Abstract
Protein arginine deiminase 4 (PAD4) is an enzyme that hydrolyzes peptidyl arginine residues to form citrulline and ammonia. This enzyme has been implicated in several disease states, e.g. rheumatoid arthritis, and therefore represents a unique target for the development of a novel therapeutic. A solution-phase synthesis of Cl-amidine, the most potent PAD4 inactivator described to date, has been developed. This synthesis proceeds in 80% yield over 4 steps at a significantly (12-fold) lower cost.
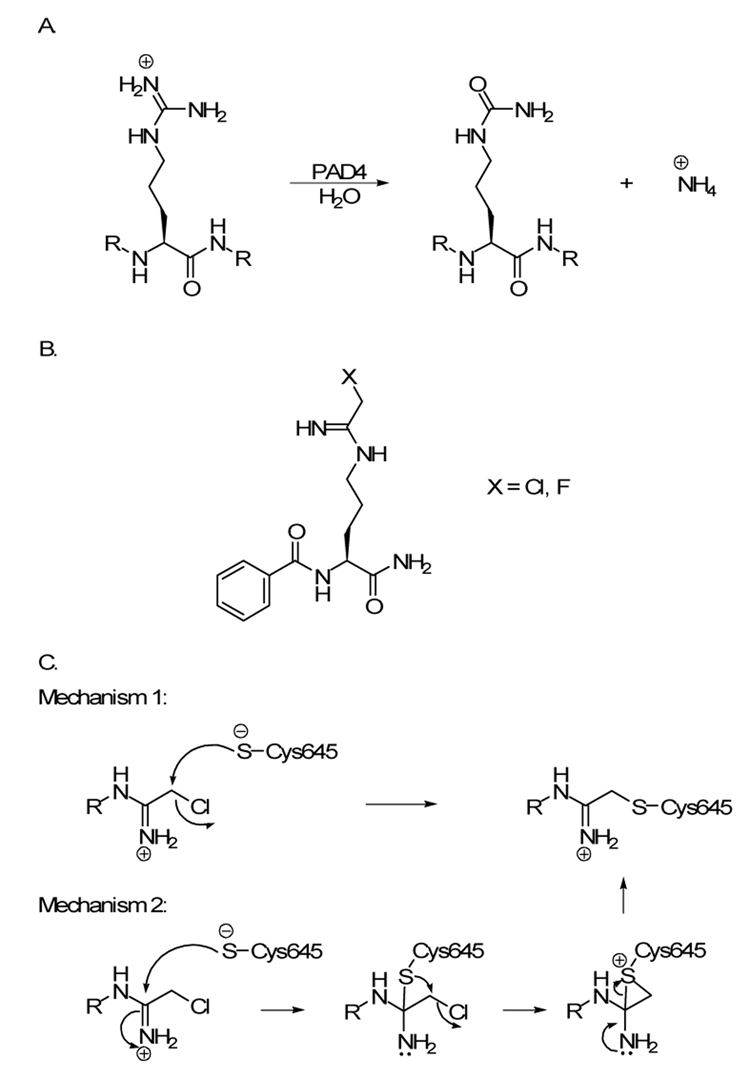
Although long considered a relatively minor post-translational modification, protein citrullination (Figure 1) has recently come to the forefront because this modification appears to be abnormally increased in numerous human diseases, including: multiple sclerosis,1 Alzheimer’s disease,2 primary open-angle glaucoma,3,4 various adenocarcinoas,5 and rheumatoid arthritis (RA).6,7. In particular, the links between abnormal protein citrullination and RA are especially strong and a significant body of evidence suggests that the enzymes responsible for this modification, i.e. the Protein Arginine Deiminases (PADs), are dysregulated in RA.6,7 This evidence includes the fact that mutations within the gene encoding PAD4, a PAD isozyme, are associated with the onset and progression of RA in Japanese, Korean, and Caucasian populations.8–11 Additional evidence includes the facts that the levels of PADs 2 and 4 are increased in the RA synovium and RA patients produce autoantibodies that recognize citrulline containing proteins.12–15 The presence of these autoantibodies in patient’s sera is highly significant because they can be detected before the onset of overt clinical symptoms and their presence in patient’s sera are correlated with a more severe disease course.14–16
Figure 1.
(A) PAD4 hydrolyzes peptidyl-arginine to form citrulline and ammonia. (B) Structure of haloacetamidine-based inactivators of PAD4. (C) Two possible mechanisms of PAD4 inactivation.
