Abstract
Background
Metabolic syndrome (MetS) predisposes to cardiovascular disease. Endothelial dysfunction is thought to be an important factor in the pathogenesis of atherosclerosis. We tested the hypothesis that both MetS and endothelial dysfunction are vascular risk factors and provide additive prognostic values in predicting cardiovascular events in a multi-ethnic community sample.
Methods
The study population consisted of 819 subjects (467 female, mean age 66.5±8.8 years, 66% Hispanic) enrolled in the Northern Manhattan Study. MetS was defined using the revised Adult Treatment Panel III criteria. Brachial artery flow-mediated dilation (FMD) was measured using high-resolution ultrasound. Endothelial dysfunction was defined as FMD < 8.44% (lower three quartiles). Cox proportional hazards models were used to assess the effect of MetS and endothelial dysfunction on risk of cardiovascular events.
Results
During 81±21 months of follow-up, events occurred in 84 subjects. MetS was independently associated with cardiovascular events in a multivariate model including cardiovascular risk factors (adjusted HR 2.08, 95% CI 1.27–3.40). Subjects with both MetS and endothelial dysfunction were at higher risk for cardiovascular events than those with either one of them alone (adjusted HR 2.60, 95% CI 1.14–5.92).
Conclusions
MetS is associated with incident cardiovascular events. Combined use of MetS and FMD identifies those who are at higher risk of cardiovascular events. MetS and non-invasive FMD testing can be used concurrently for cardiovascular risk prediction.
Introduction
The metabolic syndrome (MetS), a concurrence of impaired glucose and insulin metabolism, overweight and abdominal fat distribution, dyslipidemia, and hypertension, has been shown to predict cardiovascular and coronary heart disease mortality (1–4). Insulin resistance has been postulated as an underlying mechanism in this syndrome (5;6). To aid in the research and clinical applications, the World Health Organization (WHO) and the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III established a definition of the metabolic syndrome (7;8) The ATP III definition was later modified by the joint American Heart Association/National Heart, Lung, and Blood Institute Panel in 2005 and the threshold for impaired fasting glucose (IFG) was reduced, corresponding with revised American Diabetes Association criteria for IFG (9;10).
Endothelial function is thought to be an important factor in the pathogenesis of atherosclerosis (11). Endothelial dysfunction is both an early marker of vascular disease and a facilitative process in the development of atherosclerosis (12;13). Previous studies have shown that endothelial function assessed using intra-arterial infusion of endothelium-dependent vasodilator acetylcholine in a coronary or peripheral artery identifies individuals at increased risk for cardiovascular disease events (14–18). However, because of the invasive nature of the studies, use of these techniques has been limited to small studies, and large epidemiological studies are lacking. Instead, endothelial function assessment by brachial-artery flow-mediated dilation (FMD) has been proposed as a surrogate marker for cardiovascular risk (19–21). A previous study in our cohort showed that lower FMD was associated with cardiovascular events in the multi-ethnic cohort of Northern Manhattan (22).
Little is known about the combined effect of MetS and endothelial dysfunction in a community-based cohort. The aim of our study was therefore to test the hypothesis that both the metabolic syndrome and endothelial dysfunction are risk factors and provide additive prognostic values in predicting cardiovascular events such as myocardial infarction (MI), stroke, and vascular death in a multi-ethnic community cohort.
Method
Study population
The Northern Manhattan Study (NOMAS), the first to focus on stroke risk factors in African-Americans, Hispanics, and whites living in the same community, has been previously described (23–25). Briefly, random digit dialing of households was performed by Audits and Surveys, Inc. Community participants were enrolled in NOMAS if they: (1) had never been diagnosed with stroke, (2) were over age 40, and (3) resided in Northern Manhattan for >3 months in a household with a telephone (26). Ninety-one percent of those called participated in a telephone interview, and 75% of those who were eligible and invited to participate came to the Columbia University Medical Center (CUMC) for an in-person evaluation (overall participation rate 68%). Subjects with a history of MI at baseline were excluded from this analysis. The study was approved by the Institutional Review Board at CUMC. All patients provided written informed consent directly or through a surrogate where appropriate.
