Abstract
Equilin and equilenin, components of the hormone replacement therapy drug Premarin, can be metabolized to the catechol 4-hydroxyequilenin (4-OHEN). The quinoids produced by 4-OHEN oxidation react with dC, dA and dG to form unusual stable cyclic adducts, which have been found in human breast tumor tissue. Four stereoisomeric adducts have been identified for each base. These twelve Premarin-derived adducts provide a unique opportunity for analyzing effects of stereochemistry and base damage on DNA structure, and consequently its function. Our computational studies have shown that these adducts, with obstructed Watson-Crick hydrogen bond edges and near-perpendicular ring systems, have limited conformational flexibility, and near-mirror image conformations in stereoisomer pairs. The dC and dA adducts can adopt major and minor groove positions in the double helix, but the dG adducts are positioned only in the major groove. In all cases, opposite orientations of the equilenin rings with respect to the 5'→3' direction of the damaged strand are found in stereoisomer pairs derived from the same base, and no Watson-Crick pairing is possible. However, detailed structural properties in DNA duplexes are distinct for each stereoisomer of each damaged base. These differences may underlie observed differential stereoisomer and base-dependent mutagenicities and repair susceptibilities of these adducts.
The hormone replacement drug Premarin, whose use has been shown to increase breast cancer risk (1-6), contains the equine estrogens equilin and equilenin (Figure 1). This pair of substances can be metabolized to highly reactive 4-OHEN quinoids that can form unusual stable cyclic adducts with dC, dA and dG (7-9). There are four different stereoisomers for each base adduct (9-11), for a total of twelve distinct lesions (Figure 1). The structural properties of this set of twelve DNA adducts present a fascinating opportunity for elucidating how the stereochemical features and the nature of the damaged base differentially distort and destabilize the local structure of double-stranded DNA molecules around the lesion sites. Such differences in distortion/destablization could significantly affect the susceptibilities of the stereoisomeric 4-OHEN-DNA lesions to nucleotide excision repair (NER) in cellular environments. Furthermore, the absolute configurations and the nature of the base in these adducts can affect translesion bypass catalyzed by DNA polymerases and mutagenic specificity if bypass is successful, as well as transcription catalyzed by RNA polymerases. The biological impact of 4-OHEN-DNA adducts is of special interest because three stereoisomeric dG and two dA adducts have been found in the mammary fat pads of rats upon 4-OHEN injection (12), and dG, dA and dC adducts have been detected in human breast tissue of patients who were exposed to equilin and equilenin via hormone replacement therapy (13). Our detailed analysis of the structural features of the stereoisomeric 4-OHEN-DNA adducts reported here is important because differences in miscoding properties among the various adducts have been noted in in vitro systems (14-18) and, recently, differences in excision susceptibilities catalyzed by human and prokaryotic NER systems have been observed (17, 19, 20).
Figure 1.
(a) Chemical structures of equilin, equilenin and 4-OHEN. (b) Chemical structures and stereochemical characteristics of the 4-OHEN-G, -A and -C adducts. (c) Sequence of the 11mer B-DNA duplex for the MD simulations. The asterisk denotes the damaged position.
Estrogens are metabolized by cytochrome P450 to 2- and 4-hydroxylated catechols (21, 22). The 4-hydroxylated estrogens are likely to be more carcinogenic than the 2-hydroxylated ones. In the hamster kidney system 4-hydroxyestradiol was carcinogenic, while 2-hydroxyestradiol did not induce tumors (23). 4-hydroxyestradiol also showed much higher carcinogenic activity than 2-hydroxyestradiol in CD-1 mice (24). Furthermore, the levels of 4-hydroxyestrone/estrodiol and derived conjugates were significantly higher in breast tissue from women with breast cancer than in controls (25). Recent studies have suggested that high levels of estrogen 4-hydroxylase expression are linked to an increased risk of developing breast cancer (26-28). Potential mechanisms of estrogen quinone carcinogenesis, focusing on DNA and protein damaging pathways, have been recently reviewed (8). Increased B ring unsaturation in equilin and equilenin (Figure 1a) significantly increases the deleterious 4-hydroxylation metabolic pathway. In human breast cancer cells (MCF-7), 4-hydroxyequilenin (4-OHEN) is the major phase I metabolite of both equilin and equilenin (22, 29). 4-OHEN autooxidizes to the highly reactive and potent cytotoxic quinoids (22, 30, 31) that can cause a variety of DNA lesions in vitro and in vivo (12, 32-36), including the stable dC, dG and dA adducts (9, 10). The quinoid of 4-hydroxyequilin (4-OHEQ) derived from the metabolic activation of equilin oxidizes to the 4-OHEN o-quinone and forms the same DNA adducts as 4-OHEN (30). 4-OHEQ has been shown to be mutagenic in a supF shuttle vector plasmid system propagated in human cells, presumably due to adduct formation (37).
The chemical structures of the four stereoisomeric 4-OHEN adducts to dG, dA and dC have been determined (Figure 1) (9-11). The analogous dT adducts derived from 4-OHEN were not investigated here because none have been found experimentally at this writing (10). The Watson Crick hydrogen bonding edge of the base is obstructed by the formation of the cyclic adduct in all cases. While there are actually three chiral centers within the region connecting the nucleobase-4-OHEN ring system, the two covalent bonds formed between 4-OHEN and the base adopt only a cis configuration since the connection ring would be highly strained in a trans configuration (11). Thus, only four different stereoisomeric adducts are observed for each of the bases C, A, and G (9, 10). The chiralities at C1' and C3' determine the stereochemistry of the 4-OHEN-G adducts, while the absolute configurations of substituents at C2' and C3' determine the stereochemical features of the 4-OHEN-C and A adducts.
We have previously investigated the twelve stereoisomeric adducts on the base level by QM calculations (38), and the dC and dA adducts in DNA duplexes with MD simulations (39, 40). Here, we present new results for the 4-OHEN dG adducts in duplex DNA, and provide an integrated view with comparisons of our conformational analyses for the twelve unique 4-OHEN-G, -C, and -A adducts at the base level and in double-stranded DNA. We highlight both the common features as well as the structural distinctions of all twelve stereoisomeric adducts in the duplexes. As new biological data emerges, the specific structural hallmarks elucidated in our studies should provide a structural perspective for the functional biological differences that are beginning to emerge (14-20).
METHODS
Molecular modeling and MD simulations were carried out for the 4-OHEN-G adducts in DNA, as in previous studies of the 4-OHEN-C and A DNA adducts (39, 40). Briefly, we started with an energy minimized B-DNA structure whose sequence is given in Figure 1 and replaced the guanine with the 4-OHEN-G. All the conformations of each 4-OHENG stereoisomer on the base level (38) were modeled into the DNA duplexes. A search was then made with glycosidic torsion χ rotated continuously over its 360 degree range to locate structures with minimal close contacts. Structures with both syn and anti glycosidic torsions between base and sugar were built as initial models for the MD simulations (Table S1 and Figure S1 in the Supporting information). Figure S1 shows syn and anti glycosidic torsion conformations. The MD protocol (41-52) is given in the Supporting Information. Force field parameters added for the 4-OHEN-G adducts in DNA are given in Tables S2 and S3. The PTRAJ and CARNAL modules of the AMBER 7 package (41) were employed for structural analyses. Stacking interactions were estimated by computing the van der Waals interaction energies between adjacent base pairs, including the damaged base and partner pair, with the program ANAL of AMBER. DNA duplex groove dimensions and bend angles were computed with the MD Toolchest (53, 54) and CURVES (53) programs, respectively. The solvent accessible surface area was computed using the Connolly algorithm (55) implemented in Insight II (Accelrys Inc.) with a probe radius of 1.4 Å. The molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) method (56-59) in AMBER was employed for thermodynamic analyses. Full details of the structural and thermodynamic analysis methods are provided in (39, 40).
