Abstract
Prions are viewed as enigmatic infectious entities whose genetic properties are enciphered solely in an array of self-propagating protein aggregate conformations. Rnq1, a yeast protein with yet unknown function, forms a prion named [PIN+] for its ability to facilitate the de novo induction of another prion, [PSI+]. Here we investigate a set of RNQ1 truncations that were designed to cover major Rnq1 sequence elements similar to those important for the propagation of other yeast prions: a region rich in asparagines and glutamines and several types of oligopeptide repeats. Proteins encoded by these RNQ1 truncations were tested for their ability to (i) join (decorate) pre-existing [PIN+] aggregates made of wild-type Rnq1 and (ii) maintain the heritable aggregated state in the absence of wild-type RNQ1. While the possible involvement of particular sequence elements in the propagation of [PIN+] is discussed, the major result is that the efficiency of transmission of [PIN+] from wild-type Rnq1 to a fragment, decreased with the fragment’s length.
Keywords: Rnq1 [PIN+] yeast prion [PSI+] induction
Introduction
Many proteins can take on an alternative β-sheet-rich conformation in which they polymerize into amyloid aggregates (Chiti and Dobson 2006). Sometimes, this state is infectious and, in such cases, is referred to as a prion (Prusiner 2004). The infectivity is based upon a prion’s ability to convert soluble molecules of the same protein into the amyloid form, and the ability of these amyloid aggregates to break and segregate, resulting in multiple seeds for further conversion (Serpell et al. 1997;Borchsenius et al. 2001). Therefore, prions can be regarded as genetic elements although they are solely proteinaceous (Wickner et al. 2004). The first characterized prion, mammalian PrPSc is a pathogenic agent causing a series of neurodegenerative disorders, e.g. Creutzfeldt-Jackob disease, Gerstmann-Strasussler syndrome, fatal familial insomnia, and kuru in humans; scrapie in sheep; spongiform encephalopathy in cows (Prusiner 1998). The recent discovery of prion-like factors in other organisms: [PSI+], [URE3], and [PIN+] in Saccharomyces cerevisiae and [Het-s] in Podospora anserina (Wickner 1994; Coustou et al. 1997; Derkatch et al. 2001) facilitated the search for general criteria of prionogenicity in a protein’s primary structure.
This problem has been approached in many studies (DePace et al. 1998; Parham et al. 2001; Bradley and Liebman 2004; Osherovich et al. 2004; Ross et al. 2005b) of the [PSI+] determinant (Cox et al. 1988) which is a prion form of the translational termination factor Sup35 (eRF3) (Ter-Avanesyan et al. 1994; Zhouravleva et al. 1995; Paushkin et al. 1996). Intriguingly, the de novo appearance of [PSI+] (upon overproduction of Sup35) is dependent upon the presence of another prion, [PIN+] (Derkatch et al. 1997) which is formed by Rnq1, a protein with no known function (Derkatch et al. 2001). [PIN+] also facilitates the de novo appearance of [URE3] (Derkatch et al. 2001; Bradley et al. 2002), a prion form of Ure2 (Wickner 1994) which is a regulator of nitrogen metabolism (Courchesne and Magasanik 1988). In vitro studies using recombinant proteins have shown that Rnq1 aggregates constituting [PIN+] can promote the assembly of Sup35 into [PSI+] aggregates (Derkatch et al. 2004; Vitrenko et al. 2006).
Because prions arise from the misfolding of a prionogenic protein, the establishment and maintenance of prions is dependent on chaperone activity (Chernoff 2001). Propagation of [PIN+] requires Hsp104 (Derkatch et al. 2001) as well as some members of the Hsp40 and Hsp70 chaperone families (Bradley et al. 2002; Lopez et al. 2003; Aron et al. 2005). The dependence on Hsp104 is assumed to result from Hsp104’s primary function of fragmentation, with partitioning being a consequence of this activity. This Hsp104 activity has been shown to be crucial for the propagation of [PSI+] (Chernoff et al. 1995; Borchsenius et al. 2001; Jung et al. 2002; Kryndushkin et al. 2003) and [URE3] (Moriyama et al. 2000). Likewise, there seems to be a reverse correlation between Hsp104 activity and the size of detergent-resistant aggregates of [PSI+] (Kryndushkin et al. 2003) and [PIN+] (Bagriantsev and Liebman 2004).
Prions can exist in multiple forms producing distinct phenotypes (Derkatch et al. 1996; Prusiner 1998; Schlumpberger et al. 2001). These prion “strains” or “variants” arise from the ability of a single prionogenic protein to polymerize into structurally different aggregates (Tanaka et al. 2004; Krishnan and Lindquist 2005; Spassov et al. 2006). Similarly, [PIN+] variants have been characterized that exhibit different levels of [PSI+] induction. The variants were hence named “very high” (the most efficient [PSI+] inducer), “high”, “medium” and “low” [PIN+] (very inefficient [PSI+] inducer). Rnq1 forms aggregates in all of these strains (Bradley et al. 2002).
Regions sufficient for prionization (‘prion domains’) have been delineated for [PSI+] (Ter-Avanesyan et al. 1994), [URE3] (Masison and Wickner 1995; Maddelein and Wickner 1999; Schlumpberger et al. 2000) and [PIN+]/[RNQ+] (Sondheimer and Lindquist 2000). The primary structures of these domains display some similarities, e.g. all are rich in asparagines and glutamines (QN-rich). More than 170 QN-rich prion-like sequences were found in an analysis of the yeast genome (Harrison and Gerstein 2003). Yet no universal criteria for the prediction of prionogenic properties have been established. Interestingly, the P. anserina prion [Het-s] has no QN-rich domain but can be efficiently propagated as a prion in yeast (Taneja 2006). Likewise, PrP is not QN-rich (Oesch et al. 1985), however, the PrP sequence, like Sup35 and Rnq1, does contain imperfect oligopeptide repeats. Furthermore, altering the number of repeats in Sup35 (Liu and Lindquist 1999; Parham et al. 2001) and PrP (Rogers et al. 1993) affects the probability of conversion into the prion form. Intriguingly, randomization (scrambling) of sequences of Sup35 and Ure2 prion domains, while keeping the amino acid composition the same as in the wild-type versions, did not result in the loss of prion-forming abilities of the corresponding proteins (Ross et al. 2004; Ross et al. 2005a).
We made and characterized different fragments of Rnq1 and examined them for the ability to propagate two [PIN+] variants. Two phenotypes characteristic of [PIN+] were specifically scored for these fragments: the formation of Rnq1 aggregates and ability to facilitate the induction of [PSI+]. Differences in the efficiency with which Rnq1 fragments can be converted to a stable prion by wild-type Rnq1 aggregates appear to be linked to the fragment length.