ATP III definition of metabolic syndrome
We used the modified ATP III definition of MetS proposed by the National Cholesterol Education Program (10). The ATP III-defined metabolic syndrome required three or more of the five criteria linked to insulin resistance: (1) waist circumference (≥102 cm (40 in) in men, ≥88 cm (35 in) in women), (2) triglycerides ≥150 mg/dL, (3) high-density lipoprotein (HDL) cholesterol (<40 mg/dL in men, <50 mg/dL in women), (4) fasting plasma glucose concentration ≥100 mg/dL, and (5) blood pressure ≥130/85 mmHg.
Endothelial function study
All subjects enrolled in NOMAS between January 1998 and April 2001 were invited to undergo endothelial function study. It was not performed if subjects refused, or if ultrasonography was not technically adequate in a given individual. There was no significant difference in race-ethnicity, level of education or other risk factors between those with and without endothelial function assessment (26).
Endothelial function was assessed using brachial artery flow-mediated dilation (FMD). FMD was measured using high-resolution vascular ultrasound (Philips 5500, Andover, MA) as previously described (20;22;27). Briefly, brachial diameter was measured at rest and during reactive hyperemia 6 cm above the antecubital crease with a 15 MHz linear array transducer. Reactive hyperemia was induced by inflating a blood pressure cuff on the proximal portion of the arm to at least 50 mmHg above systolic blood pressure to occlude arterial flow for 5 minutes.
End-diastolic images were acquired and digitized by a frame grabber (model LG3, Scion Corporation) at baseline and 1 minute after cuff deflation (20;27). A blinded reader analyzed brachial artery diameters by using analysis software. Three consecutive cardiac cycles were analyzed for both baseline and hyperemia studies of each subject, and the measurements averaged. The values of FMD were calculated with the formula
Absolute intra-observer variability for FMD measurement was 1.3% (n =15) (22). A previous study in our cohort showed that lower FMD was associated with cardiovascular events (22). In the current study, endothelial dysfunction was defined as FMD <8.44% (lower three quartiles).
Assessment of cardiovascular events
Subjects were followed prospectively to a combined endpoint of stroke, MI, or vascular death. Stroke was defined by the first symptomatic occurrence of ischemic stroke as defined by the WHO criteria (28). Myocardial infarction was defined by criteria adapted from the Cardiac Arrhythmia Suppression Trial(29) and the Lipid Research Clinics Coronary Primary Prevention Trial(30) and required at least of two of the three following criteria: (a) ischemic cardiac pain determined to be typical angina; (b) cardiac enzyme abnormalities defined as abnormal CPK-MB fraction or troponin I values; and (c) electrocardiographic abnormalities (22). Vascular death was classified based on information from the family, medical records, and death certificate. These outcomes were adjudicated and confirmed by a panel of physicians.
Statistical analysis
Data are expressed as means ± standard deviations (SD) unless otherwise indicated. Univariate and multivariate analyses using Cox proportional hazards models were performed to examine the associations of MetS and endothelial dysfunction with cardiovascular events during follow-up. To control for confounding factors, the following factors were included in the model: age, gender, race, diabetes mellitus, low-density lipoprotein (LDL) value, and current cigarette smoking.
To assess correlation between FMD and the various components of MetS, Spearman’s correlation analyses were performed between FMD values and each continuous variable of MetS. To determine whether the combination of MetS and endothelial dysfunction increased the risk of cardiovascular events in an additive way, we divided the study population into four groups on the basis of the presence or absence of MetS and endothelial dysfunction and hazard ratios (HRs) of cardiovascular events were calculated in each group. Interaction was evaluated as proposed by Rothman(31) and Hallqvist et al(32). A synergy index (SI) is a means of evaluating additive interaction: a SI of 1 indicates no interaction and SI > 1 indicates positive interaction between the two variables. Kaplan-Meier event-free curves were constructed in each group and log-rank test was performed to evaluate the difference among the groups.