RESULTS and DISCUSSION
Conformations of 4-OHEN base adducts
The 4-OHEN adducts have unusual cyclic linkage sites, which limit their flexibilities even on the base level. The conformations of the 4-OHEN base adducts were computed using the quantum mechanical Density Functional Theory (DFT) method (B3LYP/6-31G*) (60-62), as described fully in (38).
Connection ring conformational families
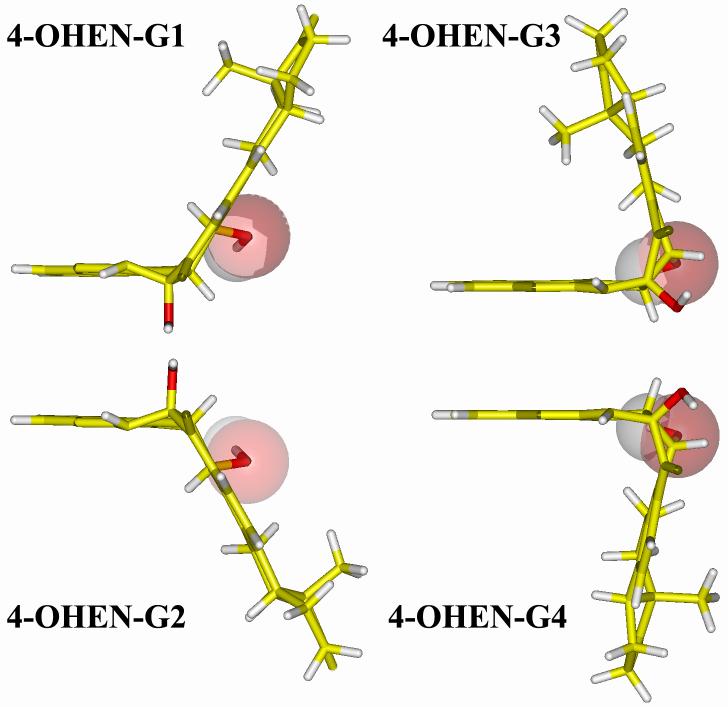
The 4-OHEN-C and 4-OHEN-A adducts have the same unsaturated bicyclo[3.3.1]nonane type linkage site, which makes the conformation highly rigid. This stems from the combined effects of the inflexible C1'-C2'-C3' bridge, the adenine or cytosine conjugated ring system and the unsaturated cyclohexene A ring. Therefore, each stereoisomer is restricted to a single conformational family (Figure 2), in which the dihedral angle θ' (C1'-N1-C6-N6) for adenine or (C1'-N1-C4-N4) for cytosine (Figure 1) is around 0°.
Figure 2.
Optimized conformations of 4-OHEN-C and 4-OHEN-A base adducts. The color code is as follows: 4-OHEN-C, blue; 4-OHEN-A, cyan; hydrogens are white and the C2' OH group is red.
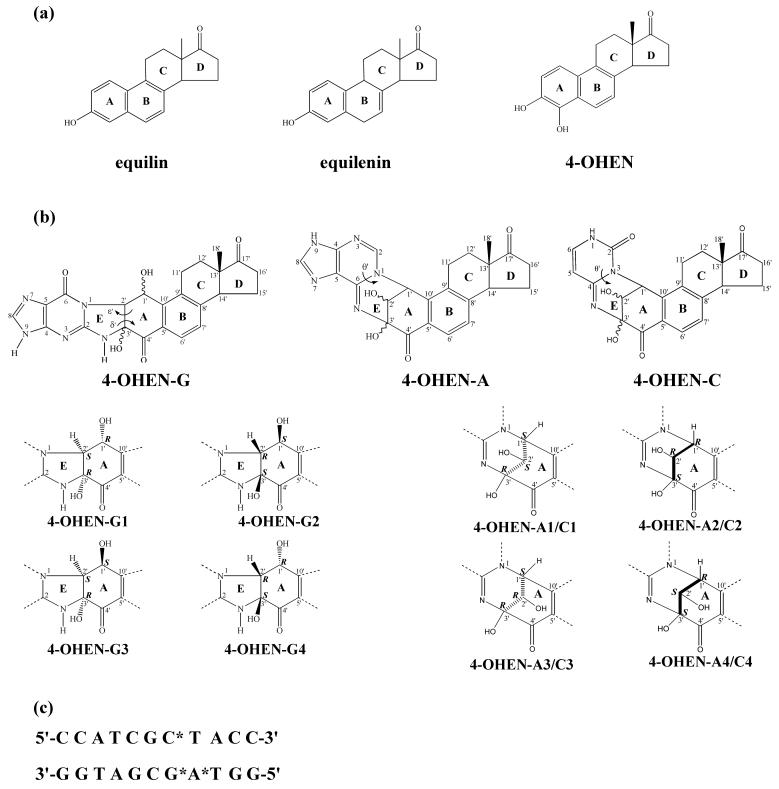
On the other hand, the reaction of 4-OHEN with guanine forms a relatively more flexible five membered connection ring. 4-OHEN-G adducts each have three base-level conformational families (except for 4-OHEN-G4 which has only two), defined by the conformations of the linking 5-membered E ring: negative envelope, positive envelope and planar conformational families. The dihedral angles δ' (N1-C2'-C3'-N2) and ε' (C1'-C2'-C3'-C4') (Figure 1) describe the conformations of the linking 5-membered E ring and the cyclohexene A ring, respectively. See Figure 2 of reference 38 for description of E and A ring conformations at the linkage site. In the negative envelope conformation, the E ring adopts an envelope conformation with negative δ' and ε' values. In the positive envelope, the E ring exhibits an envelope conformation with positive δ' and ε' values. In addition to the two envelope conformations, this 5-membered ring can also adopt a planar form with the cyclohexene A ring adopting the boat conformation. For each 4-OHEN-G stereoisomer, the energy differences between the different connection conformational families are not great (< 4 kcal/mol), suggesting each conformation might be feasible in duplex DNA. Full conformational details for the 4-OHEN-G base adduct stereoisomers are given in reference 38. Figure 3 shows the lowest energy conformations. Others are given in Figures 3-6 of reference 38.
Figure 3.
Lowest energy optimized conformations of 4-OHEN-G base adducts. The color code is as follows: 4-OHEN-G, yellow; hydrogens are white and the C1' OH group is red.
