Abstract
A concise and efficient cascade-based total synthesis of artochamins F, H, I, and J is described. The potential biogenetic connection between artochamin F, or a derivative thereof, and artochamins H, I, and J, through an unusual formal [2+2] cycloaddition process, was shown to be feasible. An alternative mechanism for this transformation is also proposed.
1. Introduction
The design and development of cascade reactions is a rapidly expanding area of research within the realm of chemical synthesis.1 The virtues of such synthetic sequences are brilliantly reflected by some of the early developments in the field such as Sir Robert Robinson’s total synthesis of tropinone (1917)2 and W. S. Johnson’s total synthesis of progesterone (1971).3 During our own endeavors in total synthesis we have demonstrated the power of cascade reactions beginning with the total synthesis of the endiandric acids (1982)4 and followed by numerous other examples, including the bisorbicillinoids (1999),5 CP-molecules (1999),6 colombiasin A (2001),7 hybocarpone (2001),8 diazonamide A (2003),9–10 1-Omethyl lateriflorone (2003),11 thiostrepton (2004),12 azaspiracids-1,13 -214a and -314b (2006), bisanthraquinones (2006),15 and biyouyanagin (2007).16 Cascade reactions are not only attractive due to their aesthetically pleasing nature, but also because of their capacity to rapidly increase molecular complexity, often efficiently and economically, from relatively simple starting materials. Furthermore, they frequently do so with savings in reagents and solvents, not to mention energy and time. As such they constitute part of the campaign of green chemistry to save and sustain the environment. In this article, we describe a detailed account of the synthesis of artochamins F, H, I, and J through cascade reactions involving a formal [2+2] cycloaddition process that proceeds thermally under a variety of conditions.
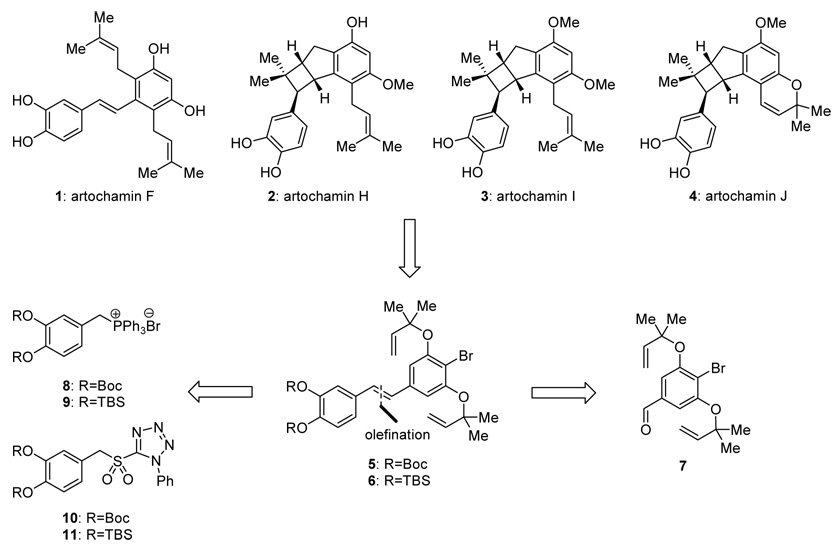
The Artocarpus genus encompasses approximately sixty species of trees that are thriving throughout the tropical lands of Asia. Belonging to the mulberry family Moraceae, several members of the species are cultivated for their fruit, edible seeds and timber. A number of these trees are historically reputed to possess medicinal properties and are utilized as folk medicines in Taiwan, Thailand, and Indonesia.17–21 Studies directed at the elucidation of the active ingredients of Artocarpus chama led to the isolation of several prenylated flavonoid compounds exhibiting cytotoxic, antiplatelet, and antibacterial activities.22 More recent investigations with the stems of the same plant revealed a series of weakly cytotoxic prenylated stilbenes and derivatives thereof.23 Artochamins, F, H, I, and J (1–4, Scheme 1) are amongst the most prominent and structurally interesting members of this class of compounds.
Scheme 1.
Structures and retrosynthetic analysis of artochamins F, H, I, and J (1–4).
Inspection of the structures of artochamins F (1) and H–J (2–4) reveals a possible biogenetic relationship between them, whereby the stilbene-like structure 1, or a derivative thereof, may serve as a precursor to the cyclobutane-containing structures 2–4 through a formal [2+2] cycloaddition reaction.24 The occurrence of artochamins H–J (2–4) as racemates is suggestive of a non-enzymatic process, but does not necessarily preclude enzymatic intervention in the formation of the bicyclo [3.2.0] heptane ring system, as will be discussed in greater detail below. Herein, we describe our studies in this area that culminated in the total syntheses of several of the artochamins and shed some light on the mechanistic aspects of the proposed formal [2+2] thermal cycloaddition reaction.25
2. Results and Discussion
2.1. Retrosynthetic analysis
The architecture of the artochamins (Scheme 1) makes them amenable to a cascade sequence which could potentially deliver them sequentially and/or selectively by fine-tuning the structure of the starting substrate. It was envisioned that a precursor such as 5 or 6 (Scheme 1), with or without protecting groups, equipped with the necessary functionality, could enter into a cascade sequence involving Claisen rearrangements to install the two prenyl groups, followed by a formal intramolecular [2+2] cycloaddition to forge the cyclobutane ring. The nature of the hydroxyl protecting groups could potentially favor or disfavor the formal [2+2] cycloaddition reaction,24a thus providing access to either artochamin F (1) or the skeletal framework of artochamins H–J (2–4). The stilbene precursors 5 and 6 were traced back to phosphonium salts 8 and 9, or sulfones 10 and 11 and aldehyde 7, respectively, with the stereoselectivity of the relevant coupling reactions to be addressed experimentally.
2.2. Synthesis of artochamin I
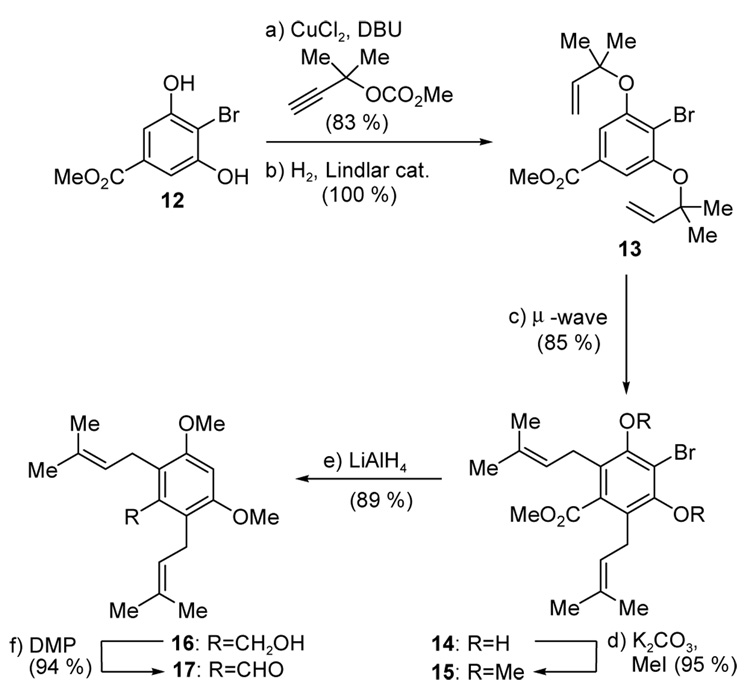
As a prelude to developing the cascade-based sequence towards the artochamins, a step-wise approach to artochamin I (3) was designed in order to explore separately the Claisen rearrangements and, in particular, the feasibility of the thermal [2+2] cycloaddition reaction. To this end, the required aldehyde 17 and phosphonium salt 9 for a Wittig-based coupling were prepared as follows. As shown in Scheme 2, copper-catalyzed etherification26 of bis-phenol 1227 with dimethylpropynyl carbonate followed by catalytic hydrogenation under Lindlar conditions led to bis-allyl ether 13 in 83 % overall yield. Microwave-promoted (180 °C) Claisen rearrangements28 then afforded the bis-prenylated ester 14 in good yield (85 %). Subsequent methylation of 14 (K2CO3, MeI) afforded bismethyl ether 15, which was subjected to the action of LiAlH4 to effect reduction of both the bromine substituent and ester functionality, affording benzylic alcohol 16. Dess–Martin oxidation of the latter compound then furnished the desired aldehyde 17 in 90 % overall yield for the two steps.
Scheme 2.
Construction of aldehyde 17. Reagents and conditions. a) methyl 1,1-dimethyl-2-propynyl carbonate (3.0 equiv), CuCl2 (0.01 equiv), DBU (3.0 equiv), CH3CN, 0 °C, 14 h; b) H2 (balloon), Lindlar catalyst (10 % w/w), quinoline (0.5 equiv), EtOAc, 23 °C, 3 h; c) µ-wave, DMF, 180 °C, 5 min; d) K2CO3 (5.0 equiv), MeI (6.0 equiv), DMF, 100 °C, 1 h; e) LiAlH4 (10 equiv), THF, 50 °C, 14 h; f) DMP (1.5 equiv), CH2Cl2, 23 °C, 30 min; DMF = N,N-dimethylformamide; TBS = tert-butyldimethylsilyl; DMP = Dess–Martin periodinane (1,1,1-triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one).
The required phosphonium salt 9 was obtained in a straightforward manner from commercially available aromatic aldehyde 18 through a four-step sequence as shown in Scheme 3. Thus, protection of aldehyde 18 as the corresponding bis-TBS ether (TBSCl, imidazole, 91 %) followed by NaBH4 reduction afforded benzylic alcohol 20 (95 %). Subsequent bromination of 20 with PBr3 and reaction of the resulting benzylic bromide 21 with PPh3 resulted in the formation of phosphonium salt 9 in 85 % yield.
Scheme 3.
Construction of phosphonium salt 9. Reagents and conditions. a) TBSCl (2.0 equiv), imidazole (3.0 equiv), DMF, 23 °C, 12 h; b) NaBH4 (1.0 equiv), EtOH, 0 → 23 °C, 10 min; c) PBr3 (0.5 equiv), CH2Cl2, 0 °C, 30 min; d) PPh3 (1.0 equiv), toluene, 110 °C, 3 h.
The Wittig coupling between the ylide derived from phosphonium salt 9 and aldehyde 17 (Scheme 4) proceeded in excellent yield (90 %) and acceptable selectivity (E:Z ca. 5:1) providing trans-stilbene E-22 as the major product. Removal of the TBS groups from E-22 (HF·py, 88 %) afforded the protected artochamin F derivative 23, setting the stage for the projected formal [2+2] cycloaddition reaction. We were pleased to observe that microwave heating of 23 in DMF at 180 °C resulted in the formation of artochamin I (3) as a single diastereoisomer in 80 % yield. Significantly, attempts to cyclize the corresponding TBS ethers (E- or Z-22) under identical conditions failed, with only trace amounts of desilylated material visible (by TLC) after prolonged microwave heating (> 45 min.). Besides constituting an efficient synthesis of artochamin I (3), this study demonstrated the feasibility of both the double Claisen rearrangement and the formal [2+2] cycloaddition reaction under essentially identical reaction conditions. The study also demonstrated the expected dependence of the formal thermal cycloaddition reaction on the free hydroxyl groups of the stilbene substrate.24c
Scheme 4.
Wittig reaction-based construction of stilbene 23 and synthesis of artochamin I (3). Reagents and conditions. a) 9 (1.2 equiv), nBuLi (1.2 equiv), −78 °C, 30 min; then 17 −78 → 23 °C, 30 min; b) HF·py (excess), THF, 0 → 23 °C, 2 h; c) µ-wave, DMF, 180 °C, 20 min; py = pyridine.
2.3. Development of the Julia–Kocieński approach to stilbenes and the cascade strategy for the total synthesis of artochamins F, H, I, and J
Having established appropriate conditions for the synthesis of the artochamins, we then proceeded to incorporate them within a cascade sequence towards an advanced common intermediate from which several of the natural products were expected to emerge. The bromine-containing bis-Boc protected stilbene derivative 5 (Scheme 1) was designed with the expectation that under thermal conditions it would undergo cleavage of the Boc groups, Claisen rearrangement of the two allylic ether moieties, and a formal [2+2] cycloaddition reaction to afford the tetracyclic core structure of artochamins H–J (2–4). Our initial route to precursor 5 involved a Wittig coupling that required aldehyde 7 (Scheme 1) and phosphonium salt 8 (Scheme 1). To this end, chemoselective reduction of methyl ester 13 with LiAlH4 provided alcohol 24, which was oxidized to aldehyde 7 in 91 % yield over the two steps as shown in Scheme 5.
Scheme 5.
Construction of aldehyde 7. Reagents and conditions. a) LiAlH4 (1.2 equiv), THF, 0 °C, 10 min; b) DMP (1.2 equiv), CH2Cl2, 23 °C, 30 min.
Next, the bis-Boc protected phosphonium salt 8 was prepared from commercially available aldehyde 18 by the same sequence as that employed to construct its bis-TBS protected counterpart (9, Scheme 3), as shown in Scheme 6. The two fragments, the ylide derived from 8 and aldehyde 7, were then combined using the Wittig reaction to afford stilbene 5 in 90 % yield as a ca. 1:1 mixture of geometric isomers (Scheme 6). This stereoselectivity setback in combination with the low-yielding bromination (26 → 27, 18 %) prompted us to search for an alternative route to the targeted stilbene substrate.
Scheme 6.
Wittig reaction-based construction of stilbene 5. Reagents and conditions. a) Boc2O, (2.0 equiv), 4-DMAP (0.05 equiv), iPr2NEt (0.1 equiv), THF, 23 °C, 2 h; b) NaBH4, (1.0 equiv), EtOH, 0 → 23 °C, 10 min; c) PBr3 (2.0 equiv), CH2Cl2, 0 °C, 1 h; d) PPh3 (1.5 equiv), toluene, 60 °C, 14 h; e) 8 (1.2 equiv), tBuOK (1.2 equiv), −78 °C, 30 min; then 7, −78 → 23 °C, 30 min; DMAP = 4-dimethylaminopyridine.
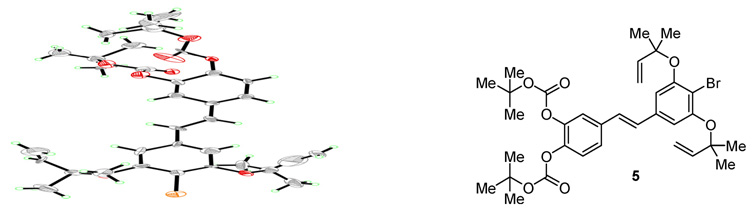
Whilst numerous examples of the Wittig reaction to prepare stilbenes can be found in the literature,29 precedent for the corresponding Julia–Kocieński olefination was lacking.30 Nevertheless, we decided to explore this alternative in the hope that we could improve upon the selectivity of the coupling reaction. Towards this end, stilbenes 5 and 6 were targeted for synthesis according to Scheme 7. Thus, the previously prepared benzylic alcohols 20 and 26 were subjected to thioetherification under Mitsunobu conditions to afford the corresponding sulfides, which were converted through molybdenum-catalyzed oxidation to the desired sulfone coupling partners 10 and 11 in 79 and 82 % yield, respectively, over the two steps. In the main event of this sequence, employing sulfone 10 as a starting material, we were pleased to observe that the Julia–Kocieński olefination31 with aldehyde 7 using KHMDS in THF afforded the bis-Boc protected stilbene 5 in 86 % yield as a single E-stereoisomer (vide infra). The bis-TBS protected stilbene 6 was obtained in a similar fashion from the corresponding sulfone (11) in 95 % yield, but as a ca. 2:1 mixture of E:Z isomers. In contrast to the TBS-protected stilbenes, whose stereochemistry could be determined by NMR spectroscopy, the geometry of the bis-Boc protected stilbene 5 could not be comfortably assigned from its 1H NMR spectrum. Fortunately, this compound yielded to x-ray crystallographic analysis which confirmed the suspected E-geometry (see ORTEP drawing, Figure 1).32
Scheme 7.
Julia–Kocieński reaction-based construction of stilbenes 5 and 6. Reagents and conditions. a) 1-phenyl-1H-tetrazole-5-thiol (1.0 equiv), DEAD (1.2 equiv), PPh3 (1.1 equiv), THF, 0 °C, 30 min; b) ammonium molybdate tetrahydrate (0.1 equiv), 30 % aqueous H2O2 (20 equiv), EtOH, 0 → 23 °C, 14 h; c) 10 or 11 (1.5 equiv), KHMDS (1.5 equiv), −78 °C, 30 min; then 7 (1.0 equiv), THF, −78 → 23 °C; Boc = tert-butylcarbonate; KHMDS = potassium bis(trimethylsilyl)amide; TBS = tert-butyldimethylsilyl.
Figure 1.
X-Ray derived ORTEP drawing of stilbene 5. Thermal ellipsoids at the 30 % probability level.
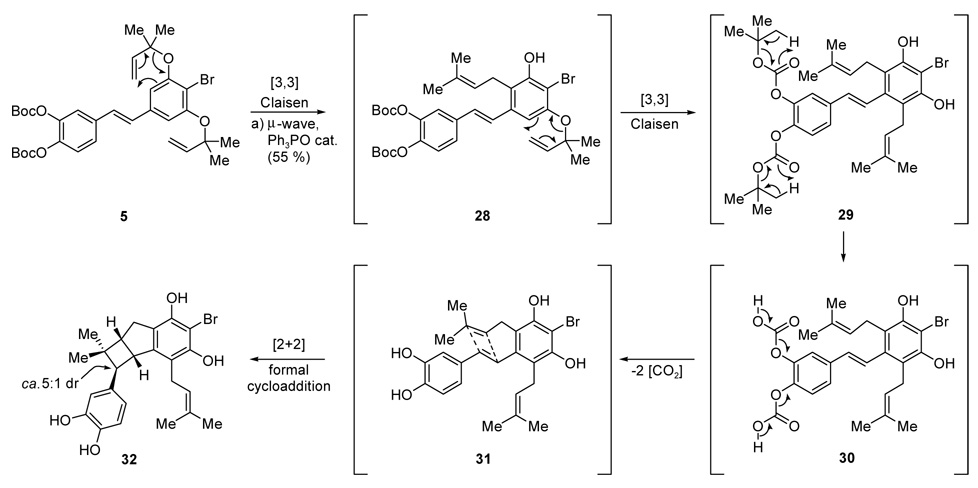
With ample quantities of stilbene 5 available through the Julia–Kocieński route, we were in a position to test our hypothesis regarding the proposed cascade sequence to the bicyclo [3.2.0] ring system of artochamins H–J (2–4). After considerable experimentation it was discovered that the desired cascade could be realized under microwave conditions33 in o-xylene at 180 °C (20 min.) in the presence of trace amounts of Ph3PO as shown in Scheme 8. The beneficial effect of Ph3PO was discovered serendipitously when a sample of precursor 5 that had been obtained through the Wittig route was used for the reaction (vide infra). The tetracyclic product 32 was obtained in 55 % yield as a ca. 5:1 mixture of diastereoisomers with respect to the benzylic stereocenter adjacent to the all-carbon quaternary center (see Scheme 8).
Scheme 8.
Cascade-based synthesis of artochamin ring framework 32. Reagents and conditions. a) µ-wave, Ph3PO (5 % wt.), o-xylene, 180 °C, 20 min.