Materials and Methods
Yeast strains and cultivation procedures
All MATa strains used are derivatives of 74-D694 (MATa ade1-14 trp1-289 his3-Δ200 leu2-3,112 ura3-52) (Chernoff et al. 1993): L1751 is [pin−] (Derkatch et al. 1997), L1749 is high [PIN+] (Derkatch et al. 1997), L1943 is low [PIN+] (Bradley et al. 2002). L2668 is MATα ade1-14 ura3-52 leu2-3,112 trp1-289 his3-200 kar1-Δ15 can1R cyh2R rnq1∷KanMX4 (Bradley and Liebman 2003). Standard cultivation procedures were followed. Yeast was incubated at 30°C unless otherwise indicated. Synthetic media containing dextrose (SD) lacking one or two amino acids were used to select for the corresponding nutritional markers, e.g. media lacking histidine or uracil are denoted, respectively, SD-His or SD-Ura. Synthetic medium containing galactose and raffinose (SGal +raffinose) instead of dextrose was used for the inducible overexpression of SUP35 from the GAL1 promoter. Copper sulfate (50 µM) was added to media to drive the expression of rnq1 alleles from the CUP1 promoter.
Plasmids
The rnq1-Δ1…Δ7, -ΔN1 alleles were generated with PCR using the primers listed in Table 1. The template, pYV10 RNQ1:GFP, was made from pID116, a centromeric HIS3-marked vector, containing RNQ1 fused to GFP under the control of the CUP1 promoter (Derkatch unpublished) in which we replaced the CUP1 promoter with the RNQ1 native promoter on an XhoI – BamHI fragment. Since attempts to transfer [PIN+] to the rnq-Δ strain L2668 expressing plasmid-born wild-type RNQ1 from the native promoter failed (data not shown) we constructed plasmids in which the rnq1 alleles were more highly expressed from the CUP1 promoter. The PCR-generated fragments were cloned into pID116 as BamHI – SacII inserts in place of the BamHI – SacII fragment carrying RNQ1, to yield the pCUP-rnq1-x:GFP constructs used in this study (x stands for one of the rnq1 alleles). pID116 was used as an RNQ1 positive control and also to make the ‘empty vector’ pCUP:GFP and ‘QN-rich region only’ construct pCUP:rnq1-ΔN2:GFP by cutting out the BamHI – SacII and BamHI – Van91I fragments, respectively. For [PSI+] induction, we used pGAL:SUP35, a centromeric, URA3-marked plasmid (Derkatch et al. 1996). pRS415 (Sikorski and Hieter 1989) was used to provide the LEU2 selectable marker when needed.
Table 1.
Primers used to make pCUP rnq1-x : GFP constructs
| Allele (x) | Primers | |
|---|---|---|
| forward | reverse | |
| Δ1 | 5′-AGGTCGACGGTATCGATAAGCTT-3′ | 5′-GATGCCGCGGGCTGCTTTGATTTTGACCTTGC-3′ |
| Δ2 | same | 5′-GATGCCGCGGATAGGATTGGTTATAGCCCTGTTGA-3′ |
| Δ3 | same | 5′-GATGCCGCGGTTGTCCCTGTTGTTGTTGGTAAC-3′ |
| Δ4 | same | 5′-GATGCCGCGGGTATTGCTGTTGGTTGGATTG-3′ |
| Δ5 | same | 5′-GATGCCGCGGGGAGTGGCCTTGTTGCTGC -3′ |
| Δ6 | same | 5′-GATGCCGCGGATTGGAGTTATTGCCCAGGTAG-3′ |
| Δ7 | same | 5′-GATGCCGCGGGTATTGCGGTCTTCCGTACT-3′ |
| ΔN1 | 5′-GACAGGATCCATGGGTTCTTTTACTGCTTTGGCG-3′ | 5′-TAGCCATCCGCGGGTAGCGGTTCTG -3′ |
GFP-based assay of Rnq1 aggregation (decoration assay)
L1751, L1749 and L1943 bearing pGAL:SUP35 (URA3) were crossed with L2668 bearing pCUP-rnq1-x:GFP (HIS3), and progeny was selected on SD-His-Ura +50 µM CuSO4. Mating type verification showed that most of this progeny are diploids. Colonies were picked to 1 µl water, and examined on glass slides using a Zeiss Axioskop 2 equipped with x40 Plan-Neofluar objective lens. Only cells emitting green fluorescence were scored and their total number was used as the total in all percentages presented.
[PIN+] transfer via cytoduction
[PIN+] was transferred from a wild-type RNQ1 strain to rnq1-Δ pCUP-rnq1-x:GFP strains by cytoduction. This technique results in mixing the cytoplasms of two strains crossed while retaining the nucleus from only one strain called, therefore, the recipient. Nuclear fusion is prevented by the kar1-1 allele present in one of the strains (Conde and Fink 1976). The recipient nucleus has cyh2R, a recessive cycloheximide resistance allele facilitating selection (on cycloheximide-containing medium) of progeny retaining only the recipient nucleus. While cytoduction is generally performed by selecting for the transfer of mitochondria from a donor to a recipient, better [PIN+] transfer was achieved when we selected for the fusion of donor and recipient cytoplasms using the plasmid-born URA3 and HIS3 markers, respectively. This technique called plasmiduction has been described previously (Natsoulis et al. 1994). For transfer of cytoplasm from [pin−] (L1751), high (L1749) and low [PIN+] (L1943) donors (transformed with pGAL:SUP35) to the [pin−] recipient L2668 (transformed with pCUP-rnq1-x:GFP), donor and recipient strains were mated on YPD + 50 µM CuSO4 overnight, and the mating patches were then replica plated onto SD-Ura-His + 50 µM CuSO4 + 3 µg / ml cycloheximide. From this medium, Ura+ His+ CyhR colonies were picked, their mating type and markers were verified to make sure they were cytoductants.
Assaying cytoductants for [PIN+]
Rnq1–x:GFP aggregation was scored directly in cytoductants by microscopic examination of GFP fluorescence as described above. To assay the cytoductants’ ability to induce [PSI+], plates of cytoductants were replicated to YPD + 50 µM CuSO4 (to obtain thick uniform patches) and then either onto SGal – His – Ura + 50 µM CuSO4 + 2% raffinose (SUP35 inducing medium) or SD – His – Ura + 50 µM CuSO4 (non-inducing medium). After two passages on these media, cytoductants were replica plated onto SD-Ade and incubated for two weeks at +20°C. We scored cytoductants as being able to induce [PSI+] only if Ade+ papillation was higher following growth on inducing vs non-inducing medium. We verified in pilot experiments (data not shown) that induced Ade+ colonies are usually GuHCl-curable. Thus, as shown previously (Chernoff et al. 1993; Derkatch et al. 1997), induced Ade+ papillation can be used to score for [PSI+] appearance and hence the inheritance of [PIN+] by cytoductants.