The apparent causal relationship between PAD dysregulation and RA disease onset thereby suggested these enzymes as a novel therapeutic target for RA that could overcome the limitations of current therapies, which focus on disease management17 rather than the treatment of an underlying cause of the disease. Towards that goal, we have recently described the synthesis and characterization of a series of haloacetamidine containing mechanism based inactivators that are the most potent PAD inhibitors described to date.18,19
The two most potent compounds, N-α-benzoyl-N5-(2-fluoro-1-iminoethyl)-L-ornithine amide (F-amidine; kinact/KI = 3000 M−1 min−1)19 and N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide (Cl-amidine; kinact/KI =13,000 M−1 min−1)18, inactivate PAD4 by covalently modifying an active site cysteine (Cys645) that is involved in nucleophilic catalysis. Although the exact mechanism of inactivation has yet to be elucidated, it presumably proceeds through one of two routes (Figure 1).
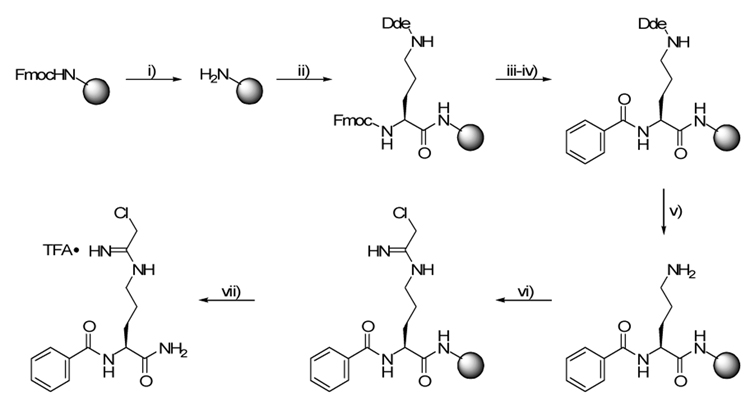
The initial syntheses of F- and Cl-amidine were carried out on the solid-phase (Rink AM amide resin) and proceeded in 7 steps with an overall yield of 70% (Scheme 1).18,19 As with all solid-phase syntheses, this route was attractive because it mitigated the need to isolate intermediates by chromatography. In addition to serving as a solid support, the Rink resin also afforded the primary carboxamide upon acidic cleavage. While this method does generate the desired product in reasonable yield, there are considerable drawbacks, the most significant being the cost of the starting materials (~US$950/g).
Scheme 1.
Reagents and conditions: i) 20% piperidine, DMF; ii) HBTU, HOBt, 0.4 M NMM, Fmoc-Orn(Dde)-OH, DMF; iii) 20% piperidine, DMF; iv) BzCl, 0.4 M NMM, DMF; v) 2% hydrazine, DMF; vi) ethyl chloroacetimidate (HCl), Et3N, DMF; vii) TFA/TIS/H2O (95/2.5/2.5), 70%.
The major cost contributor is an orthogonally protected ornithine (Fmoc-Orn(Dde)-OH) that must be used in large excess (4-fold) to ensure quantitative coupling to the resin – this stoichiometry means a 75% loss from the outset. A second drawback of the solid-phase synthesis arises when the compound is cleaved from the resin. During this step, the acidic cleavage conditions inevitably result in some unintended hydrolysis of the primary amide. Not only does this reduce the overall yield but it necessitates the separation of the carboxylic acid and amide derivatives, which is tedious and time consuming. If either F- or Cl-amidine are to become viable therapeutic candidates, a more practical synthesis is necessary; and as we moved forward to mouse trials, the need for a more cost effective method became clear. To this end, we have designed a solution-phase strategy to synthesize the more potent of these compounds, i.e. Cl-amidine in fewer steps, with higher yield, and at a significantly lower cost.
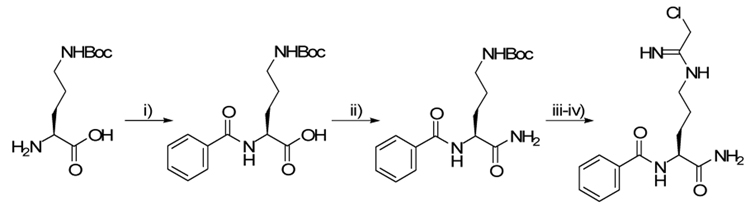
The solution-phase synthesis of Cl-amidine proceeded in 4 steps with an overall yield of 80% (Scheme 2). This route was particularly attractive because, similarly to the solid phase synthetic route, this procedure mitigates the need for time consuming intermediate chromatography because all of the intermediates are isolated and purified by simple extractive work-ups.