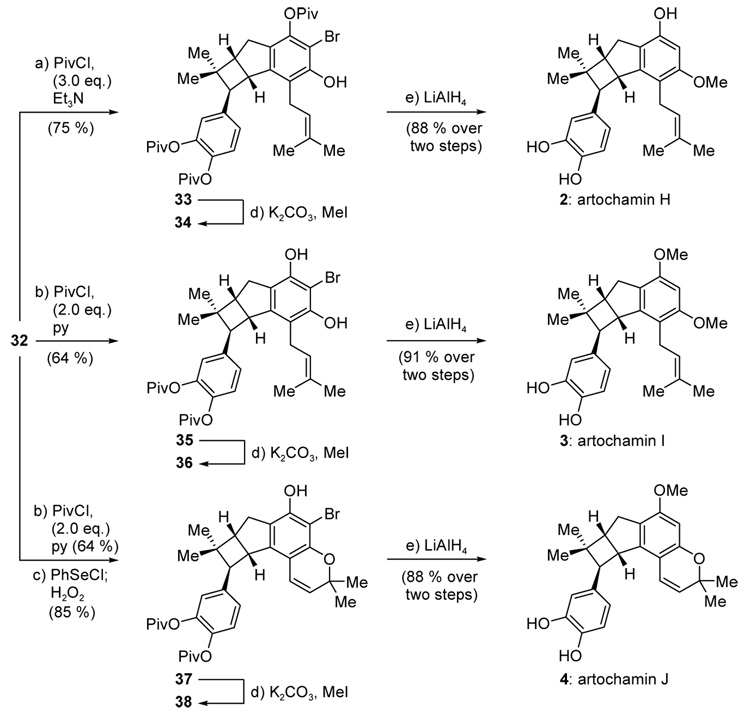
With common intermediate 32 available through the cascade sequence, the total synthesis of artochamins H–J (2–4) was completed as shown in Scheme 9. Thus, treatment of 32 with PivCl (2.0 equiv) and pyridine (6.0 equiv) in CH2Cl2 furnished the bis-pivalate 35 in 64 % yield, with recyclable mono-pivalates accounting for the remainder of the mass balance. As anticipated, steric shielding from the adjacent bromine atom disfavored acylation of the two remaining hydroxyl groups under these conditions. Subsequent methylation of the remaining hydroxyl groups (K2CO3, MeI) and simultaneous cleavage of the bromine and pivalate moieties (LiAlH4) then furnished artochamin I (3) in 91 % overall yield for the two steps. The total synthesis of artochamin J (4) was completed from common intermediate 32 via pivalate 35 by selenoetherification34 (PhSeCl) and oxidative elimination (H2O2) to afford benzopyran 37 (85 % overall yield), which was converted to artochamin J (4) by methylation and exhaustive LiAlH4 reduction as described above for artochamin I (3). Finally, artochamin H (2) was secured from common intermediate 32 through tri-pivalate 33, obtained in 75 % yield under somewhat more forcing acylation conditions (3.0 eq PivCl, 6.0 equiv Et3N). Methylation and deprotection as above provided artochamin H (2) in 88 % overall yield (see Scheme 9).
Scheme 9.
Total synthesis of artochamins H–J (2–4). Reagents and conditions. a) PivCl (3.0 equiv), Et3N (6.0 equiv), CH2Cl2, 23 °C, 30 min ; b) PivCl (2.0 equiv), py (6.0 equiv), CH2Cl2, 23 °C, 14 h; c) PhSeCl (1.0 equiv), CH2Cl2, −78 → 23 °C, 30 min; then H2O2 (20 equiv), CH2Cl2, 0 → 23 °C, 3 h; d) K2CO3 (5.0 equiv), MeI (6.0 equiv), DMF, 100 °C, 1 h; e) LiAlH4 (6.0 equiv), THF, 0 → 23 °C, 24 h; Piv = trimethylacetyl; py = pyridine.
The spectroscopic data of synthetic artochamins H–J (2–4) matched those reported for the natural products.35 Furthermore, in the case of artochamin H (2), a ROESY NMR experiment provided unequivocal support of its regiochemistry and relative stereochemistry through the observation of the appropriate ROE interactions, as shown in Figure 2.
Figure 2.
Principal ROE interactions in synthetic artochamin H (2).
Finally, based on the results obtained during the first-generation synthesis of artochamin I (3) (see Scheme 4), the total synthesis of artochamin F (1) was completed as shown in Scheme 10. Thus, the Julia–Kocieński derived stilbene 6 (see Scheme 7) was subjected to microwave heating resulting in the formation of bis-prenyl phenol 39 in excellent yield (95%). As expected, no cyclobutane-containing product was obtained from this microwave-promoted reaction. Reductive cleavage of the bromide, followed by desilylation then afforded synthetic artochamin F (1) in 81 % yield over the two steps. Subjection of artochamin F (1) to microwave heating, both in the presence or absence of Ph3PO, provided, in 82 % yield, derivative 40 containing the [3.2.0] ring system of artochamins H–J (2–4), thereby demonstrating that neither the bromine atom, nor the Ph3PO are necessary for the formal [2+2] cycloaddition reaction to take place, at least in this instance.
Scheme 10.
Total synthesis of artochamin F (1). Reagents and conditions. a) o-xylene, µ-wave, 180 °C, 5 min; b) AIBN (0.1 equiv), nBu3SnH (2.0 equiv), benzene, reflux, 1 h; c) HF·Et3N (5.0 equiv), THF, 23 °C, 1 h; d) o-xylene, µ-wave, 180 °C, 10 min. AIBN = azobisisobutylonitrile.
2.4. Mechanistic studies
The cascade sequence described above could also be conducted successfully by conventional heating (o-xylene, 165 °C, sealed tube), although one hour was required for the reaction to reach completion. TLC analysis of the reaction mixture indicated that the Claisen rearrangements proceed faster than the Boc cleavage or the formal [2+2] cycloaddition reaction. Given the fact that the free hydroxyl groups are necessary for the formal cycloaddition reaction to occur, we surmise the sequence shown in Scheme 8 as the most reasonable order of events in this cascade sequence. Thus, it is thought that rapid Claisen rearrangements are followed by slower deprotections and ring closures (5→28→29→30→31→32, Scheme 8).
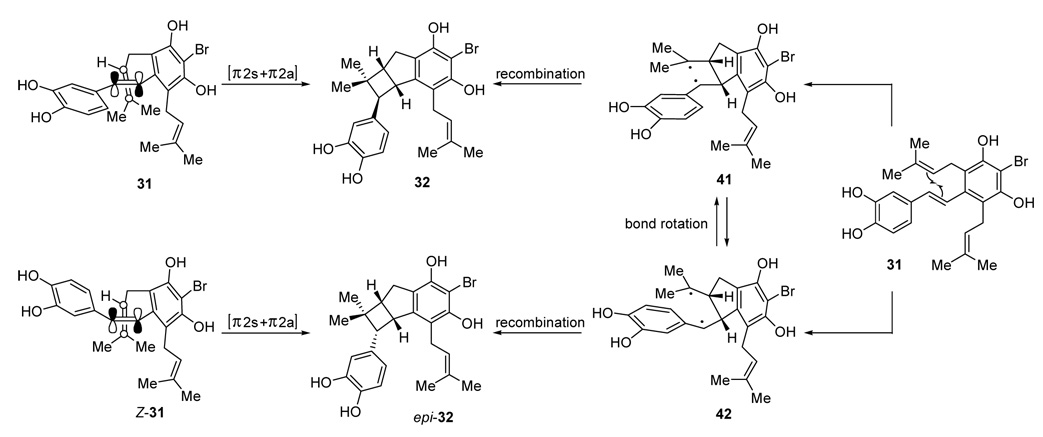
The precise mechanism of the formal [2+2] cycloaddition reaction is quite fascinating, but not fully illuminated as yet. At present, we can outline three potential alternatives for this interesting transformation, the first two of which are described in Scheme 11. The first scenario involves a concerted [π2s+π2a] cycloaddition36 between the stilbene alkene and one of the prenyl groups (31→32, Scheme 11). Such a process ought to be stereospecific with respect to the geometry of the stilbene alkene. We view this first possibility to be highly unlikely due to the difficulty in obtaining the necessary orbital overlap, especially when the conformational constraints imposed by the tether are taken into account. The second, and more reasonable scenario, may involve a stepwise radical mechanism in which an initial 5-exo cyclization is followed by rapid recombination of the biradical so obtained (31→41→32, Scheme 11). The stereochemical outcome of this process with respect to the benzylic stereocenter adjacent to the gem-dimethyl group should be governed by the relative rates of the indicated bond rotation and radical coupling processes. If the radical recombination occurs faster than the bond rotation, then the stereochemistry of the starting olefin will be retained in the product, whereas, in the reverse scenario, the formation of epi-32 will be competitive with that of 32.
Scheme 11.
Potential mechanistic pathways for the formal [2+2] cycloaddition reaction.
In an initial experiment with Wittig-derived stilbenes E- and Z-5, it was discovered that the reaction was not stereospecific with respect to the geometry of the alkene, although possible E/Z isomerization of the stilbene under the reaction conditions in the case of the Z-isomer could undermine this conclusion. These observations corroborate the stepwise biradical mechanism in which bond rotation occurs at a rate competitive with radical recombination.
The third, and most attractive, alternative is the ionic mechanism depicted in Scheme 12. This mechanism may be particularly relevant to the formation of artochamins H–J (2–4) in nature due to the ease of enzymatic generation of o-quinones from catechols.37 This alternative biosynthetic pathway appears to be more attractive than the one originally proposed based on a [2+2] cycloaddition mechanism since the conditions typically required to bring about such transformations are rather forcing.24 According to this newly proposed process, initial enzymatic37 or chemical38 oxidation of the electron-rich catechol moiety of generic stilbene 43 (Scheme 12) would furnish o-quinone 44 which could readily undergo intramolecular conjugate addition as shown, affording the intermediate 45, whose facile ring closure to tetracyclic quinone 46 would be expected. Finally, redox reaction between quinone 46 and dihydroquinone 43 (starting material) may be responsible for the completion of the cascade, leading to product 47 with concomitant re-generation of o-quinone 44, thus necessitating only a catalytic amount of the initial oxidant. This could be an enzyme,37 trace amounts of O2,39 or other oxidants such as DDQ or even Ph3PO, as will be discussed further below.
Scheme 12.
Postulated redox-based mechanistic pathway for the biogenesis of artochamins H–J from stilbene precursors.
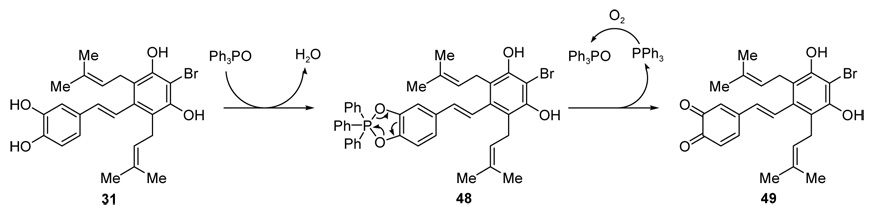
The intriguing effect of Ph3PO on the cascade reaction was first noticed when stilbene 5 derived from the Julia-Kocieński olefination failed to reproducibly undergo the cascade reaction observed previously with the Wittig-derived material. Suspecting that a trace impurity arising from the Julia–Kocieński olefination was causing the problem, we proceeded to further purify our substrate by crystallization only to observe the same unsuccessful results in inducing the desired cascade reaction. On turning our attention to possible contaminants in the Wittig-derived stilbene, we begun experimentation with Ph3PO as an additive to the Julia–Kocieński derived stilbene. These experiments led us to the discovery that 3–5 % w/w of Ph3PO was required for the desired cascade reaction to take place. Although we cannot at present define precisely the exact role played by the phosphine oxide, some speculations are possible. To recap our observations, we know that Ph3PO is not absolutely essential in every instance for the [2+2] cycloaddition reaction, nor is it required for the Claisen rearrangements or Boc cleavages. However, that it is not essential does not preclude a role in which it facilitates one or more of the events in the cascade sequence. We suspect that this additive could potentially facilitate the cycloaddition step based on the following considerations. Proton transfer from phenols to phosphine oxides both in solution and the solid state has been observed to varying degrees spectroscopically.40 In such events it is typical to observe a downfield shift of the 1H NMR signal of the hydroxyl group in the phenol-Ph3PO complex as the pKa of the phenol decreases. These results, together with a recent report24a describing a base promoted intramolecular [2+2] cycloaddition reaction between a prenyl group and a benzopyran moiety, suggest that Ph3PO may be acting as a Lewis base providing formal HOMO activation of the stilbene system towards cycloaddition. Additionally, it is noteworthy to mention the distinct color differences of the reaction mixtures of these cascade reactions in the presence and absence of Ph3PO. The latter becomes significantly darker in color, suggesting decomposition that does not occur to the same extent in the presence of the additive.
An intriguing possibility for the role of the Ph3PO in the described cascade reactions and in the context of the redox/ionic mechanism proposed above (Scheme 12) for the biogenesis of artochamins H–J is outlined in Scheme 13.
Scheme 13.
Potential role of Ph3PO in the generation of o-quinone intermediate 49.
Given that the redox-based sequence described above is dependent on the initial generation of small amounts of the o-quinone stilbene in order to initiate, we contemplated the possible role that Ph3PO might play in this oxidation process. The somewhat counterintuitive process, outlined in Scheme 13, hinges upon combining the dehydration reaction known to take place between Ph3PO and catechols41 and the chelotropic elimination of Ph3P from the so-derived phospholene.39 Thus, formation of phospholene 48 from stilbene 31 (Scheme 13) may occur under the reaction conditions; its conversion to Ph3P and o-quinone 49 (which may be reversible) could be driven by re-oxidation of the former to Ph3PO with molecular oxygen present in trace amounts in the reaction mixture. It should be reiterated that only a trace amount of the o-quinone stilbene intermediate is necessary for the cascade sequence described in Scheme 12 to become operative. The direct oxidation of stilbene 31 by molecular oxygen is another possibility39 that could account for the generation of quinone stilbene intermediate 49. This process is potentially more relevant in the conversion of stilbene 23 to artochamin I (3) (Scheme 4) or the conversion of artochamin F (1) to 40 (Scheme 10), where the stilbenes could, in principle, already contain traces of the corresponding o-quinones arising from aerial oxidation before entering into the formal cycloaddition reaction. Given that deprotection of the catechol moiety of stilbene 5 occurs during the cascade process, the Ph3PO oxidation cycle described above may also be relevant in the cascade sequence described in Scheme 8.
In order to gain further insight into these mechanistic proposals, we considered the following experiments to be relevant. The addition of a more conventional oxidant to the cascade reaction of Scheme 8 should also have a similar effect as Ph3PO, but obviously will not provide any information with respect to the differentiation of the diradical and redox/ionic mechanisms. The inclusion of 1 % by wt. DDQ in this cascade gave similar results to those obtained with Ph3PO. Interestingly, the initial deep red color of the reaction mixture was slowly bleached after heating for a few minutes at 180 °C in o-xylene. Presumably, this color change signals the fact that the DDQ persists in solution until the cleavage of both Boc groups from one or more of the cascade intermediates has occurred, after which time it is reduced by the free catechol moiety. In an attempt to observe this process spectroscopically, stilbene 31 possessing a free catechol moiety was prepared and reacted with DDQ (1.5 equiv) in CDCl3 at ambient temperature and the reaction mixture was analyzed by 1H and 13C NMR spectroscopy (Scheme 14). Exclusive formation of tetracycle 50 was observed in minutes. Formed through initial oxidation of 31 to o-quinone 49 followed by intramolecular [4+2] cycloaddition as shown in Scheme 14, this conjugated o-quinone proved too labile for isolation. However, upon removal of the solvent (CDCl3), dissociation of the residue in o-xylene and microwave irradiation at 180 °C for 30 minutes, cyclobutane 32 was formed (30 % overall yield from 31). A most likely pathway for the conversion of 50 to 32 involves thermally induced reversal of the [4+2] cycloaddition, followed by conversion of the so obtained o-quinone 49, to 32, via o-quinone 51, by the redox/ionic mechanism described above (Scheme 12). We suspect that under the acidic reaction conditions and high temperatures, Diels–Alder adduct 50 undergoes rapid tautomerisation to catechol 53, via hydroquinone 52, which may, in turn, be responsible for the reduction of o-quinone 51 to cyclobutane 32, also leading to o-quinone 54. This cycle reaches equilibrium, accounting for the loss of reactivity over longer periods of heating. Another scenario in which Diels–Alder adduct 50 would lead to 32 is by a formal [1,3]-alkyl shift giving o-quinone 51 directly. We believe this pathway to be highly unlikely given the formidable geometrical constraints to the required orbital overlap.36
Scheme 14.
Spectroscopic observation of Diels–Alder adduct 50 from direct oxidation of stilbene 31 and its conversion to cyclobutane 32. Reagents and conditions. a) DDQ (1.5 equiv), CDCl3, 23 °C, 5 min; b) µ-wave, o-xylene, 180 °C, 30 min. DDQ = 2,3-dichloro-5,6-1,4-benzoquinone.
An obvious corollary of the redox/ionic mechanism proposed in Scheme 12 is the availability of the catechol unit; Given that the methylene acetal derivative of 58 (Scheme 15) ought to be inert with respect to oxidation, any formal cycloaddition product obtained from this substrate should be derived from the diradical pathway shown in Scheme 11, since we have already rejected the concerted pathway on the grounds of insufficient orbital overlap. Furthermore, the absence of product in this experiment would provide circumstantial evidence for the redox/ionic mechanism proposed. We therefore prepared the methylene acetal stilbene 58, through intermediates 56 and 57 as depicted in Scheme 15, and subjected it to microwave heating.
Scheme 15.
Construction and microwave irradiation of methylene acetal stilbene 58. Reagents and conditions. a) 1-phenyl-1H-tetrazole-5-thiol (1.0 equiv), Et3N (1.25 equiv), THF, 23 °C; then 55 (1.25 equiv), reflux, 14 h; b) thioether (1.0 equiv), m-CPBA (3.5 equiv), CH2Cl2, 0 → 23 °C, 24 h; c) 56 (1.8 equiv), KHMDS (1.8 equiv), −78 °C, 30 min; then 7 (1.0 equiv), THF, −78 → 23 °C, 1 h, E:Z ca. 2:1; d) o-xylene, µ-wave, 180 °C, 5 min; e) o-xylene, µ-wave, 180 °C, > 30 min m-CPBA = 3-chloroperbenzoic acid.
Exposure of stilbene 58 to microwave heating failed to yield any cyclobutane–containing products; indeed this substrate was recovered quantitatively even after prolonged heating (> 30 min. at 180 °C). This provides strong circumstantial evidence for the intervention of the o-quinone intermediate in the formation of the bicylco [3.2.0] heptane ring system.