Structural features: stereochemistry and linkage
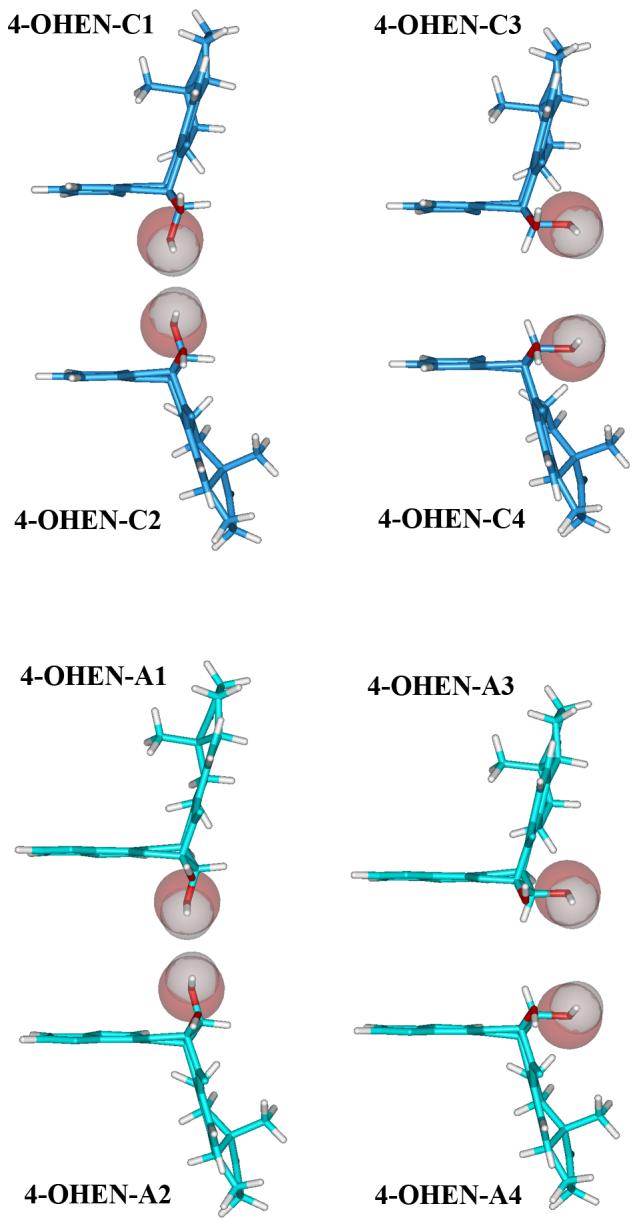
The chemical structures of the stereoisomeric adducts show that they are paired, with inverse R and S configurations at the linkage site. The four stereoisomers of each base adduct form two pairs. The chirality of the C3' atom determines the handedness of the equilenin ring systems. In each stereisomeric pair, the equilenin rings adopt opposite orientations with respect to the attached base. Therefore, each pair adopts near mirror image conformations (Figures 2 and 3), with the symmetry broken only by the D ring with it C18' methyl group on the equilenin moiety. This is consistent with the near-mirror image CD spectra of each stereoisomeric pair that has been observed experimentally (63). The two stereoisomeric pairs of each base adduct differ in the orientations of one hydroxyl group at the linkage site. For the 4-OHEN-C and A adducts, the hydroxyl group at C2' is situated close to the base in the 4-OHEN-C1/C2 and A1/A2 stereoisomer pairs, while in the C3/C4 and A3/A4 pairs, it is directed away from the base and toward the equilenin moiety. In the 4-OHEN-G adducts, the OH group at C1' points outward from the ring system in the 4-OHEN-G1/G2 pair and is directed between the base and equilenin rings in the G3/G4 pair.
The essential structural feature of these 4-OHEN base adducts is that the equilenin rings are near-perpendicular to the covalently linked base, creating very bulky and conformationally restricted structures (Figures 2 and 3). Another important and characteristic feature of these base adducts is the orientations of the stereoisomeric adducts: stereoisomer pairs, with reverse chiralities at C1', C2' and C3' atoms, have opposite orientations of the equilenin rings with respect to the attached base.
4-OHEN adducts in DNA duplexes
In order to investigate the structural effects of the 4-OHEN adducts in DNA duplexes, molecular modeling and MD simulations were carried out. For each stereoisomeric adduct, we modeled each base conformer into the 11mer B-DNA duplex in the sequence of Figure 1, which had been under experimental investigation (63). Both syn and anti glycosidic bond conformations were studied (see Figure S1). In all cases an unmodified control was also investigated.
Connection ring conformations
On the base level, as mentioned above, there is only one conformational family of the connection ring for each of the stereoisomeric 4-OHEN-C and A base adducts. During the MD simulations, these adducts retain the same conformational family in the duplexes, due to their rigidity.
For each guanine stereoisomer, there are two or three different conformational families for the connection ring, as mentioned above and detailed in reference 38. These all served as initial conformations for the MD simulations of the duplexes (see Methods). In these duplex simulations, the initial conformations of the connection ring converged to one connection ring conformational domain in the case of each stereoisomer. This is shown in the time-dependence of the linkage site torsion angles (Figure S3). The domain is the same for both syn and anti glycosidic torsions and is in one of the base level domains, with just slightly altered pucker of the E and A rings at the linkage site. However, the connection ring conformations in the duplexes vary with stereochemistry, so that the guanine-equilenin ring systems are more or less open in the different isomers (Figure 4). We computed free energies for each case using the MM-PBSA method (56-59) employed previously for the C and A adducts in DNA. The lowest free energy conformation in the syn and anti domain for each stereoisomer was selected for detailed analysis (Table S4).
Figure 4.

Conformations of the 4-OHEN-G adducts in the trajectory-average structures for 4-OHEN-G modified duplexes.
Stereochemistry governed structural features
Stereochemistry plays a key role in determining the specific structural properties of each base adduct. The 4-OHEN-C and A adducts have the same connection ring, which is close to the major groove side of the C and A bases. Therefore these adducts have similar stereochemistry-determined conformations. However, the 4-OHEN-G adducts’ connection ring is different: the linkage site of the modified guanine is close to the minor groove side of the guanine (Figure 1), resulting in distinct stereochemistry-governed structural properties for this base adduct.
The stereochemistry of the equilenin C3' and the glycosidic bond conformation determine the orientation of the bulky equilenin ring system. In the syn conformation, the equilenin rings are 3' directed in 4-OHEN-C1/A1 and C3/A3 with R stereochemistry at the C3' position, and 5' directed in 4-OHEN-C2/A2 and C4/A4 with C3' S (Figure 5). However, the equilenin rings are oriented toward the 5'-side of the modified strand in 4-OHEN-G1 and G3 with C3' R, and 3'-side in 4-OHEN-G2 and G4 with C3' S. The orientations of the equilenin rings in the anti glycosidic bond conformation adducts (Figure 6) are opposite to those of the syn structures. The stereochemistry also governs the orientations of the methyl and OH groups at the linkage site. These stereochemical characteristics of the 4-OHEN adducts are summarized in Table 1.
Figure 5.
Five base pair segments with modified bases at center in the trajectory-average structures for the 4-OHEN modified duplexes in syn conformation. 4-OHEN adducts are in CPK. The color code is as follows: 4-OHEN-C, blue; 4-OHEN-A, cyan; 4-OHEN-G, yellow; hydrogens are white, the methyl group is orange and the OH group (at C1' in 4-OHEN-G or C2' in 4-OHEN-C and A) is red.
Figure 6.
Five base pair segments with modified bases at center in the trajectory-average structures for the 4-OHEN modified duplexes in anti conformation. In 4-OHEN-A1 and — A3 6 pb segments at the 5'-end of the modified strand are shown. 4-OHEN adducts are in CPK. The color code is the same as in Figure 5.
