Abstract
Polychlorinated biphenyls (PCBs) have promoting activity in the liver, which may be brought about in part by the induction of oxidative stress. In this study we examined the effects of several antioxidant phytochemicals on the tumor promoting activity of 3,3′,4′4-tetrachlorobiphenyl (PCB-77). Female Sprague Dawley rats were first injected with diethylnitrosamine (DEN, 150 mg/kg) and one week later the rats were fed an AIN-93 based purified diet or the same diet containing ellagic acid (0.4%), β-carotene (0.5%), curcumin (0.5%), N-acetyl cysteine (NAC, 1.0%), co-enzyme CoQ10 (CoQ10, 0.4%), resveratrol (0.005%), lycopene (10% as LycoVit (Sweeny et al.), which contains 10% lycopene), or a tea extract (1%, containing 16.5% epigallocatechin-3-gallate [EGCG] and 33.4% total catechins). Rats were fed the diets for the remainder of the study. After 3 weeks, 2/3 of the control rats and all of the antioxidant diet-fed rats were injected i.p. with PCB-77 (300 μmol/kg) every other week for four injections. All rats were euthanized 10 days after the last PCB injection. The rats that received PCB-77 alone showed an increase in the number and size of placental glutathione S-transferase (PGST)-positive foci in the liver. Lycopene significantly decreased the number of foci, while curcumin and CoQ10 decreased the size of the foci. In contrast ellagic acid increased the number but decreased the size of the foci. All of the other phytochemicals showed only slight or no effects. Compared with the PCB-77 group, CoQ10 increased cell proliferation in normal hepatocytes, whereas the other antioxidants had no effect in either normal or PGST-positive hepatocytes. These findings show that none of the antioxidant phytochemicals produced a clear decrease in the promoting activity of PCB-77.
Keywords: antioxidant, PCB, phytochemicals, altered hepatic foci
Introduction
Polychlorinated biphenyls (PCBs) cause several toxic effects in the liver, including the induction of hepatocellular carcinoma (Silberhorn et al., 1990; van Birgelen et al., 1996; Robertson and Hansen, 2001). Several PCB mixtures and certain specific PCB congeners have been shown to have promoting activity in the liver (Silberhorn et al., 1990; Safe, 1994; Glauert et al., 2001). The PCB mixtures and PCB congeners that have promoting activity are described in Glauert et al. (2001). The mechanisms of the promoting activity of PCBs, however, have not been determined. A number of mechanisms have been proposed, including direct effects on signal transduction pathways, induction of oxidative stress, effects on vitamin A metabolism, and effects on intercellular communication (Glauert et al., 2001).
One mechanism by which PCBs may exert their promoting activity is by increasing hepatic oxidative stress. PCBs have been observed in many studies to induce oxidative stress. Several studies have shown that PCBs increase lipid peroxidation in the liver (Kamohara et al., 1984; Oda et al., 1987; Shara and Stohs, 1987; Dogra et al., 1988; Pelissier et al., 1990; Saito, 1990; Yamamoto et al., 1994; Fadhel et al., 2002). PCBs also can produce oxidative DNA damage, in the form of 8-hydroxydeoxyguanosine (Oakley et al., 1996). We have observed that PCBs can activate NF-κB (Tharappel et al., 2002; Lu et al., 2003; Lu et al., 2004), which is known to be activated by oxidative stress (Schreck et al., 1992; Li and Karin, 1999; vandenBerg et al., 2001), and that the promoting activity of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153) is inhibited by the deletion of the p50 subunit of NF-κB (Glauert et al., 2008).
We therefore hypothesized that the tumor promoting activities of PCBs could be inhibited by decreasing oxidative stress in the liver. The present study examines if supplementing antioxidant phytochemicals could prevent the promotion of altered hepatic foci by 3,3′,4,4′-tetrachlorobiphenyl (PCB-77). The phytochemicals selected for this study were ellagic acid, β-carotene, curcumin, N-acetyl cysteine (NAC), coenzyme Q10 (CoQ10), resveratrol, lycopene, and a tea extract containing epigallocatechin-3-gallate (EGCG). All of these agents have been found to inhibit experimental carcinogenesis in several studies (Wood et al., 1982; Mukhtar et al., 1986; Suzuki et al., 1986; Toma et al., 1995; Yang et al., 2001; Balansky et al., 2002; Nishino et al., 2002; Yang et al., 2002; Hannum, 2004; Joe et al., 2004; Russell, 2004; Bhuvaneswari and Nagini, 2005; Perumal et al., 2005; Nishikawa-Ogawa et al., 2006; Sakano et al., 2006; Thangapazham et al., 2006). Ellagic acid is a naturally occurring plant phenol that has strong scavenging ability for hydrogen peroxide, superoxide anion and hydroxy anion in vitro (Cozzi et al., 1995; Iino et al., 2001). The carotenoid β-carotene has been demonstrated to quench singlet oxygen and also scavenge peroxy radicals (Paiva and Russell, 1999). Curcumin has antioxidant activity against free radicals, and also increases the activity of antioxidant enzymes (Joe et al., 2004). NAC is a precursor of glutathione and increases glutathione levels; it also can scavenge free radicals itself (Zafarullah et al., 2003; Aitio, 2006). NAC has also been shown to modulate transcriptional activities through pathways involving c-fos/c-jun, NF-κB, and cyclin inhibitors (Zafarullah et al., 2003). CoQ10 is a component of the electron transport pathway in mitochondria (Kwong et al., 2002). The quinol form of CoQ10 is a potent antioxidant in the inner mitochondrial membrane. It inhibits lipid peroxidation by either scavenging free radicals or reducing the alpha tocopheryl radical (Turrens et al., 1985; Battino et al., 1990; Ernster and Dallner, 1995). Resveratrol is found in grapes and other plants and is a phytoalexin (antifungal agent) with strong antioxidant activity (Fauconneau et al., 1997); it has also been found to be a competitive inhibitor of Ah receptor ligands and to inhibit dioxin response element driven transcription of CYP1A1 and other phase 1 enzymes in vivo (Ciolino et al., 1998; Casper et al., 1999). Lycopene is one of the major carotenoid antioxidants in tomatoes and is known to have a significant anticancer effect with its singlet oxygen quenching ability (Di Mascio et al., 1989; Shi et al., 2004). EGCG and other polyphenols present in green tea have antioxidant activity and also prevent oxidation by chelating metal ions such as iron or copper (Cabrera et al., 2006). EGCG has also been found to modulate signal transduction pathways that inhibit cell proliferation and increase apoptosis (Khan et al., 2006).
Several models have been used to study the initiation and promotion of carcinogenesis in the liver. Initiation generally consists of the administration of a necrogenic dose of an initiating agent or a non-necrogenic dose in combination with a proliferative stimulus, such as partial hepatectomy (Glauert, 1991). Promotion consists of the long-term administration of generally non-genotoxic agents such as phenobarbital, peroxisome proliferators, or PCBs, and results in the formation of hepatocellular adenomas and carcinomas (Pitot et al., 1987; Glauert et al., 2001). In addition, foci of putative preneoplastic hepatocytes, which have altered enzyme activities or cellular functions, appear before the development of gross tumors (Glauert, 1991).
In this experiment we investigated if the above antioxidant phytochemicals would inhibit the hepatic tumor promoting activity of PCB-77. We used PCB-77 because this PCB congener has efficacious hepatic tumor promoting activity (Glauert et al., 2001). After initiation with diethylnitrosamine (DEN), rats were fed a diet containing one of these antioxidants or a control diet before receiving four biweekly injections of PCB-77. The number and volume of placental glutathione S-transferase (PGST)-positive foci were quantified, as well as the rate of cell proliferation in normal and PGST-positive hepatocytes.