Western blot analysis
To analyze the expression and aggregation levels of the Rnq1 fragments, lysates were made from 100 ml SD-His + 50 µM CuSO4 saturated cultures. Harvested cells were resuspended in 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM MgCl 0.1% Triton X-100 5 mM PMSF, 1:50 protease inhibitor cocktail (Sigma; cat. # 8215) (Bagriantsev and Liebman 2004) and vigorously shaken with an equal volume of 0.5-mm glass beads (BioSpec Products) in a vortex equipped with a tube holder for 10–60 min at +4°C. An additional dose of 5 mM PMSF was added after half of the incubation time. Lysates were precleared by centrifugation at 600 × g for 1 min and normalized for the total protein concentration using Bradford Protein Assay Reagent (Biorad). Lysates were analyzed either on SDS-polyacrylamide (after boiling for 7 min) or SDS-agarose gels (without boiling) and were then transferred to a PVDF membrane. Biorad components and recommendations were used with modifications for SDS-agarose gels described earlier (Bagriantsev and Liebman 2004). Membranes were probed with 1:5000 anti-GFP antibody (Sigma; cat. # G1544) using a Western-Star™ immunodetection kit (Applied Biosystems).
Hydrophobicity profiles
The Rnq1 sequence (Saccharomyces Genome Database #YCL028W) was analyzed by the Kyte-Doolittle algorithm (Kyte and Doolittle 1982) using the ProtScale program (http://ca.expasy.org/tools/protscale.html) with the window set at 9 aa. Analysis of hydrophobicity by other algorithms available in ProtScale gave similar profiles.
Results
Analysis of Rnq1 sequence motifs
The Rnq1 sequence can be divided into two halves: the N-terminal non-QN-rich (aa 1-152) and the C-terminal QN-rich (aa 153-405). The latter has been shown to be sufficient for prion formation (Sondheimer and Lindquist 2000). Several types of sequence regularities can be outlined in the QN-rich portion whose location, sequence and hydrophobicity are shown in Fig. 1a, b, and c, respectively. Motif A is represented by a QG stretch and was shown to contain polymorphisms in S. cerevisiae strains where Rnq1 was soluble, whereas Rnq1 is otherwise typically aggregated in strains analyzed from the wild (Resende et al. 2003). Motifs B, C, D and F are similar in that they have a hydrophobic stretch that we call “FLASAS” for the one-letter abbreviated amino acids that are aligned in each of these motifs. B and C constitute a subgroup because the “FLASAS” sequence in them is followed by the polar FM(N/H)SNN(N/Q) stretch distinguishing them from the D and F subgroup followed by the more hydrophobic mini-motif YLG(G/N)(G/N). Also noteworthy are: a tandem repeat, E, and the less polar mini-motif G consisting of two identical 7-aa sequences with a 7-aa spacer in between. Finally, we note another highly hydrophobic motif, H, at the very C terminus of the QN-rich region. The presence of several phenylalanines in such a short motif may indicate its importance in prion formation as stacking interactions between aromatic amino acids were previously suggested to play a role in stabilizing amyloid structure (Nelson et al. 2005).
Fig. 1.
Truncated alleles of the RNQ1 gene. a Scheme of deletions. Deletion names are on the left of the corresponding lines that show the part of the gene that is remaining (sequences are available in Supplemental Text). Amino acid positions are given above. Letters below the bar indicate the oligopeptide degenerate repeats and other sequences of interest, each spanning the corresponding striped or dotted region. Pluses on the right indicate the fragments ability to join (decorate) pre-existing Rnq1 aggregates and propagate [PIN+] transferred to them from wild-type Rnq1 via cytoduction. The efficiency of this transfer is displayed by the number of pluses. See Table 2 and Table 3 for details. b Sequences of the motifs are designated by letters A—H in (a). The “FLASAS” motif is underlined. Identical amino acids are given in capital letters. c Hydrophobicity profile of Rnq1. Letters indicate peaks corresponding to motifs in (b). Note the peaks of hydrophobocity linked with motifs B, C, D and F. d Western blot analysis of expression of RNQ1 alleles fused to GFP and driven by the CUP promoter. Total cell-free extracts made from L1749 transformed with the alleles listed in (a) and empty vector (ev) were separated by PAGE, blotted and probed with anti-GFP antibody. Membrane stained with Coomassie Blue shows the amount of total protein loaded. Rnq1-ΔN2 reproducibly displayed a lower immunosignal.
Design of RNQ1 alleles
To investigate the role of Rnq1 sequence motifs in the propagation of [PIN+] we designed seven C-terminal truncations such that each of them removes an additional motif (rnq1-Δ1…Δ7; Fig. 1a). To further characterize [PIN+] formed by the QN-rich region alone (capable of propagating a heritable aggregated state in vivo (Sondheimer and Lindquist 2000)) we also made a deletion of most of the non-QN-rich portion (rnq1-ΔN2) and another deletion (rnq1-ΔN1) covering the entire non-QN-rich sequence plus motif A (Fig. 1a). The amino acid sequence of all deletions is presented in Supplemental Text available on-line. Deletions as well as wild-type RNQ1 were C-terminally fused to green fluorescent protein (GFP) and expressed from the CUP1 copper-inducible promoter on a centromeric pRS316-based vector. The steady-state level of Rnq1-x:GFP determined by Western blot analysis (Fig. 1d) was similar for the constructs used in this study, although there was a reproducible slightly decreased amount of Rnq1-ΔN1:GFP. Constructs bearing RNQ1 deletions under the control of the native promoter without any fusions could not provide detectable levels of protein which was the reason for the use of the CUP1 promoter known to drive more efficient expression.
Analysis of the ability of Rnq1 fragments to join pre-existing aggregates of wild-type Rnq1
The joining of a GFP-tagged prionogenic fragment to prion aggregates made of the homologous wild-type protein is visualized by the formation of fluorescent foci in vivo (Patino et al. 1996). To analyze Rnq1 fragments by this assay, plasmids carrying rnq1-x:GFP alleles were transformed into a strain having a complete deletion of RNQ1 (L2668) and crossed to either [pin−] (L1751), high (L1749) or low (L1943) [PIN+] strains. Thus, the Rnq1-x:GFPs encoded by these plasmids were given a chance to “decorate” the wild-type Rnq1 aggregates present in these strains (Fig. 2 and (Sondheimer and Lindquist 2000)). A significant level of foci was only seen in crosses of transformants bearing rnq1-Δ4…Δ7, -ΔN1,-ΔN2 and RNQ1 alleles (fused to GFP) to high or low [PIN+] but not [pin−] strains implying the efficient decoration of pre-existing wild-type Rnq1 aggregates by the corresponding Rnq1 fragments (Table 2). There was no significant difference in the appearance of aggregates or the number of cells with aggregates in when the rnq1-Δ4…Δ7, -ΔN1, -ΔN2, RNQ1:GFP alleles were expressed.
Fig. 2.
Aggregation pattern of Rnq1-Δ3, -Δ4, and Rnq1 fused to GFP in high [PIN+], low [PIN+] but not in [pin−] strains. Shown crosses of a [ pin−] strain harboring the indicated construct to an RNQ1 strain of the indicated prion phenotype.
