Abstract
A 14-membered macrocycle with an allene and a furan strategically located at across the ring from each other is synthesized using an allene ring closing metathesis reaction. Upon treatment of the macrocycle with a catalytic amount of Pd(OAc)2 and other additives, the first transannular [4+3] cycloaddition occurred to yield 37% of a tetracyclic compound containing the ABC ring structure of the natural products cortistatins.
Carbocyclic seven-membered rings appear in a variety of natural products and have provided a strong impetus for the development of new synthetic methodology. The potent cytostatic natural product cortistatin A, which contains a center oxabicyclo[3.2.1] octene ring system (Scheme 1), further underscores the significance of the development of synthetic methodologies in this area. The intermolecular version of the [4+3] cycloaddition reaction has been widely investigated for over three decades.1–3 The intramolecular versions of [4+3] cycloadditions have also been investigated although not as widely as its intermolecular counterpart.4–6 To the best of our knowledge, unlike the Diels-Alder reaction,7–9 the transannular [4+3] cycloaddition variant has not been reported. In this communication, we report our successful implementation of the first transannular [4+3] cycloaddition reaction to the synthesis of the ABCD ring carbon frame of the cortistatins.
Scheme 1.
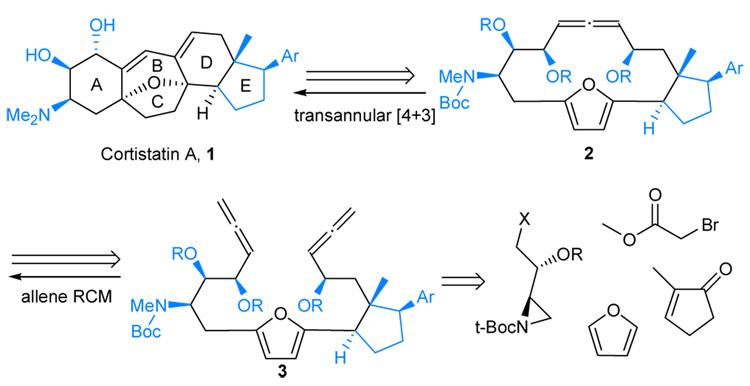
Retrosynthetic analysis for cortistatin A. The structures in black are synthesized
The cortistatins contain a central oxabicyclo[3.2.1]-octene ring system. The first synthesis of cortistatin A was recently reported starting from a steroid precursor, prednisone.10 Our retrosynthetic analysis in Scheme 1 indicates that a transannular [4+3] cycloaddition should provide an efficient synthetic route to the polycyclic structure frame of cortistatins by forming four rings in one transformation.
In order to verify the feasibility of the transannular approach, our plan is to first synthesize a simple macrocycle containing both a furan moiety and an oxy-allyl cation precursor function. Common functional groups for generating oxyallyl cations are α-halogenated ketones.1,2,4 However, at least one example was reported in which an allene oxide functional group was implicated in a [4+3] cycloaddition reaction.11 In addition, nitrogen-stabilized oxyallyl cations can be generated in situ via epoxidations of allenamides, which can undergo inter- and intramolecular [4+3] cycloadditions with dienes.12–14 Considering the advances in the chemistry of ring closing metathesis (RCM),15,16 and a report from Janssen and Krause on allene RCM reactions,17 the allene function was chosen as the functional equivalent to an allyl cation in our initial plan.
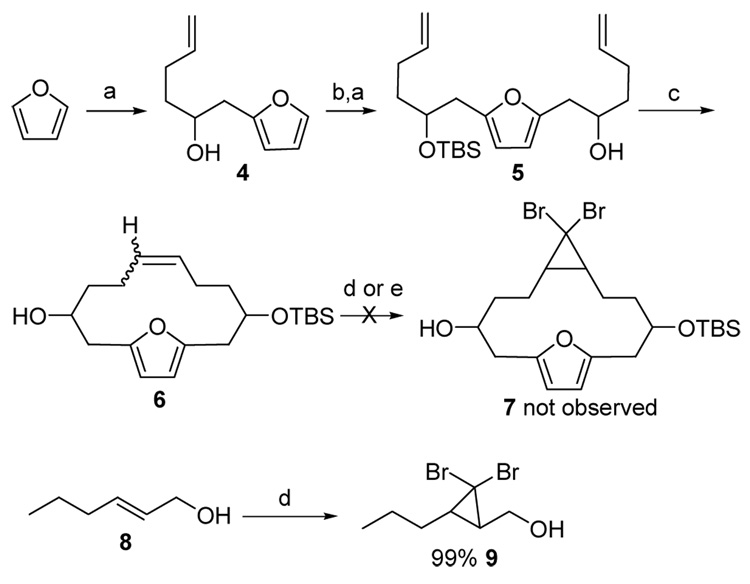
The desired macrocyclic precursor to our planned transannular [4+3] cycloaddition is required to contain both a furan and an allene unit. Our initial plan was to generate the corresponding macrocyclic furanoalkene followed by the addition of a dihalocarbene to the double bond and then base mediated allene formation.17 This plan started with the reaction between the lithiated furan and the commercially available 1,2-epoxy-5-hexene, Scheme 2, to generate the alcohol 4. After the protection of the secondary alcohol with a TBS ether, a repetition of the first step afforded the diene 5. RCM reaction was carried out using Grubbs’ first generation catalyst to afford the macrocycle 6 in 56% yield. However, the planned dibromocarbene addition reactions fail to yield the desired dibromocyclopropane 7 despite the high yield of dibromocyclopropane 9 from the simpler hexenol 8 under the same conditions.18 The total destruction of the starting material suggests competing reactions had taken place between the furan ring and the electrophilic reagents. The employment of the Seyferth reagent19 did not produce the desired product either, Scheme 2.