Materials and Methods
Chemicals
PCB-77 was synthesized and characterized as described previously (Schramm et al., 1985; Lehmler and Robertson, 2001). The purity of the PCB-77 was >99% by GC-MS. Diethylnitrosamine (DEN, catalog #N0258, purity unknown) was purchased from Sigma Chemical Company (St. Louis, MO). Anti-PGST antibody was purchased from Novocastra Laboratories (Newcastle upon Tyne, UK) and ABC and ABC-AP staining kits were purchased from Vector Laboratories (Burlingame, CA). Antigen retrieval Citra solution was obtained from Biogenex (San Ramon, CA). Alzet osmotic pumps (model 2ML1) were obtained from Alza Scientific Products (Palo Alto, CA). The anti-5-bromo-2′-deoxyuridine (BrdU) antibody was purchased from Becton-Dickinson (San Jose, CA). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Experimental Diets
Ellagic acid, N-acetyl-L-cysteine (NAC), resveratrol, β-carotene, and curcumin were purchased from Sigma Chemical Co. (St. Louis, MO). A tea extract containing 16.5% EGCG and 33.4% total catechins, was a generous gift from Jarrow Formulas, Inc. (Los Angeles, CA). Coenzyme Q10 complex 20% (Co Q10) was purchased from Tishcon Corp., Westbury, NY. Lycovit (containing 10% lycopene) was a generous gift from BASF Corporation (Shreveport, LA). Casein, corn starch, dextrose monohydrate, soybean oil, non nutritive cellulose fiber, AIN-93 mineral mix, AIN-93 vitamin mix, choline bitartrate and L-cystine were purchased from Harlan Teklad Test Diets, Madison, Wisconsin. The composition of the control diet, which was based on the AIN-93 diet (Reeves et al., 1993), is shown in Table 1. Purified diets were used because they have lower levels of naturally-occurring phytochemicals. Purified diets consist primarily of refined ingredients with added vitamin and mineral mixtures, whereas unrefined (i.e. chow) diets are composed primarily of unrefined plant and animal materials [Bieri, 1977 #54]. The experimental diets were made by mixing in the following concentrations of the phytochemicals: ellagic acid, 0.4%; NAC, 1.0%; resveratrol, 0.005%; CoQ10, 0.4% (2% of 20% CoQ10 complex); curcumin, 0.5%; tea extract, 1.0%; β-carotene, 0.5%; lycopene, 10.0% Lycovit. The doses chosen were based on previous studies using these phytochemicals (Daniel and Stoner, 1991; Hirose et al., 1995; Lubet et al., 1997; Hirose et al., 1999; Schilling et al., 2001; Thomas et al., 2001; Martin et al., 2002; Witschi et al., 2002; Jonker et al., 2003; Miura et al., 2003). Diets were prepared every other week and were stored at 4° before use. We did not check the diets for the stability of any of the ingredients; purified diets, however, are generally stable for over one month at refrigerator temperatures [Fullerton, 1982 #10965].
Table 1.
Composition of Purified Diets
| Dietary Ingredient | % of diet |
|---|---|
| Casein | 14.00 |
| Cornstarch | 46.57 |
| Dextrose monohydrate | 25.5 |
| Soybean oil | 4.00 |
| Cellulose fiber | 5.00 |
| AIN-93 mineral mix | 3.50 |
| AIN-93 vitamin mix | 1.00 |
| Choline bitartrate | 0.25 |
| L-Cystine | 0.18 |
Experimental Design
The experimental protocols and procedures that involved rats were approved by the Institutional Animal Care and Use committee of the University of Kentucky and were in accordance with all policies for the use and care of laboratory research animals as stipulated by the NIH. Eighty female weanling Sprague-Dawley rats weighing 100–125g at arrival (Harlan Sprague Dawley, Indianapolis, IN), were housed three per cage in a temperature- and light-controlled room. Upon arrival, the animals were fed an unrefined diet and allowed to adjust for one week before starting the experiment. All animals received a single dose of DEN (150 mg/kg) by oral intubation. One week after the DEN administration, rats were provided with either a purified diet alone (Table 1) or with one of the antioxidant diets ad libitum for the remainder of the study. Body weights and food consumption were measured twice per week during the experiment.
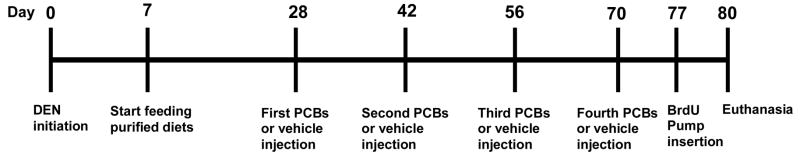
After being fed the experimental diets for 21 days, 2/3 of the control animals and all antioxidant diet-fed animals were given four i.p. injections of 300 μmol/kg of PCB 77 every 14 days (Figure 1). Ten days after the last PCB injection, all animals were euthanized, by overexposure to carbon dioxide gas. Three days before euthanasia, Alzet osmotic pumps containing BrdU solution (20 mg/ml) were subcutaneously implanted in all animals. Immediately after euthanasia, a piece of liver from each of four lobes was removed from all animals, fixed in buffered neutral formalin, and then made into paraffin blocks. The remainder of the liver was frozen in liquid nitrogen and then stored at −80°C.
Figure 1.
Experimental Design
Immunohistochemical Staining
The formalin-fixed liver tissues were paraffin-embedded, sectioned, laid on glass slides, and then double-immunostained with an anti-BrdU antibody first and an anti-PGST antibody second, to identify nuclei that had incorporated BrdU and PGST positive preneoplastic foci, respectively, and finally counterstained with hematoxylin. The Vectastain ABC and ABC-AP Kit (Vector Laboratories) were used for this staining according to the protocol provided by the manufacturer.
Quantitation of Altered Hepatic Foci
The number and volume of PGST-positive foci were measured using a computer digitizing software system developed at University of Wisconsin (Campbell et al., 1982; Campbell et al., 1986; Xu et al., 1998). The images were captured using a Nikon Eclipse E800 microscope equipped with MACRO 0.5x and 1.0x lenses. The number of foci/cm3 (Saltykov method), foci/liver (Saltykov method), the mean focal volume (Saltykov method), and the volume fraction (Delesse method) were analyzed.
Labeling Indexes
The labeling indexes were quantified in both normal hepatocytes and in PGST-positive foci. At least 3000 nuclei from normal hepatocytes were randomly counted per slide (>1000 in each of three lobes) and the labeling indexes were expressed as the percentage of number of labeled nuclei out of the total number of nuclei counted.
Statistical Analyses
The variables body and liver weights, labeling indexes, mean focal volume, and volume fraction, were analyzed by one-way analysis of variance (ANOVA). The comparisons between the treatments and the control, PCB-77 only, were based on the least squares mean effects of the diets followed by the appropriate statistical test procedure. The least square means provide better estimates of the effects when the treatments have unequal number of replicates. The number of foci per liver and number of foci per cubic centimeter data were analyzed by negative binomial regression model with logarithm as the link function. The statistical comparison was made between group receiving DEN only and the antioxidant treatment groups with the group receiving PCB-77 only. The goodness of fit of the model was assessed by Pearson χ2 value adjusted for overdispersion and the parameters of the model were estimated by the method of maximum likelihood. The Wald’s asymptotic procedure was used to determine the p values for significance of the differences between the PCB treatment groups and the control groups. We used the negative binomial regression model for the analysis of data obtained for the number of foci per liver and number of foci per cubic centimeter because of the discrete nature of these measurements (Espandiari et al., 2003). SAS version 9.1 (SAS Institute, Cary, NC) was used for the above analysis. The results were expressed as means ± standard error of the mean (SEM). The results were considered significant at p<0.05.