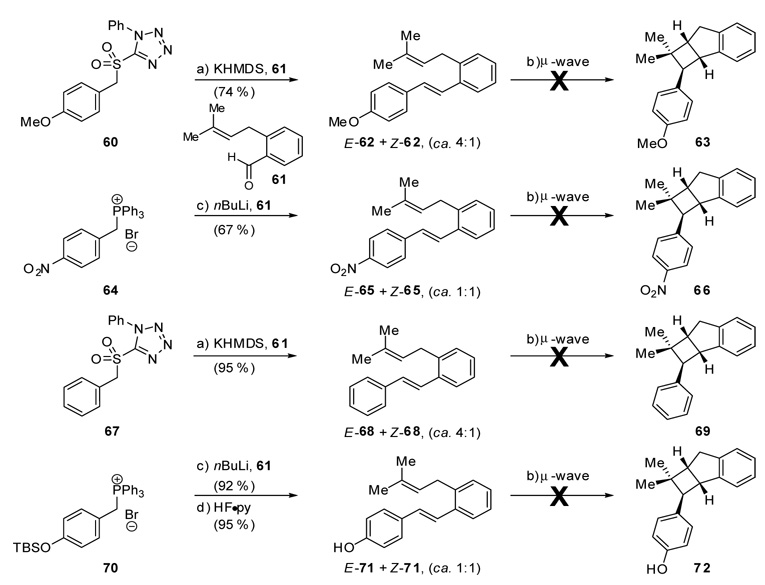
In an attempt to further investigate the mechanism of the formal cycloaddition and to probe the generality of the reaction, four simplified stilbene substrates (62, 65, 68, and 71) were prepared from known aldehyde 61 as shown in Scheme 16. After microwave heating at 180 °C (> 30 min.), stilbenes 62, 65, 68 and 71 were recovered quantitatively, clearly demonstrating their failure to undergo the formal cycloaddition reaction (Scheme 16). These negative results provide further evidence that the redox/ionic mechanism shown in Scheme 12 is the most likely scenario operating in these reactions.
Scheme 16.
Construction and microwave irradiation of stilbenes 62, 65, 68 and 71. Reagents and conditions. a) 60 or 67 (1.8 equiv), KHMDS (1.8 equiv), −78 °C, 30 min; then 61 (1.0 equiv), THF, −78 → 23 °C, 1 h, E:Z ca. 4:1 for 62 and E:Z ca. 4:1 for 68; b) o-xylene, µ-wave, 180 °C, > 30 min; c) 64 or 70 (1.2 equiv), nBuLi (1.2 equiv), THF, 0 → 23 °C, 30 min; then 58 (1.0 equiv), THF, 0 → 23 °C, 14 h, E:Z ca. 1:1 for 65 and 71; d) HF·py (excess), THF, 0 → 23 °C, 2 h.
3. Conclusions
Described herein are a series of cascade-based strategies whose implementation resulted in expeditious and efficient total syntheses of artochamins H–J (2–4) as well as artochamin F (1). The mechanism for those cascade reactions was investigated and a number of proposals are put forward, including a redox-based mechanism involving o-quinone species that may be relevant to the biosynthesis of members of this class. These cascade-based total syntheses highlight, once again, the advantages of such synthetic sequences in rapidly building molecular complexity and diversity from relatively simple starting materials in efficient and environmentally benign ways.
5. Experimental
5.1. General Experimental
All reactions were carried out under an argon atmosphere with dry solvents under anhydrous conditions, unless otherwise noted. Dry tetrahydrofuran (THF), dimethylformamide (DMF), triethylamine (Et3N), and methylene chloride (CH2Cl2) were obtained by passing commercially available predried, oxygen-free formulations through activated alumina columns. Yields refer to chromatographically and spectroscopically (1H NMR) homogeneous materials, unless otherwise stated. Reagents were purchased at the highest commercial quality and used without further purification, unless otherwise stated. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60F-254) using UV light as the visualizing agent and an ethanolic solution of anisaldehyde and sulfuric acid or an ethanolic solution of phosphomolybdic acid and cerium sulfate, and heat as developing agents. Preparative thin-layer chromatography (PTLC) separations were carried out on 0.25 or 0.50 mm E. Merck silica gel plates (60F-254). NMR spectra were recorded on Bruker DRX-600, DRX-500, or AMX-400 instruments and calibrated using residual undeuterated solvent as an internal reference. The following abbreviations were used to designate multiplicities: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, quin=quintuplet, sext=sextet, sep=septet, br=broad. IR spectra were recorded on a Perkin-Elmer 1600 series FT-IR spectrometer. Electrospray ionization (ESI) mass spectrometry (MS) experiments were performed on an API 100 Perkin Elmer SCIEX single quadrupole mass spectrometer at 4000V emitter voltage. High-resolution mass spectra (HRMS) were recorded on a VG ZAB-ZSE mass spectrometer using MALDI (matrix-assisted laser-desorption ionization) or ESI (electrospray ionization).
5.2. First generation synthesis of artochamin I (3)
5.2.1. 4-Bromo-3,5-bis-(1,1-dimethyl-allyloxy)-benzoic acid methyl ester (13)
To a solution of methyl 4-bromo-3,5-dihydroxybenzoate (12) (3.0 g; 12.1 mmol) in CH3CN (120 mL) at 0 °C was added CuCl2 (30 mg; 0.12 mmol) followed by DBU (5.46 mL; 36.3 mmol). After stirring for 15 minutes methyl 1,1-dimethyl-2-propynyl carbonate (5.19 g; 36.3 mmol) was added and the resulting mixture was stirred at 0 °C overnight. The reaction mixture was treated with H2O (50 mL) and extracted with Et2O (3 × 100 mL), then the combined organic extracts were washed with brine (50 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % EtOAc in hexanes) to give the bis-alkyne (3.32 g; 83 %) as a clear oil. Rf = 0.50 (silica gel, 30 % EtOAc in hexanes); IR νmax (film) 2982m, 1724m, 1576m, 1415m, 1382w, 1364w, 1331m, 1240m, 1134m, 1058s, 1033s, 1014m, 840w, 771w cm−1; 1H NMR (500 MHz, CDCl3) δ = 8.00 (s, 2 H), 3.91 (s, 3 H), 2.63 (s, 2 H), 1.73 (s, 12 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 166.4, 154.0, 129.0, 117.9, 116.1, 85.4, 74.5, 74.5, 52.4, 29.5 ppm; HRMS (ESI TOF) m/z calcd. for C18H19BrO4 [M+H+]: 379.0539; found 379.0544.
To a solution of bis-alkyne (2.50 g; 6.59 mmol) in EtOAc (66 mL) under an atmosphere of argon at room temperature was added quinoline (389 µL; 3.30 mmol) followed by 5 % Pd/CaCO3 (poisoned with Pb) (250 mg; 10 % w/w). The system was flushed with H2 and then stirred under an atmosphere of H2 (balloon) for 3 hours. The system was flushed with argon and the reaction mixture was filtered through celite and the filtrate was concentrated in vacuo. The residue was purified by column chromatography (10 % EtOAc in hexanes) to give the bis-alkene 13 (2.53 g; 100 %) as a colorless oil. Rf = 0.18 (silica gel, 15 % EtOAc in hexanes); IR νmax (film) 2982m, 1725s, 1571m, 1415s, 1379w, 1364w, 1334m, 1238s, 1128m, 1072m, 1005w, 927w, 772w cm−1; 1H NMR (500 MHz, CDCl3) δ = 7.46 (s, 2 H), 6.17 (dd, J = 17.6, 6.8 Hz, 2 H), 5.24 (d, J = 17.6 Hz, 2 H), 5.18 (d, J = 6.8 Hz, 2 H), 3.86 (s, 3 H), 1.53 (s, 12 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 166.6, 154.5, 143.6, 128.5, 118.4, 115.7, 114.1, 82.2, 52.3, 26.9 ppm; HRMS (ESI TOF) m/z calcd. for C18H23BrO4 [M+H+]: 383.0852; found 383.0862.
5.2.2. 4-Bromo-3,5-dihydroxy-2,6-bis-(3-methyl-but-2-enyl)-benzoic acid methyl ester (14)
A solution of bis-alkene 13 (900 mg; 0.24 mmol) in DMF (2.0 mL) was submitted to microwave heating for 5 minutes at 180 °C. The reaction mixture was diluted with EtOAc (10 mL) and H2O (2 mL) was added. The aqueous phase was acidified (pH 3) with HCl (1 M) and the layers were separated. The organic phase was further washed with H2O (4 × 2 mL) and brine (2 mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (60 % CH2Cl2 in hexanes) to give bisprenyl compound 14 (76.5 mg; 85 %) as a clear oil. Rf = 0.22 (silica gel, 60 % CH2Cl2 in hexanes); IR νmax (film) 3464brm, 2965w, 2914w, 2856w, 1717s, 1579m, 1431s, 1363s, 1320m, 1253s, 1206m, 1144s, 1092s, 1023m, 1002w, 927w, 901w, 799w, 775w, 735w, 604w cm−1; 1H NMR (500 MHz, CDCl3) δ = 5.68 (s, 2 H), 5.16 (m, 2 H), 3.86 (s, 3 H), 3.28 (d, J = 7.0 Hz, 4 H), 1.73 (s, 6 H), 1.70 (s, 6 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 169.4, 149.4, 134.3, 133.4, 121.8, 117.2, 101.4, 52.1, 27.3, 25.7, 17.8 ppm; HRMS (ESI TOF) m/z calcd. for C18H23BrO4 [M+H+]: 383.0852; found 383.0850.
5.2.3. 4-Bromo-3,5-dimethoxy-2,6-bis-(3-methyl-but-2-enyl)-benzoic acid methyl ester (15)
To a solution of bis-phenol 14 (200 mg; 0.52 mmol) in DMF (10 mL) was added K2CO3 (361 mg; 2.61 mmol) and MeI (195 µL; 3.13 mmol), and the resulting mixture was heated at 100 °C for 1 hour. Once cool, the reaction mixture was diluted with EtOAc (30 mL) and washed with H2O (5 × 5 mL) and brine (5 mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (60 % CH2Cl2 in hexanes) to give the bismethyl ether 15 (204 g; 95 %) as a clear oil. Rf = 0.38 (silica gel, 60 % CH2Cl2 in hexanes); IR νmax (film) 2934w, 2859w, 1730s, 1561w, 1450m, 1434m, 1398s, 1375w, 1319s, 1248m, 1194m, 1144m, 1093s, 1031s, 984m, 950w, 925w, 895w, 833w, 802w, 764w, 732w cm−1; 1H NMR (500 MHz, CDCl3) δ = 5.09 (m, 2 H), 3.81 (s, 3 H), 3.78 (s, 6 H), 3.35 (d, J = 6.5 Hz, 4 H), 1.70 (s, 6 H), 1.67 (d, J = 1.0 Hz, 6 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 169.0, 154.6, 135.0, 132.2, 129.6, 122.4, 114.8, 60.9, 52.0, 27.0, 25.6, 17.9 ppm; HRMS (ESI TOF) m/z calcd. for C20H27BrO4 [M+H+]: 411.1165; found 411.1164.
5.2.4. [4-Bromo-3,5-dimethoxy-2,6-bis-(3-methyl-but-2-enyl)-phenyl]-methanol (16)
To a solution of methyl ester 15 (180 mg; 0.44 mmol) in THF (4.0 mL) at 0 °C was added LiAlH4 (4.4 mL; 4.4 mmol; 1 M solution in THF) and the resulting mixture was warmed to 50 °C and stirred for 14 hours. Excess LiAlH4 was quenched by the addition of EtOAc, then H2O (1 mL), NaOH (1 mL; 1 M aqueous solution) and H2O (2 mL), and MgSO4 were added successively and the mixture was filtered. The solvent was concentrated in vacuo and the residue was purified by column chromatography (CH2Cl2) to give alcohol 16 (119 mg; 89 %) as a clear oil. Rf = 0.39 (silica gel, CH2Cl2); IR νmax (film) 3418brm, 2962m, 2913m, 2855m, 1592s, 1453m, 1437m, 1375m, 1311s, 1207m, 1158m, 1114m, 1084s, 1001m, 957w, 927w, 888w, 817m, 756w cm−1; 1H NMR (500 MHz, CDCl3) δ = 6.50 (s, 1 H), 5.13 (m, 2 H), 4.65 (s, 2 H), 3.84 (s, 6 H), 3.46 (d, J = 7.0 Hz, 4 H), 1.81 (s, 6 H), 1.69 (s, 6 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 156.4, 138.7, 131.0, 124.5, 121.9, 95.8, 59.1, 55.8, 25.7, 24.4, 17.8 ppm; HRMS (ESI TOF) m/z calcd. for C19H28O3 [M+H+]: 305.2111; found 305.2102.
5.2.5. 4-Bromo-3,5-dimethoxy-2,6-bis-(3-methyl-but-2-enyl)-benzaldehyde (17)
To a solution of alcohol 16 (100 mg; 0.33 mmol) in CH2Cl2 (5.0 mL) at room temperatue was added Dess–Martin periodinane (209 mg; 0.49 mmol). After 30 minutes the reaction mixture was diluted with Et2O (30 mL) and a solution of Na2S2O3 (840 mg) in saturated aqueous NaHCO3 (10 mL) was added, and stirring maintained until a clear biphase persisted. The aqueous phase was removed and the organic layer was washed with saturated aqueous NaHCO3 (10 mL) and brine (10mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % CH2Cl2 in hexanes) to give aldehyde 7 (93 mg; 94 %) as a clear oil. Rf = 0.32 (silica gel, 30 % CH2Cl2 in hexanes); IR νmax (film) 2951m, 2925m, 2850m, 1697m, 1603m, 1588m, 1462m, 1437m, 1375w, 1311s, 1200m, 1163w, 1119w, 1097w, 1082w, 1027w, 965w, 827w cm−1; 1H NMR (400 MHz, CDCl3) δ = 10.44 (s, 1 H), 6.64 (s, 1 H), 5.10 (m, 2 H), 3.85 (s, 6 H), 3.54 (d, J = 6.8 Hz, 4 H), 1.74 (s, 6 H), 1.66 (d, J = 0.8 Hz, 6 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 195.3, 156.4, 134.9, 131.4, 123.8, 123.5, 99.9, 56.1, 25.8, 23.8, 17.9 ppm; HRMS (ESI TOF) m/z calcd. for C19H26O3 [M+H+]: 303.1955; found 303.1943.
5.2.6. 3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-benzaldehyde (19)
Aldehyde 19 was prepared according to the literature and displayed identical physical properties to those reported.42
5.2.7. [3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-phenyl]-methanol (20)
To a solution of aldehyde 19 (3.28 g; 8.96 mmol) in EtOH (45 mL) was added NaBH4 (339 mg; 8.96 mmol) portion wise at 0 °C. After complete addition, the reaction mixture was warmed to room temperature and stirred for 10 minutes. The reaction was quenched with water, extracted with EtOAc (2 × 50 mL), dried (MgSO4), and concentrated in vacuo to yield benzyl alcohol 20 (3.23 g; 98 %). Rf = 0.22 (silica gel, 15 % EtOAc in hexanes); IR (film) νmax 3318brw, 2954m, 2930s, 2886w, 2858s, 1605w, 1578w, 1509s, 1472m, 1422m, 1295s, 1252s, 1158m, 1123m, 904s, 777s cm−1; 1H NMR (500 MHz, CDCl3) δ 6.85 (s, 1 H), 6.80 (s, 2 H), 4.55 (d, J = 6.0 Hz, 2 H), 1.47 (t, J = 6.0 Hz, 1 H), 0.99 (s, 18 H), 0.20 (s, 12 H) ppm; 13C NMR (500 MHz, CDCl3) δ = 146.9, 146.5, 134.2, 121.0, 120.2, 120.1, 65.2, 25.9, 18.5 ppm; HRMS (ESI TOF) m/z calcd. for C19H36O3Si2 [M+Na+]: 391.2095; found 391.2111.
5.2.8. 4-Bromomethyl-1,2-bis-(tert-butyl-dimethyl-silanyloxy)-benzene (21)
To solution of benzyl alcohol 20 (430 mg; 1.17 mmol) in CH2Cl2 (5 mL) at 0 °C was added a solution of PBr3 (55 µL; 0.58 mmol) in CH2Cl2 (1.5 mL) and the resulting mixture was stirred for 10 minutes. The reaction mixture was diluted with CH2Cl2 (20 mL) and washed with saturated aqueous NaHCO3 (10 mL) and H2O (10 mL), then dried (MgSO4) and concentrated in vacuo to give the corresponding benzyl bromide 21 (503 mg; 100 %) as a yellow oil, which displayed identical physical properties to those reported.43
5.2.9. [3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-benzyl]-triphenylphosphonium bromide (9)
To a solution of benzyl bromide 21 (503 mg; 1.17 mmol) in toluene (5.5 mL) was added PPh3 (300 mg; 1.17 mmol) and the resulting mixture was heated at reflux overnight. The precipitate was collected by filtration and dried under vacuum to give phosphonium bromide 9 (720 mg; 89 %) as a white solid, which displayed identical physical properties to those reported.44
5.2.10 3-{2-[3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-phenyl]-vinyl}-1,5-dimethoxy-2,4-bis-(3-methyl-but-2-enyl)-benzene (22)
To a solution of phosphonium bromide 9 (127 mg; 0.18 mmol) in THF (9.0 mL) at −78 °C was added n-BuLi (71 µL; 0.18 mmol; 2.5 M solution in hexanes) and the resulting red mixture was stirred for 30 minutes. A solution of aldehyde 17 (45 mg; 0.15 mmol) in THF (1.0 mL) was added via cannula and the reaction mixture was warmed to room temperature over 30 minutes. Saturated aqueous NaHCO3 (5 mL) was added and the mixture was extracted with Et2O (3 × 20 mL). The combined organic extracts were washed with brine (5 mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % CH2Cl2 in hexanes) to give Z-22 (17 mg; 18 %) followed by E-22 (68 mg; 72 %) as clear oils. Physical data for E-22: Rf = 0.29 (silica gel, 30 % CH2Cl2 in hexanes); IR νmax (film) 2951s, 2928s, 2857m, 1739w, 1585m, 1507s, 1462m, 1433m, 1309m, 1297m, 1253m, 1213w, 1160w, 1120w, 1081w, 991w, 908s, 839s, 806s, 782s cm−1; 1H NMR (500 MHz, CDCl3) δ = 6.94 (d, J = 2.0 Hz, 1 H), 6.89 (dd, J = 8.0, 2.0 Hz, 1 H), 6.88 (d, J = 16.5 Hz, 1 H), 6.80 (d, J = 8.0 Hz, 1 H), 6.46 (s, 1 H), 6.38 (d, J = 16.5 Hz, 1 H), 5.14 (m, 2 H), 3.85 (s, 6 H), 3.34 (d, J = 7.0 Hz, 4 H), 1.66 (d, J = 1.0 Hz, 6 H), 1.63 (s, 6 H), 1.01 (s, 18 H), 0.23 (s, 6 H), 0.22 (s, 6 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 156.3, 146.8, 146.5, 139.4, 133.9, 131.6, 130.4, 124.6, 124.1, 120.9, 120.8, 119.7, 118.9, 94.8, 55.9, 26.2, 2 6.0, 26.0, 25.8, 18.5, 18.5, 18.0, −4.1, −4.1 ppm.
5.2.11. Artochamin I (3)
To a solution of bis-TBS ether E-22 (40 mg; 62.7 µmol) in THF (500 µL) at 0 °C was added HF•py (500 µL; excess) and the resulting mixture was warmed to room temperature and stirred for an additional 2 hours. The reaction was diluted with Et2O (10 mL) and then washed successively with saturated aqueous CuSO4 (3 × 2 mL), saturated aqueous NaHCO3 (2 × 2 mL) and brine (2 mL). The organic layer was then dried (MgSO4) and concentrated in vacuo. The residue was passed through a short plug of silica gel (30 % EtOAc in hexanes) to give diol 23 (23 mg; 88 %) as a clear oil, which was used directly in the following reaction.