Table 2.
Decoration of pre-existing wild-type Rnq1 aggregates by Rnq1 fragments.
| Allele | Percentage of cells with GFP aggregates | ||
|---|---|---|---|
| [pin−] | high [PIN+] | low [PIN+] | |
| no RNQ1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Δ1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Δ2 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Δ3 | 0 ± 0 | 3.3 ± 2.6 | 1.8 ± 1.0 |
| Δ4 | 0 ± 0 | 60.5 ± 11.9 | 73.3 ± 14.9 |
| Δ5 | 0 ± 0 | 69.0 ± 18.1 | 57.8 + 23.1 |
| Δ6 | 0 ± 0 | 43.5 ± 25.9 | 77.0 ± 15.6 |
| Δ7 | 0 ± 0 | 61.5 ± 12.6 | 77.5 ± 11.3 |
| ΔN1 | 0 ± 0 | 59.3 ± 22.5 | 44.0 ± 11.7 |
| ΔN2 | 0 ± 0 | 45.5 ± 23.6 | 46.8 ± 23.6 |
| WT | 1.5 ± 1.0 | 79.5 ± 6.1 | 85.5 ± 7.9 |
Progeny from each cross of rnq-Δ pCUP:rnq1-x:GFP to wild-type RNQ1 strains harboring either [pin−], high or low [PIN+] were assayed for the presence of GFP aggregates (Fig. 2). For each cross, 100 fluorescent cells were examined in each of three samples to give the standard deviation. Rnq1-Δ4 and longer fragments can decorate [PIN+].
In the prion state, Rnq1 forms SDS-resistant particles indicative of their amyloid structure (Bagriantsev and Liebman 2004). To see whether Rnq1-x:GFPs take on an SDS-stable conformation upon contact with wild-type Rnq1 aggregates, we analyzed them using semi-denaturing agarose gel electrophoresis and immunoblotting. SDS-resistant Rnq1-x:GFP sub-particles with molecular weights much higher than 250 kD were found in crossing rnq1- Δ4…Δ7, -ΔN1, -ΔN2, and RNQ1 :GFP transformants to high and low [PIN+] (Fig. 3) but not [pin−] strains (data not shown). This is consistent with the results of the decoration assay (Table 2) and suggests that aggregation of Rnq1-x:GFP visualized by fluorescence indeed represents the acquisition of an SDS-resistant state seeded by wild-type Rnq1 aggregates.
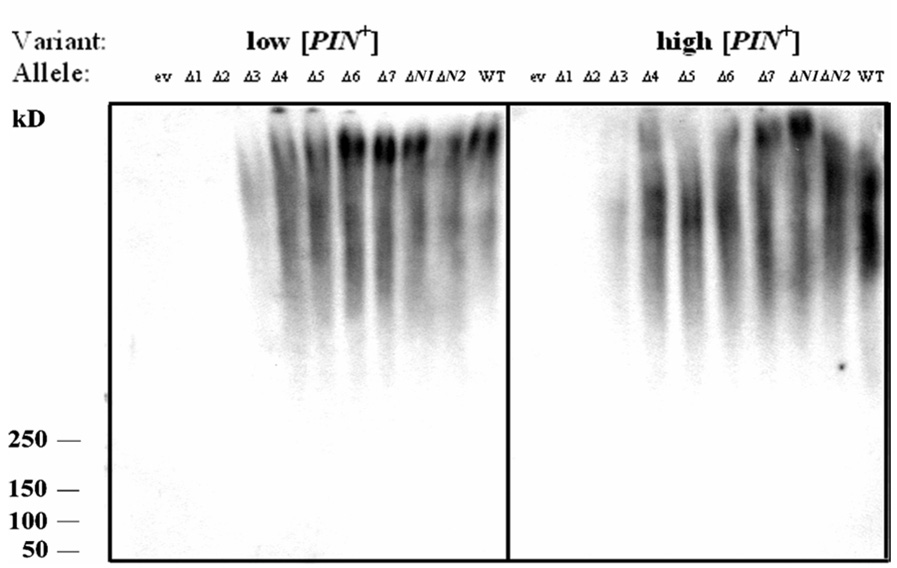
Fig. 3.
SDS resistant sub-particles formed by Rnq1 fragments fused to GFP in the presence of low and high [PIN+]. Lysates were subjected to SDS-AGE and immunoblotted with anti-GFP antibody. ev, empty vector. The monomer could be detected only for Rnq1-Δ1:GFP and Rnq1- Δ2:GFP upon a prolonged exposure time (data not shown). Irregularities of the intensity along the smears representing sub-particles are not reproducible. No SDS-resistant sub-particles were detected in [pin−] for all alleles (data not shown).
Propagation of [PIN+] by RNQ1 alleles on their own
The abilitiy to join pre-existing prion aggregates does not necessarily mean that an Rnq1 fragment can propagate the inherited prion state on its own. To distinguish which of the Rnq1-x:GFP fragments indeed propagates the prion state, [PIN+] was transferred to an rnq1-Δ strain harboring our rnq1-x:GFP constructs (Fig. 4) via cytoplasmic fusion concomitant with loss of the donor’s nucleus after mating (cytoduction, see Materials and Methods). During cytoduction, Rnq1-x:GFPs co-existed with aggregates of wild-type Rnq1 still present in the cytoplasm for some time after expelling the donor’s nucleus carrying the wild-type RNQ1 gene. Because RNQ1 alleles were cloned under the CUP1 promoter, the cytoduction and all subsequent manipulations were performed on media containing 50 µM CuSO4 so that the Rnq1-x:GFP fusion would be continually synthesized. We obtained 6 – 16 cytoductants expressing each of the rnq1-x:GFP alleles, but no longer expressing wild-type RNQ1 from each of the [pin−], high or low [PIN+] donors.
Fig. 4.
Genetic manipulations performed to analyze the ability of Rnq1 fragments to maintain [PIN+]. The rnq1-x alleles are expressed as GFP fusions on HIS-marked plasmids in the recipient. SUP35 overexpression is driven by the GAL promoter which is only turned on for the [PSI+] induction assay in cytoductants. Donors are L1751 ([pin−]), L1943 (low [PIN+]), L1749 (high [PIN+]); recipient is L2668; the wild-type RNQ1 parent is L1751. All strains are SUP35 [psi−]. Cytoductants were selected on SD-His- Ura + 50 µM Cu + 3 µg/ml cycloheximide.
