Abstract
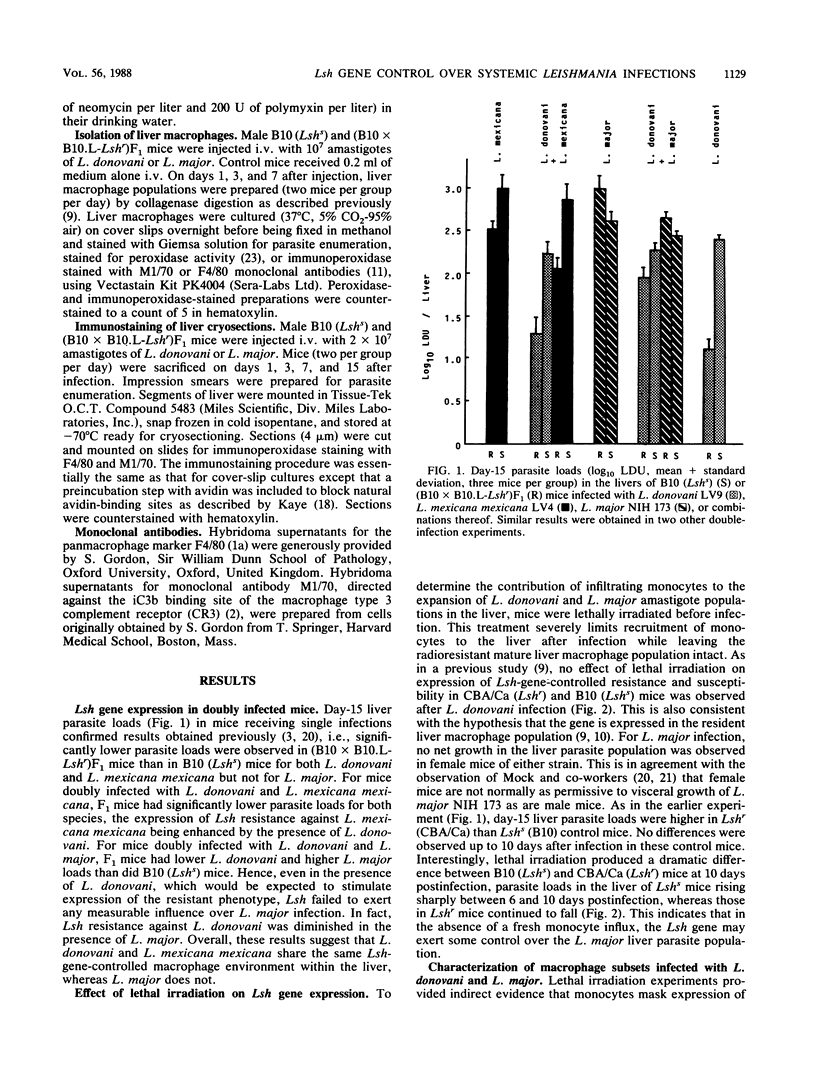
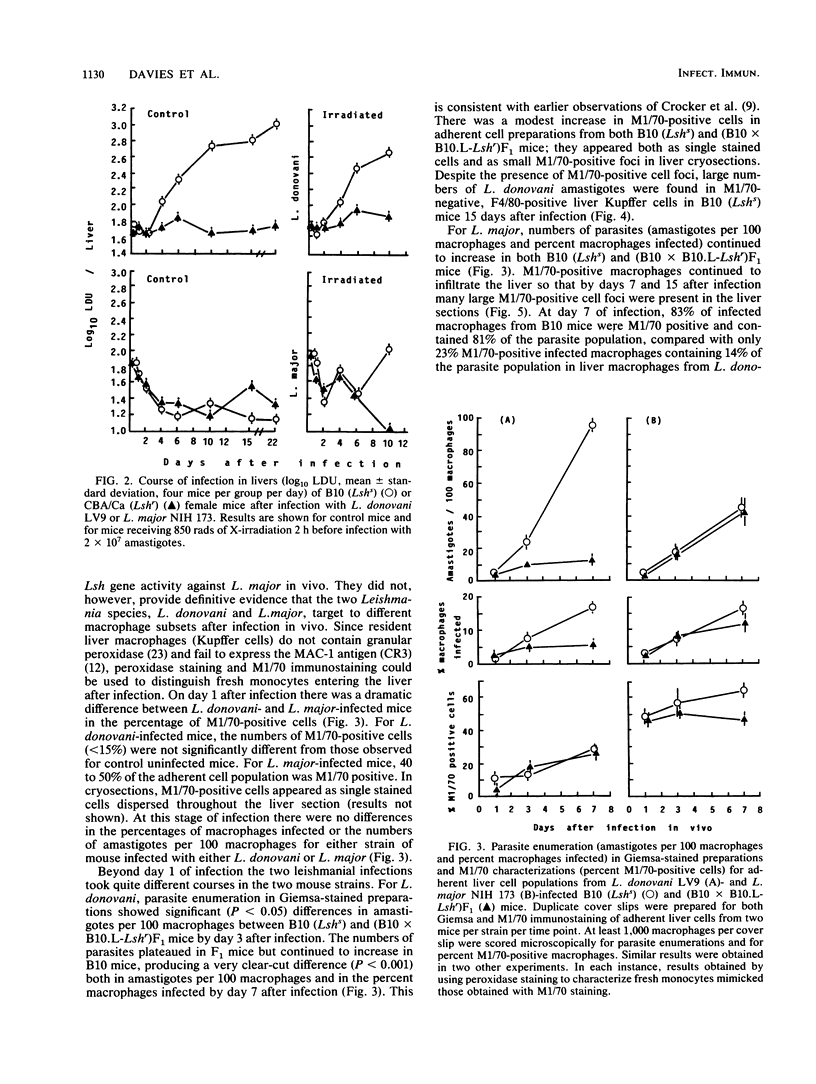
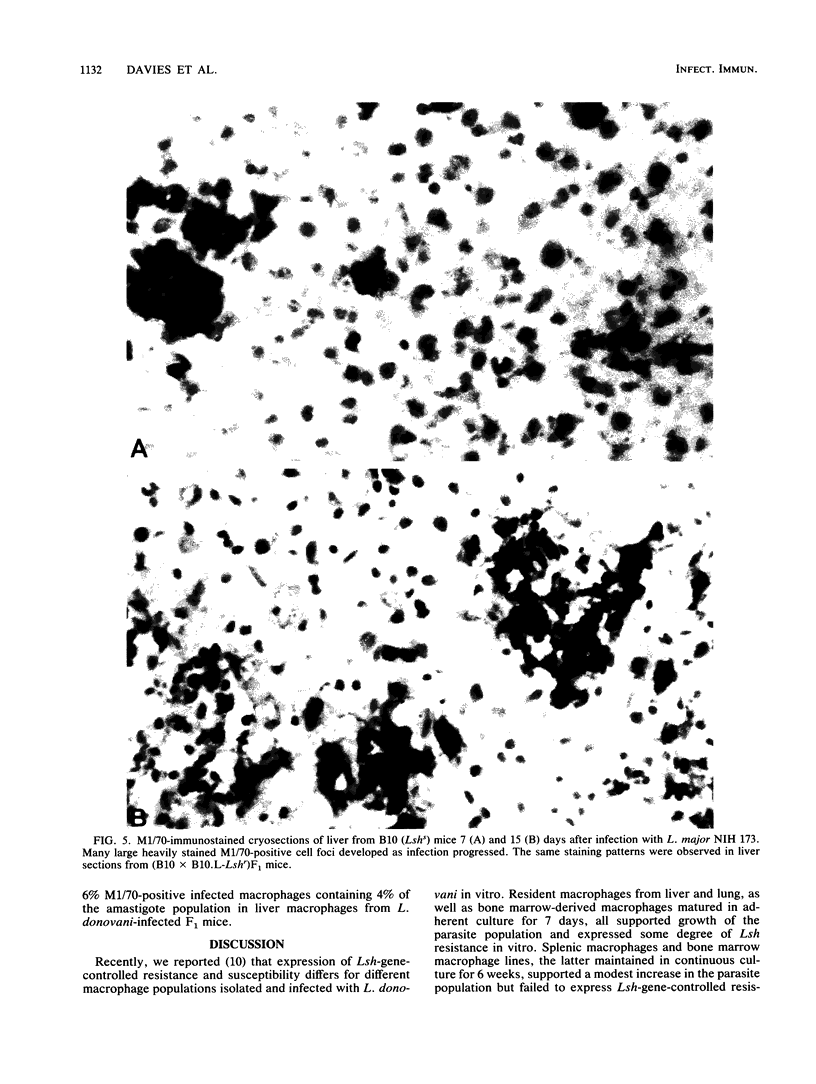
Earlier studies had shown that the viscerotropic NIH 173 strain of cutaneous Leishmania major fails to come under Lsh gene control. Visceral Leishmania donovani LV9 and another viscerotropic cutaneous strain, Leishmania mexicana mexicana LV4, are controlled by Lsh. The results of double-infection experiments presented here show that expression of Lsh resistance against L. mexicana mexicana was enhanced in the presence of L. donovani, whereas L. major still failed to come under Lsh gene control, even in the presence of L. donovani. Prior irradiation (850 rads) of mice showed that in the absence of infiltrating monocytes, Lsh did exert some influence over L. major. The presence of a higher infiltrate of fresh monocytes after L. major infection was confirmed in liver macrophage populations isolated from mice after infection in vivo and in liver cryosections immunostained with monoclonal antibody M1/70 directed against the type 3 complement receptor CR3. The results support the hypothesis that Lsh is expressed maximally in the resident tissue macrophages and poorly in the immature macrophages preferentially infected by L. major amastigotes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Glynn A. A., Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982 Sep;47(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Davies E. V., Blackwell J. M. Variable expression of the murine natural resistance gene Lsh in different macrophage populations infected in vitro with Leishmania donovani. Parasite Immunol. 