Results
In this study, the effect of antioxidant phytochemicals on the promoting activity of PCB-77 was determined. After initiation with DEN, rats were fed diets containing several antioxidants while receiving injections of PCB-77 during the promotion period. At the end of the study, the body weights of the rats were not affected by either the PCB-77 or antioxidant treatments (Table 2). The rats receiving DEN + PCB-77 had a significantly higher liver weight and liver weight/body weight ratio compared to rats receiving only DEN. The rats receiving CoQ10 had a higher liver weight and liver to body weight ratio than rats receiving PCB-77 alone. None of the other antioxidant phytochemicals affected the liver weight.
Table 2.
Body and Liver Weights
| Treatment | Rats per treatment | Body weight (gm) | Liver weight (gm) | Liver weight/body weight (%) |
|---|---|---|---|---|
| No PCB-77 | ||||
| Control diet | 4 | 266 ± 9 | 8.8 ± 0.3* | 3.3 ± 0.1* |
| PCB-77 Treated | ||||
| Control diet | 9 | 256 ± 2 | 14.0 ± 0.6 | 5.4 ± 0.2 |
| β-carotene | 8 | 255 ± 4 | 14.4 ± 0.3 | 5.7 ± 0.2 |
| CoQ10 | 9 | 256 ± 4 | 15.2 ± 0.4* | 5.9 ± 0.2* |
| Curcumin | 9 | 255 ± 4 | 14.0 ± 0.4 | 5.5 ± 0.2 |
| Tea extract | 8 | 262 ± 4 | 14.3 ± 0.3 | 5.5 ± 0.1 |
| Ellagic acid | 9 | 258 ± 5 | 13.5 ± 0.4 | 5.2 ± 0.1 |
| Lycopene | 6 | 259 ± 4 | 14.6 ± 0.2 | 5.7 ± 0.1 |
| NAC | 9 | 251 ± 3 | 13.5 ± 0.5 | 5.4 ± 0.2 |
| Resveratrol | 9 | 260 ± 4 | 13.9 ± 0.3 | 5.4 ± 0.1 |
Values are means ± SEM.
Significantly different from the PCB-77-treated control diet group (P ≤ 0.05)
The number and volume of preneoplastic foci were quantified using placental glutathione S-transferase (PGST) as a marker. The numbers of PGST-positive foci were quantified as foci/cm3 and as foci/liver. The volume of the PGST-positive foci was expressed as two endpoints: the mean volume of the foci (in mm3) and the volume fraction (the percentage of the liver that is occupied by foci), which represents the product of focal number and focal volume (Campbell et al., 1982; Campbell et al., 1986; Xu et al., 1998). The rats receiving DEN + PCB-77 with no antioxidants in the diet had a significant increase in the number and volume of PGST-positive foci compared to rats receiving only DEN (Table 3). None of the phytochemicals significantly altered the number of foci per liver, although lycopene slightly decreased (P = 0.09) and ellagic acid slightly increased (P = 0.08) the number of foci per liver. Lycopene significantly decreased and ellagic acid significantly increased the number of foci per cm3 compared to rats treated with PCB-77 alone; none of the other phytochemicals had a significant effect, although β-carotene slightly decreased the number of foci per cm3 (P = 0.09). None of the phytochemicals altered the percent of liver volume occupied by foci compared to the DEN+PCB-77 treated rats, although β-carotene slightly decreased the volume fraction (P = 0.10). However, ellagic acid, CoQ10, and curcumin all significantly decreased the mean volume of foci compared to the DEN + PCB-77 treated rats; in addition, lycopene slightly increased the mean focal volume (P = 0.06).
Table 3.
Effect of Antioxidant Phytochemicals on the Promotion of PGST-Positive Foci by PCB-77
| Treatment | Foci/Liver | Foci/cm3 | Mean Focal Volume (mm3 × 10−3) | Focal Volume (% of Liver) |
|---|---|---|---|---|
| No PCB-77 | ||||
| Control diet | 3182 ± 842* | 361 ± 93* | 4.6 ± 0.24* | 0.17 ± 0.05* |
| PCB-77 Treated | ||||
| Control diet | 29449 ± 3772 | 2123 ± 278 | 6.5 ± 0.66 | 1.3 ± 0.18 |
| β-Carotene | 21677 ± 2845 | 1511 ± 214 | 5.8 ± 0.45 | 0.85 ± 0.09 |
| CoQ10 | 28330 ± 4030 | 1878 ± 280 | 4.9 ± 0.71* | 1.1 ± 0.21 |
| Curcumin | 27108 ± 3896 | 1930 ± 261 | 4.9 ± 0.61* | 0.9 ± 0.13 |
| Tea extract | 25717 ± 6530 | 1803 ± 453 | 6.7 ± 0.52 | 1.2 ± 0.3 |
| Ellagic acid | 40940 ± 5425 | 3065 ± 431* | 4.8 ± 0.52* | 1.5 ± 0.33 |
| Lycopene | 20630 ± 2400 | 1407 ± 157* | 8.0 ± 0.69 | 1.1 ± 0.2 |
| NAC | 31210 ± 3212 | 2319 ± 240 | 5.4 ± 0.22 | 1.2 ± 0.08 |
| Resveratrol | 29241 ± 3341 | 2109 ± 240 | 5.5 ± 0.37 | 1.1 ± 0.13 |
Data are means ± standard errors.
Significantly different from the PCB-77-treated control diet group (P ≤ 0.05)
Cell proliferation was quantified by administering BrdU in Alzet osmotic pumps for three days before euthanasia and then quantifying labeling indexes in tissue sections. In PGST-negative hepatocytes, PCB-77 increased BrdU labeling about two-fold when compared to the group receiving DEN but no PCB-77, but this difference was not statistically significant (P = 0.07). The labeling index was significantly higher in the CoQ10 group compared to DEN+PCB-77 group, but no other phytochemicals produced a significant effect. Labeling indexes were higher in PGST-positive foci than in normal hepatocytes. The rats receiving DEN but no PCB-77 had a lower labeling index in PGST-positive foci than the rats receiving DEN + PCB-77, but there were no significant differences between the PCB-77 only group and the other groups.
Discussion
In this study we have examined the ability of several antioxidant phytochemicals to inhibit the hepatic promoting activity of PCB-77. None of the agents tested produced a clear decrease in both the number and volume of foci induced. Lycopene and β-carotene decreased the number of foci induced, but either did not affect or increased the mean volume of the foci. Ellagic acid, CoQ10, and curcumin all significantly decreased the mean volume of foci, but either did not affect or increased the number of foci induced. A number of other agents have been observed to have opposite effects on focal number and focal size. Stemm et al. (2008) found that dietary selenium increased the number of foci but decreased their size in PCB-treated rats. Kobusch et al. (1989), however, found that PCB-77 increased the size of N-nitrosomorpholine-initiated foci but decreased the number induced.
β-Carotene produced slight decreases in the number of foci induced as well as in the volume fraction. These results are in general agreement with other experimental studies examining β-carotene. Several studies have examined the effect of β-carotene on liver carcinogenesis using p.o. injections or feeding it in the diet or drinking water at concentrations ranging from 0.01–0.1%, and most, but not all, observed a protective effect (Moreno et al., 1991; Murakoshi et al., 1992; Sarkar et al., 1994; Hirose et al., 1995; Astorg et al., 1996; Sadek and Hayat, 1996; Tsuda et al., 1996; Rizzi et al., 1997; Dagli et al., 1998; Gradelet et al., 1998; Bishayee et al., 2000; Moreno et al., 2002; Takasuka et al., 2002; Chattopadhyay et al., 2004; de Almeida Vasconcelos Fonseca et al., 2005). The dietary concentration used in the present study (0.5%) was higher than in the other dietary studies, but did not result in a greater inhibition of carcinogenesis. In experimental carcinogenesis studies in other tissues, most studies have observed a protective effect of β-carotene (Toma et al., 1995; Nishino et al., 2002; Russell, 2004). However, in the human ATBC and CARET clinical trials, β-carotene was found to enhance the development of lung cancer in smokers (Heinonen et al., 1994; Omenn et al., 1996a; Omenn et al., 1996b).