Scheme 2.
Initial attempted synthesis of the macrocyclic allene
Key: a) (i) BuLi, THF, −78 C; (ii) 1,2-epoxy-5-hexene, 57%; b) TBSCl, imid., DMF, rt, 6h, 90%; c) Grubbs catalyst 1st gen. CH2Cl2, reflux, 19h, 56%; d) CHBr3, Bn(Et)3NCl, 50% aq. NaOH, e) PhHgCBr3
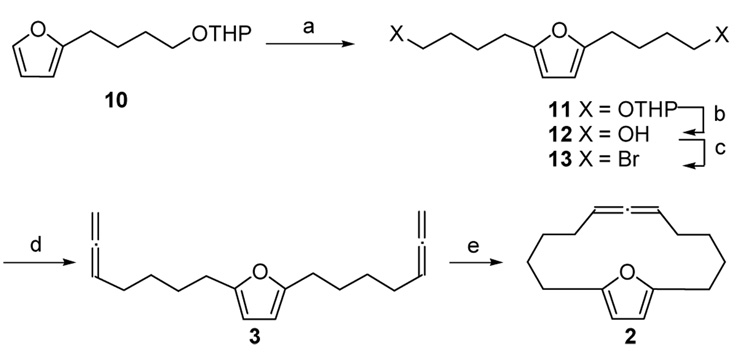
To accomplish our goal of examining the transannular [4+3] cycloaddition, we needed a rapid entry to the macrocyclic precursor. We turned our attention to preparing the allene function directly and form the macrocyclic allene using an allene RCM reaction, Scheme 3.17 This plan started with the known monoalkylated furan 10,20 which was lithiated and alkylated with 2-(4-Bromo-butoxy)-tetrahydropyran to give the bis THP ether 11. The removal of the THP protecting group from 11 followed by treatment of the corresponding diol 12 with CBr4 and Ph3P produced dibromide 13. Allenylation of 13 with lithiated allene afforded diallene 3. Finally RCM reaction using Grubbs’ first generation catalyst under high dilution condition gave macrocycle 2 in 47% yield.
Scheme 3.
Synthesis of macrocycle 2
Key: a) (i) BuLi, THF, −78 °C; (ii) 2-(4-Bromo-butoxy)-tetrahydropyran, 87%; b) p-TsOH, MeOH, rt, 6h, 91%; c) CBr4, Ph3P, CH2Cl2, 0 °C, 90%; d) allenyl lithium, THF, HMPA, −78 °C, 2h, 76%; e) Grubbs catalyst (1st generation), 47%.