1987 Nov;9(6):705–719. doi: 10.1111/j.1365-3024.1987.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med. 1985 Sep 1;162(3):993–1014. doi: 10.1084/jem.162.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. J., Springer T. A., Thorbecke G. J. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983 Apr;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Hoover D. L., Nacy C. A. Intracellular replication of Leishmania tropica in mouse peritoneal macrophages: amastigote infection of resident cells and inflammatory exudate macrophages. Infect Immun. 1982 Dec;38(3):1304–1308. doi: 10.1128/iai.38.3.1304-1308.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nakamura R. M., Takahashi H., Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984 Oct;46(1):135–140. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Berger M., Nacy C. A., Hockmeyer W. T., Meltzer M. S. Killing of Leishmania tropica amastigotes by factors in normal human serum. J Immunol. 1984 Feb;132(2):893–897. [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Kaye P. M. Inflammatory cells in murine visceral leishmaniasis express a dendritic cell marker. Clin Exp Immunol. 1987 Dec;70(3):515–519. [PMC free article] [PubMed] [Google Scholar]
- Mirkovich A. M., Galelli A., Allison A. C., Modabber F. Z. Increased myelopoiesis during Leishmania major infection in mice: generation of 'safe targets', a possible way to evade the effector immune mechanism. Clin Exp Immunol. 1986 Apr;64(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Mock B. A., Fortier A. H., Potter M., Blackwell J., Nacy C. A. Genetic control of systemic Leishmania major infection: identification of subline differences for susceptibility to disease. Curr Top Microbiol Immunol. 1985;122:115–121. doi: 10.1007/978-3-642-70740-7_17. [DOI] [PubMed] [Google Scholar]
- Mock B. A., Fortier A. H., Potter M., Nacy C. A. Genetic control of systemic Leishmania major infections: dissociation of intrahepatic amastigote replication from control by the Lsh gene. Infect Immun. 1985 Nov;50(2):588–591. doi: 10.1128/iai.50.2.588-591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Pappas M. G., Henry R. R. Susceptibility of inbred mice to Leishmania tropica infection: correlation of susceptibility with in vitro defective macrophage microbicidal activities. Cell Immunol. 1983 Apr 15;77(2):298–307. doi: 10.1016/0008-8749(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Page D. T., Garvey J. S. Isolation and characterization of hepatocytes and Kupffer cells. J Immunol Methods. 1979;27(2):159–173. doi: 10.1016/0022-1759(79)90262-x. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Patel P. J., Nesbitt M. N. Regulation of resistance to leprosy by chromosome 1 locus in the mouse. Immunogenetics. 1984;19(2):117–124. doi: 10.1007/BF00387854. [DOI] [PubMed] [Google Scholar]