Table 1.
Stereochemistry-Determined Structural Properties of 4-OHEN Adducts
| Stereochemistry |
Syn Conformation |
Anti Conformation |
|||||
|---|---|---|---|---|---|---|---|
| Equilenin rings orientationa | C18' methyl groupb | OH groupb,c,d | Equilenin rings orientationa | C18' methyl groupb | OH groupb,c,d | ||
| 4-OHEN-A/C1 | 2'S-3'R | 3' | M | A | 5' | M | A |
| 4-OHEN-A/C2 | 2'R-3'S | 5' | S | A | 3' | P | A |
| 4-OHEN-A/C3 | 2'R-3'R | 3' | M | E | 5' | M | E |
| 4-OHEN-A/C4 |
2'S-3'S |
5' |
S |
E |
3' |
P |
E |
| 4-OHEN-G1 | 1'R-3'R | 5' | M | S | 3' | S | P |
| 4-OHEN-G2 | 1'S-3'S | 3' | S | S | 5' | S | P |
| 4-OHEN-G3 | 1'S-3'R | 5' | M | M | 3' | S | M |
| 4-OHEN-G4 | 1'R-3'S | 3' | S | M | 5' | S | M |
with respect to the modified strand
S, toward solvent; M, toward modified strand; P, toward partner strand
OH at C2' in 4-OHEN-C and A; OH at C1' in 4-OHEN-G
A, near axial; E, near equatorial
Syn and anti conformations: structures and energetics
In the syn conformation, in all cases the equilenin rings are situated externally in the DNA major groove without much duplex distortion (Table 2): Watson-Crick hydrogen bonding of the adjacent base pairs is retained and less disturbed; base pair stacking is mainly retained; and groove dimensions and bending are comparable to the unmodified control. However, the equilenin rings are mostly exposed to solvent (Figure 7 and Table S5). On the other hand, less solvent exposure and larger duplex distortions are observed in the anti case. Table 2 and Figure 7 show these differences between the syn and anti conformations. Experimental thermal melting data in the same sequence contexts we investigated here indicate that these adducts are quite destabilizing as compared to unmodified DNA (63), which is consistent with our structural analyses. The structural perturbations summarized in Table 2 can account for the destabilizations of the 4-OHEN-modified duplexes.
Table 2.
Comparison of Structural Features of 4-OHEN-C, Aa and G Adducts with the Unmodified Duplex
| Adjacent Base Pairb | Number of Adduct Hydrogen Bondsc | Stacking Energy Differenced(kcal/mol) | Bend Angle Differencee (degrees) | Major Groove Distortionf (Å) | Minor Groove Distortionf (Å) | |
|---|---|---|---|---|---|---|
| Color code: most distorted, red; somewhat distorted, orange; least distorted, cyan. | ||||||
| syn | ||||||
| 4-OHEN-C1 | intact | 0 | 5.5 | 11.9 | 3.1 | 0.7 |
| 4-OHEN-A1 | intact | 0 | 5.9 | 14.3 | 1.8 | -1.3 |
| 4-OHEN-G1 | intact | 2 | 3.2 | 4.2 | -5.7 | 1.6 |
| 4-OHEN-C2 | intact | 1 | 4.6 | 11.0 | 3.1 | 2.0 |
| 4-OHEN-A2 | intact | 2 | 4.7 | 8.7 | 2.3 | 2.1 |
| 4-OHEN-G2 | distorted | 1 | 5.4 | 0.7 | 3.0 | 2.4 |
| 4-OHEN-C3 | intact | 0 | 5.3 | -0.7 | -2.2 | -2.3 |
| 4-OHEN-A3 | intact | 1 | 6.6 | 15.6 | 1.0 | 1.8 |
| 4-OHEN-G3 | distorted | 3 | 5.1 | -2.5 | 2.8 | 3.6 |
| 4-OHEN-C4 | distorted | 2 | 9.2 | 0.1 | 5.3 | -6.0 |
| 4-OHEN-A4 | intact | 2 | 3.1 | 7.5 | 2.9 | 2.7 |
| 4-OHEN-G4 |
distorted |
2 |
3.4 |
4.3 |
4.0 |
2.1 |
| anti | ||||||
| 4-OHEN-C1 | distorted | 0 | 22.2 | 4.1 | 5.2 | 8.8 |
| 4-OHEN-A1 | broken | 2 | _g | 26.3 | 1.4 | 6.2 |
| 4-OHEN-G1 | broken | 3 | 12.8 | 13.5 | 10.7 | 2.2 |
| 4-OHEN-C2 | broken | 1 | 19.7 | 15.8 | 3.7 | -2.6 |
| 4-OHEN-A2 | broken | 2 | 22.1 | 38.9 | 4.1 | 9.5 |
| 4-OHEN-G2 | broken | 4 | 11.3 | 7.0 | 4.7 | 3.5 |
| 4-OHEN-C3 | broken | 3 | 22.6 | 38.4 | 2.2 | 8.2 |
| 4-OHEN-A3 | broken | 1 | _g | 32.9 | 4.7 | 6.1 |
| 4-OHEN-G3 | distorted | 3 | 12.6 | 26.4 | 7.1 | 2.7 |
| 4-OHEN-C4 | broken | 2 | 15.9 | 40.1 | 2.8 | 1.6 |
| 4-OHEN-A4 | broken | 0 | 20.9 | 39.8 | 13.4 | -1.9 |
| 4-OHEN-G4 | broken | 4 | 20.8 | 28.5 | 5.4 | 3.2 |
Watson-Crick hydrogen bonds: intact, occupancy of all > 90%; distorted, occupancy of any one 50%-90%; broken, occupancy of all 0%. Distorted or broken hydrogen bonds are at the base pair adjacent to the lesion in the direction of the equilenin ring orientation in 20 of 24 cases, excepting syn 4-OHEN-G2, G4 and C4, and anti C1; for these the disturbance is at the base pair adjacent to the lesion in the direction opposite to the equilenin ring orientation due to specific hydrogen bonding and stacking interactions (39). The unmodified duplex has all base pairs intact.
Number of hydrogen bonds involving 4-OHEN base adduct with occupancy > 50% in stable time frame of trajectory (see Methods and Table S6).
Difference between stacking interaction energy of modified duplex and its unmodified counterpart (Table S7). Larger energies show more perturbed stacking.
Difference between the trajectory average bend angle of the adduct and the unmodified duplex (Table S8).
Using the unmodified duplex as reference, we calculated the groove distortions of the modified duplexes as (d-d0), where d is a groove dimension of the modified duplex, and d0 is the corresponding value for the unmodified duplex (Figure S4). The groove dimension difference with the largest absolute value is shown. A negative sign indicates groove closing compared to the unmodified control.
Stacking cannot be calculated due to the dangling end.
Figure 7.
