Abstract
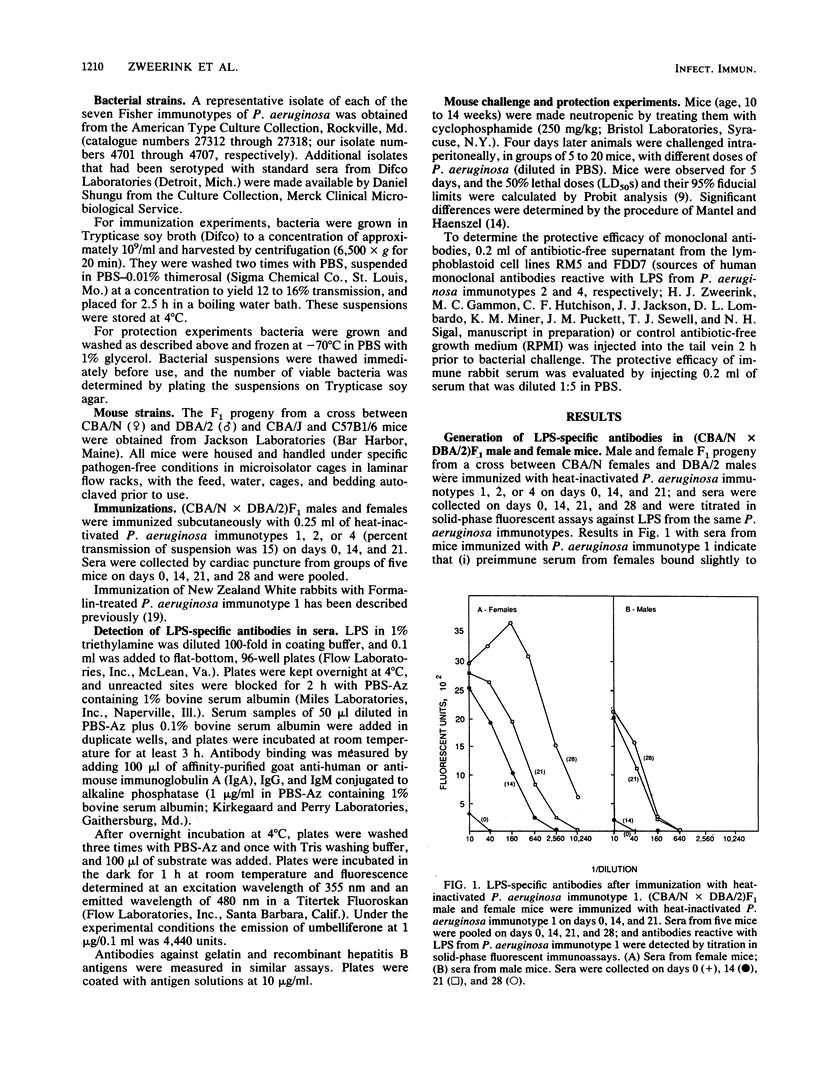
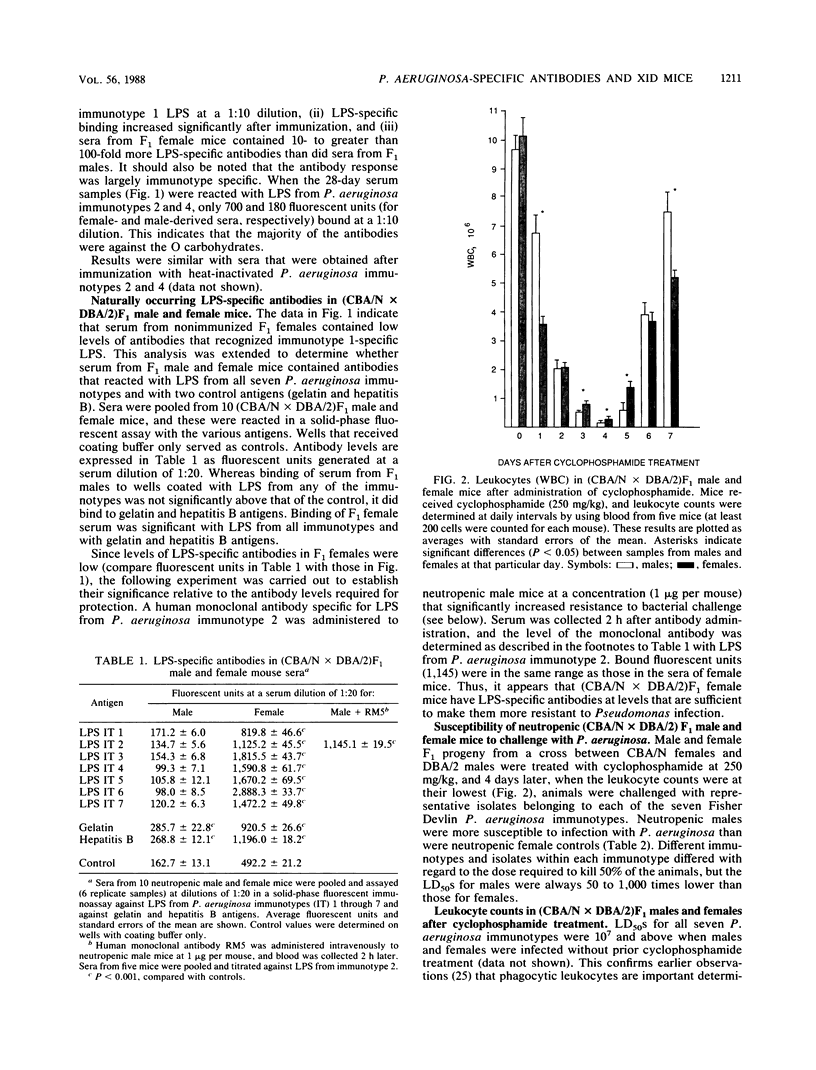
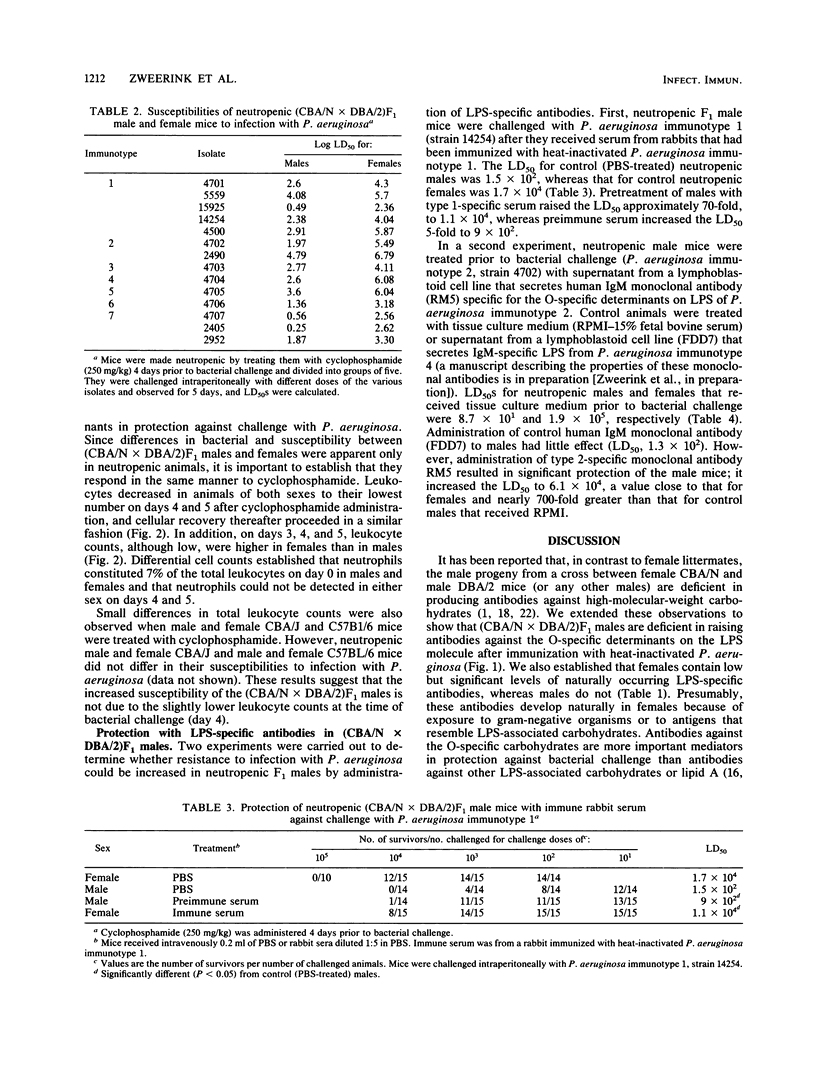
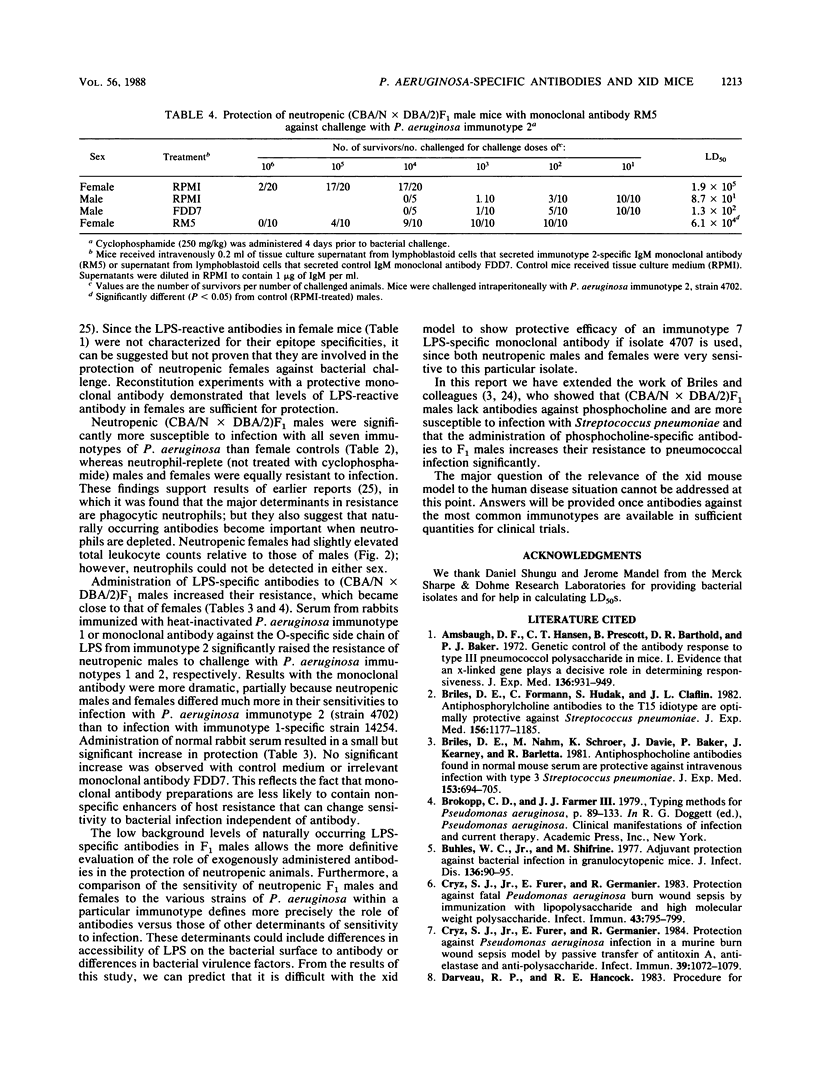
(DBA/N[female] X CBA/2[male])F1 males have been reported to be deficient in producing antibodies against a number of antigens, including carbohydrates (I. Scher, Adv. Immunol. 35:1-71, 1982). We show that F1 male mice, in contrast to females, made less lipopolysaccharide (LPS)-specific antibodies after immunization with heat-inactivated Pseudomonas aeruginosa and had significantly less naturally occurring LPS-specific antibodies. Furthermore, neutropenic males were 50 to 1,000 times more sensitive to challenge with representative isolates belonging to the seven Fisher immunotypes. Administration to neutropenic F1 males of a human monoclonal antibody specific for the O carbohydrates of P. aeruginosa immunotype 2 LPS or administration of serum from rabbits immunized with heat-inactivated P. aeruginosa immunotype 1 raised the level of