Ellagic acid was found to increase the number of foci but to decrease their mean volume. In other studies examining the liver, ellagic acid (1% in diet) was similarly found to increase the number of PGST-positive foci in a multi-organ carcinogenesis model (Akagi et al., 1995). Ellagic acid (0.04% in diet), however, was found to inhibit 2-acetylaminofluorene (AAF)-induced liver tumors (Tanaka et al., 1988). Therefore the intermediate dose used in the present study (0.4% in diet) is more in agreement with the high-dose study. Ellagic acid additionally has been found to be anti-carcinogenic in other tissues (Wood et al., 1982; Mukhtar et al., 1986; Hannum, 2004).
Curcumin did not affect the number of foci induced, but significantly decreased their mean volume. In previous studies in the liver, curcumin has been found to inhibit the induction of altered hepatic foci by DEN (0.2% curcumin in diet) (Chuang et al., 2000) or by DEN/AAF (200 mg curcumin/kg body weight for 5 days) (Shukla and Arora, 2003). However, curcumin (0.5% in diet) was found to enhance the promotion of altered hepatic foci by 2-amino-3,4-dimethylimidazo[4,5-f ]quinoline (MeIQ) (Hirose et al., 1999). Dietary curcumin (0.5% in diet) did not affect the incidence of spontaneous liver tumors in Long-Evans Cinnamon rats (Frank et al., 2003), which could be related to an elevation in lipid peroxidation-related DNA adducts in curcumin-fed rats (Nair et al., 2005). The dose used in the present study (0.5%) was similar to that used in these other studies. Curcumin (0.1–0.2% in diet) was found to prevent colon carcinogenesis in several studies (Rao et al., 1995; Kawamori et al., 1999; Mahmoud et al., 2000). Most other carcinogenesis studies in other tissues have also observed an inhibition in tumor induction after curcumin feeding (Joe et al., 2004; Thangapazham et al., 2006).
Lycopene decreased the number of foci induced but slightly increased their volume. Other studies that examined the role of lycopene in hepatocarcinogenesis saw mixed results. Astorg and colleagues found that lycopene (0.03% in diet) decreased the initiating activity of DEN but not that of 2-nitropropane or aflatoxin B1 (Astorg et al., 1997; Gradelet et al., 1998). Lycopene (0.2 mg p.o. 3x/wk) was found to decrease the incidence and multiplicity of spontaneous liver tumors in C3H mice (Nishino, 1997), but did not significantly affect the incidence of spontaneous liver tumors in Long-Evans Cinnamon rats (when fed at 0.005% of diet) (Watanabe et al., 2001). Breinholt et al. (2003), however, found that lycopene (0.005% in diet) induced a low level of PGST-positive foci in rats. The use of the Lycovit in the present study makes the present study somewhat difficult to compare with the other studies, but the higher dose used may have been effective in lycopene’s ability to decrease the number of foci induced. In other tissues, most studies have observed a chemopreventive effect of lycopene (Bhuvaneswari and Nagini, 2005).
Co-enzyme Q10 decreased the mean volume of foci but did not affect the number induced. No other studies have examined the effect of coenzyme Q10 on liver carcinogenesis. In studies in other tissues, coenzyme Q10 was found to reduce the volume of DMBA-induced mammary tumors (40 mg/kg body wt/day p.o. for 28 days) (Perumal et al., 2005), the number of colon tumors induced by dimethylhydrazine (200 μg/day for 26 wk) (Suzuki et al., 1986), and the number of aberrant crypt foci induced by azoxymethane (0.02% or 0.05% in diet) (Sakano et al., 2006).
Although the tea extract did not affect the promotion of foci by PCB-77 in this study, most other studies have found tea extracts and EGCG to be inhibitory in liver carcinogenesis models. Both green tea (0.63 or 1.25% in drinking water for 40 wk) and black tea (1.25% in drinking water) were found to inhibit the induction of hepatic tumors by DEN in C3H mice, but no association between EGCG content and chemopreventive effect was observed (Cao et al., 1996). Green tea (2% in drinking water) was found to prevent the promotion of hepatic tumors by pentachlorophenol (PCP) in mice (Umemura et al., 2003). Green tea catechins (1% in diet) were found to inhibit the promotion of altered hepatic foci by MeIQ or 2-amino-6-methyldipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-1) in rats (Hirose et al., 1995; Hirose et al., 1999). In addition, Mao (1993) found that an epicatechin complex inhibited the initiation of hepatocarcinogenesis by DEN. Purified EGCG as well as other purified catechins and tea extracts (0.05 or 0.1% catechin in diet) were found to inhibit the induction of PGST-positive foci by DEN and phenobarbital (Matsumoto et al., 1996). However, green tea catechins (1% in diet) increased the induction of PGST-positive foci using a multi-organ rat carcinogenesis model (Hirose et al., 1993). A green tea extract (0.01 or 0.1% in drinking water) was found to inhibit the number but not the size of DEN-induced tumors in rats but did not affect tumors induced by choline deficiency (Tamura et al., 1997). There does not appear to be a correlation between dose and the effect of tea components in the published studies, but comparisons are difficult due to differences in the tea extracts and route of administration (feed vs. drinking water). In other tissues, EGCG as well as tea or tea extracts generally were found to inhibit carcinogenesis (Yang et al., 2002).
Neither NAC nor resveratrol had an effect in this study. In other liver carcinogenesis models, NAC (10–100 mg/kg body weight p.o. 5x/wk) inhibited the induction of PGST-positive foci by 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in rats (Nishikawa-Ogawa et al., 2006), but did not affect the induction of liver tumors in rats treated with DEN and diethyldithiocarbamate when administered at 400 mg/kg body weight/day in drinking water (Balansky et al., 2002). Resveratrol, injected at a dose of 1 mg/kg body weight/day i.p. for 7 days (Carbo et al., 1999) or at 5, 10 or 15 mg/kg body weight/day i.p. for 10 days (Wu et al., 2004), was found to decrease the growth of a transplantable hepatoma. It is difficult to compare these results with those from the present study since we fed these agents in the diet whereas the other studies administered them in the drinking water or by injection. In other tissues, resveratrol inhibited chemically-induced carcinogenesis in the skin, mammary gland, and colon, but had no effect in the lung (Yang et al., 2001).
Other dietary antioxidants have been examined for their effect on the promotion of liver carcinogenesis by PCBs. Stemm et al. (2008) found that selenium supplementation increased the number of PGST-positive foci in PCB-77-treated rats, but that it reduced the mean focal volume of the foci in untreated, PCB-77-treated, and PCB-153-treated rats. Glauert et al. (2005) found that dietary vitamin E did not affect the promotion of PGST-positive foci by either PCB-77 or PCB-153.
The only phytochemical that affected cell proliferation was CoQ10, which increased the labeling index in normal hepatocytes (but which did not affect the labeling index in PGST-positive foci). This increase in normal hepatocytes correlated with CoQ10’s effect on the liver weight, which also was increased. It is possible that these two endpoints indicate toxicity from the CoQ10 but they could also represent additive hyperplasia. However, body weight was not affected by CoQ10 or any of the other phytochemicals. Several of the phytochemicals (CoQ10, curcumin, and ellagic acid) significantly decreased the mean focal volume and one (lycopene) slightly increased it, but none of these phytochemicals affected the labeling index in PGST-positive foci (P > 0.30 for all phytochemicals). The rats not receiving PCB-77 had a lower labeling index in both normal hepatocytes and PGST-positive foci, which correlated with the decrease both in the liver weight and in the number and volume of foci induced.