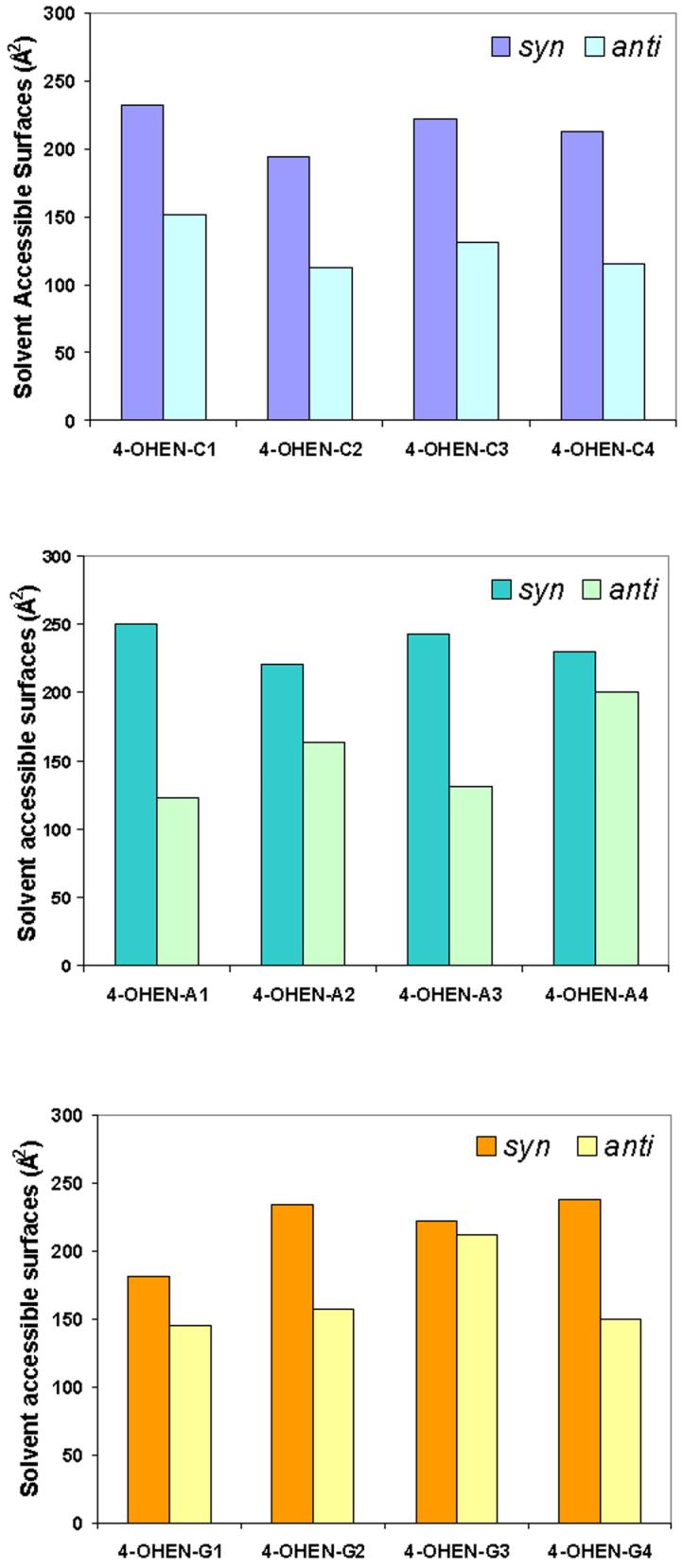
Trajectory-average solvent accessible surfaces of the equilenin rings in 4-OHEN Adducts modified 11mer duplexes.
The 4-OHEN-C and A adducts in DNA occupy similar syn/major groove and anti/minor groove positions (Figures 5 and 6). However, there are subtle differences that are governed by the nature of the damaged base. Compared to the C adducts, the equilenin rings of the A adducts are positioned further away from the DNA in the major groove due to the larger purine ring.(40) Thus, the equilenin rings are more solvent exposed and distort the major grooves less. Generally the larger 4-OHEN-A adducts disturb the stacking more and the DNA duplexes bend more toward the major groove. In the anti conformation, the equilenin rings insert well into the smaller minor groove, providing favorable hydrophobic interactions for both A and C adducts. However, the 4-OHEN-A adducts often cause the nearby bases to tilt in order to accommodate their larger bulk (Figure 6), causing weaker stacking interactions and larger groove distortions. In the anti 4-OHEN-A1 and -A3 cases, a dangling end is observed because the 5'-directed adducts are positioned only three base pairs away from the terminus in the current sequence context (Figure 6).
The structural features of the 4-OHEN-G adducts in DNA are distinct from those of the C and A adducts, since the connection ring differs and the linkage site is closer to the minor groove side of the base (Figure 1). The specific orientations of the 4-OHEN-G adducts are opposite to the 4-OHEN-A and C adducts with the same stereochemistry at C3', (Table 1). Also, the equilenin rings are in the major groove in both syn and anti conformations; this arises because the equilenin distal rings are oriented toward the guanine major groove edge while being oriented toward the minor groove edge in C and A adducts (Figure 1). The syn guanine adducts mainly disturb the 5' side base pair of the modified strand, and the disturbance is greater than for the cytosine and adenine adducts, because the purine ring of the 5' side adenine stacks with the syn guanine. The details of the hydrogen bonding, van der Waals interactions and bending angles in 4-OHEN-G modified duplexes are shown in Tables S6-8. In the anti 4-OHEN-G adducts, the equilenin rings protrude into and widen the major groove and there are no favorable and compensating hydrophobic interactions (Figure 6). The two hydroxyl groups and the carbonyl group at the linkage site of the guanine adducts all participate in hydrogen bonding interactions with the partner C and nearby bases (Table S6).
Thermodynamic analyses of the simulated structures, using the MM-PBSA method (56-59), were carried out. Relative free energies of syn and anti conformations were calculated to compare their conformational stabilities (Table 3). The MM-PBSA free energy components for 4-OHEN-G modified duplexes are given in Table S4. The syn conformers are mainly favored by less distortion; the anti structures are favored by diminished solvent exposure. Moreover, the anti/minor groove conformations of 4-OHEN-C and A adducts have enhanced favorable hydrophobic contacts. However, each stereoisomer of each base adduct perturbs the structure of the DNA duplex differently. Each adduct selects a different balance between the following competing features: distortion, adduct solvent exposure, and favorable hydrophobic contacts. Generally, the 4-OHEN-A adducts mainly favor the anti/minor groove conformation due to less solvent exposure with attendant favorable hydrophobic interactions. The 4-OHEN-G adducts prefer the syn/major groove conformation due to lesser extents of distortion of the duplexes. This effect arises because the more distorting anti conformation, in contrast to 4-OHEN-C and A adducts, places the equilenin residue in the major groove where its ring systems cannot participate in the attendant favorable hydrophobic interactions. For the 4-OHEN-C case, in C2 and C4 the favorable hydrophobic contacts in anti dominate; in C1 and C3 the lack of distortion with favorable van der Waals interactions between the inward-facing equilenin methyl group and adjacent base in syn dominate (Figure 5).
Table 3.
| 4-OHEN-C1 | 4-OHEN-C2 | 4-OHEN-C3 | 4-OHEN-C4 | |
| syn | 0.0 | 3.9 | 0.0 | 5.0 |
|
anti |
1.5 |
0.0 |
6.8 |
0.0 |
| 4-OHEN-A1 | 4-OHEN-A2 | 4-OHEN-A3 | 4-OHEN-A4 | |
| syn | 3.6 | 0 | 5.4 | 0.6 |
|
anti |
0 |
6.3 |
0 |
0 |
| 4-OHEN-G1 | 4-OHEN-G2 | 4-OHEN-G3 | 4-OHEN-G4 | |
| syn | 0 | 0 | 0 | 0 |
|
anti |
1.0 |
7.0 |
6.2 |
5.0 |
For each stereoisomeric adduct, the conformation with the lower energy is assigned ΔG = 0. Energies are in kcal/mol.