These cytoductants were assayed for two [PIN+]-related phenotypes: the formation of Rnq1:GFP aggregates and the ability to facilitate [PSI+] induction following overexpression of SUP35. In addition, the cytoductants with fluorescent foci had SDS-resistant subparticles Rnq1-x (Fig. S1) similar to those shown in Fig. 3. We found that the high [PIN+] variant was transferred more efficiently than low [PIN+] to the Rnq1-x C-terminal truncations (Table 3). Indeed, even wild-type Rnq1:GFP was aggregated in fewer low [PIN+] than high [PIN+] cytoductants. It is also clear that the efficiency of [PIN+] transfer decreases as deletions are shortened from the C terminus. The aggregated state could be reliably transmitted from high [PIN+] to an Rnq1 fragment as short as 1-341 aa (Rnq1-Δ6, -Δ7, and wild-type); from low [PIN+], to a fragment as short as 1-372 aa (Rnq1-Δ7 and wild-type). In addition, occasional [PIN+] cytoductants were found among recipients expressing Rnq1-Δ5 (aa 1-321) and Δ4 (aa 1-289). These cytoductants were verified to contain the correct Rnq1-x:GFP fragment, no wild-type RNQ1, and to have the reported [PIN+] phenotype. Thus, it is clear that once these fragments (including Rnq1-Δ5 (aa 1-321) and Δ4 (aa 1-289)) inherit [PIN+] they can maintain it in the absence of wild-type Rnq1.
Table 3.
Maintenance of [PIN+] in rnq1-Δ pCUP:rnq1-x:GFP cytoductant
|
rnq1 allele |
Number of cytoductants obtained from the following donor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [pin−] |
High [PIN+] |
Low [PIN+] a) |
|||||||
| Total | GFP foci | [PSI+] induction | Total | GFP foci | [PSI+] induction | Total | GFP foci | ||
| no RNQ1 | 15 | 0 | 0 | 15 | 0 | 0 | 6 | 0 | |
| C | Δ4 | 9 | 1 | 1 | 12 | 1 | 1 | 9 | 0 |
| Δ5 | 16 | 0 | 0 | 14 | 2 | 2 | 9 | 0 | |
| Δ6 | 15 | 0 | 0 | 14 | 7 | 7 | 16 | 2 | |
| Δ7 | 14 | 0 | 0 | 15 | 8 | 10 | 15 | 6 | |
| WT | 14 | 1 | 1 | 14 | 12 | 11 | 16 | 10 | |
| N | ΔN2 | 10 | 0 | 0 | 11 | 6 | 3 | 7 | 5 |
| ΔN1 | 12 | 4 | 3 | 12 | 10 | 8 | 12 | 9 | |
A cytoductant was considered to have GFP foci if more than 10% of the fluorescent cells had aggregates. Three samples of ~100 cells were assayed for each cytoductant. [PSI+] induction was scored as an increase of Ade+ in response to overexpression of SUP35 (Fig. 5). “N” and “C” indicate N- and C-terminal deletions, respectively.
Transmission of low [PIN+] could not be scored by [PSI+] induction in the recipient strain background
Surprisingly, [PIN+] was only transferred with low efficiency to the N-terminal truncation Rnq1-ΔN2:GFP (aa 133-405) which contained the entire presumptive prion domain. In contrast, transfer of [PIN+] to the N-terminal truncation Rnq1-ΔN1:GFP (aa 172-405), that lacked motif A suggested to be important for aggregation in an earlier polymorphism study, was quite efficient (Resende et al. 2003). Interestingly, Rnq1-ΔN1:GFP did not respond differently to the two [PIN+] variants and produced aggregates even in some cytoductants from a [pin−] donor (compared to the almost zero background of aggregation in [pin−] cytoductants for all other alleles). This suggests Rnq1-ΔN1’s higher propensity to form [PIN+] spontaneously.
The [PSI+] induction assay is another way to score for [PIN+] in addition to GFP fluorescence. To facilitate controllable SUP35 overexpression required for this assay, each cytoductant inherited a galactose-inducible SUP35 construct used in earlier [PIN+] studies (Derkatch et al. 1997). Because [PSI+] results in loss-of-function of the translational termination factor Sup35 and consequent nonsense suppression (Ter-Avanesyan et al. 1994), the appearance of [PSI+] is scored by the rescue of adenine prototrophy in cytoductants bearing the nonsense mutation ade1-14 (Cox et al. 1988). While many cytoductants derived from the high [PIN+] donor displayed induced Ade+ papillation of the same density as the donor high [PIN+] strain (Fig. 5) this was not the case for low [PIN+]. Perhaps, the recipient genetic background was such that low [PIN+]-facilitated [PSI+] induction could not be reliably scored by the assay used (Table 3).
Fig. 5.
Induction of Ade+ papillation by overexpressed SUP35 in donors and representative cytoductants. Shown are patches on SD-Ade from non-inducing (upper) and inducing (lower) media featuring low and high SUP35 levels, respectively, as labeled. The donors of [pin−] (L1751), low [PIN+] (L1943) and high [PIN+] (L1749) are on the left. On the right are shown representative rnq1-Δ cytoductants harboring pCUP:RNQ1:GFP that received cytoplasm from low and high [PIN+]. Only high [PIN+] cytoductants produced an easily scorable increase of Ade+ papillation after SUP35 overexpression.
As expected, generally both or neither phenotype was present in a given cytoductant indicating the presence or absence of high [PIN+], respectively. The arbitrarily set 10% boundary for scoring cytoductants as containing GFP foci and the qualitative nature of the [PSI+] induction assay could be the cause of some of these exceptions.
Discussion
Sequence analysis
The goal of the molecular dissection of Rnq1 was to determine what primary structural elements are required for the acquisition of the prion state. We looked at the Rnq1 amino acid sequence in the light of the importance of oligopeptide repeats postulated for other prions (Prusiner et al. 1993; Liu and Lindquist 1999; Parham et al. 2001). While only one type of imperfect oligopeptide repeat is found in Sup35 (Kushnirov et al. 1988) and PrP (Oesch et al. 1985), Rnq1 contains different types of repeats (Fig.1b). It has been suggested that oligopeptide repeats of Sup35 mediate the interaction of its aggregated state with Hsp104 (Crist et al. 2003; Osherovich et al. 2004), a chaperone required for the maintenance of [PSI+] (Chernoff et al. 1995). Likewise, some of the Rnq1 repeats may be a target for chaperones shown to interact with Rnq1 (Bradley et al. 2002; Lopez et al. 2003; Aron et al. 2005). Taking a different view of the function of the repeats, we also note that [PIN+] is a template for the de novo formation of [PSI+] (Derkatch et al. 1997) and speculate that some of the repeats, when exposed on the surface of [PIN+] aggregates could specifically bind to, and stabilize, prionogenic Sup35 oligomers. Finally, we were intrigued to notice that, both in Sup35 and Rnq1, the position of the oligopeptide repeats could be easily revealed in hydrophobicity profiles, as the level of hydrophobicity is quite distinct from that of flanking sequence. Previous reports suggested that hydrophobic interactions are a driving force in oligomerization, preceding the establishment of the amyloid structure (Zanuy et al. 2003; Chen et al. 2005; Krishnan and Lindquist 2005; Nelson et al. 2005; Wu et al. 2005). For all these reasons, we used oligopeptide and tandem repeats as end points when designing C-terminal deletions. Removing them one by one we attempted to reveal those that are crucial for [PIN+] propagation.