95% confidence intervals (CIs) were constructed; a value of P < 0.05 was considered significant. Statistical analyses were conducted with SAS 9.1 (SAS Institute, Cary, NC).
Results
Population characteristics
The full NOMAS cohort comprises 3298 stroke-free individuals age ≥40 years. Of the 1223 participants who underwent FMD measurement, 919 (75%) participants had images that were of sufficient quality for analysis. Participants with a history of MI (n = 74) or with missing values on basic risk profiles, components of MetS or follow-up for outcomes (n = 26) were excluded from our study. For this analysis, 819 subjects (467 female, mean age: 66.5±8.8 years, 17% African-American, 66% Hispanic, 15% white) were enrolled from January 1998 through April 2001. The clinical characteristics of participants with and without MetS are summarized in Table 1. Those with MetS are more likely to be female and Hispanic and have history of diabetes mellitus compared with those without MetS. FMD was lower in those with MetS (P <0.0001). There was no difference in age and smoking status. Participants were prospectively followed for 81 ± 21 months.
Table 1.
Clinical characteristics
| Variable (n = 819) | No MetS (n=442) | MetS (n=377) | P value |
|---|---|---|---|
| Age (years) | 66.5 (9.3) | 66.6 (8.3) | 0.87 |
| Sex (% female) | 217 (49.1) | 250 (66.3) | <0.0001 |
| Race | <0.005 | ||
| African-American (%) | 81 (18) | 55 (15) | |
| Hispanic (%) | 267 (60) | 275 (73) | |
| White (%) | 80 (18) | 42 (11) | |
| Diabetes mellitus (%) | 47 (11) | 134 (36) | <0.0001 |
| Hypertension (%) | 248 (56) | 293 (78) | <0.0001 |
| Hypercholesterolemia (%) | 194 (44) | 204 (54) | <0.005 |
| Current smoking (%) | 72 (16) | 55 (15) | 0.51 |
| Coronary artery disease (%) | 64 (15) | 66 (18) | 0.237 |
| Peripheral vascular disease (%) | 59 (14) | 68 (18) | 0.065 |
| Flow-mediated dilation (%) | 6.29 (3.76) | 5.26 (3.70) | <0.0001 |
| Body mass index (kg/m2) | 26.2 (4.1) | 30.1 (5.0) | <0.0001 |
| Waist circumference (in) | 35.2 (4.1) | 38.6 (4.2) | <0.0001 |
| Total cholesterol (mg/dL) | 200 (38) | 207 (41) | <0.05 |
| Triglyceride (mg/dL) | 101 (41) | 172 (99) | <0.0001 |
| High-density lipoprotein (mg/dL) | 51 (15) | 40 (10) | <0.0001 |
| Low-density lipoprotein (mg/dL) | 129 (36) | 134 (36) | 0.06 |
| Fasting plasma glucose (mg/dL) | 90 (32) | 121 (59) | <0.0001 |
| Systolic blood pressure (mmHg) | 138 (19) | 148 (19) | <0.0001 |
| Diastolic blood pressure (mmHg) | 82 (11) | 86 (10) | <0.0001 |
Diabetes mellitus was defined by a fasting blood glucose level greater than 126 mg/dL, the subject’s self-report of a history of diabetes mellitus, or use of insulin or oral hypoglycemic agents. Hypertension is defined as a systolic blood pressure of 140 mmHg or higher or a diastolic blood pressure of 90 mmHg or higher or the subject’s self-report of such a history or use of antihypertensive medication. Hypercholesterolemia was defined as a history of elevated cholesterol, taking medications for elevated cholesterol or a total cholesterol level greater than 240 mg/dL. Metabolic syndrome was defined using the modified ATP III criteria. Coronary artery disease was defined as history of angina, history of bypass surgery, bypass surgerty or angioplasty. Peripheral vascular disease was defined as self-reported peripheral arterial disease.