The calculated free energy values and errors in the mean for each entry are given in Table S9.
A subtle interplay of adduct-induced distortion and adduct solvation and hydrophobic interactions determine the conformational preference of each stereoisomeric adduct. Hence, environmental conditions, such as salt concentration, base sequence context, duplex length and lesion position in the duplex (eg. central or close to an end), could readily influence the preferred glycosidic domain and attendant positioning of the equilenin moiety.
Stereoisomer and base effects in lesion processing
The twelve 4-OHEN adducts investigated share the obstructed Watson-Crick hydrogen bond edge and near-perpendicular ring systems. However, our modeling studies indicate structural properties in DNA duplexes that are distinct for each stereoisomeric adduct and its attached nucleobase. These structural differences can produce differences in biochemical function. In vitro primer extension studies conducted with several Y-family bypass polymerases indicate that various 4-OHEN-C and A DNA lesions are differentially bypassed, depending on stereochemistry and the base damaged (14-17, 19). Bypass efficiency in pol η is highly dependent on adduct stereochemistry. Specifically, the bypass frequency in this enzyme differed by two orders of magnitude in the members of a pair of 4-OHEN-dC stereoisomers whose CD spectra were opposite in sign (14). With 4-OHEN-dA adducts, the bypass frequency past one stereoisomer was approximately 3 times higher than for another (15). Using pol κ and the pair of 4-OHEN-dC adducts with opposite sign CD spectra, mismatched dCMP and dAMP were inserted opposite both stereoisomers; however, chain extension with dCMP was much higher than with dAMP (14). Insertion of dGMP, the correct base, was highly inefficient. For this 4-OHEN-dC pair with pol η, insertion of dAMP and subsequent extension were both higher than for the correct base dGMP. By contrast, for the pair of 4-OHEN-dA adducts with opposite sign CD spectra, both pols κ and η preferentially incorporated dTMP, the correct base, opposite these lesions; mismatched dAMP and dCMP were also incorporated by pol κ and η, respectively (15). Clearly the nature of the polymerase also governs the biological effects. Current studies also suggest damaged base-specific repair susceptibilities, with certain dA adducts less repaired than certain dC adducts in prokaryotic and eukaryotic nucleotide excision repair (NER) assays (17, 19, 20) (Kropachev, K., Chen, D., Kolbanovskiy, M. and Geacintov, N., to be published).
CONCLUSION
Conformations of the 4-OHEN base adducts have been determined using quantum mechanical methods, followed by structural and thermodynamic studies of these adducts in 11-mer DNA duplexes using molecular modeling and MD simulations. Adduct stereochemistry-governed unifying conformational features were delineated involving opposite orientations of stereoisomeric pairs of adducts; these characteristics are found in all modified bases. The absence of Watson-Crick base pairing due to the obstructed edge is also common to all adducts. However, each individual stereoisomer and base adduct has a unique impact on DNA structure which is manifested in an accumulating set of experimental biochemical data revealing different lesion-specific responses to DNA repair systems and polymerases. The emerging integrated structure-function knowledge base could potentially stimulate the design of improved hormone replacement therapy agents with lower reactivities of their metabolites with DNA and with lower mutagenic potentials, thus diminishing the genotoxic impact. Furthermore, an improved understanding of structure-function relationships may permit the biomonitoring of those Premarin-derived DNA lesions that are most genotoxic and mutagenic, and thus pose a greater risk for developing human cancers.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by NIH Grants CA-75449 and CA-28038 to S.B. and R.S., and NIH Grant CA-112412 to N.E.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Computations were carried out on our own cluster of Silicon Graphic Origin supercomputers and Octane workstations, as well as at the New York University Information Technology Services supercomputers.
Abbreviations
- 4-OHEN
4-hydroxyequilenin
- 4-OHEN-G
4-hydroxyequilenin-guanine
- 4-OHEN-A
4-hydroxyequilenin-adenine
- 4-OHEN-C
4-hydroxyequilenin-cytosine
- dC
2'-deoxycytosine
- dA
2'-deoxyadenosine
- dG
2'-deoxyguanosine
- DFT
Density Functional Theory
- MD
molecular dynamics
- QM
quantum mechanics
- RMSD
root-mean-square deviations
- MM-PBSA
molecular mechanics Poisson-Boltzmann surface area
- NER
nucleotide excision repair
Footnotes
Supporting Information Available: Details of the molecular dynamics protocol of 4-OHEN-G adducts in duplexes are provided. Table S1 gives glycosidic torsion χ values of the modified guanine, box sizes and numbers of waters in the MD simulation starting models. Table S2 gives added force field parameters for the modified guanine. Table S3 gives AMBER atom type, connection type, and partial charge assignments for the 4-OHEN-G adducts. Table S4 gives MM-PBSA free energy components of the lowest free energy conformation for each 4-OHEN-G stereoisomer modified duplexes. Table S5 gives solvent accessible surfaces of the equilenin rings of 4-OHEN adducts in 11mer duplexes. Table S6 gives hydrogen bonds and occupancies at the lesion sites of 4-OHEN-G modified duplexes. Table S7 gives van der Waals interaction energies between the base pairs of the 4-OHEN-G modified duplexes. Table S8 gives trajectory average bending angles of the 4-OHEN-G modified DNA duplexes. Table S9 gives free energies for syn and anti conformations of each stereoisomeric adduct. Figure S1 shows starting structures for the MD simulations of the 4-OHEN-G stereoisomers in the DNA duplexes. Figure S2 shows RMSD vs time plots for each molecular dynamics simulation of the 4-OHEN-G adducts. Figure S3 shows torsion angle δ' (N1-C2'-C3'-N2) (degrees) vs time plots for each molecular dynamics simulation of 4-OHEN-G adducts. Figure S4 shows trajectory-average groove dimensions of 4-OHEN-G modified duplexes. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B, Speizer FE. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- (2).Lupulescu A. Estrogen use and cancer incidence: a review. Cancer Invest. 1995;13:287–295. doi: 10.3109/07357909509094464. [DOI] [PubMed] [Google Scholar]
- (3).Zumoff B. Does postmenopausal estrogen administration increase the risk of breast cancer? Contributions of animal, biochemical, and clinical investigative studies to a resolution of the controversy. Proc Soc Exp Biol Med. 1998;217:30–37. doi: 10.3181/00379727-217-44202. [DOI] [PubMed] [Google Scholar]
