MB. Expression and localization in the fish retina of a homologue of the Alzheimer’s related PS1 gene. Neuroreport. 1998;9:2049–56. doi: 10.1097/00001756-199806220-00026. [DOI] [PubMed] [Google Scholar]
- 93.Loffler KU, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci. 1995;36:24–31. [PubMed] [Google Scholar]
- 94.Vickers JC, Lazzarini RA, Riederer BM, Morrison JH. Intraperikaryal neurofilamentous accumulations in a subset of retinal ganglion cells in aged mice that express a human neurofilament gene. Exp Neurol. 1995;136:266–9. doi: 10.1006/exnr.1995.1104. [DOI] [PubMed] [Google Scholar]
- 95.Tezel G, Hernandez R, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 2000;118:511–18. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- 96.Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:1982–92. [PubMed] [Google Scholar]
- 97.Uchida S, Fujiki M, Nagai Y, Abe T, Kobayashi H. Geranylgeranylacetone, a noninvasive heat shock protein inducer, induces protein kinase C and leads to neuroprotection against cerebral infarction in rats. Neurosci Lett. 2006;396:220–4. doi: 10.1016/j.neulet.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 98.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–14. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Costa VP, Harris A, Stefansson E, Flammer J, Krieglstein GK, Orzalesi N, Heijl A, Renard JP, Serra LM. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog Retin Eye Res. 2003;22:769–805. doi: 10.1016/s1350-9462(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 100.Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 101.Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005;39:365–73. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 102.Birich TV, Birich TA, Marchenko LN, Remizonova DB, Fedulov AS. Lipid peroxidation in the blood of primary glaucoma patients. Vestn Oftalmol. 1986;102:13–15. [PubMed] [Google Scholar]
- 103.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–9. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 104.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–83. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- 105.Aydemir O, Naziroglu M, Celebi S, Yilmaz T, Kukner AS. Antioxidant effects of alpha-, gamma-and succinate-tocopherols in guinea pig retina during ischemia-reperfusion injury. Pathophysiology. 2004;11:167–71. doi: 10.1016/j.pathophys.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 106.Cellini M, Caramazza N, Mangiafico P, Possati GL, Caramazza R. Fatty acid use in glaucomatous optic neuropathy treatment. Acta Ophthalmol Scand Suppl. 1998;227:41–2. doi: 10.1111/j.1600-0420.1998.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 107.Dilsiz N, Sahaboglu A, Yildiz MZ, Reichenbach A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefes Arch Clin Exp Ophthalmol. 2006;244:627–33. doi: 10.1007/s00417-005-0084-6. [DOI] [PubMed] [Google Scholar]
- 108.Cheung ZH, So KF, Lu Q, Yip HK, Wu W, Shan JJ, Pang PK, Chen CF. Enhanced survival and regeneration of axotomized retinal ganglion cells by a mixture of herbal extracts. J Neurotrauma. 2002;19:369–78. doi: 10.1089/089771502753594936. [DOI] [PubMed] [Google Scholar]
- 109.Kim SY, Kwak JS, Shin JP, Lee SH. The protection of the retina from ischemic injury by the free radical scavenger EGb 761 and zinc in the cat retina. Ophthalmologica. 1998;212:268–74. doi: 10.1159/000027305. [DOI] [PubMed] [Google Scholar]
- 110.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 111.Moalem G, Yoles E, Leibowitz-Amit R, Muller-Gilor S, Mor F, Cohen IR, Schwartz M. Autoimmune T cells retard the loss of function in injured rat optic nerves. J Neuroimmunol. 2000;106:189–97. doi: 10.1016/s0165-5728(00)00240-x. [DOI] [PubMed] [Google Scholar]
- 112.Moalem G, Monsonego A, Shani Y, Cohen IR, Schwartz M. Differential T cell response in central and peripheral nerve injury: connection with immune privilege. FASEB J. 1999;13:1207–17. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- 113.Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20:6421–30. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartz M. Neuroprotection as a treatment for glaucoma: pharmacological and immunological approaches. Eur J Ophthalmol. 2001;11(suppl 2):S7–S11. doi: 10.1177/112067210101102s01. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz M. Physiological approaches to neuroprotection. Boosting of protective autoimmunity. Surv Ophthalmol. 2001;45(Suppl 3):S256–S260. doi: 10.1016/s0039-6257(01)00208-9. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz M. Neurodegeneration and neuroprotection in glaucoma: development of a therapeutic neuroprotective vaccine: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2003;44:1407–11. doi: 10.1167/iovs.02-0594. [DOI] [PubMed] [Google Scholar]
- 117.Schori H, Kipnis J, Yoles E, WoldeMussie E, Ruiz G, Wheeler LA, Schwartz M. Vaccination for protection of retinal ganglion cells against death from glutamate cytotoxicity and ocular hypertension: implications for glaucoma. Proc Natl Acad Sci USA. 2001;98:3398–403. doi: 10.1073/pnas.041609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–8. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ben Simon GJ, Bakalash S, Aloni E, Rosner M. A rat model for acute rise in intraocular pressure: immune modulation as a therapeutic strategy. Am J Ophthalmol. 2006;141:1105–11. doi: 10.1016/j.ajo.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 120.Kipnis J, Yoles E, Porat Z, Cohen A, Mor F, Sela M, Cohen IR, Schwartz M. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc Natl Acad Sci USA. 2000;97:7446–51. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Botchkina GI, Meistrell ME, III, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–81. [PMC free article] [PubMed] [Google Scholar]
- 122.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–8. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 123.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–44. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 124.Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–94. [PubMed] [Google Scholar]
- 125.Laengle UW, Markstein R, Pralet D, Seewald W, Roman D. Effect of GLC756, a novel mixed dopamine D1 receptor antagonist and dopamine D2 receptor agonist, on TNF-alpha release in vitro from activated rat mast cells. Exp Eye Res. 2006;83:1335–9. doi: 10.1016/j.exer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 126.Laengle UW, Trendelenburg AU, Markstein R, Nogues V, Provencher-Bollinger A, Roman D. GLC756 decreases TNF-alpha via an alpha2 and beta2 adrenoceptor related mechanism. Exp Eye Res. 2006;83:1246–51. doi: 10.1016/j.exer.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ip NY, Ibanez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, Le Beau MM, Espinosa R, III, Squinto SP, Persson H, Yaneopolous GD. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA. 1992;89:3060–4. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–83. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]