Metabolic syndrome and endothelial function study results
We first evaluated the associations of MetS and endothelial dysfunction with cardiovascular events individually. For the entire group of subjects, 377 (46.0%) subjects had the MetS. MetS was independently associated with cardiovascular events in a multivariate model including endothelial dysfunction, age, gender, race, diabetes mellitus, LDL value, and current smoking (adjusted HR 2.08, 95% CI 1.27–3.40), while endothelial dysfunction was not (adjusted HR 1.43, 95% CI 0.78–2.60, Table 2).
Table 2.
Hazard ratios for cardiovascular events by metabolic syndrome and endothelial dysfunction
| Unadjusted HR (95% CI) | P value | Adjusted HR* (95% CI) | P value | |
|---|---|---|---|---|
| Metabolic syndrome | 2.12 (1.35–3.33) | 0.001 | 2.08 (1.27–3.40) | 0.004 |
| Endothelial dysfunction | 1.64 (0.92–2.92) | 0.09 | 1.43 (0.78–2.60) | 0.247 |
adjusted by either endothelial dysfunction (for MetS) or MetS (for endothelial dysfunction), age, gender, race, diabetes mellitus, LDL level, and current smoking
Interrelationships between metabolic syndrome, endothelial dysfunction, and cardiovascular events
Spearman’s correlation coefficients between FMD and each continuous variable of MetS are shown in Table 3. There was a statistically significant negative correlation between FMD values and waist circumference, fasting glucose level, and systolic blood pressure. The strongest correlation (r = −0.16) was observed between FMD and waist circumference and systolic blood pressure.
Table 3.
Spearman’s rank correlations between FMD and components of MetS
| Components of MetS | FMD (%) | P value |
|---|---|---|
| Waist circumference (in) | −0.16 | <0.0001 |
| Triglyceride (mg/dL) | −0.03 | 0.44 |
| High-density lipoprotein (mg/dL) | 0.05 | 0.14 |
| Fasting plasma glucose (mg/dL) | −0.09 | <0.01 |
| Systolic blood pressure (mmHg) | −0.16 | <0.0001 |
HRs for cardiovascular events categorized by presence or absence of MetS or endothelial dysfunction are shown in Table 4. HRs in those with either MetS or endothelial dysfunction were 43% and 24 % greater than HR in those with neither of them, respectively. When MetS and endothelial dysfunction coexisted, the hazard ratio almost tripled. The HR of those with both of MetS and endothelial dysfunction was 2.60 after adjustment for cardiovascular risk factors. The Rothman’s synergy index between MetS and endothelial dysfunction was 2.9, suggesting that there was a 190% relative excess risk compared with that expected under an additive model (95% CI −74% to +3034%).
Table 4.
Hazard ratios for cardiovascular events categorized by presence of metabolic syndrome or endothelial dysfunction