In summary, we observed that some antioxidant phytochemicals can influence the promoting activity of PCB-77. However, none of the agents produced a clear decrease in both the number and volume of PGST-positive foci induced. Therefore the activity of these antioxidant phytochemicals as chemopreventive agents in PCB-induced carcinogenesis would appear to be limited. Only one of the PCBs that have promoting activity in the liver was studied, however, so it is possible that one or more of these phytochemicals could have a major effect on another PCB that has promoting activity. The results of this study, in combination with the studies showing that vitamin E and selenium also do not clearly decrease the promoting activities of PCB-77 and PCB-153 (Glauert et al., 2005; Stemm et al., 2008), imply that dietary antioxidants are not effective at inhibiting tumor promotion by PCBs. These results also indicate that the induction of oxidative stress by PCBs may not be a mechanism in the promoting activities of these agents. Other mechanisms, such as direct effects on signal transduction pathways or effects on vitamin A metabolism, may be more important (Glauert et al., 2001).
Table 4.
Effect of Antioxidant Phytochemicals on Cell Proliferation in Normal and PGST-Positive Hepatocytes
| Treatment | Labeling index: non-focal hepatocytes (%) | Labeling index: PGST-positive hepatocytes (%) |
|---|---|---|
| No PCB-77 | ||
| Control diet | 2.58 ± 1.14 | 10.0 ± 2.1* |
| PCB-77 Treated | ||
| Control diet | 5.84 ± 0.46 | 17.8 ± 1.4 |
| β-carotene | 5.43 ± 0.69 | 15.0 ± 1.3 |
| CoQ10 | 13.06 ± 1.88* | 20.1 ± 1.7 |
| Curcumin | 5.51 ± 0.85 | 18.8 ± 2.6 |
| Tea extract | 6.68 ± 0.96 | 20.1 ± 2.6 |
| Ellagic acid | 5.25 ± 0.47 | 14.7 ± 1.7 |
| Lycopene | 4.85 ± 0.30 | 15.7 ± 1.5 |
| NAC | 5.21 ± 0.44 | 20.1 ± 2.5 |
| Resveratrol | 6.81 ± 1.41 | 21.5 ± 3.4 |
Data are means ± standard errors.
Significantly different from the PCB-77-treated control diet group (P ≤ 0.05)
Acknowledgments
The authors would like to thank Dr. Zaineb Fadhel, Amy Dugan, Amita Kumar, Jill Cholewa, and Sam Patel for their assistance with the study. This work was supported by grants from the National Institutes of Health (ES07380, ES012475, and ES013661) and by the Kentucky Agricultural Experiment Station.
Abbreviations
- AAF
2-acetylaminofluorene
- ANOVA
analysis of variance
- BrdU
bromodeoxyuridine
- CoQ10
co-enzyme CoQ10
- DEN
diethylnitrosamine
- EGCG
epigallocatechin-3-gallate
- GC-MS
gas chromatography-mass spectrometry
- Glu-P-1,
2-amino-6-methyldipyrido[1,2-a: 3′,2′-d]imidazole
- MeIQ
2-amino-3,4-dimethylimidazo[4,5-f ]quinoline
- NAC
N-acetyl cysteine
- PCB-77
3,3′,4′4-tetrachlorobiphenyl
- PCBs
polychlorinated biphenyls
- PGST
placental glutathione S-transferase
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K, Hirose M, Hoshiya T, Mizoguchi Y, Ito N, Shirai T. Modulating effects of ellagic acid, vanillin and quercetin in a rat medium term multi-organ carcinogenesis model. Cancer Lett. 1995;94:113–121. doi: 10.1016/0304-3835(95)03833-i. [DOI] [PubMed] [Google Scholar]
- Astorg P, Gradelet S, Berges R, Suschetet M. No evidence for an inhibitory effect of beta-carotene or of canthaxanthin on the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutrition and Cancer. 1996;25:27–34. doi: 10.1080/01635589609514425. [DOI] [PubMed] [Google Scholar]
- Astorg P, Gradelet S, Berges R, Suschetet M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutrition and Cancer. 1997;29:60–68. doi: 10.1080/01635589709514603. [DOI] [PubMed] [Google Scholar]
- Balansky RM, Ganchev G, D’Agostini F, De Flora S. Effects of N-acetylcysteine in an esophageal carcinogenesis model in rats treated with diethylnitrosamine and diethyldithiocarbamate. Int J Cancer. 2002;98:493–497. doi: 10.1002/ijc.10215. [DOI] [PubMed] [Google Scholar]
- Battino M, Fato R, Parenti-Castelli G, Lenaz G. Coenzyme Q can control the efficiency of oxidative phosphorylation. Int J Tissue React. 1990;12:137–144. [PubMed] [Google Scholar]
- Bhuvaneswari V, Nagini S. Lycopene: a review of its potential as an anticancer agent. Curr Med Chem Anticancer Agents. 2005;5:627–635. doi: 10.2174/156801105774574667. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Sarkar A, Chatterjee M. Further evidence for chemopreventive potential of beta-carotene against experimental carcinogenesis: Diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis is prevented more effectively by beta-carotene than by retinoic acid. Nutrition and Cancer. 2000;37:89–98. doi: 10.1207/S15327914NC3701_12. [DOI] [PubMed] [Google Scholar]
- Breinholt VM, Molck AM, Svendsen GW, Daneshvar B, Vinggaard AM, Poulsen M, Dragsted LO. Effects of dietary antioxidants and 2-amino-3-methylimidazo[4,5-f]-quinoline (IQ) on preneoplastic lesions and on oxidative damage, hormonal status, and detoxification capacity in the rat. Food Chem Toxicol. 2003;41:1315–1323. doi: 10.1016/s0278-6915(03)00122-4. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Campbell HA, Pitot HC, Potter VR, Laishes BA. Application of quantitative stereology to the evaluation of enzyme-altered foci in rat liver. Cancer Research. 1982;42:465–472. [PubMed] [Google Scholar]
- Campbell HA, Xu YD, Hanigan MH, Pitot HC. Application of quantitative stereology to the evaluation of phenotypically heterogeneous enzyme-altered foci in the rat liver. Journal of the National Cancer Institute. 1986;76:751–767. doi: 10.1093/jnci/76.4.751. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu Y, Chen JS, Klaunig JE. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Fundamental and Applied Toxicology. 1996;29:244–250. doi: 10.1006/faat.1996.0028. [DOI] [PubMed] [Google Scholar]
- Carbo N, Costelli P, Baccino FM, Lopez-Soriano FJ, Argiles JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999;254:739–743. doi: 10.1006/bbrc.1998.9916. [DOI] [PubMed] [Google Scholar]
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- Chattopadhyay MBCBM, Kanna PS, Ray RS, Roy S, Chatterjee M. Combined supplementation of vanadium and beta-carotene suppresses placental glutathione S-transferase-positive foci and enhances antioxidant functions during the inhibition of diethylnitrosamine-induced rat liver carcinogenesis. J Gastroenterol Hepatol. 2004;19:683–693. doi: 10.1111/j.1440-1746.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000;21:331–335. doi: 10.1093/carcin/21.2.331. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- Cozzi R, Ricordy R, Bartolini F, Ramadori L, Perticone P, De Salvia R. Taurine and ellagic acid: two differently-acting natural antioxidants. Environ Mol Mutagen. 1995;26:248–254. doi: 10.1002/em.2850260310. [DOI] [PubMed] [Google Scholar]
- Dagli MLZ, Guerra JL, Sinhorini IL, Wu TS, Rizzi MBSL, Penteado MVC, Moreno FS. Beta-carotene reduces the ductular (oval) cell reaction in the liver of Wistar rats submitted to the resistant hepatocyte model of carcinogenesis. Pathology. 1998;30:259–266. doi: 10.1080/00313029800169416. [DOI] [PubMed] [Google Scholar]