Scheme 2.
Reagents and condition: i) BzCl, NaOH, H2O, 90%; ii) HOTT, DIPEA, NH4Cl, DMF, 89%; iii) TFA, 100%; iv) ethyl chloroacetamidate (HCl), 100%.
The first challenge in this synthesis is differentiating between the two amino groups of ornithine. This challenge was easily met by choosing the mono-protected ornithine, H-Orn(Boc)-OH, a commodity chemical that is available at a considerably lower (~5-fold) cost than the ornithine derivative used previously. Benzoylation of the α-amine was accomplished with benzoyl chloride and afforded pure BzNH-Orn(Boc)-OH in 90% yield.20 Next, we chose to convert the carboxylic acid into a primary amide. Using methodology developed by Nájera et al21 conversion to the primary amide was achieved using the coupling reagent, HOTT, with ammonium chloride.22 In the final steps, the amidine moiety was installed on the δ-amine. Removal of the Boc-group with neat TFA proceeded in quantitative yield,23 thus enabling the coupling of the free amine to ethyl chloroaceamidate hydrochloride in the presence of triethylamine.24 This coupling procedure also proceeded quantitatively to afford the title compound in a total of four steps and 80% yield.
In conclusion, we have developed an efficient solution-phase synthesis of Cl-amidine. Using this method, the inactivator can be produced in fewer steps, with higher yields, and at a fraction of the previous cost (~US$75/g).
Supplementary Material
Acknowledgments
We thank the NIH (GM079357) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. J. Neurochem. 2002;81:335–343. doi: 10.1046/j.1471-4159.2002.00834.x. [DOI] [PubMed] [Google Scholar]
- 2.Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, Murayama S, Asaga H, Toda T, Kimura N, Maruyama N. J Neurosci Res. 2005;80:120–128. doi: 10.1002/jnr.20431. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya SK, Crabb JS, Bonilha VL, Gu X, Takahara H, Crabb JW. Invest Ophthalmol Vis Sci. 2006;47:2508–2514. doi: 10.1167/iovs.05-1499. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya SK, Bhat MB, Takahara H. Curr Eye Res. 2006;31:1063–1071. doi: 10.1080/02713680600991437. [DOI] [PubMed] [Google Scholar]
- 5.Chang X, Han J. Mol. Carcinog. 2006;45:183–196. doi: 10.1002/mc.20169. [DOI] [PubMed] [Google Scholar]
- 6.Vossenaar ER, Van Venrooij WJ. Arthritis Res. Ther. 2004;6:107–111. doi: 10.1186/ar1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono M, Ohtsuki M, Furukawa H, Yoshino S, Yukioka M, Tohma S, Matsubara T, Wakitani S, Teshima R, Nishioka Y, Sekine A, Iida A, Takahashi A, Tsunoda T, Nakamura Y, Yamamoto K. Nat. Genet. 2003;34:395–402. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- 9.Kang CP, Lee HS, Ju H, Cho H, Kang C, Bae SC. Arthritis Rheum. 2006;54:90–96. doi: 10.1002/art.21536. [DOI] [PubMed] [Google Scholar]
- 10.Lee YH, Rho YH, Choi SJ, Ji JD, Song GG. Rheumatol Int. 2007;27:827–833. doi: 10.1007/s00296-007-0320-y. [DOI] [PubMed] [Google Scholar]
- 11.Plenge RM, Padyukov L, Remmers EF, Purcell S, Lee AT, Karlson EW, Wolfe F, Kastner DL, Alfredsson L, Altshuler D, Gregersen PK, Klareskog L, Rioux JD. Am J Hum Genet. 2005;77:1044–1060. doi: 10.1086/498651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, Zendman AJ, Harris HE. Arthritis Res Ther. 2005;7:R458–R467. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, Vincent C, Nachat R, Yamada M, Takahara H, Simon M, Guerrin M, Serre G. Arthritis Rheum. 2007;56:3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 14.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. J. Clin. Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, Uffmann M, Smolen JS. Rheumatology (Oxford) 2007;46:342–349. doi: 10.1093/rheumatology/kel237. [DOI] [PubMed] [Google Scholar]