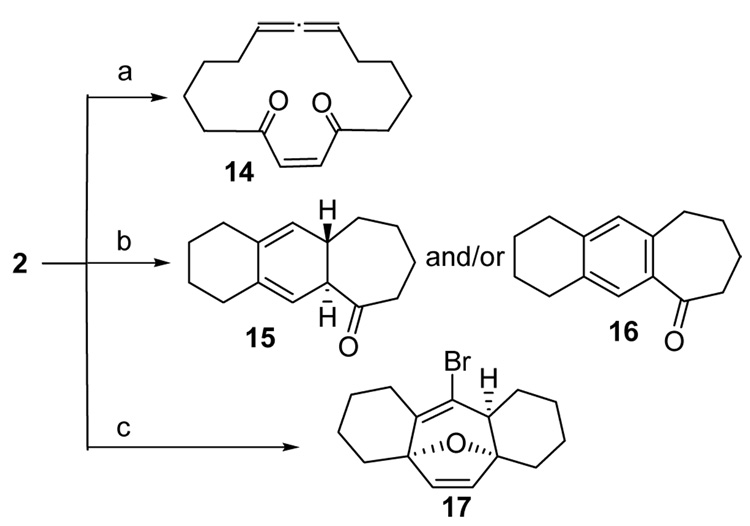
With macrocyclic allene 2 in hand, the stage was set for the transannular [4+3] cycloadditon. Although furans are reactive to oxidants,21 Tanis has reported the epoxidation of trisubstituted double bonds in the presence of furan rings.22 Prompted by the examples of [4+3] cycloadditions involving the oxidation of allenamides,23 dimethyldioxirane (DMDO) was first tried. The reaction of macrocycle 5 with DMDO in acetone was completed in two hours at rt. to give a single product in 83% yield, which was identified as the macrocyclic diketone 14, Scheme 4. The disubstituted furan ring is apparently much more reactive towards DMDO than the allene function. Next we tried the Jacobsen epoxidation conditions because an α,β-unsaturated ester was transformed into α,β-epoxyester under these conditions.24 The same diketone was obtained.
Scheme 4.
Conditions to obtain macrocyclic diketone 14, tricyclic ketones 15/16, and tetracyclic diene 17
Key: Reagents and conditions: a) DMDO, acetone, CH2Cl2, 2h, 83%; b) PtCl2, toluene, 80%; c) 10% Pd(OAc)2, 5 eq. LiBr, 2 eq. Cu(OAc)2, 1.2 eq. K2CO3, CH3CN, 37%.
During our exploration of suitable conditions for the transannular [4+3] cycloaddition reaction, Mascarenas and coworkers reported a platinum-catalyzed intramolecular [4+3] cycloaddition between dienes and allenes.25 Unfortunately only ketones 15 and/or 16 (at high temperature) were isolated when macrocycle 5 was treated with PtCl2 in either CH2Cl2 or toluene. Based on the mechanism proposed by Mascarenas, a transannular [4+3] cycloaddition probably did occur, but was followed by a cationic rearrangement to give the product 15. Aromatization at a higher temperature produced 16, which is a known compound.26
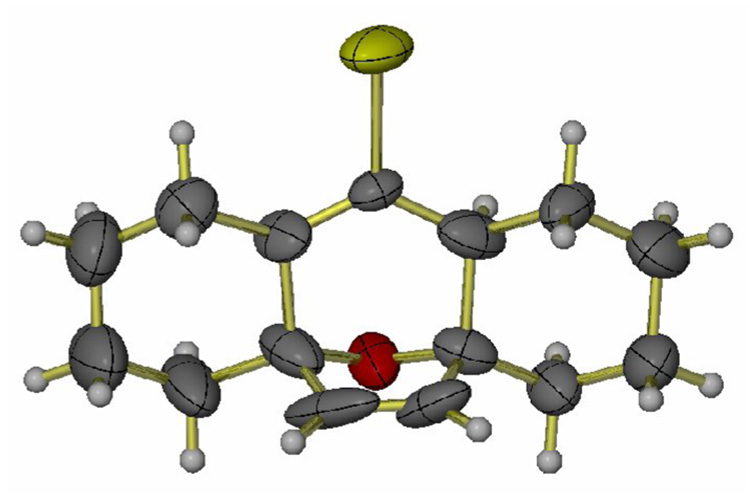
Although the PtCl2-catalyzed reactions of 5 produced disappointing results, the ability of the transition metal to activate the allene in the presence of a furan led us to the work by Backvall and coworkers.27 Palladium(II)-catalyzed intramolecular 1,2-additions to allenes substituted with an internal nucleophile were reported.27,28 Although heteroatoms such as oxygen and nitrogen were the internal nucleophiles in the palladium-catalyzed reactions, an allylpalladium intermediate was identified in the reaction. We reasoned that the furan ring in macrocycle 5 may serve as the internal nucleophile and may react with the allylpalladium intermediate to give the desired transannular [4+3] cycloaddition product. To our delight, when macrocycle 5 was treated with 5% Pd(OAc)2 in the presence of LiBr,27 the tetracyclic compound 17 was isolated in 37% yield (Scheme 4, condition c). When 17 was allowed to stand at rt. in a mixed solvent system (EtOAc:hexanes:MeOH=1:49:50) and the solvents were allowed to slowly evaporate, a crystal suitable for X-ray analysis was obtained (Figure 1). The stereochemistry of 17 is consistent with an endo-compact transition state for the cycloaddition.3
Figure 1.