A solution of stilbene 23 (10 mg; 24.2 µmol) in DMF (2 mL) was subjected to microwave heating at 180 °C for 20 minutes. The reaction mixture was diluted with EtOAc (10 mL) and H2O (2 mL) was added. The aqueous phase was acidified (pH 3) with HCl (1 M) and the layers were separated. The organic phase was further washed with H2O (4 × 2 mL) and brine (2 mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (50 % EtOAc in hexanes) to give artochamin I (3) (8.0 mg; 80 %) as a clear oil. Rf = 0.17 (silica gel, 30 % EtOAc in hexanes); IR νmax (film) 3381brm, 2926s, 2856m, 1701w, 1600s, 1518m, 1492m, 1461s, 1451s, 1436s, 1365m, 1312s, 1282m, 1260m, 1202m, 1119s, 1086s, 1033w, 867w, 806m cm−1; 1H NMR (500 MHz, (CD3)2CO) δ = 7.64 (brs, 2 H), 6.79 (d, J = 8.0 Hz, 1 H), 6.76 (d, J = 2.0 Hz, 1 H), 6.64 (dd, J = 8.0, 2.0 Hz, 1 H), 6.47 (s, 1 H), 4.92 (m, 1 H), 3.95 (dd, J = 8.0, 6.0, Hz, 1 H), 3.84 (s, 3 H), 3.79 (s, 3 H), 3.03 (dd, J = 16.0, 3.0 Hz, 1 H), 2.94–2.91 (m, 1 H), 2.88 (dd, J = 17.0, 9.5 Hz, 1 H), 2.84 (d, J = 5.5 Hz, 1 H), 2.76 (m, 2 H), 1.51 (d, J = 1.5 Hz, 3 H), 1.42 (s, 3 H), 1.01 (s, 3 H), 0.75 (s, 3 H) ppm; 13C NMR (150 MHz, (CD3)2CO) δ = 159.6, 156.4, 150.9, 146.6, 145.2, 135.2, 131.5, 125.6, 125.6, 121.0, 119.0, 117.1, 116.7, 95.9, 59.6, 57.3, 56.5, 46.6, 46.4, 40.1, 31.7, 28.4, 27.6, 27.2, 26.8, 18.8 ppm; HRMS (ESI TOF) m/z calcd. for C26H32O4 [M+H+]: 409.2373; found 409.2374.
5.3. Julia–Kocieński approach to stilbenes and cascade strategy for artochamins H–J (2–4)
5.3.1. [4-Bromo-3,5-bis-(1,1-dimethyl-allyloxy)-phenyl]-methanol (24)
To a solution of methyl ester 13 (1.90 g; 4.96 mmol) in THF (50 mL) at 0 °C was added a solution of LiAlH4 (5.95 mL; 5.95 mmol; 1 M solution in THF). After 10 minutes the reaction was quenched by the addition of H2O (2 mL), NaOH (2 mL; 1 M aqueous solution) and H2O (3 mL), then MgSO4 was added and the mixture was filtered. The solvent was concentrated in vacuo and the residue was purified by column chromatography (40 % Et2O in hexanes) to give alcohol 24 (1.67 g; 95 %) as a clear oil. Rf = 0.36 (silica gel, 60 % Et2O in hexanes); IR νmax (film) 3404brw, 2981w, 1572m, 1422s, 1378m, 1363m, 1217w, 1127s, 1057s, 999m, 923m, 864m, 704m cm−1; 1H NMR (500 MHz, CDCl3) δ = 6.82 (s, 2 H), 6.18 (dd, J = 15.0, 9.0 Hz, 2 H), 5.22 (d, J = 15.0 Hz, 2 H), 5.14 (d, J = 9.0 Hz, 2 H), 4.52 (d, J = 5.0 Hz, 2 H), 1.51 (s, 12 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 154.6, 144.2, 139.6, 113.9, 113.6, 111.9, 81.8, 65.0, 26.9 ppm; HRMS (ESI TOF) m/z calcd. for C17H23BrO3 [M+H+]: 355.0903; found 355.0906.
5.3.2. [4-Bromo-3,5-bis-(1,1-dimethyl-allyloxy)]-benzaldehyde (7)
To a solution of alcohol 24 (1.66 g; 4.67 mmol) in CH2Cl2 (25 mL) at room temperature was added Dess–Martin periodinane (2.4 g; 5.61 mmol). After 30 minutes the reaction mixture was diluted with Et2O (100 mL) and a solution of Na2S2O3 (9.6 g) in saturated aqueous NaHCO3 (20 mL) was added and stirring maintained until a clear biphase persisted. The aqueous phase was removed and the organic layer was washed with saturated aqueous NaHCO3 (20 mL) and brine (20 mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % Et2O in hexanes) to give aldehyde 7 (1.60 g; 97 %) as a clear oil. Rf = 0.34 (silica gel, 30 % Et2O in hexanes); IR νmax (film) 2982w, 1696s, 1570s, 1421s, 1379s, 1364m, 1325m, 1217w, 1124s, 1072s, 1035s, 1001m, 924m, 827m, 757m, 704m cm−1; 1H NMR (500 MHz, CDCl3): δ = 9.78 (s, 1 H), 7.27 (s, 2 H), 6.18 (dd, J = 17.5, 11.0 Hz, 2 H), 5.27 (d, J = 17.5 Hz, 2 H), 5.21 (d, J = 11.0 Hz, 2 H), 1.55 (s, 12 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 191.6, 155.7, 144.0, 135.3, 121.0, 115.1, 114.8, 82.8, 27.4 ppm; HRMS (ESI TOF): m/z calcd. for C17H21BrO3 [M+H+]: 353.0747; found 353.0746.
5.3.3. Carbonic acid 2-tert-butoxycarbonyloxy-4-formylphenyl ester tert-butyl ester (25)
To a solution of 3,4-dihydroxybenzaldehyde 18 (2.0 g; 14.5 mmol) in THF (50 mL) at room temperature was added di-tert-butyl dicarbonate (6.32 g; 29.0 mmol), iPr2NEt (203 µL; 1.45 mmol) and DMAP (89 mg; 0.72 mmol). After 2 hours the reaction mixture was diluted with EtOAc (100 mL) and washed with NaOH (50 mL; 1 M aqueous solution) and brine, then dried (MgSO4), concentrated in vacuo to give aldehyde 25 (4.90 g; 100 %), which was used in the next reaction without further purification. Rf = 0.38 (silica gel, 30 % EtOAc in hexanes); IR νmax (film) 2978w, 2936w, 2868w, 1767m, 1740m, 1704m, 1371m, 1243s, 1151s, 1136s, 1106s, 1045m, 886m, 865w, 776m, 728m, 634m cm−1; 1H NMR (500 MHz, CDCl3) δ = 9.95 (s, 1 H), 7.79 (d, J = 2.0 Hz, 1 H), 7.76 (dd, J = 8.5, 2.0 Hz, 1 H), 7.44 (d, J = 8.5 Hz, 1 H), 1.55 (s, 18 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 190.0, 150.3, 149.8, 147.3, 143.2, 134.5, 127.9, 124.0, 123.7, 84.5, 84.4, 27.5 ppm; HRMS (ESI TOF) m/z calcd. for C17H22O7 [M+Na+]: 361.1258; found 361.1259.
5.3.4. Carbonic acid 2-tert-butoxycarbonyloxy-4-hydroxymethyl-phenyl ester tert-butyl ester (26)
To a solution of aldehyde 25 (4.44 g; 13.1 mmol) in EtOH (70 mL) at 0 °C was added NaBH4 (548 mg; 13.1 mmol) and the reaction mixture was warmed to room temperature and stirred for 10 minutes. Saturated aqueous NH4Cl (50 mL) was added and the mixture was extracted with Et2O (3 × 100 mL). The combined organic extracts were washed with brine (50 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % EtOAc in hexanes) to give alcohol 26 (4.24 g; 95 %) as a clear oil. Rf = 0.38 (silica gel, 30 % EtOAc in hexanes); IR νmax (film) 3377brs, 2976w, 2934w, 2874w, 1766m, 1371m, 1252s, 1154s, 1113s, 1046m, 880m, 777m, 734m cm−1; 1H NMR (500 MHz, CDCl3) δ = 7.31 (d, J = 8.5 Hz, 1 H), 7.30 (d, J = 2.0 Hz, 1 H), 7.11 (dd, J = 8.5, 2.0 Hz, 1 H), 4.67 (s, 2 H), 1.58 (s, 18 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 150.8, 150.8, 142.4, 141.6, 139.8, 124.5, 122.9, 121.4, 83.8, 83.7, 27.6 ppm; HRMS (ESI TOF) m/z calcd. for C24H24O7 [M+Na+]: 363.1414; found 363.1414.
5.3.5. Carbonic acid 4-bromomethyl-2-tert-butoxycarbonyloxy-phenyl ester tert-butyl ester (27)
To a solution of alcohol 26 (500 mg; 1.47 mmol) in CH2Cl2 (10 mL) was added PBr3 (0.28 mL; 2.94 mmol) dropwise and the resulting solution was stirred at 0 °C for one hour. The reaction was quenched with water (3 mL), and then extracted with Et2O (3 × 5 mL), and the combined organic extracts were washed with saturated aqueous NaHCO3 (3 mL) and brine (3 mL), then dried (K2CO3) and concentrated in vacuo to yield the corresponding bromide (104 mg; 18 %) as an orange oil. Rf = 0.69 (silica gel, 50 % EtOAc in hexanes); IR (film) νmax 2981w, 2935w, 1761s, 1610w, 1508m, 1457w, 1433w, 1395m, 1370m, 1248s, 1143s, 1109s, 1046w, 1014w, 888m cm−1; 1H NMR (500 MHz, CDCl3) δ 7.34 (d, J = 1.5 Hz, 1 H), 7.30–7.26 (m, 2 H), 4.47 (s, 2 H), 1.58 (s, 9 H), 1.58 (s, 9 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 150.5, 150.4, 142.3, 142.2, 136.1, 126.9, 123.7, 123.2, 83.9, 31.9, 27.5 ppm; HRMS (ESI TOF) m/z calcd. for C17H23BrO6 [M+Na+]: 425.0570; found 425.0564.
5.3.6. [3,4-Bis-(tert-butoxycarbonyloxy)-benzyl]-triphenylphosphonium bromide (8)
A mixture of bromide 27 (170 mg; 0.42 mmol) and triphenylphosphine (166 mg; 0.63 mmol) in toluene (3.0 mL) was heated to 60 °C and stirred overnight. The solvent was then removed in vacuo to give phosphonium bromide 8 (151 mg; 62 %) as a white solid. IR (film) νmax 2981w, 2870w, 2773w, 1769s, 1755s, 1589w, 1505w, 1438m, 1371m, 1284m, 1251s, 1157s, 1137m, 1113s, 886m cm−1; 1H NMR (500 MHz, DMSO-d6) δ 7.93–7.90 (m, 5 H), 7.77–7.62 (m, 10 H), 7.17 (d, J = 8.0 Hz, 1 H), 6.96 (d, J = 8.5 Hz, 1 H), 6.89 (s, 1 H), 5.25 (d, J = 15.5 Hz, 2 H), 1.46 (s, 9 H), 1.44 (s, 9 H) ppm; 13C NMR (150 MHz, DMSO-d6) δ = 149.6 (d, J = 8.6 Hz), 149.5 (d, J = 8.1 Hz), 142.0 (d, J = 4.2 Hz), 141.7 (d, J = 3.6 Hz), 135.1 (d, J = 2.7 Hz), 134.0 (d, J = 9.9 Hz), 130.1 (d, J = 12.5 Hz), 126.7 (d, J = 8.6 Hz), 125.5 (d, J = 5.4 Hz), 123.6 (d, J = 2.9 Hz), 117.3 (d, J = 85.1 Hz), 84.0 (s), 83.9 (s), 27.5 (d, J = 47.0 Hz), 27.0 (s), 26.9 (s) ppm; HRMS (ESI TOF) m/z calcd. for C35H38O6P [M+•]: 585.2406; found 585.2392.
5.3.7. Carbonic acid 4-{2-[4-bromo-3,5-bis-(1,1-dimethyl-allyloxy)-phenyl]-vinyl}-2-tert-butoxycarbonyloxy-phenyl ester tert-butyl ester (5)
To a solution of phosphonium bromide 8 (200 mg; 0.30 mmol) in THF (1.2 mL) at −78 °C was added t-BuOK (34 mg; 0.30 mmol) and the resulting red mixture was stirred for 30 minutes. A solution of aldehyde 7 (88 mg; 0.25 mmol) in THF (500 µL) was added via cannula and the reaction mixture was warmed to room temperature over 30 minutes. Saturated aqueous NaHCO3 (5 mL) was added and the mixture was extracted with Et2O (3 × 15 mL). The combined organic extracts were washed with brine (5 mL), then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % CH2Cl2 in hexanes) to give Z-22 (16.5 mg; 44 %) followed by E-22 (17.5 mg; 46 %) as clear oils. Physical data for E-22: Rf = 0.23 (silica gel, 15 % Et2O in hexanes); IR νmax (film) 2981w, 1767s, 1582w, 1506w, 1414w, 1370w, 1253s, 1156s, 1115m, 1072w cm−1; 1H NMR (400 MHz, CDCl3) δ = 7.37 (d, J = 2.4 Hz, 1 H), 7.32 (dd, J = 8.4, 2.4 Hz, 1 H), 7.23 (d, J = 8.4 Hz, 1 H), 6.95 (s, 2 H), 6.83 (s, 2 H), 6.22 (dd, J = 17.6, 10.8 Hz, 2 H), 5.25 (dd, J = 17.6, 0.8 Hz, 2 H), 5.19 (dd, J = 10.8, 0.8 Hz, 2 H), 1.56 (s, 9 H), 1.55 (s, 9 H) 1.53 (s, 12 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 150.8, 150.7, 144.6, 144.4, 144.3, 144.3, 142.8, 141.9, 135.9, 135.5, 129.5, 127.4, 127.3, 124.6, 123.3, 120.9, 120.9, 120.8, 113.9, 113.8, 112.7, 83.9, 82.0, 27.8, 27.7, 27.0, 26.9 ppm; HRMS (ESI TOF) m/z calcd. for C34H43BrO8 [M+H+]: 659.2214; found 659.2197.
5.3.8. Carbonic acid 2-tert-butoxycarbonyloxy-4-(1-phenyl-1H-tetrazole-5-sulfonylmethyl)-phenyl ester tert-butyl ester (10)
To a solution of alcohol 26 (5.4 g; 17.5 mmol) in THF (175 mL) at 0 °C was added PPh3 (5.1 g; 19.3 mmol), 1-phenyl-1H-tetrazole-5-thiol (3.12 g; 17.5 mmol) and DEAD (3.31 mL; 21.0 mmol). After stirring for 30 minutes at 0 °C saturated aqueous NaHCO3 (75 mL) was added and the reaction mixture was extracted with Et2O (3 × 100 mL). The combined organic extracts were washed with brine (75 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % EtOAc in hexanes) to give the sulfide (6.74 g; 77 %) as a yellow oil.
To a solution of the sulfide (5.04 g; 10.1 mmol) in EtOH (120 mL) at room temperature was added a cooled (0 °C) solution of ammonium molybdate tetrahydrate (1.25 g; 0.10 mmol) in H2O2 (120 mL). The resulting yellow mixture was allowed to stir for 14 hours before being diluted with H2O (100 mL) and extracted with EtOAc (3 × 100 mL). The combined organic extracts were washed with brine (100 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (40 % EtOAc in hexanes) to give sulfone 10 (5.31 g; 99 %) a white powder. Rf = 0.44 (silica gel, 40 % EtOAc in hexanes); IR νmax (film) 2982w, 2935w, 1763s, 1508w, 1497w, 1371m, 1356m, 1249s, 1148s, 1112s, 1046m, 1014m, 890m, 763m, 733m, 688m, 535m cm−1; 1H NMR (500 MHz, CDCl3) δ = 7.56–7.52 (m, 1 H), 7.50–7.47 (m, 2 H), 7.33–7.31 (m, 2H), 7.22 (d, J = 2.0 Hz, 1 H), 7.22 (d, J = 8.5 Hz, 1 H), 7.16 (dd, J = 8.5, 2.0 Hz, 1 H), 4.87 (s, 2 H), 1.54 (s, 9 H), 1.53 (s, 9 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 152.6, 150.2, 143.8, 142.8, 132.6, 131.3, 129.5, 129.4, 126.3, 125.4, 123.8, 123.3, 84.2, 61.4, 27.5 ppm; HRMS (ESI TOF) m/z calcd. for C24H28N4O8S [M+Na+]: 555.1520; found 555.1512.
5.3.9. 5-[3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-phenylmethanesulfonyl]-1-phenyl-1H-tetrazole (11)
To a solution of benzyl alcohol 20 (2.14 g; 5.80 mmol), Ph3P (1.67 g; 6.39 mmol), and 1-phenyl-1H-tetrazole-5-thiol (1.03 g; 5.80 mmol) at 0 °C in THF (58 mL) was added DEAD (3.17 mL; 6.97 mmol). The mixture was stirred for 30 minutes at 0 °C and then treated with saturated aqueous NaHCO3 (30 mL) and diluted with Et2O (55 mL). The phases were separated and the aqueous phase was extracted with Et2O (2 × 40 mL). The combined organic extracts were washed with brine (30 mL), dried (MgSO4) and concentrated in vacuo to yield the sulfide as a yellow oil.
A solution of the sulfide prepared above (4.47 g; 8.45 mmol) in EtOH (84.5 mL), THF (16.9 mL), and H2O2 (31.7 mL) was treated with ammonium molybdate tetrahydrate (2.1 g; 1.69 mmol) at room temperature. The mixture was stirred overnight, then partitioned with CH2Cl2 (60 mL) and saturated aqueous NH4Cl (30 mL). The layers were separated and the aqueous phase was extracted with CH2Cl2 (2 × 30 mL) and the combined organic extracts were dried (MgSO4) and concentrated in vacuo to yield 11 (4.09 g; 86 %, over two steps) as a white foam. Rf = 0.26 (silica gel, 10 % EtOAc in hexanes); IR (film) νmax 2954w, 2930m, 2887w, 2858m, 1599w, 1577w, 1509s, 1472m, 1424m, 1346s, 1304s, 1253s, 1154s, 1137s, 985s, 905s, 780s, 761s cm−1; 1H NMR (500 MHz, CDCl3) δ 7.59–7.55 (m, 1 H), 7.52–7.49 (m, 2 H), 7.38 (m, 2 H), 6.82 (d, J = 2.5 Hz, 1 H) 6.78 (d, J = 8.5 Hz, 1 H), 6.74 (dd, J = 8.0, 2.0 Hz, 1 H), 4.81 (s, 2 H), 0.97 (s, 9 H), 0.96 (s, 9 H), 0.19 (s, 6 H), 0.15 (s, 6 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 153.0, 148.6, 147.3, 132.9, 131.3, 129.4, 125.3, 124.7, 124.1, 121.2, 117.0, 62.0, 25.8, 25.8, 18.4, 18.3, −4.1, −4.2 ppm; HRMS (ESI TOF) m/z calcd. for C26H40N4O4SSi2 [M+H+]: 561.2381; found 561.2379.
5.3.10. Carbonic acid 4-{2-[4-bromo-3,5-bis-(1,1-dimethyl-allyloxy)-phenyl]-vinyl}-2-tert-butoxycarbonyloxy-phenyl ester tert-butyl ester (5)
To a solution of sulfone 10 (1.13 g; 2.12 mmol) in THF (20 mL) at −78 °C was added KHMDS (4.24 mL; 2.12 mmol; 0.5 M solution in toluene) and the resulting bright yellow solution was stirred for 30 minutes. A solution of aldehyde 7 (500 mg; 1.42 mmol) in THF (5 mL) was then added via cannula and the reaction mixture was slowly warmed to room temperature. The reaction was quenched by the addition of saturated aqueous NaHCO3 (20 mL) and then extracted with Et2O (3 × 30 mL). The combined organic extracts were washed with brine (20 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (15 % Et2O in hexanes) to give stilbene 5 (747 mg; 80 %; 100 % E) as a white powder. For physical data see 5.3.7.