resistance to bacterial challenge close to that of females. The results show that the X-linked immunodeficient mouse is an excellent model with which to test the protective efficacy of P. aeruginosa-specific monoclonal antibodies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Adjuvant protection against bacterial infection in granulocytopenic mice. J Infect Dis. 1977 Jul;136(1):90–95. doi: 10.1093/infdis/136.1.90. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect Immun. 1984 Mar;43(3):795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. M., Bullen J. J. The effect of passage and iron on the virulence of Pseudomonas aeruginosa. J Clin Pathol. 1972 Jan;25(1):65–68. doi: 10.1136/jcp.25.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. R., Young P. R., Lee S., Dixon J., Zuckerman A. J., McAleer W. J., Lehman E. D. Hepatitis B surface antigen polypeptide micelles from antigen expressed in Saccharomyces cerevisiae. J Virol Methods. 1986 Aug;14(1):25–35. doi: 10.1016/0166-0934(86)90004-2. [DOI] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- MacIntyre S., Lucken R., Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Apr;52(1):76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Metcalf E. S. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981 Apr;126(4):1368–1372. [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Weinblatt A. C., O'Brien A. D. Genetic control of resistance to infection in mice. Crit Rev Immunol. 1982 Jun;3(4):263–330. [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. Characterization of a human monoclonal antibody to lipopolysaccharides of Pseudomonas aeruginosa serotype 5: a possible candidate as an immunotherapeutic agent for infections with P. aeruginosa. J Infect Dis. 1985 Nov;152(5):965–970. doi: 10.1093/infdis/152.5.965. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Yother J., Forman C., Gray B. M., Briles D. E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982 Apr;36(1):184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]