Crystal structure for the tetracyclic compound 17. Carbon: gray; oxygen: red; hydrogen: pale gray; bromine: gold.
The structure of 17 was further confirmed with the following transformations. Selective hydrogenation of one double bond in 17 was achieved under one atmospheric H2 in the presence of 5% Pd/C, Scheme 5. Only the double bond derived from the furan ring in 17 was saturated. The bromine atom in 18 was removed by stirring with LiAlH4 at rt to yield the tetracyclic ring structure 19.
Scheme 5.
Transformation of 17
Key: a) H2, Pd/C, EtOAc, 3h, 96%; b) LiAlH4, THF, rt, 23h, 63%
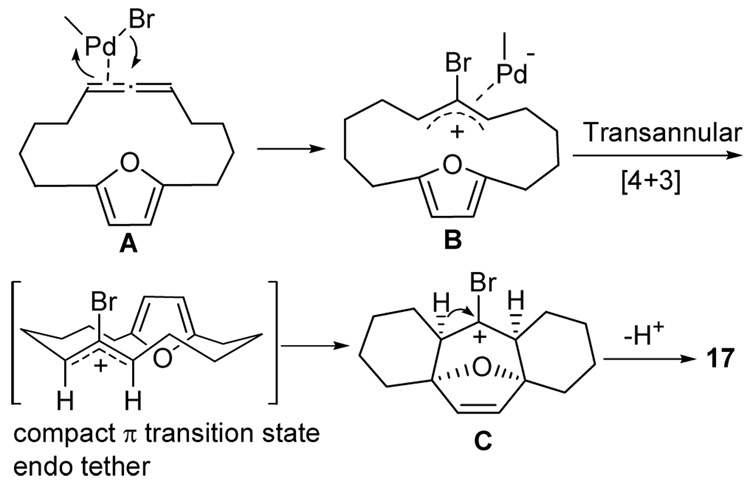
A likely mechanism for the Pd(II) initiated activation of allenes followed by a [4+3] cycloaddition is depicted in Figure 2. Our proposed mechanism is based on Backvall’s original proposal, which involves a (π-allyl)palladium intermediate (B). The initial (allene)-palladium complex A produces a σ-allylpalladium intermediate, which rapidly equilibrates to the corresponding (π-allyl)-palladium intermediate (B). Intramolecular nucleophilic attack by the furan ring is possibly induced by ligand coordination on palladium to give the [4+3] cycloaddition product (C). The stereochemistry of the transannular product 17 is consistent with a compact π transition state with endo tether. The cationic intermediate C loses a proton in the presence of K2CO3 to produce the desired tetracyclic compound 17.
Figure 2.
Proposed mechanism for the formation of 17.
The most important conclusion from this preliminary study is that a (π-allyl)palladium intermediate is able to serve as the three carbon component in a [4+3] cycloaddition reaction. It sould be mentioned that Trost reported intermolecular [4+3] cycloadditions of dienes with Pd-stabilized trimethylenemethane intermediates in 1987.29 The current three carbon fragment has a bromine atom in place of a methylene group. As shown in Scheme 4, different transition metals produce divergent results. This conclusion is consistent with literature precedents.30–32 Furthermore, solvents and co-catalysts or additives have also been shown to play important roles in transition metal catalyzed reactions.30,31 In order to find a set of optimal conditions for the transannular [4+3] cycloaddition reactions, our plan to survey various transition metals and reaction conditions is underway in our laboratories.