5.3.11. 5-{2-[3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-phenyl]-vinyl}-2-bromo-1,3-bis-(1,1-dimethyl-allyloxy)-benzene (6)
To a solution of sulfone 11 (163 mg; 0.29 mmol) in DME (26 mL) at −78 °C was added KHMDS (0.6 mL; 0.30 mmol; 0.5 M solution in toluene) and the resulting bright yellow solution was stirred for 30 minutes. A solution of aldehyde 7 (57.0 mg; 0.16 mmol) in DME (1.4 mL) was then added via cannula and the reaction mixture was slowly warmed to room temperature. The reaction was quenched by the addition of saturated aqueous NaHCO3 (3 mL) and the mixture was extracted with Et2O (3 × 6 mL). The combined organic extracts were washed with brine (3 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (25 % benzene in hexanes) to give stilbene 6 (107 mg; 95 %; E/Z ca. 2:1) as a yellow oil. Rf = 0.57 (silica, 10 % EtOAc in hexanes); IR (film) νmax 2951m, 2930s, 2858m, 1566m, 1508s, 1472w, 1421m, 1291m, 1253m, 1128m, 1069m, 994w, 906m, 839s, 804w, 782s cm−1; 1H NMR (500 MHz, CDCl3) δ 6.98–6.91 (m, 4 H), 6.81–6.79 (m, 1 H), 6.76 (d, J = 16.2 Hz, 1 H), 6.68 (d, J = 16.2 Hz, 1 H), 6.23 (dd, J = 17.4, 10.8 Hz, 2 H), 5.24 (d, J = 17.4 Hz, 2 H), 5.17 (d, J = 17.4 Hz, 2 H), 1.54 (s, 6 H), 1.53 (s, 6 H), 1.01 (s, 9 H), 0.98 (s, 9 H), 0.22 (s, 6 H), 0.21 (s, 6 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 154.5, 147.1, 147.0, 144.4, 136.3, 130.7, 129.0, 126.4, 121.2, 120.1, 119.2, 113.8, 113.5, 112.1, 81.9, 26.8, 26.0, 25.9, 18.4, −4.1, −4.1 ppm; HRMS (ESI TOF) m/z calcd. for C36H55BrO4Si2 [M+H+]: 687.2895; found 687.2879.
5.3.12. 5-Bromo-2-(3,4-dihydroxy-phenyl)-1,1-dimethyl-3-(3-methyl-but-2-enyl)-2,2a,7,7a-tetrahydro-1H-cyclobuta[a]indene-4,6-diol (32)
Stilbene 31 (12.2 mg; 18.5 µmol) and Ph3PO (0.6 mg; 5 % w/w) were dissolved in o-xylene (1.5 mL) and the resulting solution was subjected to microwave heating at 180 °C for 20 minutes. Alternatively, heating could be conducted conventionally with an oil bath at 165 °C for 1 hour. The cooled reaction mixture was directly loaded onto a silica gel column packed with hexanes as the eluant and the o-xylene was run off. The eluant was switched to 10 % Et2O in CH2Cl2 and the cycloaddition product 32 (4.7 mg; 55 %) was obtained as an inseparable 5:1 mixture of diastereomers. Major diastereomer Rf = 0.13 (silica gel, 5 % MeOH in CH2Cl2); IR νmax (film) 3475brs, 2956m, 2928m, 2855m, 1719w, 1600m, 1517m, 1441s, 1367m, 1285s, 1249s, 1188m, 1158m, 1105m, 1093m, 966w, 804w, 786w, 766w, 741w cm−1; 1H NMR (500 MHz, CDCl3) δ = 6.82 (d, J = 8.0 Hz, 1 H), 6.76 (d, J = 2.0 Hz, 1 H), 6.67 (dd, J = 8.0, 2.0 Hz, 1 H), 5.48 (s, 1 H), 5.25 (s, 1 H), 5.11 (s, 1 H), 5.00 (m, 1 H), 4.97 (s, 1 H), 3.95 (ddd, J = 8.0, 6.0, 1.0 Hz, 1 H), 3.13 (ddd, J = 16.0, 3.0, 1.0 Hz, 1 H), 3.08 (d, J = 7.0 Hz, 1 H), 3.01 (dd, J = 16.5, 9.5 Hz, 1 H), 3.00 (m, 1 H), 2.89 (d, J = 6.0 Hz, 1 H), 2.80 (m, 1 H), 1.61 (d, J = 1.0 Hz, 3 H), 1.54 (s, 3 H), 1.05 (s, 3 H), 0.76 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 149.8, 149.3, 146.6, 143.2, 141.6, 134.6, 133.5, 122.8, 121.9, 120.4, 115.6, 115.1, 115.0, 97.6, 57.4, 44.7, 44.6, 38.7, 30.5, 27.3, 27.0, 25.8, 25.7, 17.5 ppm; HRMS (ESI TOF) m/z calcd. for C24H27BrO4 [M+H+]: 459.1165; found 459.1145.
5.3.13. 2,2-Dimethyl-propionic acid 5-[5-bromo-6-(2,2-dimethyl-propionyloxy)-4-hydroxy-1,1-dimethyl-2,2a,7,7a-tetrahydro-1H-cyclobuta[a]inden-2-yl]-2-(2,2-dimethyl-propionyloxy)-phenyl ester (33)
To a solution of tetraol 32 (16.0 mg; 34.8 µmol) in CH2Cl2 (300 µL) at room temperature was added Et3N (29 µL; 209 µmol) followed by trimethylacetyl chloride (105 µL; 104.5 µmol; 1 M solution in CH2Cl2). After 30 minutes the reaction mixture was diluted with EtOAc (2 mL) and the aqueous layer was acidified to pH 3 with aqueous HCl (1 M). The layers were separated and the aqueous phase was further extracted with EtOAc (3 × 2 mL). The combined extracts were washed with brine (1 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (10 % to 20 % Et2O in hexanes) to afford the tris-protected compound 33 (18.6 mg; 75 %) as a white foam. Rf = 0.23 (silica gel, 20 % Et2O in hexanes); IR νmax (film) 3473brs, 2962m, 2929m, 2855m, 1760s, 1505m, 1480m, 1460m, 1397s, 1368s, 1271m, 1259m, 1115s, 1029s, 890w cm−1; 1H NMR (500 MHz, CDCl3) δ = 7.10–7.07 (m, 2 H), 6.96 (d, J = 2.0 Hz, 1 H), 5.56 (s, 1 H), 4.99 (m, 1 H), 4.00 (dd, J = 7.0, 5.0 Hz, 1 H), 3.03 (dd, J = 16.0, 4.5 Hz, 1 H), 2.99 (d, J = 5.0 Hz, 1 H), 2.96–2.86 (m, 3 H), 2.82 (m, 1 H), 1.60 (d, J = 1.0 Hz, 3 H), 1.47 (s, 3 H), 1.42 (s, 9 H), 1.36 (s, 9 H), 1.35 (s, 9 H), 1.05 (s, 3 H), 0.75 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 176.0, 175.7, 175.2, 150.3, 147.9, 142.4, 142.2, 140.8, 139.7, 133.4, 130.0, 125.5, 125.4, 122.8, 122.5, 121.2, 103.2, 57.0, 44.7, 44.6, 39.4, 39.1, 38.9, 30.3, 29.7, 27.3, 25.6, 17.8 ppm; HRMS (ESI TOF) m/z calcd. for C39H51BrO7 [M+Na+]: 733.2710; found 733.2724.
5.3.14. 2,2-Dimethyl-propionic acid 5-[5-bromo-6-(2,2-dimethyl-propionyloxy)-4-methoxy-1,1-dimethyl-2,2a,7,7a-tetrahydro-1H-cyclobuta[a]inden-2-yl]-2-(2,2-dimethyl-propionyloxy)-phenyl ester (34)
To a solution of phenol 33 (5.5 mg; 7.73 µmol) in DMF (250 µL) was added K2CO3 (5.3 mg; 38.5 µmol) followed by MeI (3.0 µL; 46.2 µmol) and the resulting mixture was stirred at 100 °C for 1 hour. Upon cooling the reaction mixture was diluted with EtOAc (2 mL), washed with H2O (5 × 1 mL), then brine (1 mL), and finally dried (MgSO4) before removal of the solvent in vacuo. The residue was purified by column chromatography (20 % Et2O in hexanes) to afford the methylated compound 34 (5.3 mg; 95 %) as a white foam. Rf = 0.36 (silica gel, 20 % Et2O in hexanes); IR νmax (film) 2956m, 2925m, 2860m, 1760s, 1501w, 1476w, 1458w, 1395w, 1365w, 1319w, 1260m, 1111s, 1026m, 800w cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.08 (d, J = 8.0 Hz, 1 H), 7.02 (dd, J = 8.0, 2.0 Hz, 1 H), 6.94 (d, J = 2.0 Hz, 1 H), 4.91 (m, 1 H), 4.02 (dd, J = 8.0, 6.0 Hz, 1 H), 3.76 (s, 3 H), 3.04–2.87 (m, 5 H), 2.81 (m, 1 H), 1.56 (s, 3 H), 1.43 (s, 9 H), 1.42 (s, 3 H), 1.35 (s, 9 H), 1.35 (s, 9 H), 1.06 (s, 3 H), 0.75 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 176.0, 175.7, 175.3, 155.1, 148.1, 143.2, 142.2, 140.8, 139.6, 134.5, 132.5, 129.8, 125.3, 122.8, 122.4, 121.9, 110.1, 61.1, 56.9, 44.6, 44.4, 39.4, 39.1, 39.1, 38.9, 31.0, 30.3, 27.3, 27.3, 27.3, 25.8, 25.5, 17.8 ppm; HRMS (ESI TOF): m/z calcd. for C40H53BrO7 [M+H+]: 725.3047; found 725.3042.
5.3.15. Artochamin H (2)
To a solution of compound 34 (4.0 mg; 5.51 µmol) in THF (100 µL) at 0 °C was added LiAlH4 (33 µL; 33.1 µmol; 1 M solution in THF). The resulting mixture was warmed to room temperature and allowed to stir for 24 hours. Excess LiAlH4 was destroyed by the addition of EtOAc and the mixture was acidified to pH 3 with aqueous HCl (1 M). The layers were separated and the aqueous phase was extracted with EtOAc (3 × 2 mL). The combined organic extracts were washed with brine (1 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (50 % EtOAc in hexanes) to afford artochamin H (2) (2.0 mg; 93 %) as a clear oil. Rf = 0.43 (silica gel, 50 % EtOAc in hexanes); IR νmax (film) 3366s, 2955m, 2925m, 2856m, 1691m, 1599s, 1518m, 1445s, 1365m, 1347m, 1309m, 1280s, 1256s, 1191s, 1160m, 1109s, 1059m, 960w, 868m, 806m, 784m cm−1; 1H NMR (600 MHz, (CD3)2CO) δ = 7.79 (brs, 1 H), 7.66 (brs, 2 H), 6.79 (d, J = 7.8 Hz, 1 H), 6.78 (d, J = 1.8 Hz, 1 H), 6.64 (dd, J = 7.8, 1.8 Hz, 1 H), 6.31 (s, 1 H), 4.91 (m, 1 H), 3.96 (dd, J = 7.8, 6.0 Hz, 1 H), 3.70 (s, 3 H), 3.07 (ddd, J = 16.8, 3.0, 1.2 Hz, 1 H), 3.01 (dd, J = 14.4, 6.0 Hz, 1 H), 2.90 (dd, J = 16.8, 9.6 Hz, 1 H), 2.80 (m, 2 H), 2.75 (m, 1 H), 1.50 (d, J = 1.2 Hz, 3 H), 1.41 (s, 3 H), 1.03 (s, 3 H), 0.75 (s, 3 H) ppm; 13C NMR (150 MHz, (CD3)2CO) δ = 159.5, 153.6, 151.1, 146.5, 145.1, 135.3, 131.3, 125.8, 124.0, 121.2, 118.2, 117.1, 116.6, 99.4, 59.6, 57.0, 46.7, 46.5, 40.1, 31.4, 28.5, 27.6, 27.2, 26.8, 18.7 ppm; HRMS (ESI TOF) m/z calcd. for C25H30O4 [M−H−]: 393.2071; found 393.2079.
5.3.16. 2,2-Dimethyl-propionic acid 5-(5-bromo-4,6-dihydroxy-1,1-dimethyl-2,2a,7,7a-tetrahydro-1H-cyclobuta[a]inden-2-yl)-2-(2,2-dimethyl-propionyloxy)-phenyl ester (35)
To a solution of tetraol 32 (15.0 mg; 32.7 µmol) in CH2Cl2 (300 µL) at room temperature was added pyridine (16.0 µL; 196 µmol) followed by trimethylacetyl chloride (66 µL; 66.0 µmol; 1 M solution in CH2Cl2). After stirring at room temperature overnight, the reaction mixture was diluted with EtOAc (2 mL) and the aqueous layer was acidified to pH 3 with aqueous HCl (1 M). The layers were separated and the aqueous phase was further extracted with EtOAc (3 × 2 mL). The combined extracts were washed with brine (1 mL), dried (MgSO4) and then concentrated in vacuo. The residue was purified by column chromatography (30 % to 40 % Et2O in hexanes) to afford the bis-protected compound 35 (13.1 mg; 64 %) as a white foam. Rf = 0.24 (silica gel, 30 % Et2O in hexanes); IR νmax (film) 3450brm, 2970m, 2931m, 2865w, 1758m, 1737m, 1595w, 1505w, 1480w, 1458w, 1367w, 1276w, 1259m, 1228m, 1117s, 1032w, 891w, 790w cm−1; 1H NMR (600 MHz, CDCl3) δ = 7.10–7.07 (m, 2 H), 6.96 (d, J = 1.2 Hz, 1 H), 5.51 (s, 1 H), 5.26 (s, 1 H), 5.02 (m, 1 H), 3.95 (dd, J = 7.8, 5.4 Hz, 1 H), 3.14–3.09 (m, 2 H), 3.04–2.98 (m, 3 H), 2.85 (m, 1 H), 1.62 (d, J = 1.2 Hz, 3 H), 1.54 (s, 3 H), 1.35 (s, 9 H), 1.35 (s, 9 H), 1.07 (s, 3 H), 0.77 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 176.0, 175.7, 149.9, 147.9, 146.6, 142.2, 140.7, 139.8, 133.9, 125.4, 122.8, 122.8, 122.5, 121.7, 115.7, 97.8, 57.1, 44.7, 44.6, 39.1, 39.1, 39.0, 30.5, 27.3, 27.3, 27.3, 27.1, 25.9, 25.7, 17.8 ppm; HRMS (ESI TOF) m/z calcd. for C34H43BrO6 [M+Na+]: 649.2135; found 649.2123.
5.3.17. Artochamin I (3)
To a solution of bis-phenol 35 (6.0 mg; 9.56 µmol) in DMF (300 µL) was added K2CO3 (6.6 mg; 47.8 µmol) followed by MeI (3.6 µL; 57.4 µmol) and the resulting mixture was stirred at 100 °C for 1 hour. Upon cooling the reaction mixture was diluted with EtOAc (2 mL), washed with H2O (5 × 1 mL), then brine (1 mL), and finally dried (MgSO4) before removal of the solvent in vacuo. The residue was purified by column chromatography (20 % Et2O in hexanes) to afford the methylated compound 36 (6.0 mg; 96 %) as a foam, which was used directly in the following reaction.
To a solution of compound 36 (3.7 mg; 5.64 µmol) in THF (100 µL) at 0 °C was added LiAlH4 (33.6 µL; 33.6 µmol; 1 M solution in THF). The resulting mixture was warmed to room temperature and allowed to stir for 24 hours. Excess LiAlH4 was destroyed by the addition of EtOAc, and the mixture was acidified to pH 3 with aqueous HCl (1 M). The layers were separated and the aqueous phase was extracted with EtOAc (3 × 2 mL). The combined organic extracts were washed with brine (1 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % EtOAc in hexanes) to afford artochamin I (3) (2.2 mg; 95 %) as a clear oil. Rf = 0.17 (silica gel, 30 % EtOAc in hexanes); IR νmax (film) 3381brm, 2926s, 2856m, 1701w, 1600s, 1518m, 1492m, 1461s, 1451s, 1436s, 1365m, 1312s, 1282m, 1260m, 1202m, 1119s, 1086s, 1033w, 867w, 806m cm−1; 1H NMR (500 MHz, (CD3)2CO) δ = 7.64 (brs, 2 H), 6.79 (d, J = 8.0 Hz, 1 H), 6.76 (d, J = 2.0 Hz, 1 H), 6.64 (dd, J = 8.0, 2.0 Hz, 1 H), 6.47 (s, 1 H), 4.92 (m, 1 H), 3.95 (dd, J = 8.0, 6.0 Hz, 1 H), 3.84 (s, 3 H), 3.79 (s, 3 H), 3.03 (dd, J = 16.0, 3.0 Hz, 1 H), 2.94–2.91 (m, 1 H), 2.88 (dd, J = 17.0, 9.5 Hz, 1 H), 2.84 (d, J = 5.5 Hz, 1 H), 2.76 (m, 2 H), 1.51 (d, J = 1.5 Hz, 3 H), 1.42 (s, 3 H), 1.01 (s, 3 H), 0.75 (s, 3 H) ppm; 13C NMR (150 MHz, (CD3)2CO) δ = 159.6, 156.4, 150.9, 146.6, 145.2, 135.2, 131.5, 125.6, 125.6, 121.0, 119.0, 117.1, 116.7, 95.9, 59.6, 56.5, 46.6, 46.4, 40.1, 31.7, 28.4, 27.6, 27.2, 26.8, 18.8 ppm; HRMS (ESI TOF) m/z calcd. for C26H32O4 [M+H+]: 409.2373; found 409.2374.
5.3.19. Benzopyran 37
To a solution of the bis-protected compound 35 (8.6 mg; 13.7 µmol) in CH2Cl2 (350 µL) at −78 °C was added a solution of PhSeCl (3.0 mg; 13.7 µmol) in CH2Cl2 (50 µL). After 30 minutes the reaction mixture was briefly warmed to room temperature before being diluted with CH2Cl2 (2 mL) and washed with H2O (1 mL). The organic phase was dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (20 % Et2O in hexanes) to afford the corresponding selenide (10.2 mg; 95 %) a yellow oil which was submitted to the oxidation without further analysis.