| MetS | Endothelial dysfunction | Incidence rates per 1000 person-years | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) |
|---|---|---|---|---|
| (−) | (−) | 8.35 | 1 (ref) | 1 (ref) |
| (−) | (+) | 10.36 | 1.24 (0.53–2.91) | 1.17 (0.49–2.77) |
| (+) | (−) | 12.17 | 1.43 (0.50–4.08) | 1.53 (0.50–4.68) |
| (+) | (+) | 24.31 | 2.89 (1.31–6.38) | 2.60 (1.14–5.92) |
adjusted by age, gender, race, diabetes mellitus, LDL level, and current smoking
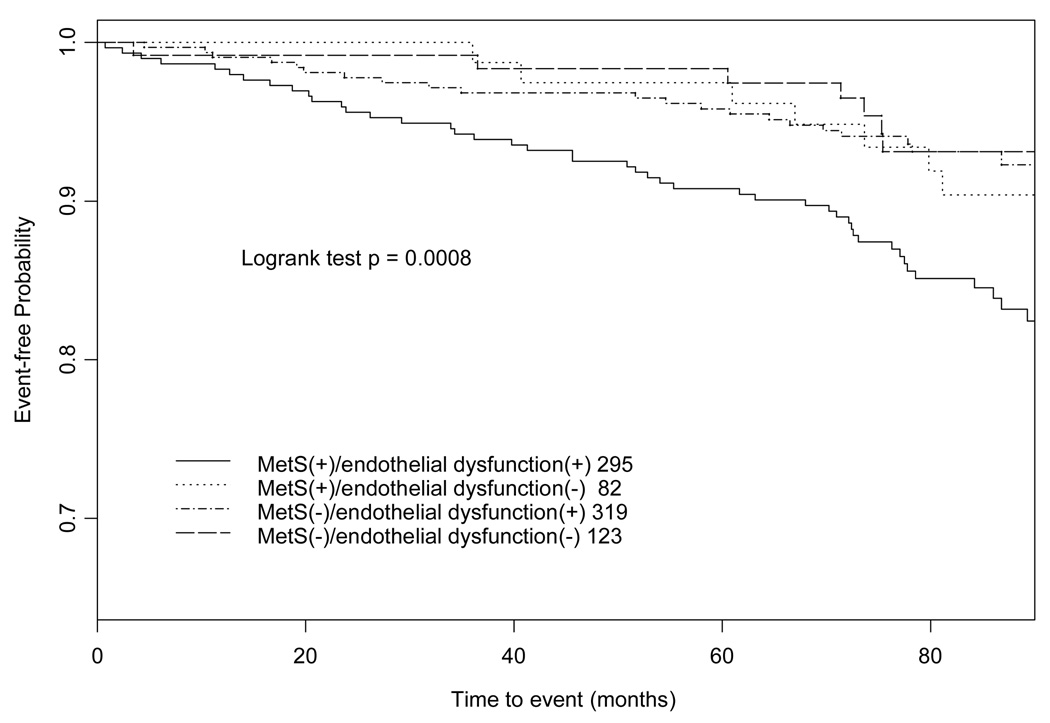
Figure 1 shows Kaplan-Meier event-free curves categorized by presence or absence of MetS and endothelial dysfunction. Logrank test in the Kaplan-Meier curves showed significant differences in cumulative survival rate between the four groups (P <0.001).
Figure 1.
Kaplan-Meier event-free curves categorized based on MetS and endothelial dysfunction
Discussion
This study tests the hypothesis that concurrent finding of MetS and poor FMD identifies individuals that may be at a higher risk than those with only one of these findings. To our knowledge, this is the first study to combine these two important risk factors, especially in a multi-ethnic community population.
The importance of MetS and endothelial dysfunction has been discussed previously. Epidemiological studies have shown an increased risk of cardiovascular mortality in subjects with MetS (33–35). In the Atherosclerosis Risks in Communities study, subjects with MetS were at ~1.5–2 times increased risk for long-term cardiovascular outcomes (34). In the Cardiovascular Health Study (CHS), MetS was associated with ~1.4 fold increase risk of cardiovascular disease (35). In a European population, MetS was associated with an approximate 2-fold increased risk of incident cardiovascular morbidity and mortality (33). Our study is in agreement with these previous studies. Several previous studies showed the association between endothelial dysfunction and cardiovascular events in community-based cohorts (22;36). Shimbo et al showed previously in our population that lower FMD was associated with incident cardiovascular events (22). Recently, FMD has been shown to be a predictor of future cardiovascular events in 2791 older adults in the CHS (36). Our study didn’t show significant association between FMD and cardiovascular events. This could be because of lack of power in our study as previously discussed (22). Or it may be attributable to racial difference between the CHS and NOMAS. Our study cohort is different from the CHS in that our cohort has a predominantly Hispanic population. There is age difference between the two cohorts: mean age was 78 years old in the CHS and 66 years old in our cohort. Despite these differences, both studies suggest that FMD may give long-term prognostic information in a community-based study population.