- (4).Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Jama. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- (5).Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- (6).Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, Aragaki AK, Shumaker SA, Brzyski RG, LaCroix AZ, Granek IA, Valanis BG. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- (7).Bolton JL, Pisha E, Zhang F, Qiu S. Role of quinoids in estrogen carcinogenesis. Chem Res Toxicol. 1998;11:1113–1127. doi: 10.1021/tx9801007. [DOI] [PubMed] [Google Scholar]
- (8).Bolton JL, Thatcher GR. Potential Mechanisms of Estrogen Quinone Carcinogenesis. 2007 doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Shen L, Qiu SX, vanBreemen RB, Zhang FG, Chen YM, Bolton JL. Reaction of the Premarin metabolite 4-hydroxyequilenin semiquinone radical with 2'-deoxyguanosine: Formation of unusual cyclic adducts. J Am Chem Soc. 1997;119:11126–11127. [Google Scholar]
- (10).Shen L, Qiu S, Chen Y, Zhang F, van Breemen RB, Nikolic D, Bolton JL. Alkylation of 2'-deoxynucleosides and DNA by the Premarin metabolite 4-hydroxyequilenin semiquinone radical. Chem Res Toxicol. 1998;11:94–101. doi: 10.1021/tx970181r. [DOI] [PubMed] [Google Scholar]
- (11).Embrechts J, Lemiere F, Van Dongen W, Esmans EL. Equilenin-2'-deoxynucleoside adducts: analysis with nano-liquid chromatography coupled to nano-electrospray tandem mass spectrometry. J Mass Spectrom. 2001;36:317–328. doi: 10.1002/jms.136. [DOI] [PubMed] [Google Scholar]
- (12).Zhang F, Swanson SM, van Breemen RB, Liu X, Yang Y, Gu C, Bolton JL. Equine estrogen metabolite 4-hydroxyequilenin induces DNA damage in the rat mammary tissues: formation of single-strand breaks, apurinic sites, stable adducts, and oxidized bases. Chem Res Toxicol. 2001;14:1654–1659. doi: 10.1021/tx010158c. [DOI] [PubMed] [Google Scholar]
- (13).Embrechts J, Lemiere F, Van Dongen W, Esmans EL, Buytaert P, Van Marck E, Kockx M, Makar A. Detection of estrogen DNA-adducts in human breast tumor tissue and healthy tissue by combined nano LC-nano ES tandem mass spectrometry. J Am Soc Mass Spectrom. 2003;14:482–491. doi: 10.1016/S1044-0305(03)00130-2. [DOI] [PubMed] [Google Scholar]
- (14).Suzuki N, Yasui M, Santosh Laxmi YR, Ohmori H, Hanaoka F, Shibutani S. Translesion synthesis past equine estrogen-derived 2'-deoxycytidine DNA adducts by human DNA polymerases eta and kappa. Biochemistry. 2004;43:11312–11320. doi: 10.1021/bi049273n. [DOI] [PubMed] [Google Scholar]
- (15).Yasui M, Laxmi YR, Ananthoju SR, Suzuki N, Kim SY, Shibutani S. Translesion Synthesis Past Equine Estrogen-Derived 2'-Deoxyadenosine DNA Adducts by Human DNA Polymerases eta and kappa. Biochemistry. 2006;45:6187–6194. doi: 10.1021/bi0525324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen D, Kolbanovskiy A, Shastry A, Chang M, Bolton JL, Geacintov NE. Replication of DNA sequences with equine estrogen metabolite 4-OHEN-dC adducts catalyzed by A- and Y- family polymerases in vitro. Abstracts of Papers of the American Chemical Society. 2005;230:U1853–U1853. [Google Scholar]
- (17).Chen D. Department of Chemistry. New York University; New York: 2006. Nucleotide Excision Repair and Translesion Synthesis of DNA Adducts Derived From the Equine Estrogen Metabolite 4-hydroxyequilenin. [Google Scholar]
- (18).Yasui M, Suzuki N, Liu X, Okamoto Y, Kim SY, Laxmi YR, Shibutani S. Mechanism of translesion synthesis past an equine estrogen-DNA adduct by Y-family DNA polymerases. Journal of molecular biology. 2007;371:1151–1162. doi: 10.1016/j.jmb.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chen DD, Oum L, Kolbanovskiy A, Kuzmin V, Shastry A, Chang MS, Bolton JL, Geacintov N. Translesion synthesis and nucleotide excision repair of site specifically modified oligodeoxyribonucleotides containing single lesions derived from the equine estrogen metabolite 4-OHEN. Chemical Research in Toxicology. 2004;17:1782–1782. [Google Scholar]
- (20).Chen DD, Liu TM, Ruan Q, Zou Y, Kuzmin V, Kolbanovskiy A, Chang MS, Bolton JL, Geacintov N. Nucleotide excision repair of site specific cytidine adducts derived from the equine estrogen metabolite 4-OHEN in DNA by UvrABC proteins from Escherichia coli. Chemical Research in Toxicology. 2003;16:1682–1683. [Google Scholar]
- (21).Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57:237–257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- (22).Bolton JL, Pisha E, Zhang F, Qiu S. Role of quinoids in estrogen carcinogenesis. Chemical research in toxicology. 1998;11:1113–1127. doi: 10.1021/tx9801007. [DOI] [PubMed] [Google Scholar]
- (23).Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- (24).Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- (25).Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, Higginbotham SM, Cavalieri EL. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- (26).Zheng W, Xie DW, Jin F, Cheng JR, Dai Q, Wen WQ, Shu XO, Gao YT. Genetic polymorphism of cytochrome P450-1B1 and risk of breast cancer. Cancer Epidemiology Biomarkers & Prevention. 2000;9:147–150. [PubMed] [Google Scholar]
- (27).Kisselev P, Schunck WH, Roots I, Schwarz D. Association of CYP1A1 polymorphisms with differential metabolic activation of 17 beta-estradiol and estrone. Cancer Res. 2005;65:2972–2978. doi: 10.1158/0008-5472.CAN-04-3543. [DOI] [PubMed] [Google Scholar]
- (28).Wen WQ, Ren ZF, Shu XO, Cai QY, Ye CZ, Gao YT, Zheng W. Expression of cytochrome P450 1B1 and catechol-O-methyltransferase in breast tissue and their associations with breast cancer risk. Cancer Epidemiology Biomarkers & Prevention. 2007;16:917–920. doi: 10.1158/1055-9965.EPI-06-1032. [DOI] [PubMed] [Google Scholar]
- (29).Spink DC, Zhang F, Hussain MM, Katz BH, Liu X, Hilker DR, Bolton JL. Metabolism of equilenin in MCF-7 and MDA-MB-231 human breast cancer cells. Chemical research in toxicology. 2001;14:572–581. doi: 10.1021/tx000219r. [DOI] [PubMed] [Google Scholar]
- (30).Zhang F, Chen Y, Pisha E, Shen L, Xiong Y, van Breemen RB, Bolton JL. The major metabolite of equilin, 4-hydroxyequilin, autoxidizes to an o-quinone which isomerizes to the potent cytotoxin 4-hydroxyequilenin-o-quinone. Chem Res Toxicol. 1999;12:204–213. doi: 10.1021/tx980217v. [DOI] [PubMed] [Google Scholar]
- (31).Shen L, Pisha E, Huang Z, Pezzuto JM, Krol E, Alam Z, van Breemen RB, Bolton JL. Bioreductive activation of catechol estrogen-orthoquinones: aromatization of the B ring in 4-hydroxyequilenin markedly alters quinoid formation and reactivity. Carcinogenesis. 1997;18:1093–1101. doi: 10.1093/carcin/18.5.1093. [DOI] [PubMed] [Google Scholar]
- (32).Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- (33).Chen Y, Liu X, Pisha E, Constantinou AI, Hua Y, Shen L, van Breemen RB, Elguindi EC, Blond SY, Zhang F, Bolton JL. A metabolite of equine estrogens, 4-hydroxyequilenin, induces DNA damage and apoptosis in breast cancer cell lines. Chem Res Toxicol. 2000;13:342–350. doi: 10.1021/tx990186j. [DOI] [PubMed] [Google Scholar]