To a solution of the above selenide (10.2 mg; 13.0 µmol) in CH2Cl2 (200 µL) at 0 °C was added H2O2 (16.0 µL; 30 % aq.). The resulting biphase was warmed to room temperature and stirred rapidly for 3 hours before being diluted with CH2Cl2 (2 mL), washed with H2O (1 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (20 % Et2O in hexanes) to afford benzopyran 37 (7.3 mg; 89 %) as a white foam. Rf = 0.46 (silica gel, 20 % Et2O in hexanes); IR νmax (film) 3483brw, 2956m, 2929m, 2860m, 1716m, 1596w, 1505w, 1479w, 1462w, 1393w, 1367w, 1259w, 1226w, 1201w, 1120s, 1029w, 943w, 895w cm−1; 1H NMR (500 MHz, CDCl3) δ = 7.10 (m, 2 H), 6.98 (m, 1 H), 5.92 (d, J = 10.2 Hz, 1 H), 5.61 (s, 1 H), 5.36 (d, J = 10.2 Hz, 1 H), 3.98 (t, J = 6.0 Hz, 1 H), 3.12 (dd, J = 16.5, 2.0 Hz, 1 H), 3.02 (d, J = 6.0 Hz, 1 H), 3.00 (dd, J = 16.5, 9.6 Hz, 1 H), 2.85 (m, 1 H), 1.38 (s, 3 H), 1.36 (s, 9 H), 1.35 (s, 9 H), 1.35 (s, 3 H), 1.09 (s, 3 H), 0.77 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 176.1, 175.9, 149.3, 148.3, 144.2, 142.2, 140.7, 139.7, 135.7, 128.3, 125.5, 125.3, 123.0, 122.7, 122.4, 119.5, 110.1, 97.7, 56.7, 45.0, 43.4, 39.2, 39.2, 34.2, 30.4, 30.3, 29.7, 28.1, 27.5, 27.3, 27.3, 27.1, 25.8, 16.2 ppm; HRMS (ESI TOF) m/z calcd. for C34H41BrO6 [M+H+]: 625.2159; found 625.2141.
5.3.20. Methylbenzopyran 38
To a solution of pyran 37 (5.0 mg; 7.99 µmol) in DMF (150 µL) was added K2CO3 (5.5 mg; 40.0 µmol) followed by MeI (3.0 µL; 48.0 µmol), and the resulting mixture was stirred at 100 °C for 1 hour. Upon cooling the reaction mixture was diluted with EtOAc (2 mL), washed with H2O (5 × 1 mL), then brine (1 mL) and finally dried (MgSO4) before removal of the solvent in vacuo. The residue was purified by column chromatography (10 % Et2O in hexanes) to afford the methylated compound 38 (4.8 mg; 94 %) as a foam. Rf = 0.36 (silica gel, 20 % Et2O in hexanes); IR νmax (film) 2962m, 2933m, 2870w, 1760s, 1590w, 1505w, 1480w, 1460w, 1417w, 1397w, 1367w, 1258m, 1119s, 1097s, 1029w, 993w, 891w cm−1; 1H NMR (600 MHz, CDCl3) δ = 7.12–708 (m, 2 H), 6.99 (d, J = 1.8 Hz, 1 H), 5.93 (d, J = 10.2 Hz, 1 H), 5.45 (d, J = 10.2 Hz, 1 H), 3.96 (dd, J = 8.4, 5.4 Hz, 1 H), 3.87 (s, 3 H), 3.17 (dd, J = 16.8, 1.8 Hz, 1 H), 3.07 (dd, J = 16.8, 9.6 Hz, 1 H), 3.00 (d, J = 5.4 Hz, 1 H), 2.85 (m, 1 H), 1.45 (s, 3 H), 1.39 (s, 3 H), 1.36 (s, 9 H), 1.35 (s, 9 H), 1.07 (s, 3 H), 0.76 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 176.1, 175.9, 153.1, 149.9, 144.1, 142.2, 140.7, 139.7, 135.8, 130.1, 129.8, 125.5, 125.4, 122.9, 122.5, 119.4, 114.2, 103.9, 59.9, 56.7, 44.9, 43.3, 39.2, 39.1, 34.7, 34.2, 30.9, 30.3, 28.1, 27.6, 27.3, 27.3, 27.1, 25.8, 14.1 ppm; HRMS (ESI TOF) m/z calcd. for C35H43BrO6 [M+H+]: 639.2316; found 639.2319.
5.3.21. Artochamin J (4)
To a solution of methylbenzopyran 38 (4.3 mg; 6.72 µmol) in THF (100 µL) at 0 °C was added LiAlH4 (40 µL; 40.2 µmol; 1 M solution in THF). The resulting mixture was warmed to room temperature and allowed to stir for 24 hours. Excess LiAlH4 was destroyed by the addition of EtOAc and the mixture was acidified to pH 3 with aqueous HCl (1 M). The layers were separated and the aqueous phase was extracted with EtOAc (3 × 2 mL). The combined organic extracts were washed with brine (1 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (40 % EtOAc in hexanes) to afford artochamin J (4) (2.5 mg; 94 %) as a clear oil. Rf = 0.32 (silica gel, 40 % EtOAc in hexanes); IR νmax (film) 3387s, 2951s, 2926s, 2855m, 1706w, 1605s, 1519m, 1483m, 1461m, 1440m, 1364m, 1317m, 1259m, 1199m, 1124s, 1065w, 1019w, 910w, 857w, 802m cm−1; 1H NMR (600 MHz, (CD3)2CO) δ = 7.72 (brs, 2 H), 6.81 (d, J = 7.8 Hz, 1 H), 6.79 (d, J = 1.8 Hz, 1 H), 6.66 (dd, J = 7.8, 1.8 Hz, 1 H), 6.20 (s, 1 H), 6.00 (d, J = 10.2 Hz, 1 H), 5.37 (d, J = 10.2 Hz, 1 H), 3.96 (dd, J = 7.8, 6.6 Hz, 1 H), 3.80 (s, 3 H), 3.01 (dd, J = 16.8, 1.8 Hz, 1 H), 2.89–2.76 (m, 2 H), 2.75 (m, 1 H), 1.33 (s, 3 H), 1.31 (s, 3 H), 1.05 (s, 3 H), 0.76 (s, 3 H) ppm; 13C NMR (150 MHz, (CD3)2CO) δ = 157.4, 154.7, 146.6, 145.8, 144.4, 133.8, 128.1, 125.3, 120.7, 120.0, 115.9, 115.9, 110.5, 98.6, 76.5, 58.3, 55.6, 45.8, 44.5, 39.4, 30.8, 28.4, 27.8, 27.4, 26.1 ppm; HRMS (ESI TOF) m/z calcd. for C25H28O4 [M+H+]: 393.2060; found 393.2062.
5.3.22. 5-{2-[3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-phenyl]-vinyl}-2-bromo-4,6-bis-(3-methyl-but-2-enyl)-benzene-1,3-diol (39)
Stilbene 6 (10.0 mg; 14.5 µmol) was dissolved in o-xylene (1.0 mL) and the resulting solution was subjected to microwave heating at 180 °C for 5 minutes. The reaction mixture was then directly loaded onto a silica gel column packed with hexanes as the eluant and the o-xylene was run off. The eluant was switched to 2 % EtOAc in hexanes and the Claisen product 39 (9.5 mg; 95 %) was obtained as a yellow oil. Rf = 0.11 (silica gel, 4 % EtOAc in hexanes); IR (film) νmax 3423brm, 2956m, 2929m, 2858m, 1569m, 1507s, 1472w, 1440w, 1299s, 1252s, 1151m, 1123m, 1091m, 981m, 905s, 837s, 805s, 732s cm−1; 1H NMR (600 MHz, CDCl3) δ 6.93–6.90 (m, 2 H), 6.82 (d, J = 16.2 Hz, 1 H), 6.80 (d, J = 7.8 Hz, 1 H), 6.36 (d, J = 16.8 Hz, 1 H), 5.54 (s, 2 H), 5.18 (m, 2 H), 3.42 (d, J = 6.6 Hz, 4 H), 1.70 (s, 6 H), 1.68 (s, 6 H), 1.00 (s, 9 H), 0.99 (s, 9 H), 0.22 (s, 6 H), 0.21 (s, 6 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 149.1, 146.9, 146.8, 1138.4, 134.7, 132.8, 130.9, 125.5, 123.7, 122.7, 121.0, 119.8, 118.9, 118.8, 98.7, 30.3, 27.4, 25.9, 25.8, 18.5, 18.4, 18.0 ppm; HRMS (ESI TOF) m/z calcd. for C36H55BrO4Si2 [M+H+]: 687.2895; found 687.2896.
5.3.23. Artochamin F (1)
A solution of Claisen product 39 (10.0 mg, 14.5 µmol) and Bu3SnH (8.0 µL, 30.0 µmol) in benzene (200 µL) at room temperature was stirred for 30 minutes. Then a catalytic amount of AIBN was added and the mixture was heated at reflux for one hour. The reaction mixture was concentrated in vacuo and the residue was purified by column chromatography (silica gel, 30 % EtOAc in hexanes) to give the de-brominated material (8.0 mg, 88 %) as a brown oil. Rf = 0.22 (silica gel, 30 % EtOAc in hexanes); IR (film) νmax 3427brs, 2929s, 2858s, 1594s, 1508s, 1472m, 1301s, 1253s, 1160m, 1124m, 1085m, 981m, 907s, 839s, 781s cm−1; 1H NMR (600 MHz, CDCl3) δ 6.93–6.91 (m, 2 H), 6.86 (d, J = 16.2 Hz, 1 H), 6.80 (d, J = 8.4 Hz, 1 H), 6.33 (d, J = 16.8 Hz, 2 H), 5.21–5.19 (m, 2 H), 5.07 (s, 2 H), 3.37 (d, J = 6.6 Hz, 4 H), 1.73 (s, 12 H), 1.54 (s, 18 H), 1.00 (s, 6 H), 0.99 (s, 6 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 154.7, 146.8, 139.0, 134.5, 133.8, 131.0, 125.5, 124.6, 122.8, 121.0, 119.7, 118.9, 117.7, 102.7, 30.3, 26.9, 25.9, 25.8, 18.5, 18.4, 18.0 ppm; HRMS (ESI TOF) m/z calcd. for C36H56O4Si2 [M+H+]: 609.3790; found 609.3780.
To a solution of bis-silyl ether 39 (8.0 mg; 13.1 µmol) in THF (1.0 mL) at room temperature was added HF•NEt3 (13 µL; excess) and the reaction mixture was stirred for two hours. The reaction mixture was quenched with saturated aqueous NaHCO3 (3 mL), extracted with EtOAc (3 × 4 mL), and the combined organic extracts were washed with brine (3 mL), dried (MgSO4) and concentrated in vacuo to yield artochamin F (1) (4.6 mg; 92 %) as a white foam. Rf = 0.14 (silica gel, 35 % EtOAc in hexanes); IR νmax (film) 3358brs, 2951m, 2925s, 2855m, 1718m, 1602m, 1512w, 1444s, 1260s, 1088s, 802m cm−1; 1H NMR (500 MHz, (CD3)2CO) δ = 7.90 (brs, 1 H), 7.88 (brs, 1 H), 7.78 (s, 1 H), 7.04 (d, J = 1.5 Hz, 1 H), 6.91 (d, J = 16.5 Hz, 1 H), 6.84 (dd, J = 6.5, 2.0 Hz, 1 H), 6.82 (d, J = 8.0 Hz, 1 H), 6.42 (s, 1 H), 6.36 (d, J = 16.5 Hz, 1 H), 5.18 (brt, J = 6.5 Hz, 2 H), 3.32 (d, J = 7.0 Hz, 4 H), 1.64 (brs, 6 H), 1.62 (brs, 6 H) ppm; 13C NMR (150 MHz, (CD3)2CO) δ = 154.7, 146.5, 146.2, 140.8, 134.9, 131.6, 130.2, 126.2, 125.5, 120.0, 118.8, 116.6, 114.0, 102.6, 27.2, 26.3, 18.6 ppm; HRMS (ESI TOF) m/z calcd. for C24H28O4 [M+H+]: 381.2060; found 381.2043.
5.3.24. 2-(3,4-Dihydroxy-phenyl)-1,1-dimethyl-3-(3-methyl-but-2-enyl)-2,2a,7,7a-tetrahydro-1H-cyclobuta[a]indene-4,6-diol (40)
Artochamin F (1) (5.0 mg; 13.1 µmol) was dissolved in o-xylene (0.5 mL) and the resulting solution was subjected to microwave heating at 180 °C for 10 minutes. The reaction mixture was then purified by preparatory thin-layer chromatography (50 % EtOAc in hexanes, few drops AcOH) to yield cycloadduct 40 (4.0 mg, 82 %) as a clear oil. Rf = 0.11 (silica gel, 55 % EtOAc in hexanes + 0.5 % AcOH); IR νmax (film) 3364brs, 2956m, 2925s, 2856w, 2504brw, 1697s, 1602s, 1516s, 1440s, 1365s, 1255s, 1193m, 1160m, 1109m, 1088s, 971w cm−1, 1H NMR (600 MHz, (CD3)2CO) δ = 7.67 (s, 1 H), 7.66 (s, 1 H), 7.41 (brs, 1 H), 6.77 (s, 1 H), 6.76 (d, J = 8.4 Hz, 1 H), 6.59 (dd, J = 8.4, 1.5 Hz, 1 H), 6.25 (s, 1 H), 4.97 (brt, 1 H), 3.89 (t, J = 6.6 Hz, 1 H), 3.01–2.98 (m, 2 H), 2.87–2.79 (m, 3 H), 2.70 (brt, J = 6.6 Hz, 1 H), 1.49 (brs, 3 H), 1.38 (brs, 3 H), 0.99 (brs, 3 H), 0.71 (brs, 3 H) ppm; 13C NMR (150 MHz, (CD3)2CO) δ = 156.6, 153.5, 151.2, 146.5, 145.1, 135.4, 131.2, 126.0, 123.3, 121.2, 117.1, 116.6, 103.0, 59.6, 46.7, 46.6, 40.0, 29.7, 28.5, 27.6, 27.2, 26.8, 18.8 ppm; HRMS (ESI TOF) m/z calcd. for C24H28O4 [M+H+]: 381.2060; found 381.2051.
5.3.25. 5-Bromo-2-(3,4-dihydroxy-phenyl)-1,1-dimethyl-3-(3-methyl-but-2-enyl)-2,2a,7,7a-tetrahydro-1H-cyclobuta[a]indene-4,6-diol (32)
To a solution of bis-silyl ether 39 (82.2 mg; 0.12 mmol) in THF (500 µL) at 0 °C was added HF•py (200 µL; excess) and the reaction mixture was warmed to ambient temperature and stirred for two hours. The reaction was diluted with Et2O (20 mL) and then washed successively with saturated aqueous CuSO4 (3 × 5 mL), saturated aqueous NaHCO3 (2 × 5 mL) and brine (5 mL). The organic layer was then dried (MgSO4) and concentrated in vacuo. The residue was passed through a short plug of silica gel (30 % EtOAc in hexanes) to give diol 31 (52 mg; 95 %) as a clear oil, which was used directly in the following reaction. Rf = 0.20 (silica gel, 30 % EtOAc in hexanes); IR νmax (film) 3440brs, 2974m, 2913m, 1595m, 1574m, 1515m, 1436s, 1404w, 1375w, 1348w, 1315w, 1283s, 1254s, 1191m, 1151m, 1090s, 1029w, 957w, 908w, 874w, 814w, 786m, 661w cm−1;1H NMR (500 MHz, CDCl3) δ 6.68 (d, J = 8.5 Hz, 1 H), 6.56–6.52 (m, 3 H), 6.33 (d, J = 12.5 Hz, 1 H), 5.66 (s, 2 H), 5.56 (brs, 1 H), 5.47 (brs, 1 H), 5.00–4.96 (m, 2 H), 3.30 (d, J = 7.0 Hz, 4 H), 1.68 (s, 6 H), 1.60 (d, J = 1.0 Hz, 6 H), ppm; 13C NMR (125 MHz, CDCl3) δ = 149.5, 143.4, 142.8, 137.8, 133.2, 131.2, 130.1, 125.5, 122.5, 122.1, 118.1, 115.2, 115.0, 98.6, 27.4, 25.7, 17.8 ppm; HRMS (ESI TOF) m/z calcd. for C24H27BrO4 [M+H+]: 459.1165; found 459.1166.
To a solution of stilbene 31 (5.9 mg; 12.8 µmol) in CDCl3 (400 µL) was added a solution of DDQ (4.4 mg; 19.2 µmol) in CDCl3 (200 µL) at ambient temperature. After 5 minutes the mixture was filtered through cotton wool and the 1H and 13C NMR spectra were recorded. 1H NMR (500 MHz, CDCl3) δ 6.27 (dd, J = 12.0, 1.0 Hz, 1 H), 6.50 (d, J = 12.0 Hz, 1 H), 6.42 (dd, J = 7.0, 1.5 Hz, 1 H), 5.46 (s, 1 H), 5.34 (s, 1 H), 5.10–5.07 (m, 1 H), 3.50–3.46 (m, 1 H), 3.44 (dd, J = 3.5, 2.0 Hz, 1 H), 3.39 (dd, J = 16.0, 7.0 Hz, 1 H), 3.16 (d, J = 6.5 Hz, 1 H), 3.12 (dd, J = 13.0, 5.0 Hz, 1 H), 2.35 (ddd, J = 13.0, 5.0, 3.5 Hz, 1 H), 2.13 (t, J = 13.0 Hz, 1 H), 1.79 (s, 3 H), 1.71 (d, J = 1.0 Hz, 3 H), 1.15 (s, 3 H), 1.09 (s, 3 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 193.6, 190.0, 149.4, 148.9, 148.1, 144.5, 136.3, 133.6, 128.3, 128.1, 127.2, 121.3, 119.8, 117.3, 100.4, 62.3, 46.7, 44.5, 30.0, 28.4, 27.0, 25.7, 25.4, 18.0 ppm.
The crude reaction mixture from above was concentrated in vacuo, then re-dissolved in o-xylene (2 mL) and heated at 180 °C for 30 minutes by microwave irradiation. The reaction mixture was concentrated in vacuo and purified by column chromatography (10 % Et2O in CH2Cl2) to give cyclobutane 32 (2.4 mg; 40 %, 2 steps), which displayed identical physical properties to those reported above.
5.3.26. 5-(Benzo[1,3]dioxol-5-ylmetanesulfonyl)-1-phenyl-1H-tetrazole (56)
To a solution of 1-phenyl-1H-tetrazole-5-thiol (432 mg; 2.42 mmol) in dry THF (9.0 mL) was added Et3N (0.42 mL; 3.03 mmol), and the mixture was stirred at room temperature. After 40 minutes, 5-bromomethyl-benzo[1.3] dioxole 55 (651 mg; 3.03 mmol) was added and the reaction mixture was heated at reflux overnight, then diluted with water (20 mL), and extracted with Et2O (3 × 20 mL). The combined organic extracts were dried (MgSO4) and concentrated in vacuo to give the crude thioether.
m-CPBA (251 mg; 1.12 mmol; 77 % w/w) was added in small portions to a solution of the crude thioether (100 mg; 0.32 mmol) in CH2Cl2 (2.0 mL) at 0 °C, and the mixture was then stirred at room temperature for 24 hours. The reaction mixture was washed with saturated aqueous Na2S2O3 (2 × 5 mL) and saturated aqueous NaHCO3 (3 × 2 mL). The organic layer was then dried (MgSO4) and the solvent removed in vacuo to yield 56 (107 mg, 97 %) as a white foam. Rf = 0.30 (silica gel, 30 % EtOAc in hexanes); IR (film) νmax 3072w, 2997w, 2912w, 1765w, 1595w, 1489s, 1445s, 1339s, 1245s, 1216w, 1154s, 1134m, 1101m, 1015m, 1035s, 925s, 812s, 761s cm−1; 1H NMR (500 MHz, CDCl3) δ 7.56 (tt, J = 7.5, 1.5 Hz, 1 H), 7.50 (m, 2 H), 7.42 (m, 2 H), 6.80 (d, J = 1.5 Hz, 1 H), 6.77 (dd, J = 8.0, 2.0 Hz, 1 H), 6.74 (d, J = 8.0 Hz, 1 H), 5.94 (s, 2 H), 4.83 (s, 2 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 152.9, 148.9, 148.1, 132.7, 131.2, 129.3, 125.7, 125.1, 117.6, 111.4, 108.6, 101.5, 62.0 ppm; HRMS (ESI TOF) m/z calcd. for C15H12N4O4S [M+H+]: 345.0652; found 345.0656.