Our study showed that FMD may provide incremental value over MetS in predicting risk of cardiovascular events in a multi-ethnic community cohort. The pathophysiology of MetS remains inconclusive, but insulin resistance is thought to be the basis of most if not all of the features of the syndrome (37). Previous studies revealed that insulin resistance was related to endothelial dysfunction (38;39). As such, MetS and endothelial dysfunction are likely to be a consequence of insulin resistance. Intriguingly, however, the combination of MetS and endothelial dysfunction could increase the risk of cardiovascular events. Thus, we were able to identify a subset at a higher risk of CVD. This finding is relevant in clinical and research settings since MetS and non-invasive FMD can be readily available and have a potential to identify those subjects at higher risk. Further study is needed to verify the combined use of MetS and non-invasive FMD testing in different populations. Further study is also warranted to validate measures of endothelial function in a clinically useful way, since FMD assessment has not been standardized across institutions.
Limitations of this study need to be considered. First, the cutoff points of FMD were arbitrary. FMD was examined as a continuous value in the previous paper (22). In the current study, we set an arbitrary cutpoint of FMD to examine interaction between MetS and FMD. There is no consensus on the cutpoint of FMD. Universal cutoff point might help utilize FMD as a tool for risk stratification. Second, we did not measure endothelium-independent (nitrate-induced) dilation. Use of nitroglycerin might have added further information on endothelial function. Lastly, this study did not include novel inflammatory risk factors such as highsensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-α. Unfortunately, these inflammatory markers were not available in all of our study participants. These markers might add prognostic information in the population.
Conclusion
This is the first study to show that a combination of MetS and endothelial dysfunction may identify subjects at higher risk of cardiovascular events in a multi-ethnic community cohort. Both metabolic syndrome and endothelial dysfunction are risk factors for cardiovascular events. Measurement of endothelial function may be helpful in predicting the level of risk in patients with metabolic syndrome in a community cohort.
Acknowledgements
The authors have no financial conflicts of interest. This work was supported by grants R01 NS-29993, K24 NS02241, and K23 HL072866 from the National Institutes of Health, and a Clinically Applied Research Grant from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, Kannel WB, Silbershatz H, et al. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159(10):1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E, Haffner SM, Mitchell BD, et al. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34(6):416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 7.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 12.Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37(4):1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 14.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 15.Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 16.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104(2):191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 18.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 20.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41(10):1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106(6):640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 22.Shimbo D, Grahame-Clarke C, Miyake Y, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192(1):197–203. doi: 10.1016/j.atherosclerosis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Roberts JK, Boden-Albala B, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28(5):929–935. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 25.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 26.Elkind MS, Sciacca RR, Boden-Albala B, et al. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181(2):329–338. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86(2):207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 28.Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 29.Greene HL, Richardson DW, Barker AH, et al. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study) Am J Cardiol. 1989;63(1):1–6. doi: 10.1016/0002-9149(89)91065-5. [DOI] [PubMed] [Google Scholar]
- 30.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease. The Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. Jama. 1994;272(18):1439–1441. doi: 10.1001/jama.272.18.1439. [DOI] [PubMed] [Google Scholar]
- 31.Rothman K. Modern Epidemiology. Boston: Little, Brown; 2006. [Google Scholar]
- 32.Hallqvist J, Ahlbom A, Diderichsen F, et al. How to evaluate interaction between causes: a review of practices in cardiovascular epidemiology. J Intern Med. 1996;239(5):377–382. doi: 10.1046/j.1365-2796.1996.431782000.x. [DOI] [PubMed] [Google Scholar]
- 33.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112(5):666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 34.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28(2):385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 35.Scuteri A, Najjar SS, Morrell CH, et al. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005;28(4):882–887. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 36.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 37.Dandona P, Aljada A, Chaudhuri A, et al. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 38.Petrie JR, Ueda S, Webb DJ, et al. Endothelial nitric oxide production and insulin sensitivity. A physiological link with implications for pathogenesis of cardiovascular disease. Circulation. 1996;93(7):1331–1333. doi: 10.1161/01.cir.93.7.1331. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]