- (34).Pisha E, Lui X, Constantinou AI, Bolton JL. Evidence that a metabolite of equine estrogens, 4-hydroxyequilenin, induces cellular transformation in vitro. Chem Res Toxicol. 2001;14:82–90. doi: 10.1021/tx000168y. [DOI] [PubMed] [Google Scholar]
- (35).Bolton JL. Quinoids, quinoid radicals, and phenoxyl radicals formed from estrogens and antiestrogens. Toxicology. 2002;177:55–65. doi: 10.1016/s0300-483x(02)00195-6. [DOI] [PubMed] [Google Scholar]
- (36).Liu X, Yao J, Pisha E, Yang Y, Hua Y, van Breemen RB, Bolton JL. Oxidative DNA damage induced by equine estrogen metabolites: role of estrogen receptor alpha. Chem Res Toxicol. 2002;15:512–519. doi: 10.1021/tx0101649. [DOI] [PubMed] [Google Scholar]
- (37).Yasui M, Matsui S, Laxmi YR, Suzuki N, Kim SY, Shibutani S, Matsuda T. Mutagenic events induced by 4-hydroxyequilin in supF shuttle vector plasmid propagated in human cells. Carcinogenesis. 2003;24:911–917. doi: 10.1093/carcin/bgg029. [DOI] [PubMed] [Google Scholar]
- (38).Ding S, Shapiro R, Geacintov NE, Broyde S. Conformations of stereoisomeric base adducts to 4-hydroxyequilenin. Chem Res Toxicol. 2003;16:695–707. doi: 10.1021/tx0340246. [DOI] [PubMed] [Google Scholar]
- (39).Ding S, Shapiro R, Geacintov NE, Broyde S. Equilenin-derived DNA adducts to cytosine in DNA duplexes: structures and thermodynamics. Biochemistry. 2005;44:14565–14576. doi: 10.1021/bi051090t. [DOI] [PubMed] [Google Scholar]
- (40).Ding S, Shapiro R, Geacintov NE, Broyde S. 4-hydroxyequilenin-adenine lesions in DNA duplexes: stereochemistry, damage site, and structure. Biochemistry. 2007;46:182–191. doi: 10.1021/bi061652o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Case DA, Pearlman DA, Caldwell JW, Cheatham TE, III, Wang J, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, Cheng AL, Vincent JJ, Crowley M, Tsui V, Gohlke H, Radmer RJ, Duan Y, Pitera J, Massova I, Seibel GL, Singh UC, Weiner PK, Kollman PA. AMBER 7. University of California; San Francisco, CA: 2002. [Google Scholar]
- (42).Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A 2nd Generation Force-Field for the Simulation of Proteins, Nucleic-Acids, and Organic-Molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- (43).Cheatham TE, Cieplak P, Kollman PA. A modified version of the Cornell et al. force field with improved sugar pucker phases and helical repeat. J Biomol Struct Dyn. 1999;16:845–862. doi: 10.1080/07391102.1999.10508297. [DOI] [PubMed] [Google Scholar]
- (44).Cieplak P, Cornell WD, Bayly C, Kollman PA. Application of the Multimolecule and Multiconformational Resp Methodology to Biopolymers - Charge Derivation for DNA, Rna, and Proteins. J Comput Chem. 1995;16:1357–1377. [Google Scholar]
- (45).Bayly CI, Cieplak P, Cornell WD, Kollman PA. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges - the Resp Model. J Phys Chem-Us. 1993;97:10269–10280. [Google Scholar]
- (46).Frisch JM, Trucks WG, Schlegel BH, Scuseria EG, Robb AM, Cheeseman RJ, Zakrzewski GV, Montgomery AJ, Stratmann ER, Burant CJ, Dappprich S, Millam MJ, Daniels DA, Kudin NK, Strain CM, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson AG, Ayala YP, Cui Q, Morokuma K, Malick KD, Rabuck DA, Raghavachari K, Foresman BJ, Cioslowski J, Ortiz VJ, Baboul GA, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Comperts R, Martin LR, Fox JD, Keith T, Al-Laham AM, Peng YC, Nanayakkara A, Gonzalez C, Challacombe M, Gill WMP, Johnson B, Chen W, Wong WM, Andres LJ, Head-Gordon M, Replogle SE, Pople AJ. Gaussian 98. Gaussian, Inc.; Pittsburgh: 1998. [Google Scholar]
- (47).Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of Simple Potential Functions for Simulating Liquid Water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- (48).Darden T, York D, Pedersen L. Particle Mesh Ewald - an N.Log(N) Method for Ewald Sums in Large Systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- (49).Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A Smooth Particle Mesh Ewald Method. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- (50).Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-Integration of Cartesian Equations of Motion of a System with Constraints - Molecular-Dynamics of N-Alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- (51).Harvey SC, Tan RKZ, Cheatham TE. The flying ice cube: Velocity rescaling in molecular dynamics leads to violation of energy equipartition. J Comput Chem. 1998;19:726–740. [Google Scholar]
- (52).Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular-Dynamics with Coupling to an External Bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- (53).Ravishanker G, Swaminathan S, Beveridge DL, Lavery R, Sklenar H. Conformational and helicoidal analysis of 30 PS of molecular dynamics on the d(CGCGAATTCGCG) double helix: “curves”, dials and windows. J Biomol Struct Dyn. 1989;6:669–699. doi: 10.1080/07391102.1989.10507729. [DOI] [PubMed] [Google Scholar]
- (54).Ravishanker G, Wang W, Beveridge DL. MD Toolchest, Wesleyan University, Middletown, CT.
- (55).Connolly ML. Solvent-accessible surfaces of proteins and nucleic acids. Science. 1983;221:709–713. doi: 10.1126/science.6879170. [DOI] [PubMed] [Google Scholar]
- (56).Honig B, Nicholls A. Classical electrostatics in biology and chemistry. Science. 1995;268:1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- (57).Connolly ML. Analytical Molecular-Surface Calculation. Journal of Applied Crystallography. 1983;16:548–558. [Google Scholar]
- (58).Srinivasan J, Cheatham TE, Cieplak P, Kollman PA, Case DA. Continuum solvent studies of the stability of DNA, RNA, and phosphoramidate - DNA helices. J Am Chem Soc. 1998;120:9401–9409. [Google Scholar]
- (59).Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, Lee M, Lee T, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, Cheatham TE., 3rd. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res. 2000;33:889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- (60).Becke AD. Density-Functional Exchange-Energy Approximation with Correct Asymptotic-Behavior. Physical Review A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- (61).Vosko SH, Wilk L, Nusair M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin-Density Calculations - a Critical Analysis. Canadian Journal of Physics. 1980;58:1200–1211. [Google Scholar]
- (62).Lee CT, Yang WT, Parr RG. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Physical Review B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- (63).Kolbanovskiy A, Kuzmin V, Shastry A, Kolbanovskaya M, Chen D, Chang M, Bolton JL, Geacintov NE. Base selectivity and effects of sequence and DNA secondary structure on the formation of covalent adducts derived from the equine estrogen metabolite 4-hydroxyequilenin. Chem Res Toxicol. 2005;18:1737–1747. doi: 10.1021/tx050190x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.