Limitations of the deletion analysis
Of course, differences in prion conversion could be observed between two neighboring deletions not because of one extra oligopeptide repeat’s presence but simply because of the difference in the protein’s length. Indeed, Ross et al (2005) demonstrated that a high QN content, not any particular sequence was the main requirement for Sup35 and Ure2 prionogenicity. Therefore, the amount of QN retained in Rnq1 fragments may explain their ability to maintain [PIN+]. While, in general we can not rule out this explanation, it does not seem to be applicable for all of the Rnq1 fragments. For example, Rnq-Δ4 and -Δ3 are distinguished from each other by a 29-aa segment having only 6 Q’s and 1 N; Rnq-Δ5 and -Δ6 are distinguished by a 19-bp segment having no Q’s and only 3 N’s. Thus the distinguishing sequence for these pairs of neighboring deletions is not QN rich yet they differ in the efficiency of [PIN+] inheritance (Table 2 and Table 3). In these two examples, the frequency of Q and N in the extensions is as low as in the non-QN-rich domain. It, therefore, appears that some other features of the amino acid composition contribute to the higher prionogenicity of longer Rnq1 fragments in addition to the QN content. These could be the oligopeptide repeats and the non-random alteration of polar and hydrophobic stretches throughout Rnq1.
Motifs covered by C-terminal deletions
The first striking result of our analysis is that a shorter region is sufficient for joining pre-existing aggregates (Table 2 and Fig. 3) than is required to be efficiently infected with [PIN+] and to maintain it following the loss of the wild-type Rnq1 (Table 3). Perhaps, the three hydrophobic repeats retained in Rnq-Δ4:GFP (Fig.1) suffice to position and polymerize the protein on wild-type Rnq1 aggregates, but the efficient maintenance of the prion state in the absence of wild-type Rnq1 requires all four “FLASAS” repeats (Rnq1-Δ6). We hypothesize that the number, high hydrophobicity index and spacing of these motifs might be crucial for aggregation and prion propagation. Indeed, chaperones are known to recognize exposed hydrophobic surfaces on protein aggregates (Stirling et al. 2003). We further speculate that such a dependence of Rnq1 prionization on chaperones may make it sensitive to the environment and the intracellular level of protein misfolding. This would offer a mechanism to regulate the so far unknown function of Rnq1 as well as a series of downstream effects like facilitating the appearance of two other yeast prions, [PSI+] and [URE3] (Derkatch et al. 2001), and the aggregation of other poly-Q- rich proteins (Meriin et al. 2003; Osherovich et al. 2004).
We did not see any difference in the efficiency of decoration of pre-existing aggregates between low and high [PIN+] for all Rnq1 fragments (Table 2) while the efficiency of transfer of these two variants was dramatically different (Table 3). The transfer efficiency could be encrypted in the variant-specific conformation of prion aggregates per se as has been previously proposed for [PSI+] (Tanaka et al. 2004; Krishnan and Lindquist 2005).
The addition of a short sequence containing a spaced tandem repeat (motif G; compare Rnq1-Δ6 and -Δ7) appears to increase the infectivity of both high and low [PIN+] (Table 3). The sequence of motif G suggests its ability to form a mini β sheet and further stabilize the amyloid structure via “polar zipper”-like interactions (Perutz et al. 1993). In the light of hypotheses proposed in a structural study of a short Sup35 peptide (Nelson et al. 2005), this exemplifies the necessity of a balance between hydrophobic and polar interactions for the formation of properly folded and replicated prion aggregates.
N-terminal deletions
The results obtained for the two N-terminal deletions Rnq1-ΔN2 and Rnq1-ΔN1 were surprising. Rnq1-ΔN1:GFP, the shorter fragment, formed [PIN+] quite efficiently (Table 3). Because Rnq-ΔN1:GFP produced [PIN+] in some cytoductants that received [pin−] cytoplasm, we suggest that Rnq1-ΔN1:GFP frequently folds de novo into [PIN+] aggregates. It seemed contradictory that Rnq1-ΔN2:GFP, which is longer, did not increase the spontaneous appearance of [PIN+]. One possibility is that this is due to a lower level of the Rnq1-ΔN2:GFP protein (Fig. 1d) which might reflect a lower stability of Rnq1-ΔN2:GFP lacking the N terminus. The difference could be associated with motif A, a QG stretch deleted in Rnq1-ΔN1:GFP (Fig. 1). Without this motif, Rnq1-ΔN1:GFP might aggregate faster, bypassing protein misfolding control and associated degradation. In contrast, Rnq1-ΔN2:GFP, containing motif A, might aggregate slowly allowing misfolded precursors to be recognized and eliminated by the misfolding control machinery. Perhaps, wild-type Rnq1:GFP and [PIN+]-competent C-terminal deletions that also retain motif A are stabilized by other regions of the N terminal domain of Rnq1 that are missing in Rnq1-ΔN2:GFP. Our results indicate the involvement of the non-QN rich region in [PIN+] propagation.
Significance for structural studies
A major direction of prion studies is the determination of the prion structure. While significant progress has been made for [PSI+] (Balbirnie et al. 2001; Krishnan and Lindquist 2005; Nelson et al. 2005);(Shewmaker et al. 2006) and [URE3] (Bousset et al. 2002) (Baxa et al. 2003) (Fay et al. 2005), such studies will be more difficult for [PIN+] partially because aggregates of wild-type Rnq1 and its C-terminal domain are globular (Vitrenko et al. 2006) unlike those of the other prions which have fiber-like aggregates (Glover et al. 1997; Taylor et al. 1999). This makes Rnq1 less amenable to structural studies always benefiting from a more ordered composition of the substrate. In view of this, one of the shorter Rnq1 fragments presented here could be a better model for the structural analyses in vitro, as it is expected to aggregate into higher ordered species. Indeed, most information on prion structure has been collected from shorter fragments of prion domains (Balbirnie et al. 2001; Laws et al. 2001; Nelson et al. 2005). The molecular dissection of Rnq1 and in vivo characterization of the resulting fragments is an important step towards understanding prion phenomena both from basic and applied perspectives.
Supplementary Material
Fig. S1 Cytoductants with no wild-type Rnq1 that were scored as [PIN+] have SDS-resistant subparticles of Rnq1:x-GFP. Lysates of representative cytoductants scored as [pin−] or high [PIN+] as labeled were subjected to SDS-AGE and immunoblotted with anti-GFP antibody. Lysates were made from cultures containing GFP aggregates in more than 50° of the cells. The Rnq1-x:GFP fragments expressed in the cytoductants are as labeled.
Acknowledgments
We thank Irina Derkatch for the pID116 plasmid. This work was supported by a grant from the National Institutes of Health (GM56350 to S.W.L).