- Daniel EM, Stoner GD. The effects of ellagic acid and 13-cis-retinoic acid on N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats. Cancer Lett. 1991;56:117–124. doi: 10.1016/0304-3835(91)90085-v. [DOI] [PubMed] [Google Scholar]
- de Almeida Vasconcelos Fonseca EM, Chagas CE, Mazzantini RP, Heidor R, Ong TP, Moreno FS. All-trans and 9-cis retinoic acids, retinol and beta-carotene chemopreventive activities during the initial phases of hepatocarcinogenesis involve distinct actions on glutathione S-transferase positive preneoplastic lesions remodeling and DNA damage. Carcinogenesis. 2005;26:1940–1946. doi: 10.1093/carcin/bgi161. [DOI] [PubMed] [Google Scholar]
- Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Dogra S, Filser JG, Cojocel C, Greim H, Regel U, Oesch F, Robertson LW. Long-term effects of commercial and congeneric polychlorinated biphenyls on ethane production and malondialdehyde levels, indicators of in vivo lipid peroxidation. Archives of Toxicology. 1988;62:369–374. doi: 10.1007/BF00293625. [DOI] [PubMed] [Google Scholar]
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol. 2003;186:55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Fadhel Z, Lu Z, Robertson LW, Glauert HP. Effect of 3,3′,4,4′-tetrachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl on the induction of hepatic lipid peroxidation and cytochrome P-450 associated enzyme activities in rats. Toxicology. 2002;175:15–25. doi: 10.1016/s0300-483x(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- Frank N, Knauft J, Amelung F, Nair J, Wesch H, Bartsch H. No prevention of liver and kidney tumors in Long-Evans Cinnamon rats by dietary curcumin, but inhibition at other sites and of metastases. Mutat Res. 2003;523–524:127–135. doi: 10.1016/s0027-5107(02)00328-7. [DOI] [PubMed] [Google Scholar]
- Glauert HP. Histochemical and stereological analysis of putative preneoplastic hepatic lesions. Prog Histochem Cytochem. 1991;23:84–90. doi: 10.1016/s0079-6336(11)80172-5. [DOI] [PubMed] [Google Scholar]
- Glauert HP, Kumar A, Lu Z, Patel S, Tharappel JC, Stemm DN, Bunaciu RP, Lee EY, Lehmler HJ, Robertson LW, Spear BT. Dietary vitamin E does not inhibit the promotion of liver carcinogenesis by polychlorinated biphenyls in rats. J Nutr. 2005;135:283–286. doi: 10.1093/jn/135.2.283. [DOI] [PubMed] [Google Scholar]
- Glauert HP, Robertson LW, Silberhorn EM. PCBs and tumor promotion. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington, KY: 2001. pp. 355–371. [Google Scholar]
- Glauert HP, Tharappel JC, Banerjee S, Chan LS, Kania-Korwel I, Lehmler HJ, Lee EY, Robertson LW, Spear BT. Inhibition of the promotion of hepatocarcinogenesis by 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153) by the deletion of the p50 subunit of NF-κB in mice. Toxicol Appl Pharmacol. 2008 doi: 10.1016/j.taap.2008.06.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradelet S, Le Bon AM, Berges R, Suschetet M, Astorg P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis. 1998;19:403–411. doi: 10.1093/carcin/19.3.403. [DOI] [PubMed] [Google Scholar]
- Hannum SM. Potential impact of strawberries on human health: a review of the science. Crit Rev Food Sci Nutr. 2004;44:1–17. doi: 10.1080/10408690490263756. [DOI] [PubMed] [Google Scholar]
- Heinonen OP, Huttunen JK, Albanes D, Haapakoski J, Palmgren J, Pietinen P, Pikkarainen J, Rautalahti M, Virtamo J, Edwards BK, Greenwald P, Hartman AM, Taylor PR, Haukka J, Jarvinen P, Malila N, Rapola S, Jokinen P, Karjalainen J, Lauronen J, Mutikainen J, Sarjakoski M, Suorsa A, Tiainen M, Verkasalo M, Barrett M, Alfthan G, Ehnholm C, Gref CG, Sundvall J, Haapa E, Ovaskainen ML, Palvaalhola M, Roos E, Pukkala E, Teppo L, Frick H, Pasternack A, Brown BW, Demets DL, Kokkola K, Tala E, Aalto E, Maenpaa V, Tienhaara L, Jarvinen M, Kuuliala I, Linko L, Mikkola E. Effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. New England Journal of Medicine. 1994;330:1029–1035. [Google Scholar]
- Hirose M, Hasegawa R, Kimura J, Akagi K, Yoshida Y, Tanaka H, Miki T, Satoh T, Wakabayashi K, Ito N, et al. Inhibitory effects of 1-O-hexyl-2,3,5-trimethylhydroquinone (HTHQ), green tea catechins and other antioxidants on 2-amino-6-methyldipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-1)-induced rat hepatocarcinogenesis and dose-dependent inhibition by HTHQ of lesion induction by Glu-P-1 or 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) Carcinogenesis. 1995;16:3049–3055. doi: 10.1093/carcin/16.12.3049. [DOI] [PubMed] [Google Scholar]
- Hirose M, Hoshiya T, Akagi K, Takahashi S, Hara Y, Ito N. Effects of green tea catechins in a rat multi-organ carcinogenesis model. Carcinogenesis. 1993;14:1549–1553. doi: 10.1093/carcin/14.8.1549. [DOI] [PubMed] [Google Scholar]
- Hirose M, Takahashi S, Ogawa K, Futakuchi M, Shirai T. Phenolics: blocking agents for heterocyclic amine-induced carcinogenesis. Food Chem Toxicol. 1999;37:985–992. doi: 10.1016/s0278-6915(99)00092-7. [DOI] [PubMed] [Google Scholar]
- Iino T, Nakahara K, Miki W, Kiso Y, Ogawa Y, Kato S, Takeuchi K. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Role of ellagic acid, the nonalcoholic component. Digestion. 2001;64:214–221. doi: 10.1159/000048864. [DOI] [PubMed] [Google Scholar]
- Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- Jonker D, Kuper CF, Fraile N, Estrella A, Rodriguez Otero C. Ninety-day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul Toxicol Pharmacol. 2003;37:396–406. doi: 10.1016/s0273-2300(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Kamohara K, Yagi N, Itokawa Y. Mechanism of lipid peroxide formation in polychlorinated biphenyls (PCB) and dichlorodiphenyltrichloroethane (DDT)-poisoned rats. Environmental Research. 1984;34:18–23. doi: 10.1016/0013-9351(84)90071-9. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kobusch AB, Fischer G, Bock KW. Tumor-promoting activity and cytotoxicity of 3,4,3′,4′-tetrachlorobiphenyl on N-nitrosomorpholine-induced murine liver foci. Journal of Cancer Research and Clinical Oncology. 1989;115:247–252. doi: 10.1007/BF00391697. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Kamzalov S, Rebrin I, Bayne AC, Jana CK, Morris P, Forster MJ, Sohal RS. Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic Biol Med. 2002;33:627–638. doi: 10.1016/s0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW. Synthesis of polychlorinated biphenyls (PCBs) using the Suzuki-coupling. Chemosphere. 2001;45:137–143. doi: 10.1016/s0045-6535(00)00546-4. [DOI] [PubMed] [Google Scholar]
- Li NX, Karin M. Is NF-kappa B the sensor of oxidative stress? FASEB Journal. 1999;13:1137–1143. [PubMed] [Google Scholar]
- Lu Z, Lee EY, Robertson LW, Glauert HP, Spear BT. Effect of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153) on hepatocyte proliferation and apoptosis in mice deficient in the p50 subunit of the transcription factor NF-kB. Toxicol Sci. 2004;81:35–42. doi: 10.1093/toxsci/kfh193. [DOI] [PubMed] [Google Scholar]