- 17.Smolen JS, Steiner G. Nat. Rev. Drug. Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, Thompson PR. Biochemistry. 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Knuckley B, Lee YH, Stallcup MR, Thompson PR. J Am Chem Soc. 2006;128:1092–1093. doi: 10.1021/ja0576233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synthesis of N-α-benzoyl-N5-(tert-butoxycarbonyl)-Lornithine. N5-(tert-butoxycarbonyl)-L-ornithine (929 mg, 4.0 mmol) was dissolved in a biphasic mixture of aqueous NaOH (3.5 M, 1.16 mL, 4.0 mmol) and ether (4 mL). The mixture was cooled to 0 °C and alternating portions of benzoyl chloride (4.0 mmol, 0.464 mL) and aqueous NaOH (8.5 M, 4.0 mmol, 0.464 mL) were added every 5 mins for 30 mins. The reaction mixture was allowed to warm to rt and stir overnight. The reaction was acidified (pH = 1) with conc. HCl and extracted with dichloromethane. The organics were combined, washed with brine, dried over MgSO4 and concentrated to afford the product as a white powder (1.2 g, 90%). 1H NMR (D2O): δ 7.60 (d, 2H), 7.45-7.30 (m, 3H), 4.15 (m, 1H), 2.85 (m, 2H), 1.8-1.3 (m, 4H), 1.15 (s, 9H). 13C NMR (D2O): δ 179.05, 170.34, 158.34, 133.65, 132.26, 128.87, 127.28, 80.90 55.64, 39.83, 29.17, 27.80, 25.85. HRMS (C17H25N2O5+): calculated 337.1763, observed 337.1761.
- 21.Bailen MA, Chinchilla R, Dodsworth DJ, Najera C. Tetrahedron Letters. 2000;41:2809–9813. [Google Scholar]
- 22.Synthesis of N-α-benzoyl-N5-(tert-butoxycarbonyl)-L-ornithine amide. N-α-benzoyl-N5-(tert-butoxycarbonyl)-L-ornithine (420 mg, 1.24 mmol), HOTT (690 mg, 1.86 mmol) and DIPEA (0.44 mL, 2.5 mmol) were dissolved in DMF (5 mL). After stirring for 10 min, ammonium chloride (135 mg, 2.5 mmol) was added and stirring continued for 45 min. The reaction was partitioned between EtOAc (25 mL) and brine (60 mL). The layers were separated and the brine extracted twice more with EtOAc. The organics were conbined and washed with 2N HCl (2 × 12 mL), water (2 × 12 mL), saturated NaHCO3 (2 × 12), water (3 × 12 mL), and brine (12 mL). The organics were then dried over MgSO4 and concentrated to afford the product as a white powder (370 mg, 89%). 1H NMR (DMSO-d6): δ 8.28 (d, 1H), 7.85 (d, 2H), 7.6-7.4 (m, 3H), 7.35 (bs, 1H), 7.0 (bs, 1H), 6.78 (bs, 1H), 4.35 (m, 1H), 2.85 (m, 2H), 1.8-1.4 (m, 4H), 1.25 (s, 9H). 13C NMR (DMSO-d6): δ 174.54, 167.0, 156.29, 134.89, 131.92, 128.84, 128.17, 78.1, 53.65, 29.72 28.95, 27.1. HRMS (C17H26N3O4+): calculated 336.1923, observed 336.1927.
- 23.Synthesis of N-α-benzoyl-L-ornithine amide. N-α-benzoyl-N5-(tert-butoxycarbonyl)-L-ornithine amide (220 mg, 0.66 mmol) was dissolved in cold TFA (6 mL) and stirred at 0 °C for 45 min. After warming to rt, the TFA was removed and the residue dissolved in water (5 mL) and washed with ether (5 mL) before being lyophilized. 1H NMR (D2O): δ 7.59 (m, 2H), 7.4-7.2 (m, 3H), 4.25 (m, 1H), 2.85 (m, 2H), 1.9-1.5 (m, 4H) ppm. 13C NMR (D2O): δ 176.42, 171.06, 132.83, 132.63, 128.88, 127.40, 53.77, 38.99, 28.06, 23.59. HRMS (C12H18N3O2+): calculated 236.1399, observed 236.1397.
- 24.Synthesis of Cl-amidine. N-α-benzoy)-L-ornithine amide-TFA (247 mg, 0.7 mmol) was dissolved in MeOH (2 mL). Triethyl amine (0.29 mL, 2.1 mmol) and ethyl chloroacetimidate-HCl (221 mg, 1.4 mmol) were added and the mixture stirred overnight. The reaction was quenched with water (5 mL) and acidified with TFA. The product was isolated by RP-HPLC as a white powder. Spectral data was consistent with previous reports.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.