References
- Aron R, Lopez N, Walter W, Craig EA, Johnson J. In vivo bipartite interaction between the Hsp40 Sis1 and Hsp70 in Saccharomyces cerevisiae. Genetics. 2005;169:1873–1882. doi: 10.1534/genetics.104.037242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev S, Liebman SW. Specificity of prion assembly in vivo. [PSI+] and [PIN+] form separate structures in yeast. J Biol Chem. 2004;279:51042–51048. doi: 10.1074/jbc.M410611200. [DOI] [PubMed] [Google Scholar]
- Balbirnie M, Grothe R, Eisenberg DS. An amyloid-forming peptide from the yeast prion Sup35 reveals a dehydrated beta-sheet structure for amyloid. Proc Natl Acad Sci U S A. 2001;98:2375–2380. doi: 10.1073/pnas.041617698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxa U, Taylor KL, Wall JS, Simon MN, Cheng N, Wickner RB, Steven AC. Architecture of Ure2p prion filaments: the N-terminal domains form a central core fiber. J Biol Chem. 2003;278:43717–43727. doi: 10.1074/jbc.M306004200. [DOI] [PubMed] [Google Scholar]
- Borchsenius AS, Wegrzyn RD, Newnam GP, Inge-Vechtomov SG, Chernoff YO. Yeast prion protein derivative defective in aggregate shearing and production of new 'seeds'. Embo J. 2001;20:6683–6691. doi: 10.1093/emboj/20.23.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset L, Thual C, Belrhali H, Morera S, Melki R. Structure and assembly properties of the yeast prion Ure2p. C R Biol. 2002;325:3–8. doi: 10.1016/s1631-0691(02)01385-9. [DOI] [PubMed] [Google Scholar]
- Bradley ME, Edskes HK, Hong JY, Wickner RB, Liebman SW. Interactions among prions and prion "strains" in yeast. Proc Natl Acad Sci U S A. 2002;99 Suppl 4:16392–16399. doi: 10.1073/pnas.152330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley ME, Liebman SW. Destabilizing interactions among [PSI(+)] and [PIN(+)] yeast prion variants. Genetics. 2003;165:1675–1685. doi: 10.1093/genetics/165.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley ME, Liebman SW. The Sup35 domains required for maintenance of weak, strong or undifferentiated yeast [PSI+] prions. Mol Microbiol. 2004;51:1649–1659. doi: 10.1111/j.1365-2958.2003.03955.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Krause G, Reif B. Structure and orientation of peptide inhibitors bound to beta-amyloid fibrils. J Mol Biol. 2005;354:760–776. doi: 10.1016/j.jmb.2005.09.055. [DOI] [PubMed] [Google Scholar]
- Chernoff YO. Mutation processes at the protein level: is Lamarck back? Mutat Res. 2001;488:39–64. doi: 10.1016/s1383-5742(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Derkach IL, Inge-Vechtomov SG. Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr Genet. 1993;24:268–270. doi: 10.1007/BF00351802. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne WE, Magasanik B. Regulation of nitrogen assimilation in Saccharomyces cerevisiae: roles of the URE2 and GLN3 genes. J Bacteriol. 1988;170:708–713. doi: 10.1128/jb.170.2.708-713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci U S A. 1997;94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BS, Tuite MF, McLaughlin CS. The psi factor of yeast: a problem in inheritance. Yeast. 1988;4:159–178. doi: 10.1002/yea.320040302. [DOI] [PubMed] [Google Scholar]
- Crist CG, Nakayashiki T, Kurahashi H, Nakamura Y. [PHI+], a novel Sup35-prion variant propagated with non-Gln/Asn oligopeptide repeats in the absence of the chaperone protein Hsp104. Genes Cells. 2003;8:603–618. doi: 10.1046/j.1365-2443.2003.00661.x. [DOI] [PubMed] [Google Scholar]
- DePace AH, Santoso A, Hillner P, Weissman JS. A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell. 1998;93:1241–1252. doi: 10.1016/s0092-8674(00)81467-1. [DOI] [PubMed] [Google Scholar]
- Derkatch IL. (unpublished) In: [Google Scholar]
- Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: the story of [PIN(+)] Cell. 2001;106:171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics. 1997;147:507–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Uptain SM, Outeiro TF, Krishnan R, Lindquist SL, Liebman SW. Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proc Natl Acad Sci U S A. 2004;101:12934–12939. doi: 10.1073/pnas.0404968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay N, Redeker V, Savistchenko J, Dubois S, Bousset L, Melki R. Structure of the prion Ure2p in protein fibrils assembled in vitro. J Biol Chem. 2005;280:37149–37158. doi: 10.1074/jbc.M506917200. [DOI] [PubMed] [Google Scholar]
- Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 1997;89:811–819. doi: 10.1016/s0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Harrison PM, Gerstein M. A method to assess compositional bias in biological sequences and its application to prion-like glutamine/asparagine-rich domains in eukaryotic proteomes. Genome Biol. 2003;4:R40. doi: 10.1186/gb-2003-4-6-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G, Jones G, Masison DC. Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc Natl Acad Sci U S A. 2002;99:9936–9941. doi: 10.1073/pnas.152333299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R, Lindquist SL. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature. 2005;435:765–772. doi: 10.1038/nature03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem. 2003;278:49636–49643. doi: 10.1074/jbc.M307996200. [DOI] [PubMed] [Google Scholar]
- Kushnirov VV, Ter-Avanesyan MD, Telckov MV, Surguchov AP, Smirnov VN, Inge-Vechtomov SG. Nucleotide sequence of the SUP2 (SUP35) gene of Saccharomyces cerevisiae. Gene. 1988;66:45–54. doi: 10.1016/0378-1119(88)90223-5. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laws DD, Bitter HM, Liu K, Ball HL, Kaneko K, Wille H, Cohen FE, Prusiner SB, Pines A, Wemmer DE. Solid-state NMR studies of the secondary structure of a mutant prion protein fragment of 55 residues that induces neurodegeneration. Proc Natl Acad Sci U S A. 2001;98:11686–11690. doi: 10.1073/pnas.201404298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Lindquist S. Oligopeptide-repeat expansions modulate 'protein-only' inheritance in yeast. Nature. 1999;400:573–576. doi: 10.1038/23048. [DOI] [PubMed] [Google Scholar]