- Lu Z, Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Effect of a single dose of polychlorinated biphenyls on hepatic cell proliferation and the DNA binding activity of NF-kappaB and AP-1 in rats. Mol Carcinog. 2003;37:171–180. doi: 10.1002/mc.10135. [DOI] [PubMed] [Google Scholar]
- Lubet RA, Steele VE, Eto I, Juliana MM, Kelloff GJ, Grubbs CJ. Chemopreventive efficacy of anethole trithione, N-acetyl-L-cysteine, miconazole and phenethylisothiocyanate in the DMBA-induced rat mammary cancer model. Int J Cancer. 1997;72:95–101. doi: 10.1002/(sici)1097-0215(19970703)72:1<95::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921–927. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- Mao R. The inhibitory effects of epicatechin complex on diethylnitrosamine induced initiation of hepatocarcinogenesis in rats. Zhonghua Yu Fang Yi Xue Za Zhi. 1993;27:201–204. [PubMed] [Google Scholar]
- Martin KR, Saulnier MJ, Kari FW, Barrett JC, French JE. Timing of supplementation with the antioxidant N-acetyl-L-cysteine reduces tumor multiplicity in novel, cancer-prone p53 haploinsufficient Tg. AC (v-Ha-ras) transgenic mice but has no impact on malignant progression. Nutr Cancer. 2002;43:59–66. doi: 10.1207/S15327914NC431_7. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Kohri T, Okushio K, Hara Y. Inhibitory effects of tea catechins, black tea extract and oolong tea extract on hepatocarcinogenesis in rat. Jpn J Cancer Res. 1996;87:1034–1038. doi: 10.1111/j.1349-7006.1996.tb03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/s0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- Moreno FS, Rizzi MB, Dagli ML, Penteado MV. Inhibitory effects of beta-carotene on preneoplastic lesions induced in Wistar rats by the resistant hepatocyte model. Carcinogenesis. 1991;12:1817–1822. doi: 10.1093/carcin/12.10.1817. [DOI] [PubMed] [Google Scholar]
- Moreno FS, T SW, Naves MM, Silveira ER, Oloris SC, da Costa MA, Dagli ML, Ong TP. Inhibitory effects of beta-carotene and vitamin a during the progression phase of hepatocarcinogenesis involve inhibition of cell proliferation but not alterations in DNA methylation. Nutr Cancer. 2002;44:80–88. doi: 10.1207/S15327914NC441_11. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Das M, Bickers DR. Inhibition of 3-methylcholanthrene-induced skin tumorigenicity in BALB/c mice by chronic oral feeding of trace amounts of ellagic acid in drinking water. Cancer Res. 1986;46:2262–2265. [PubMed] [Google Scholar]
- Murakoshi M, Nishino H, Satomi Y, Takayasu J, Hasegawa T, Tokuda H, Iwashima A, Okuzumi J, Okabe H, Kitano H, et al. Potent preventive action of alpha-carotene against carcinogenesis: spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by alpha-carotene than by beta-carotene. Cancer Res. 1992;52:6583–6587. [PubMed] [Google Scholar]
- Nair J, Strand S, Frank N, Knauft J, Wesch H, Galle PR, Bartsch H. Apoptosis and age-dependant induction of nuclear and mitochondrial etheno-DNA adducts in Long-Evans Cinnamon (LEC) rats: enhanced DNA damage by dietary curcumin upon copper accumulation. Carcinogenesis. 2005;26:1307–1315. doi: 10.1093/carcin/bgi073. [DOI] [PubMed] [Google Scholar]
- Nishikawa-Ogawa M, Wanibuchi H, Morimura K, Kinoshita A, Nishikawa T, Hayashi S, Yano Y, Fukushima S. N-acetylcysteine and S-methylcysteine inhibit MeIQx rat hepatocarcinogenesis in the post-initiation stage. Carcinogenesis. 2006;27:982–988. doi: 10.1093/carcin/bgi277. [DOI] [PubMed] [Google Scholar]
- Nishino H. Cancer prevention by natural carotenoids. J Cell Biochem Suppl. 1997;27:86–91. [PubMed] [Google Scholar]
- Nishino H, Murakosh M, Ii T, Takemura M, Kuchide M, Kanazawa M, Mou XY, Wada S, Masuda M, Ohsaka Y, Yogosawa S, Satomi Y, Jinno K. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:257–264. doi: 10.1023/a:1021206826750. [DOI] [PubMed] [Google Scholar]
- Oakley GG, Devanaboyina U, Robertson LW, Gupta RC. Oxidative DNA damage induced by activation of polychlorinated biphenyls (PCBs): implications for PCB-induced oxidative stress in breast cancer. Chem Res Toxicol. 1996;9:1285–1292. doi: 10.1021/tx960103o. [DOI] [PubMed] [Google Scholar]
- Oda H, Yamashita K, Sasaki S, Horio F, Yoshida A. Long-term effects of dietary polychlorinated biphenyl and high level of vitamin E on ascorbic acid and lipid metabolism in rats. J Nutr. 1987;117:1217–1223. doi: 10.1093/jn/117.7.1217. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996a;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996b;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Paiva SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18:426–433. doi: 10.1080/07315724.1999.10718880. [DOI] [PubMed] [Google Scholar]
- Pelissier MA, Boisset M, Atteba S, Albrecht R. Lipid peroxidation of rat liver microsomes membranes related to a protein deficiency and/or a PCB treatment. Food Addit Contam. 1990;7:S172–177. doi: 10.1080/02652039009373875. [DOI] [PubMed] [Google Scholar]
- Perumal SS, Shanthi P, Sachdanandam P. Combined efficacy of tamoxifen and coenzyme Q10 on the status of lipid peroxidation and antioxidants in DMBA induced breast cancer. Mol Cell Biochem. 2005;273:151–160. doi: 10.1007/s11010-005-0325-3. [DOI] [PubMed] [Google Scholar]
- Pitot HC, Goldsworthy TL, Moran S, Kennan W, Glauert HP, Maronpot RR, Campbell HA. A method to quantitate the relative initiating and promoting potencies of hepatocarcinogenic agents in their dose-response relationships to altered hepatic foci. Carcinogenesis. 1987;8:1491–1499. doi: 10.1093/carcin/8.10.1491. [DOI] [PubMed] [Google Scholar]
- Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rizzi MBSL, Dagli MLZ, Jordao AA, Penteado MVC, Moreno FS. beta-carotene inhibits persistent and stimulates remodeling gamma GT-positive preneoplastic lesions during early promotion of hepatocarcinogenesis. International Journal of Vitamin and Nutrition Research. 1997;67:415–422. [PubMed] [Google Scholar]
- Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington, KY: 2001. [Google Scholar]
- Russell RM. The enigma of beta-carotene in carcinogenesis: what can be learned from animal studies. J Nutr. 2004;134:262S–268S. doi: 10.1093/jn/134.1.262S. [DOI] [PubMed] [Google Scholar]
- Sadek IA, Hayat LG. Initiation and post-initiation chemopreventive effects of beta-carotene in toad liver carcinogenesis. Histol Histopathol. 1996;11:357–360. [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Saito M. Polychlorinated biphenyls-induced lipid peroxidation as measured by thiobarbituric acid-reactive substances in liver subcellular fractions of rats. Biochimica et Biophysica Acta. 1990;1046:301–308. doi: 10.1016/0005-2760(90)90245-s. [DOI] [PubMed] [Google Scholar]
- Sakano K, Takahashi M, Kitano M, Sugimura T, Wakabayashi K. Suppression of azoxymethane-induced colonic premalignant lesion formation by coenzyme Q10 in rats. Asian Pac J Cancer Prev. 2006;7:599–603. [PubMed] [Google Scholar]