5.3.27. 5-{2-[4-Bromo-3,5-bis-(1,1-dimethyl-alloxy)-phenyl]-vinyl}-benzo[1,3]dioxole (57)
To a solution of sulfone 56 (87.7 mg; 0.26 mmol) in THF (2.6 mL) at −78 °C was added KHMDS (540 µL; 0.27 mmol, 0.5 M solution in toluene) and the resulting bright yellow solution was stirred for 30 minutes. A solution of aldehyde 7 (50 mg; 0.14 mmol) in THF (1.4 mL) was then added via cannula and the reaction mixture was slowly warmed to room temperature. The reaction was quenched by the addition of saturated aqueous NaHCO3 (10 mL) and then extracted with Et2O (3 × 15 mL). The combined organic extracts were washed with brine (10 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (5 to 10 % Et2O in hexanes) to give stilbene 57 (57 mg; 85 %, 2:1 E:Z) as a white powder. Rf = 0.15 (silica, 10 % Et2O in hexanes); IR (film) νmax 2981m, 2931w, 1578m, 1560s, 1503s, 1489s, 1445s, 1413s, 1378m, 1363m, 1331w, 1252s, 1235m, 1196w, 1161w, 1128s, 1069m, 1039s, 1003w, 956m, 930m, 858w, 823w, 798w cm−1; 1H NMR (500 MHz, CDCl3) δ 7.03 (d, J = 1.5 Hz, 1 H), 6.94 (s, 2 H), 6.91 (dd, J = 8.0, 1.5 Hz, 1 H), 6.81 (d, J = 16.0 Hz, 1 H), 6.79 (d, J = 8.0 Hz, 1 H), 6.73 (d, J = 16.0 Hz, 1 H), 6.23 (dd, J = 17.5, 11.0 Hz, 2 H), 5.97 (s, 2 H), 5.52 (dd, J = 17.5, 1.0 Hz, 2 H), 5.18 (dd, J = 17.5, 1.0 Hz, 2 H), 1.53 (s, 12 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 154.6, 148.2, 147.4, 144.4, 136.0, 131.5, 128.7, 126.6, 121.6, 113.7, 113.6, 112.1, 108.4, 105.5, 101.2, 81.8, 26.8 ppm; HRMS (ESI TOF) m/z calcd. for C25H27BrO4 [M+H+]: 471.1165; found 471.1144.
5.3.28. 5-(2-Benzo[1,3]dioxol-5-yl-vinyl)-2-bromo-4,6-bis-(3-methyl-but-2-enyl)-benzene-1,3-diol (58)
Stilbene 57 (15 mg; 25.3 µmol) was dissolved in o-xylene (2 mL) and the resulting solution was subjected to microwave heating at 180 °C for 5 minutes. The reaction mixture was then directly loaded onto a silica gel column packed with hexanes as the eluant and the o-xylene was run off. The eluant was switched to 10–30 % Et2O in hexanes and the Claisen product 58 (10 mg; 65 %) was obtained as a white powder. Rf = 0.21 (silica, 20 % Et2O in hexanes); IR (film) νmax 3452brm, 2956w, 2920w, 2855w, 1723w, 1568w, 1502m, 1487s, 1444s, 1365w, 1314w, 1253s, 1194m, 1155m, 1086m, 1040m, 975m, 933m, 869w, 801m cm−1; 1H NMR (500 MHz, CDCl3) δ 7.02 (d, J = 1.5 Hz, 1 H), 6.86 (d, J = 16.5 Hz, 1 H), 6.85 (dd, J = 8.0, 1.5 Hz 1 H), 6.80 (d, J = 8.0 Hz, 1 H), 6.41 (d, J = 16.5 Hz, 1 H), 5.98 (s, 2 H), 5.54 (s, 2 H), 5.17 (m, 2 H), 3.41 (d, J = 7.0 Hz, 4 H), 1.71 (d, J = 1.0 Hz, 6 H), 1.69 (s, 6 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 149.1, 148.1, 147.4, 138.2, 134.6, 132.8, 131.7, 124.0, 122.7, 121.3, 118.9, 108.5, 105.4, 101.1, 98.8, 27.4, 25.8, 18.0 ppm; HRMS (ESI TOF) m/z calcd. for C25H27BrO4 [M−H−]: 469.1009; found 469.1014.
5.3.29. 5-(4-Methoxy-phenylmethanesulfonyl)-1-phenyl-1H-tetrazole (60)
To a solution of p-methoxybenzyl alcohol (500 mg; 3.62 mmol), Ph3P (1.04 g; 3.98 mmol), and 1-phenyl-1H-tetrazole-5-thiol (645 mg; 3.62 mmol) at 0 °C in THF (36 mL) was added DEAD (2.0 mL; 4.34 mmol). The mixture was stirred for 30 minutes at 0 °C, treated with saturated aqueous NaHCO3 (15 mL) and diluted with Et2O (20 mL). The layers were separated and the aqueous phase was extracted with Et2O (2 × 20 mL). The combined organic extracts were washed with brine (15 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (15 % EtOAc in hexanes) to yield the corresponding sulfide (975 mg; 90 %) as a yellow oil.
A solution of the sulfide from above (975 mg, 3.27 mmol) in EtOH (33 mL), THF (6.5 mL), and H2O2 (12.6 mL) was treated with ammonium molybdate (808 mg; 0.65 mmol) at room temperature. The mixture was stirred overnight and then partitioned between CH2Cl2 (15 mL) and saturated aqueous NH4Cl (15 mL). The layers were separated and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The combined organic extracts were dried (MgSO4) and then concentrated in vacuo to yield sulfone 60 (1.09 g; 100 %) as a white solid. Rf = 0.26 (silica gel, 20 % EtOAc in hexanes); IR (film) νmax 2970w, 2913w, 1611m, 1512m, 1493m, 1463w, 1446w, 1352s, 1307m, 1251s, 1180m, 1154s, 1137s, 1025s, 878m, 849m, 838m, 754s, 684s cm−1; 1H NMR (500 MHz, CDCl3) δ 7.56 (m, 1 H), 7.49 (m, 2 H), 7.36 (d, J = 8.0 Hz, 2 H), 7.24 (d, J = 8.5 Hz, 2 H), 6.86 (d, J = 8.5 Hz, 2 H), 4.87 (s, 2 H), 3.80 (s, 3 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 160.8, 152.9, 132.9, 131.3, 129.3, 125.2, 116.3, 114.6, 61.8, 55.4 ppm; HRMS (ESI TOF) m/z calcd. for C15H14N4O3S [M+H+]: 331.0859; found 331.0859.
5.3.30. 2-(3-Methyl-but-2-enyl)-benzaldehyde (61)
Aldehyde 61 was prepared according to the literature and displayed identical physical properties to those reported.45
5.3.31. 1-(3-Methyl-but-2-enyl)-2-(4-methoxystyryl)-benzene (62)
To a solution of sulfone 60 (228 mg; 0.69 mmol) in THF (70 mL) at −78 °C was added KHMDS (1.4 mL; 0.69 mmol, 0.5 M solution in toluene) and the resulting solution was stirred for 30 minutes. A solution of aldehyde 61 (100 mg; 0.57 mmol) in THF (6.0 mL) was then added via cannula and the reaction mixture was slowly warmed to room temperature. The reaction was quenched by the addition of saturated aqueous NaHCO3 (30 mL) and then extracted with Et2O (3 × 40 mL). The combined organic extracts were washed with brine (30 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (2 % Et2O in hexanes) to give stilbene 62 (118 mg, 74 %, E/Z 4:1) as a yellow oil. Rf = 0.08 (silica gel, 10 % CH2Cl2 in hexanes); IR (film) νmax 2911w, 2854w, 2835w, 1605s, 1509s, 1450m, 1440m, 1294m, 1247s, 1173s, 1033s, 960s, 816s, 746s cm−1; 1H NMR (600 MHz, CDCl3) δ 7.58 (d, J = 6.6 Hz, 1 H), 7.44 (d, J = 8.4 Hz, 2 H), 7.23 (d, J = 15.6 Hz, 1 H), 7.21–7.18 (m, 3 H), 6.94 (d, J = 16.2 Hz, 1 H), 6.91 (d, J = 9.0 Hz, 2 H), 5.29-5.26 (t, J = 7.2 Hz, 1 H), 3.84 (s, 3 H), 3.46 (d, J = 7.2 Hz, 2 H), 1.77 (s, 3 H), 1.74 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 159.2, 139.1, 132.2, 130.6, 129.6, 129.3, 127.7, 127.3, 126.2, 125.5, 124.4, 123.0, 114.1, 55.3, 32.4, 25.7, 18.0 ppm; HRMS (ESI TOF) m/z calcd. for C20H22O [M+H+]: 278.1706; found 279.1706.
5.3.32. (4-Nitro-benzyl)-triphenylphosphonium bromide (64)
Phosphonium bromide 64 was purchased from Aldrich Chemical Co.
5.3.33. 1-(3-Methyl-but-2-enyl)-2-(4-nitrostyryl)-benzene (65)
To a solution of (4-nitrobenzyl)triphenylphosphonium bromide (64) (329 mg; 0.69 mmol) in THF (7.0 mL) at 0 °C was added n-BuLi (274 µL; 0.69 mmol; 2.5 M solution in hexanes) dropwise, and the resulting mixture was warmed to room temperature and stirred for 30 minutes. The bright red solution was re-cooled to 0 °C and a solution of aldehyde 61 (100 mg; 0.57 mmol) in THF (6 mL) was added via cannula, and the reaction mixture was warmed to room temperature and stirred overnight. The reaction was quenched with addition of water (3 mL) and then extracted with Et2O (3 × 10 mL). The organic phase was washed with brine (3 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (25 % benzene in hexanes) to yield stilbene 65 (113 mg; 67 %; E/Z 1:1). Rf = 0.24 (silica gel, 30 % benzene in hexanes); IR (film) νmax 2968w, 2926w, 2852w, 1593s, 1513s, 1449w, 1338s, 1182w, 1109m, 965w, 856m, 756m cm−1; 1H NMR (600 MHz, CDCl3) δ 8.23 (d, J = 10.2 Hz, 2 H), 7.63–7.60 (m, 3 H), 7.55 (d, J = 19.2 Hz, 1 H), 7.30–7.28 (m, 1 H), 7.26–7.22 (m, 2 H), 7.03 (d, J = 19.2 Hz, 1 H), 5.27-5.24 (t, J = 9.0 Hz, 1 H), 3.49 (d, J = 8.4 Hz, 2 H), 1.78 (s, 3 H), 1.74 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 144.2, 140.1, 135.0, 132.6, 131.2, 129.7, 128.8, 127.6, 126.9, 126.5, 125.9, 124.2, 123.4, 122.8, 32.4, 25.7, 18.0 ppm; HRMS (ESI TOF) m/z calcd. for C19H19NO2 [M+H+]: 294.1488; found 294.1480.
5.3.34. 1-Phenyl- 5-phenylmethanesulfonyl-1H-tetrazole (67)
Sulfone 67 was prepared according to the literature and displayed identical physical properties to those reported.46
5.3.35. 1-(3-Methyl-but-2-enyl)-2-styryl-benzene (68)
To a solution of sulfone 67 (310 mg; 1.03 mmol) in THF (103 mL) at −78 °C was added KHMDS (2.2 mL; 1.1 mmol, 0.5 M solution in toluene) and the resulting solution was stirred for 30 minutes. A solution of aldehyde 61 (100 mg; 0.57 mmol) in THF (6.0 mL) was then added via cannula and the reaction mixture was slowly warmed to room temperature. The reaction was quenched by the addition of saturated aqueous NaHCO3 (30 mL) and then extracted with Et2O (3 × 40 mL). The combined organic extracts were washed with brine (30 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (2 % Et2O in hexanes) to give stilbene 68 (136 mg; 95 %; E/Z 4:1) as a yellow oil. Rf = 0.28 (silica gel, 2 % Et2O in hexanes); IR (film) νmax 3025w, 2967w, 2912w, 2854w, 1599m, 1577w, 1494m, 1480w, 1449m, 1375m, 959s, 757s, 689s cm−1; 1H NMR (600 MHz, CDCl3) δ 7.62–7.61 (m, 1 H), 7.51 (d, J = 7.8 Hz, 2 H), 7.40–7.37 (m, 2 H), 7.37 (d, J = 15.6 Hz, 1 H), 7.28–7.27 (m, 1 H), 7.23–7.21 (m, 3 H), 6.99 (d, J = 16.2 Hz, 1 H), 5.28 (t, J = 7.2 Hz, 1 H), 3.48 (d, J = 7.2 Hz, 2 H), 1.77 (s, 3 H), 1.74 (s, 3 H) ppm; 13C NMR (150 MHz, CDCl3) δ = 139.4, 137.7, 136.1, 132.3, 130.0, 129.3, 128.7, 127.7, 127.6, 127.5, 126.6, 126.5, 126.4, 126.3, 125.7, 123.0, 32.4, 25.7, 18.0 ppm; HRMS (ESI TOF) m/z calcd. for C19H20 [M+H+]: 248.1638; found 249.1632.
5.3.36. [4-[(tert-Butyldimethysilyl)oxy]-benzyl]triphenylphosphonium bromide (70)
Phosphonium bromide 70 was prepared according to the literature and displayed identical physical properties to those reported.47
5.3.37. 1-(3-Methyl-but-2-enyl)-2-styryl-benzene (71)
To a solution of phosphonium bromide 70 (388 mg; 0.69 mmol) in THF (10.0 mL) at −78 °C was added n-BuLi (275 µL; 0.69 mmol; 2.5 M solution in hexanes) dropwise, and the resulting mixture was stirred for 30 minutes. To the bright red solution was added a solution of aldehyde 61 (100 mg; 0.57 mmol) in THF (2 mL) via cannula, and the reaction mixture was warmed to room temperature over 30 minutes. The reaction was quenched with addition of saturated aqueous NaHCO3 (10 mL) and then extracted with Et2O (3 × 20 mL). The organic phase was washed with brine (10 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (5 % CH2Cl2 in hexanes) to yield first the cis- and then the trans-stilbenes (206 mg; 92 %; E/Z 1:1). Rf = 0.20 (silica gel, 5 % CH2Cl2 in hexanes); IR (film) νmax 2956w, 2929w, 2857w, 1602m, 1507s, 1471w, 1463w, 1449w, 1390w, 1376w, 1362w, 1254s, 1168m, 1101w, 1048w, 1007w, 961w, 909s, 837s, 821s, 803m, 779s, 749m cm−1; 1H NMR (500 MHz, CDCl3) δ 7.61–7.59 (m, 1 H), 7.41 (d, J = 8.5 Hz, 2 H), 7.26 (d, J = 16.0 Hz, 1 H), 7.24–7.20 (m, 3 H), 6.95 (d, J = 16.0 Hz, 1 H), 6.86 (d, J = 8.5 Hz, 2 H), 5.31 (m, 1 H), 3.48 (d, J = 7.0 Hz, 2 H), 1.79 (s, 3 H), 1.77 (d, J = 1.0 Hz, 3 H), 1.03 (s, 9 H), 0.25 (s, 6 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 155.4, 139.2, 136.4, 132.2, 131.1, 130.1, 129.7, 129.3, 127.7, 127.3, 126.3, 125.5, 124.5, 123.1, 120.3, 32.4, 25.7, 25.7, 18.2, 18.0, −4.4 ppm; HRMS (ESI TOF) m/z calcd. for C25H34OSi [M+H+]: 379.2452; found 379.2458.
To a solution of trans-stilbene from above (59 mg; 0.16 mmol) in THF (500 µL) at 0 °C was added HF•py (300 µL; excess) and the resulting mixture was warmed to room temperature and stirred for an additional 2 hours. The reaction was diluted with Et2O (20 mL) and then washed successively with saturated aqueous CuSO4 (3 × 3 mL), saturated aqueous NaHCO3 (2 × 3 mL) and brine (3 mL). The organic layer was then dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography (30 % Et2O in hexanes) to give stilbene 71 (43 mg; 95 %) as a white powder. Rf = 0.24 (silica gel, 30 % Et2O in hexanes); IR (film) νmax 3362brm, 3025w, 2968w, 2912w, 1607m, 1591m, 1510s, 1481w, 1449m, 1375m, 1238s, 1171s, 1103m, 1047m, 963m, 923w, 856w, 838w, 815m, 777w, 750m cm−1; 1H NMR (500 MHz, CDCl3) δ 7.61–7.59 (m, 1 H), 7.42 (d, J = 8.5 Hz, 2 H), 7.25 (d, J = 16.0 Hz, 1 H), 7.24–7.20 (m, 3 H), 6.95 (d, J = 16.0 Hz, 1 H), 6.85 (d, J = 8.5 Hz, 2 H), 5.30 (m, 1 H), 5.04 (brs, 1 H), 3.50 (d, J = 7.0 Hz, 2 H), 1.79 (s, 3 H), 1.76 (s, 3 H) ppm; 13C NMR (125 MHz, CDCl3) δ = 155.1, 139.2, 136.3, 132.2, 130.8, 129.5, 129.3, 127.9, 127.4, 126.3, 125.5, 124.5, 123.0, 115.6, 32.3, 25.7, 17.7 ppm; HRMS (ESI TOF) m/z calcd. for C19H20O [M+H+]: 265.1587; found 265.1587.
4. Aknowledgements
We wish to thank Dr. D. H. Huang, Dr. G. Siuzdak and Dr. R. J. Chadha for assistance with NMR spectroscopy, mass spectrometry, and x-ray crystallography, respectively. We also wish to thank Dr. W. Robert Tao for helpful discussions. Financial support for this work was provided by grants from the National Institutes of Health (USA) and the National Science Foundation (No. 06032187), the Skaggs Institute for Chemical Biology, and a National Science Foundation predoctoral fellowship (to C.F.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.For recent reviews on cascade reactions in total synthesis, see: Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551–564. doi: 10.1039/b209440c. Nicolaou KC, Snyder SA. Actualite Chimique. 2003;4–5:83–88. Tietze LF. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e. Tietze LF, Beifuss U. Angew. Chem. Int. Ed. Engl. 1993;32:131–163. Pellissier H. Tetrahedron. 2006;62:1619–1665. Pellissier H. Tetrahedron. 2006;62:2142–2173. Bunce RA. Tetrahedron. 1995;51:13103–13159. Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Weinheim: Wiley-VCH; 2006. p. 631. Hao T-L. Tandem Organic Reactions. New York: Wiley; 1992. p. 502.