- Lopez N, Aron R, Craig EA. Specificity of class II Hsp40 Sis1 in maintenance of yeast prion [RNQ+] Mol Biol Cell. 2003;14:1172–1181. doi: 10.1091/mbc.E02-09-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddelein ML, Wickner RB. Two prion-inducing regions of Ure2p are nonoverlapping. Mol Cell Biol. 1999;19:4516–4524. doi: 10.1128/mcb.19.6.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masison DC, Wickner RB. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science. 1995;270:93–95. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, Miliaras NB, Kazantsev A, Chernoff YO, McCaffery JM, Wendland B, Sherman MY. Aggregation of expanded polyglutamine domain in yeast leads to defects in endocytosis. Mol Cell Biol. 2003;23:7554–7565. doi: 10.1128/MCB.23.21.7554-7565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama H, Edskes HK, Wickner RB. [URE3] prion propagation in Saccharomyces cerevisiae: requirement for chaperone Hsp104 and curing by overexpressed chaperone Ydj1p. Mol Cell Biol. 2000;20:8916–8922. doi: 10.1128/mcb.20.23.8916-8922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsoulis G, Winston F, Boeke JD. The SPT10 and SPT21 genes of Saccharomyces cerevisiae. Genetics. 1994;136:93–105. doi: 10.1093/genetics/136.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B, Westaway D, Walchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE, et al. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Osherovich LZ, Cox BS, Tuite MF, Weissman JS. Dissection and design of yeast prions. PLoS Biol. 2004;2:E86. doi: 10.1371/journal.pbio.0020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham SN, Resende CG, Tuite MF. Oligopeptide repeats in the yeast protein Sup35p stabilize intermolecular prion interactions. Embo J. 2001;20:2111–2119. doi: 10.1093/emboj/20.9.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino MM, Liu JJ, Glover JR, Lindquist S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273:622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. Embo J. 1996;15:3127–3134. [PMC free article] [PubMed] [Google Scholar]
- Perutz MF, Staden R, Moens L, De Baere I. Polar zippers. Curr Biol. 1993;3:249–253. doi: 10.1016/0960-9822(93)90174-m. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. In: Prion Biology and Diseases. 2nd edn. Prusine SB, editor. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2004. pp. 1–61. [Google Scholar]
- Prusiner SB, Fuzi M, Scott M, Serban D, Serban H, Taraboulos A, Gabriel JM, Wells GA, Wilesmith JW, Bradley R, et al. Immunologic and molecular biologic studies of prion proteins in bovine spongiform encephalopathy. J Infect Dis. 1993;167:602–613. doi: 10.1093/infdis/167.3.602. [DOI] [PubMed] [Google Scholar]
- Resende CG, Outeiro TF, Sands L, Lindquist S, Tuite MF. Prion protein gene polymorphisms in Saccharomyces cerevisiae. Mol Microbiol. 2003;49:1005–1017. doi: 10.1046/j.1365-2958.2003.03608.x. [DOI] [PubMed] [Google Scholar]
- Rogers M, Yehiely F, Scott M, Prusiner SB. Conversion of truncated and elongated prion proteins into the scrapie isoform in cultured cells. Proc Natl Acad Sci U S A. 1993;90:3182–3186. doi: 10.1073/pnas.90.8.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED, Baxa U, Wickner RB. Scrambled prion domains form prions and amyloid. Mol Cell Biol. 2004;24:7206–7213. doi: 10.1128/MCB.24.16.7206-7213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED, Edskes HK, Terry MJ, Wickner RB. Primary sequence independence for prion formation. Proc Natl Acad Sci U S A. 2005a;102:12825–12830. doi: 10.1073/pnas.0506136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED, Minton A, Wickner RB. Prion domains: sequences, structures and interactions. Nat Cell Biol. 2005b;7:1039–1044. doi: 10.1038/ncb1105-1039. [DOI] [PubMed] [Google Scholar]
- Schlumpberger M, Prusiner SB, Herskowitz I. Induction of distinct [URE3] yeast prion strains. Mol Cell Biol. 2001;21:7035–7046. doi: 10.1128/MCB.21.20.7035-7046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpberger M, Wille H, Baldwin MA, Butler DA, Herskowitz I, Prusiner SB. The prion domain of yeast Ure2p induces autocatalytic formation of amyloid fibers by a recombinant fusion protein. Protein Sci. 2000;9:440–451. doi: 10.1110/ps.9.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell LC, Sunde M, Blake CC. The molecular basis of amyloidosis. Cell Mol Life Sci. 1997;53:871–887. doi: 10.1007/s000180050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmaker F, Wickner RB, Tycko R. Amyloid of the prion domain of Sup35p has an in-register parallel beta-sheet structure. Proc Natl Acad Sci U S A. 2006;103:19754–19759. doi: 10.1073/pnas.0609638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Lindquist S. Destruction or potentiation of different prions catalyzed by similar Hsp104 remodeling activities. Mol Cell. 2006;23:425–438. doi: 10.1016/j.molcel.2006.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer N, Lindquist S. Rnq1: an epigenetic modifier of protein function in yeast. Mol Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- Spassov S, Beekes M, Naumann D. Structural differences between TSEs strains investigated by FT-IR spectroscopy. Biochim Biophys Acta. 2006;1760:1138–1149. doi: 10.1016/j.bbagen.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Stirling PC, Lundin VF, Leroux MR. Getting a grip on non-native proteins. EMBO Rep. 2003;4:565–570. doi: 10.1038/sj.embor.embor869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- Taneja V, Liebman SW. 2006 Submitted. [Google Scholar]
- Taylor KL, Cheng N, Williams RW, Steven AC, Wickner RB. Prion domain initiation of amyloid formation in vitro from native Ure2p. Science. 1999;283:1339–1343. doi: 10.1126/science.283.5406.1339. [DOI] [PubMed] [Google Scholar]
- Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics. 1994;137:671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrenko YA, Gracheva EO, Richmond JE, Liebman SW. Visualization of aggregation of the RNQ1 prion domain and cross-seeding interactions with Sup35NM. J Biol Chem. 2006;282:1779–1787. doi: 10.1074/jbc.M609269200. Epub 2006 Nov 22. [DOI] [PubMed] [Google Scholar]
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Roberts BT, Baxa U, Pierce MM, Ross ED, Brachmann A. Prions: proteins as genes and infectious entities. Genes Dev. 2004;18:470–485. doi: 10.1101/gad.1177104. [DOI] [PubMed] [Google Scholar]
- Wu C, Lei H, Duan Y. The role of Phe in the formation of well-ordered oligomers of amyloidogenic hexapeptide (NFGAIL) observed in molecular dynamics simulations with explicit solvent. Biophys J. 2005;88:2897–2906. doi: 10.1529/biophysj.104.055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanuy D, Ma B, Nussinov R. Short peptide amyloid organization: stabilities and conformations of the islet amyloid peptide NFGAIL. Biophys J. 2003;84:1884–1894. doi: 10.1016/S0006-3495(03)74996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhouravleva G, Frolova L, Le Goff X, Le Guellec R, Inge-Vechtomov S, Kisselev L, Philippe M. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. Embo J. 1995;14:4065–4072. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Cytoductants with no wild-type Rnq1 that were scored as [PIN+] have SDS-resistant subparticles of Rnq1:x-GFP. Lysates of representative cytoductants scored as [pin−] or high [PIN+] as labeled were subjected to SDS-AGE and immunoblotted with anti-GFP antibody. Lysates were made from cultures containing GFP aggregates in more than 50° of the cells. The Rnq1-x:GFP fragments expressed in the cytoductants are as labeled.