Supplementary Material
Supplementary data associated with this article (experimental procedures, NMR spectra) can be found in the online version at
Acknowledgment
Financial support from the National Institutes of Health (GM069441) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noyori R, Hayakawa Y. Organic Reactions. 1983;29:163. [Google Scholar]
- 2.Hoffmann HMR. Angew. Chem.-Int. Edit. Engl. 1984;23:1. [Google Scholar]
- 3.Hartung IV, Hoffmann HMR. Angew. Chem.-Int. Edit. 2004;43:1934. doi: 10.1002/anie.200300622. [DOI] [PubMed] [Google Scholar]
- 4.Fohlisch B, Herter R. Chem. Ber.-Recueil. 1984;117:2580. [Google Scholar]
- 5.Harmata M. Accounts Chem. Res. 2001;34:595. doi: 10.1021/ar000064e. [DOI] [PubMed] [Google Scholar]
- 6.Harmata M. Advanced Synthesis & Catalysis. 2006;348:2297. [Google Scholar]
- 7.Marsault E, Toro A, Nowak P, Deslongchamps P. Tetrahedron. 2001;57:4243. [Google Scholar]
- 8.Mergott DJ, Frank SA, Roush WR. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11955. doi: 10.1073/pnas.0401247101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balskus EP, Jacobsen EN. Science (Washington, DC, U. S.) 2007;317:1736. doi: 10.1126/science.1146939. [DOI] [PubMed] [Google Scholar]
- 10.Shenvi RA, Guerrero CA, Shi JLC-C, Baran PS. J. Am. Chem. Soc. 2008;130:7241. doi: 10.1021/ja8023466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong BS, Chan T-H. Heterocycles. 1977;7:913. [Google Scholar]
- 12.Xiong H, Hsung RP, Berry CR, Rameshkumar C. J. Am. Chem. Soc. 2001;123:7174. doi: 10.1021/ja0108638. [DOI] [PubMed] [Google Scholar]
- 13.Xiong H, Huang J, Ghosh SK, Hsung RP. J. Am. Chem. Soc. 2003;125:12694. doi: 10.1021/ja030416n. [DOI] [PubMed] [Google Scholar]
- 14.Rameshkumar C, Hsung RP. Angew. Chem.-Int. Edit. 2004;43:615. doi: 10.1002/anie.200352632. [DOI] [PubMed] [Google Scholar]
- 15.Gradillas A, Perez-Castells J. Angew. Chem.-Int. Edit. 2006;45:6086. doi: 10.1002/anie.200600641. [DOI] [PubMed] [Google Scholar]
- 16.Grubbs RH. Angew. Chem.-Int. Edit. 2006;45:3760. doi: 10.1002/anie.200600680. [DOI] [PubMed] [Google Scholar]
- 17.Janssen CE, Krause N. Eur. J. Org. Chem. 2005:2322. [Google Scholar]
- 18.Gassman PG, Bonser SM, Mlinaricmajerski K. J. Am. Chem. Soc. 1989;111:2652. [Google Scholar]
- 19.Seyferth D. Accounts Chem. Res. 1972;5:65. [Google Scholar]
- 20.Lin CC, Chen LH, Wu HJ. J. Chin. Chem. Soc. (Taipei) 1991;38:613. [Google Scholar]
- 21.Kraehenbuehl K, Picasso S, Vogel P. Bioorganic & Medicinal Chemistry Letters. 1997;7:893. doi: 10.1016/s0960-894x(98)00722-7. [DOI] [PubMed] [Google Scholar]
- 22.Tanis SP, Herrinton PM. J. Org. Chem. 1983;48:4572. [Google Scholar]
- 23.Huang J, Hsung RP. J. Am. Chem. Soc. 2005;127:50. doi: 10.1021/ja044760b. [DOI] [PubMed] [Google Scholar]
- 24.Deng L, Jacobsen EN. J. Org. Chem. 1992;57:4320. [Google Scholar]
- 25.Trillo B, Lopez F, Gulias M, Castedo L, Mascarenas JL. Angew. Chem.-Int. Edit. 2008;47:951. doi: 10.1002/anie.200704566. [DOI] [PubMed] [Google Scholar]
- 26.Sambaiah T, Li L-P, Huang D-J, Lin C-H, Rayabarapu DK, Cheng C-H. J. Org. Chem. 1999;64:3663. doi: 10.1021/jo9900580. [DOI] [PubMed] [Google Scholar]
- 27.Jonasson C, Horvath A, Backvall JE. J. Am. Chem. Soc. 2000;122:9600. [Google Scholar]
- 28.Dorange I, Lofstedt J, Narhi K, Franzen J, Backvall JE. Chem.-Eur. J. 2003;9:3445. doi: 10.1002/chem.200305056. [DOI] [PubMed] [Google Scholar]
- 29.Trost BM, MacPherson DT. J. Am. Chem. Soc. 1987;109:3483. [Google Scholar]
- 30.Mendez M, Munoz MP, Nevado C, Cardenas DJ, Echavarren AM. J. Am. Chem. Soc. 2001;123:10511. doi: 10.1021/ja0112184. [DOI] [PubMed] [Google Scholar]
- 31.Chianese AR, Lee SJ, Gagne MR. Angew. Chem.-Int. Edit. 2007;46:4042. doi: 10.1002/anie.200603954. [DOI] [PubMed] [Google Scholar]
- 32.Zhang GZ, Catalano VJ, Zhang LM. J. Am. Chem. Soc. 2007;129:11358. doi: 10.1021/ja074536x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article (experimental procedures, NMR spectra) can be found in the online version at