- Sarkar A, Mukherjee B, Chatterjee M. Inhibitory effect of beta-carotene on chronic 2-acetylaminofluorene induced hepatocarcinogenesis in rat: reflection in hepatic drug metabolism. Carcinogenesis. 1994;15:1055–1060. doi: 10.1093/carcin/15.5.1055. [DOI] [PubMed] [Google Scholar]
- Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington’s disease transgenic mouse model. Neurosci Lett. 2001;315:149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- Schramm H, Robertson LW, Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochemical Pharmacology. 1985;34:3735–3739. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Shara MA, Stohs SJ. Biochemical and toxicological effects of 2,3,7,8-tetrachlorodibenzo-p- dioxin (TCDD) congeners in female rats. Arch Environ Contam Toxicol. 1987;16:599–605. doi: 10.1007/BF01055816. [DOI] [PubMed] [Google Scholar]
- Shi J, Qu Q, Kakuda Y, Yeung D, Jiang Y. Stability and synergistic effect of antioxidative properties of lycopene and other active components. Crit Rev Food Sci Nutr. 2004;44:559–573. doi: 10.1080/15417060490908962. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Arora A. Suppression of altered hepatic foci development by curcumin in wistar rats. Nutr Cancer. 2003;45:53–59. doi: 10.1207/S15327914NC4501_7. [DOI] [PubMed] [Google Scholar]
- Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:440–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Stemm DN, Tharappel JC, Srinivasan C, Morris JS, Spate VL, Lehmler HJ, Robertson LW, Spear BT, Glauert HP. Effects of dietary selenium on the promotion of hepatocarcinogenesis by 3,3′,4,4′-tetrachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl. Exp Biol Med. 2008;233:366–376. doi: 10.3181/0708-RM-211. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Yamamoto J, Iwata Y, Matsumoto K, Iriyama K. Effects of immunostimulation with OK432, coenzyme Q10, or levamisole on dimethylhydrazine-induced colonic carcinogenesis in rats. Jpn J Surg. 1986;16:152–155. doi: 10.1007/BF02471087. [DOI] [PubMed] [Google Scholar]
- Sweeny GD, Jones KG, Cole FM, Basford D, Krestynski F. Iron deficiency prevents liver toxicity of 2,3,7,8-tetrachlorodibenzo-p- dioxin. Science. 1979;204:332–335. doi: 10.1126/science.432648. [DOI] [PubMed] [Google Scholar]
- Takasuka N, Naito A, Fukamachi K, Murakoshi M, Nishino H, Tsuda H. Modifying effects of carotenoids in a rat multi-organ carcinogenesis model - Inhibition in the liver but promotion of lung tumor development. Proc Jpn Acad B. 2002;78:33–37. [Google Scholar]
- Tamura K, Nakae D, Horiguchi K, Akai H, Kobayashi Y, Satoh H, Tsujiuchi T, Denda A, Konishi Y. Inhibition by green tea extract of diethylnitrosamine-initiated but not choline-deficient, L-amino acid-defined diet-associated development of putative preneoplastic, glutathione S-transferase placental form-positive lesions in rat liver. Jpn J Cancer Res. 1997;88:356–362. doi: 10.1111/j.1349-7006.1997.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Iwata H, Niwa K, Mori Y, Mori H. Inhibitory effect of ellagic acid on N-2-fluorenylacetamide-induced liver carcinogenesis in male ACI/N rats. Jpn J Cancer Res. 1988;79:1297–1303. doi: 10.1111/j.1349-7006.1988.tb01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. Aaps J. 2006;8:E443–449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Regulation of cell proliferation, apoptosis, and transcription factor activities during the promotion of liver carcinogenesis by polychlorinated biphenyls. Toxicol Appl Pharmacol. 2002;179:172–184. doi: 10.1006/taap.2001.9360. [DOI] [PubMed] [Google Scholar]
- Thomas SR, Leichtweis SB, Pettersson K, Croft KD, Mori TA, Brown AJ, Stocker R. Dietary cosupplementation with vitamin E and coenzyme Q(10) inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:585–593. doi: 10.1161/01.atv.21.4.585. [DOI] [PubMed] [Google Scholar]
- Toma S, Losardo PL, Vincent M, Palumbo R. Effectiveness of beta-carotene in cancer chemoprevention. Eur J Cancer Prev. 1995;4:213–224. doi: 10.1097/00008469-199506000-00002. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Iwahori Y, Asamoto M, Baba-Toriyama H, Hori T, Kim DJ, Uehara N, Iigo M, Takasuka N, Murakoshi M, Nishino H, Kakizoe T, Araki E, Yazawa K. Demonstration of organotropic effects of chemopreventive agents in multiorgan carcinogenesis models. IARC Sci Publ. 1996;139:143–150. [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Umemura T, Kai S, Hasegawa R, Kanki K, Kitamura Y, Nishikawa A, Hirose M. Prevention of dual promoting effects of pentachlorophenol, an environmental pollutant, on diethylnitrosamine-induced hepato- and cholangiocarcinogenesis in mice by green tea infusion. Carcinogenesis. 2003;24:1105–1109. doi: 10.1093/carcin/bgg053. [DOI] [PubMed] [Google Scholar]
- van Birgelen AP, Ross DG, DeVito MJ, Birnbaum LS. Interactive effects between 2,3,7,8-tetrachlorodibenzo-p-dioxin and 2,2′,4,4′,5,5′-hexachlorobiphenyl in female B6C3F1 mice: tissue distribution and tissue-specific enzyme induction. Fundam Appl Toxicol. 1996;34:118–131. doi: 10.1006/faat.1996.0182. [DOI] [PubMed] [Google Scholar]
- vandenBerg R, Haenen GRMM, vandenBerg H, Bast A. Transcription factor NF-kappa B as a potential biomarker for oxidative stress. Brit J Nutr. 2001;86:S121–S127. doi: 10.1079/bjn2001340. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kitade Y, Masaki T, Nishioka M, Satoh K, Nishino H. Effects of lycopene and Sho-saiko-to on hepatocarcinogenesis in a rat model of spontaneous liver cancer. Nutrition and Cancer. 2001;39:96–101. doi: 10.1207/S15327914nc391_13. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Suffia M, Pinkerton KE. Expression of cyclin D1/2 in the lungs of strain A/J mice fed chemopreventive agents. Carcinogenesis. 2002;23:289–294. doi: 10.1093/carcin/23.2.289. [DOI] [PubMed] [Google Scholar]
- Wood AW, Huang MT, Chang RL, Newmark HL, Lehr RE, Yagi H, Sayer JM, Jerina DM, Conney AH. Inhibition of the mutagenicity of bay-region diol epoxides of polycyclic aromatic hydrocarbons by naturally occurring plant phenols: exceptional activity of ellagic acid. Proc Natl Acad Sci U S A. 1982;79:5513–5517. doi: 10.1073/pnas.79.18.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL, Pan CE. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048–3052. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Dragan YP, Campbell HA, Pitot HC. STEREO: A program on a PC-Windows 95 platform for recording and evaluating quantitative stereologic investigations of multistage hepatocarcinogenesis. Computer Methods and Programs in Biomedicine. 1998;56:49–63. doi: 10.1016/s0169-2607(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Fukuda N, Shiroi S, Shiotsuki Y, Nagata Y, Tani T, Sakai T. Ameliorative effect of dietary probucol on polychlorinated biphenyls- induced hypercholesterolemia and lipid peroxidation in the rat. Life Sci. 1994;54:1019–1026. doi: 10.1016/0024-3205(94)00504-4. [DOI] [PubMed] [Google Scholar]
- Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]