- 2.Robinson R. J. Chem. Soc. Trans. 1917:762–768. [Google Scholar]
- 3.(a) Johnson WS, Gravestock MB, McCarry BE. J. Am. Chem. Soc. 1971;93:4332–4334. doi: 10.1021/ja00746a062. [DOI] [PubMed] [Google Scholar]; (b) Gravestock MB, Johnson WS, McCarry BE, Parry RJ, Ratcliffe BE. J. Am. Chem. Soc. 1978;100:4274–4282. [Google Scholar]
- 4.(a) Bandaranayake WM, Banfield JE, Black DStC. J. Chem. Soc. Chem. Commun. 1980;19:902–903. (isolation) [Google Scholar]; (b) Nicolaou KC, Petasis NA, Zipkin RE, Uenishi J. J. Am. Chem. Soc. 1982;104:5555–5557. [Google Scholar]; (c) Nicolaou KC, Petasis NA, Uenishi J, Zipkin RE. J. Am. Chem. Soc. 1982;104:5557–5558. [Google Scholar]; (d) Nicolaou KC, Zipkin RE, Petasis NA. J. Am. Chem. Soc. 1982;104:5558–5560. [Google Scholar]; (e) Nicolaou KC, Petasis NA, Zipkin RE. J. Am. Chem. Soc. 1982;104:5560–5562. (total synthesis) [Google Scholar]
- 5.For total syntheses, see Nicolaou KC, Simonsen KB, Vassilikogiannakis G, Baran PS, Viladi VP, Pitsinos EN, Couladouros EA. Angew. Chem. Int. Ed. 1999;38:3555–3559. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. Nicolaou KC, Vassilikogiannakis G, Simonsen KB, Baran PS, Zhong Y-L, Viladi VP. J. Am. Chem. Soc. 2000;122:3071–3079. Barnes-Seeman D, Corey EJ. Org. Lett. 1999;1:1503–1504. doi: 10.1021/ol991070h..; for biosynthetic proposals for the oxidative dimerization route to the bisorbicillinoids, see: Abe N, Murata T, Hirota A. Biosci. Biotechnol. Biochem. 1998;62:2120–2126. doi: 10.1271/bbb.62.2120. Nicolaou KC, Jautelat R, Vassilikogiannakis G, Baran PS, Simonsen KB. Chem. Eur. 1999;5:3651–3665.
- 6. Nicolaou KC, Baran PS, Zhong Y-L, Fong KC, He Y, Yoon WH, Choi H-S. Angew. Chem. Int. Ed. 1999;38:1669–1675. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1669::AID-ANIE1669>3.0.CO;2-D. Nicolaou KC, Baran PS, Zhong Y-L, Fong KC, He Y, Yoon WH, Choi H-S. Angew. Chem. Int. Ed. 1999;38:1676–1678. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1676::AID-ANIE1676>3.0.CO;2-T. Nicolaou KC, Jung J-K, Yoon WH, He Y. Angew. Chem. Int. Ed. 2000;39:1829–1832. doi: 10.1002/(sici)1521-3773(20000515)39:10<1829::aid-anie1829>3.0.co;2-6. Nicolaou KC, Jung J-K, Yoon WH, Fong KC, Choi H-S, Zhong Y-L, Baran PS. J. Am. Chem. Soc. 2002;124:2183–2189. doi: 10.1021/ja012010l. Nicolaou KC, Baran PS, Zhong Y-L, Fong KC, Choi H-S. J. Am. Chem. Soc. 2002;124:2190–2201. doi: 10.1021/ja012011d. Nicolaou KC, Zhong Y-L, Baran PS, Jung J-K, Choi H-S, Yoon WH. J. Am. Chem. Soc. 2002;124:2202–2211. doi: 10.1021/ja0120126.; the Shair group has also completed a total synthesis featuring a cascade reaction, see: Chen C, Layton ME, Sheehan SM, Shair MD. J. Am. Chem. Soc. 2000;122:7424–7425.
- 7.(a) Nicolaou KC, Vassilikogiannakis G, Mägerlein W, Kranich R. Angew. Chem. Int. Ed. 2001;40:2482–2486. doi: 10.1002/1521-3773(20010702)40:13<2482::AID-ANIE2482>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Vassilikogiannakis G, Mägerlein W, Kranich R. Chem. Eur. J. 2001;7:5359–5371. doi: 10.1002/1521-3765(20011217)7:24<5359::aid-chem5359>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (c) Boezio AA, Jarvo ER, Lawrence BM, Jacobsen ER. Angew. Chem. Int. Ed. 2005;44:6046–6050. doi: 10.1002/anie.200502178. [DOI] [PubMed] [Google Scholar]; (d) Harrowven DC, Pascoe DD, Demurtas D, Bourne HO. Angew. Chem. Int. Ed. 2005;44:1221–1222. doi: 10.1002/anie.200462268. [DOI] [PubMed] [Google Scholar]; (e) Davies HML, Dai X, Long MS. J. Am. Chem. Soc. 2006;128:2485–2490. doi: 10.1021/ja056877l. [DOI] [PubMed] [Google Scholar]; (f) Kim AI, Rychnovsky SD. Angew. Chem. Int. Ed. 2003;42:1267–1270. doi: 10.1002/anie.200390325. [DOI] [PubMed] [Google Scholar]
- 8.(a) Nicolaou KC, Gray DLF. Angew. Chem. Int. Ed. 2001;40:761–763. [PubMed] [Google Scholar]; (b) Nicolaou KC, Gray DLF. J. Am. Chem. Soc. 2004;126:607–612. doi: 10.1021/ja030497n. [DOI] [PubMed] [Google Scholar]
- 9.For the first total synthesis of diazonamide, see: Nicolaou KC, Bella M, Chen DY-K, Huang X, Ling T, Snyder SA. Angew. Chem. Int. Ed. 2002;41:3495–3499. doi: 10.1002/1521-3773(20020916)41:18<3495::AID-ANIE3495>3.0.CO;2-7. Nicolaou KC, Chen DY-K, Huang X, Ling T, Bella M, Snyder SA. J. Am. Chem. Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i.; for the second total synthesis of diazonamide, see: Nicolaou KC, Bheema Roa P, Hao J, Reddy MV, Rassias G, Huang X, Chen DY-K, Snyder SA. Angew. Chem. Int. Ed. 2003;42:1753–1758. doi: 10.1002/anie.200351112. Nicolaou KC, Hao J, Reddy MV, Bheema Roa P, Rassias G, Snyder SA, Huang X, Chen DY-K, Brenzovich WE, Giuseppone N, Giannakakou P, O’Brate A. J. Am. Chem. Soc. 2004;126:12897–12906. doi: 10.1021/ja040093a.
- 10.For the total synthesis of the proposed structure of diazonamide A, see: Li J, Jeong S, Esser L, Harran PG. Angew. Chem. Int. Ed. 2001;40:4765–4770. doi: 10.1002/1521-3773(20011217)40:24<4765::aid-anie4765>3.0.co;2-1. Li J, Burgett AWG, Esser L, Amezcua C, Harran PG. Angew. Chem. Int. Ed. 2001;40:4770–4773. doi: 10.1002/1521-3773(20011217)40:24<4770::aid-anie4770>3.0.co;2-t.; (c) for total synthesis of the corrected structure, see: Burgett AWG, Li Q, Wei Q, Harran PG. Angew. Chem. Int. Ed. 2003;42:4961–4966. doi: 10.1002/anie.200352577.
- 11.Nicolaou KC, Sasmal PK, Xu H, Namoto K, Ritzén A. Angew. Chem. Int. Ed. 2003;42:4255–4299. doi: 10.1002/anie.200351805. [DOI] [PubMed] [Google Scholar]; Nicolaou KC, Sasmal PK, Xu H. J. Am. Chem. Soc. 2004;126:5493–5501. doi: 10.1021/ja040037+. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaou KC, Nevalainen M, Safina BS, Zak M, Bulat S. Angew. Chem. Int. Ed. 2002;41:1941–1945. [PubMed] [Google Scholar]; (b) Nicolaou KC, Safina BS, Zak M, Estrada AA, Lee SH. Angew. Chem. Int. Ed. 2004;43:5087–5092. doi: 10.1002/anie.200461340. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Safina BS, Zak M, Lee SH, Estrada AA. Angew. Chem. Int. Ed. 2004;43:5092–5097. doi: 10.1002/anie.200461341. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Safina BS, Zak M, Estrada AA, Lee SH, Nevalainen M, Bella M, Estrada AA, Funke C, Zécri FJ, Bulat S. J. Am. Chem. Soc. 2005;127:11159–11175. doi: 10.1021/ja0529337. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J. Am. Chem. Soc. 2005;127:11176–11183. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- 13.For the total synthesis and structural revision of azaspiracid-1, see: Nicolaou KC, Pihko PM, Diedrichs N, Zou N, Bernal F. Angew. Chem. Int. Ed. 2001;40:1262–1265. Nicolaou KC, Qian W, Bernal F, Uesaka N, Pihko PM, Hinrichs J. Angew. Chem. Int. Ed. 2001;40:4068–4071. doi: 10.1002/1521-3773(20011105)40:21<4068::AID-ANIE4068>3.0.CO;2-Y. Nicolaou KC, Li Y, Uesaka N, Koftis TV, Vyskocil S, Ling T, Govindasamy M, Qian W, Bernal F, Chen DY-K. Angew. Chem. Int. Ed. 2003;42:3643–3648. doi: 10.1002/anie.200351825. Nicolaou KC, Chen DY-K, Li Y, Ling T, Vyskocil S, Koftis TV, Govindasamy M, Uesaka N. Angew. Chem. Int. Ed. 2003;42:3649–3653. doi: 10.1002/anie.200351826. Nicolaou KC, Vyskocil S, Koftis TV, Yamada YMA, Ling T, Chen DY-K, Tang W, Petrovic G, Frederick MO, Li Y, Satake M. Angew. Chem. Int. Ed. 2004;43:4312–4318. doi: 10.1002/anie.200460695. Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Ling T, Yamada YMA, Tang W, Frederick MO. Angew. Chem. Int. Ed. 2004;43:4318–4324. doi: 10.1002/anie.200460696. Nicolaou KC, Pihko PM, Bernal F, Frederick MO, Qian W, Uesaka N, Diedrichs N, Hinrichs J, Koftis TV, Loizidou E, Petrovic G, Rodriquez M, Sarlah D, Zou N. J. Am. Chem. Soc. 2006;128:2244–2257. doi: 10.1021/ja0547477. Nicolaou KC, Chen DY-K, Li Y, Uesaka N, Petrovic G, Koftis TV, Bernal F, Frederick MO, Govindasamy M, Ling T, Pihko PM, Tang W, Vyskocil S. J. Am. Chem. Soc. 2006;128:2258–2267. doi: 10.1021/ja054748z. Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Tang W, Frederick MO, Chen DY-K, Li Y, Ling T, Yamada YMA. J. Am. Chem. Soc. 2006;128:2258–2267. doi: 10.1021/ja054748z.
- 14.For the total synthesis and structural confirmation of azaspiracids-2 and -3, see Nicolaou KC, Frederick MO, Petrovic G, Cole PM, Loizidou EZ. Angew. Chem. Int. Ed. 2006;45:2609–2615. doi: 10.1002/anie.200600295. Nicolaou KC, Frederick MO, Louzidou E, Petrovic G, Cole KP, Koftis TV, Yamada Y, Yoichi MA. Chem. Asian J. 2006;1:245–263. doi: 10.1002/asia.200600059.
- 15.For the total synthesis of rugolosin, and related natural products, see: Nicolaou KC, Papageorgiou CD, Piper JL, Chadha RK. Angew. Chem. Int. Ed. 2005;44:5846–5851. doi: 10.1002/anie.200502011. Nicolaou KC, Lim YH, Papageorgiou CD, Piper JL. Angew. Chem. Int. Ed. 2005;44:7917–7921. doi: 10.1002/anie.200503678. Nicolaou KC, Lim YH, Piper JL, Papageorgiou CD. J. Am. Chem. Soc. 2007;129:4001–4013. doi: 10.1021/ja0685708. Snider BB, Gao X. J. Org. Chem. 2005;70:6863–6869. doi: 10.1021/jo0508898.
- 16.Nicolaou KC, Sarlah D, Shaw DM. Angew. Chem. Int. Ed. 2007;46:4708–4711. doi: 10.1002/anie.200701552. [DOI] [PubMed] [Google Scholar]
- 17.Chen C-C, Huang Y-L, Ou J-C. J. Nat. Prod. 1993;56:1594–1597. [Google Scholar]
- 18.Hakim EH, Asnizar, Yurnawilis, Aimi N, Kitajima M, Takayama H. Fitoterapia. 2002;73:668–673. doi: 10.1016/s0367-326x(02)00226-5. [DOI] [PubMed] [Google Scholar]
- 19.Fernando MR, Nalinie Wickramasinghe SMD, Thabrew MI, Ariyananda PL, Karunanayake EH. Ethnopharmacol. 1991;31:277–282. doi: 10.1016/0378-8741(91)90012-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang YH, Hou AJ, Chen L, Chen DF, Sun HD, Zhao QS, Bastow KF, Nakanish Y, Wang XH, Lee KH. J. Nat. Prod. 2004;67:757–761. doi: 10.1021/np030467y. [DOI] [PubMed] [Google Scholar]
- 21.Boonlaksiri C, Oonanant W, Kongsaeree P, Kittakoop P, Tanticharoen M, Thebtaranonth Y. Phytochemistry. 2000;54:415–417. doi: 10.1016/s0031-9422(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, Hou AJ, Chen L, Chen DF, Sun HD, Zhao QS, Bastow KF, Nakanish Y, Wang XH, Lee KH. J. Nat. Prod. 2004;67:757–761. doi: 10.1021/np030467y. [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Hou AJ, Chen DF, Weiller M, Wendel A, Staples RJ. Eur. J. Org. Chem. 2006;15:3457–3463. [Google Scholar]
- 24.For related formal cycloaddition reactions involving a benzopyran and a prenyl group, see: Mondal M, Puranik VG, Argade NP. J. Org. Chem. 2007;72:2068–2076. doi: 10.1021/jo0624344.; for a related formal [2+2] cycloaddition reaction involving an allylic cation and a benzopyran, see: Kurdyumov AV, Hsung RP. J. Am. Chem. Soc. 2006;128:6272–6273. doi: 10.1021/ja054872i.; The [2+2] cycloaddition chemistry of allenes and ketenes has been studied in great detail, see: Ohno H, Mizutani T, Kadoh Y, Aso A, Miyamura K, Fujii N, Tanaka T. J. Org. Chem. 2007;72:4378–4389. doi: 10.1021/jo0700528. Murakami M, Matsuda T. In: Modern Allene Chemistry. Krause N, Hashmi ASK, editors. Vol. 2. Weinheim: Wiley-VCH; 2004. pp. 727–815. Wang X, Houk KN. J. Am. Chem. Soc. 1990;112:1754.
- 25.For a preliminary communication of this work, see: Nicolaou KC, Lister T, Denton RM, Gelin CF. Angew. Chem. Int. Ed. 2007 doi: 10.1002/anie.200702363.
- 26. Godfrey JD, Jr, Mueller RH, Sedergran TC, Soundararajan N, Colandrea V. Tetrahedron Lett. 1994;35:6405–6408.. This copper-catalyzed protocol was found to be superior to procedures involving in situ generation of dimethylpropynyltrilfate.
- 27.Prepared from the commercially available acid, see: Wang Q, Huang Q, Chen B, Lu J, Wang H, She X, Pan X. Angew. Chem. Int. Ed. 2006;45:3651–3653. doi: 10.1002/anie.200600006.
- 28.For other synthetic applications of the microwave-promoted Claisen rearrangement, see: Davis CJ, Moody CJ. Synlett. 2002;11:1874–1876. Davis CJ, Hurst TE, Jacob AM, Moody CJ. J. Org. Chem. 2005;70:4414–4422. doi: 10.1021/jo050336x. Durand-Reville T, Gobbi LB, Gray BL, Ley SV, Scott JS. Org. Lett. 2002;4:3847–3850. doi: 10.1021/ol0201557.
- 29.For Wittig approaches to oxygenated stilbenes, see: Harrowven DC, Pascoe DD, Guy IL. Angew. Chem. Int. Ed. 2007;46:425–428. doi: 10.1002/anie.200603538. Li W, Li H, Li Y, Hou Z. Angew. Chem. Int. Ed. 2006;45:7609–7611. doi: 10.1002/anie.200603097. Gao M, Wang M, Miller KD, Sledge GW, Hutchins GD, Zheng Q-H. Bioorg. Med. Chem. Lett. 2006;16:5767–5772. doi: 10.1016/j.bmcl.2006.08.088.; for an interesting alternative to the Wittig reaction, see: (d) An interesting alternative approach to stilbenes was recently described, see: Robinson JE, Taylor RJK. Chem. Commun. 2007:1617–1619. doi: 10.1039/b702411h.
- 30.The use of 3,5-bis(trifluoromethylphenylsulfones) in the Julia–Kocienski olefination with aromatic aldehydes has been reported, see: Alonso DA, Fuensanta M, Najera C, Varea M. J. Org. Chem. 2005;70:6404–6416. doi: 10.1021/jo050852n.
- 31.Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26–28. [Google Scholar]
- 32.CCDC-648446 contains the supplementary crystallographic data for this paper. This data can be obtained free of charge via www.ccdc.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB21EZ, UK; fax: (+44) 1223-336-033; or deposit@ccdc.cam.co.uk).
- 33.A Discovery System model number 908005 was used for all microwave reactions.
- 34.(a) Nicolaou KC, Pfefferkorn JA, Cao G-Q. Angew. Chem. Int. Ed. 2000;39:734–739. [PubMed] [Google Scholar]; (b) Nicolaou KC, Cao G-Q, Pfefferkorn JA. Angew. Chem. Int. Ed. 2000;39:739–743. [PubMed] [Google Scholar]; (c) Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao G-Q, Barluenga S, Mitchell HJ. J. Am. Chem. Soc. 2000;122:9939–9953. [Google Scholar]
- 35.We are grateful to Dr. Ai-Jun Hou for kindly providing the 1H and 13C NMR spectra of natural artochamins F, H, I, and J for comparison purposes.
- 36.(a) Woodward RB, Hoffmann R. Angew. Chem. Int. Ed. 1969;8:781–853. [Google Scholar]; (b) Woodward RB, Hoffman R. The Conversvation of Orbital Symmetry. New York: Academic Press; 1970. [Google Scholar]; (c) Fleming I. Frontier Orbitals and Organic Chemical Reactions. Wiley: Chichester; 1976. [Google Scholar]; (d) Houk KN, Yi L, Evanseck JD. Angew. Chem. Int. Ed. Engl. 1992;31:682. [Google Scholar]; (e) Houk KN, Gonzàlez J, Li Y. Acc. Chem. Res. 1995;28:81. [Google Scholar]
- 37.Eicken C, Krebs B, Sacchettini JC. Current Opin. Struct. Biol. 1999;9:677–683. doi: 10.1016/s0959-440x(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 38.Saeed M, Rogan E, Cavalieri E. Tetrahedron Lett. 2005;46:4449–4451. [Google Scholar]
- 39.Kelly JW, Robinson PL, Evans SA. J. Org. Chem. 1986;51:4473–4475. [Google Scholar]
- 40.Lagier CM, Scheler U, Mc George G, Gonzalez M, Alejandro CO, Harris RK. J. Chem. Soc. Perkin Trans. 1996;2:1325–1329. [Google Scholar]
- 41.Bidan TA. Russian J. Gen. Chem. 2001;71:1545–1546. [Google Scholar]
- 42.Cardona ML, Fernandez MI, Garcia MB, Pedro JR. Tetrahedron. 1986;42(10):2725–2730. [Google Scholar]
- 43.Berkowitz DB, Smith MK. J. Org. Chem. 1995;60:1233–1238. doi: 10.1021/jo00110a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh SB, Pettit GR. J. Org. Chem. 1989;54(17):4105–4114. [Google Scholar]
- 45.Padwa A, Lipka H, Watterson SH, Murphree SS. J. Org. Chem. 2003;68:6238–6250. doi: 10.1021/jo0345796. [DOI] [PubMed] [Google Scholar]
- 46.de Fátima Â, Kohn LK, Antônio MA, de Carvalho JE, Pilli RA. Bioorg. Med. Chem. 2005;13:2927–2933. doi: 10.1016/j.bmc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Olszewski JD, Marshalla M, Sabat M, Sundberg RJ. J. Org. Chem. 1994;59:4285–4296. [Google Scholar]